Abstract
Xanthophyll carotenoids, such as lutein, zeaxanthin and β-cryptoxanthin, may provide potential health benefits against chronic and degenerative diseases. Investigating pathways of xanthophyll metabolism are important to understanding their biological functions. Carotene-15,15’-monooxygenase (CMO1) has been shown to be involved in vitamin A formation, while recent studies suggest that carotene-9’,10’-monooxygenase (CMO2) may have a broader substrate specificity than previously recognized. In this in vitro study, we investigated baculovirus-generated recombinant ferret CMO2 cleavage activity towards the carotenoid substrates zeaxanthin, lutein and β-cryptoxanthin. Utilizing HPLC, LC-MS and GC-MS, we identified both volatile and non-volatile apocarotenoid products including 3-OH-β-ionone, 3-OH-α-ionone, β-ionone, 3-OH-α-apo-10’-carotenal, 3-OH-β-apo-10’-carotenal, and β-apo-10’-carotenal, indicating cleavage at both the 9,10 and 9’,10’ carbon-carbon double bond. Enzyme kinetic analysis indicated the xanthophylls zeaxanthin and lutein are preferentially cleaved over β-cryptoxanthin, indicating a key role of CMO2 in non-provitamin A carotenoid metabolism. Furthermore, incubation of 3-OH-β-apo-10’-carotenal with CMO2 lysate resulted in the formation of 3-OH-β-ionone. In the presence of NAD+, in vitro incubation of 3-OH-β-apo-10’-carotenal with ferret hepatic homogenates formed 3-OH-β-apo-10’-carotenoic acid. Since apo-carotenoids serve as important signaling molecules in a variety of biological processes, enzymatic cleavage of xanthophylls by mammalian CMO2 represents a new avenue of research regarding vertebrate carotenoid metabolism and biological function.
Keywords: Xanthophyll, metabolism, CMO1, CMO2, Apo-carotenoid
1. Introduction
Many epidemiological studies have indicated that increased dietary intake of carotenoids may offer protection against the development of several chronic and degenerative diseases, including cardiovascular disease [1], age-related macular degeneration [2] and certain cancers [3]. There are six major carotenoids that can be routinely found in human plasma and tissues [4, 5], which can be divided into two major structural groups: 1) xanthophylls, which include the oxygenated carotenoids lutein, zeaxanthin, and β-cryptoxanthin; and 2) carotenes, which include hydrocarbon carotenoids that are either cyclized, such as β-carotene or α-carotene, or linear, like lycopene. The vast amount of carotenoid research efforts have focused on the provitamin A carotenoids, which includes β-carotene, α-carotene, and β-cryptoxanthin, due to their ability to be metabolized to the essential nutrient vitamin A. The non-provitamin A carotenoids, which includes lutein, zeaxanthin, and lycopene, cannot be metabolized to vitamin A but have demonstrated potentially significant impact on human health and disease prevention and progression.
Carotenoids are lipophilic polyisoprenoid plant pigments typically containing a series of conjugated double bonds in the central chain of the molecule, making them susceptible to oxidative cleavage. Although any of the conjugated double bonds within the carotenoid molecule can be cleaved, many biologically active apocarotenoids are formed via site-specific cleavage [6, 7]. Oxidative cleavage of carotenoids results in the formation of apocarotenoid metabolites, which may have important biological roles different than their parent compound. The biological significance of carotenoid metabolite generation, especially the differential effects of small and large quantities of oxidative metabolites, has been recently reviewed [8, 9].
Carotenoid cleavage oxygenases (CCOs) mediate the site-specific cleavage of carotenoid substrates, forming important apocarotenoid products. CCOs belong to an ancient and highly conserved family with members in plants, animals and bacteria. The maize 9-cis-epoxycarotenoid dioxygenase Viviparous14 (VP14) was the first CCO to be cloned and characterized [10]. VP14 catalyzes asymmetric cleavage of the 11,12 double bond of neoxanthin and/or violaxanthin forming abscisic acid, which acts as a hormone in plants, promoting senescence and abscission of leaves and dormancy induction in buds and seeds. Sequence homology with VP14 led to the cloning and characterization of the Drosophila carotene-15,15’-monooxygenase (CMO1), which is responsible for vitamin A biosynthesis from β-carotene [11]. CMO1 orthologues have since been cloned and characterized in several species, including mice and humans [12–16]. The presence of at least one unsubstituted β-ionone ring has been recognized as a requisite for cleavage by CMO1 [12], limiting cleavage to provitamin A carotenoid substrates such as β-carotene and β-cryptoxanthin and identifying central cleavage via CMO1 as the major pathway leading to vitamin A formation. Indeed, no cleavage activity was detected when lycopene or zeaxanthin was used as a substrate [14].
An alternative metabolic pathway for β-carotene, termed the excentric cleavage pathway, was proposed [17]. Existence of the excentric cleavage pathway was confirmed by the isolation of a second carotenoid cleaving enzyme, termed carotene-9’,10’-monooxygenase (CMO2), which has been identified in humans, mice and ferrets [18, 19]. CMO2 cleaves β-carotene at the 9’,10’ double bond forming β-apo-10’-carotenal and β-ionone. β-Apo-10’-carotenal can be further oxidized to β-apo-10’-carotenoic acid [20], which can be shortened to retinoic acid via a mechanism similar to β-oxidation [21]. This suggests excentric cleavage of β-carotene as an alternative pathway in retinoic acid formation [22]. The contribution of CMO2 in vitamin A biosynthesis remains a controversial issue [23]. Recently a quantitative trait locus (QTL) associated with yellow adipose tissue and milk color was identified to contain a premature stop codon mutation in the bovine CMO2 gene. This results in increased adipose, serum, and milk β-carotene concentrations and decreased liver retinol compared to wild types, yet no developmental or physiologic abnormalities in CMO2 mutants were observed [24, 25]. In addition to β-carotene, CMO2 cleaves cis-isomers of lycopene generating apo-10’-lycopenoids [18], which have displayed unique biological activities in vitro and in vivo [26–28]. A series of apo-lycopenals, including apo-10’-lycopenal, have recently been identified in human plasma, yet whether they originate from enzymatic cleavage or from consumption of apo-lycopenal-containing fruits and vegetables is unclear [29]. The cleavage of both β-carotene and lycopene suggests that CMO2 may accept a wider variety of substrates than previously recognized [18, 19].
Recent genetic analyses have provided further evidence that CMO2 plays a broader role in carotenoid metabolism. A single nucleotide polymorphism (SNP) in the sheep (Ovis aries) CMO2 gene, resulting in a premature stop codon, was shown to be associated with an increase in adipose carotenoid accumulation [30]. Lutein and flavoxanthin are the predominant carotenoids accumulated within sheep adipose tissue [31]. A SNP in the CMO2 gene was also identified in domestic chickens (Gallus gallus) leading to lower tissue specific expression of CMO2 in the skin [32]. The decrease in skin CMO2 leads to the yellow skin pigmentation of domestic chickens, suggesting a decreased ability to cleave the xanthophylls lutein and zeaxanthin, which are the major accumulated carotenoids in chicken skin [33]. However, no biochemical evidence is available demonstrating cleavage of hydroxy carotenoids by CMO2, in particular the xanthophylls lutein and zeaxanthin, which are concentrated in human macula and retina of the eye and provide potential protection against age-related macular degeneration [2, 34].
In the present study, using recombinant ferret CMO2, we identified cleavage products via HPLC, LC-MS and GC-MS and characterized the kinetic properties of CMO2 using zeaxanthin, lutein and β-cryptoxanthin as substrates in vitro. Furthermore, we identified and characterized the oxidation of the 3-OH-β-apo-10’-carotenal to 3-OH-β-apo-10’-carotenoic acid in vitro.
2. Materials and Methods
2.1 Materials
(3R)-β-Cryptoxanthin (96.8%), zeaxanthin (96%), β-apo-10’-carotenal, and 3-hydroxy(OH)-β-apo-10’-carotenal were kindly provided by BASF Inc., Ludwigshafen, Germany. (3R,3’R)-Lutein (96.4%) was purchased from Chromadex, Inc. (Irvine, CA). β-Ionone (96.4%) was purchased from Spectrum Chemical (Gardena, CA). Ammonium acetate was purchased from Sigma Chemical (St. Louis, MO). HPLC-grade solvents were obtained from J. T. Baker Chemical (Philipsburg, NJ) and Sigma-Aldrich Chemical (Milwaukee, WI). Substrate stock solutions were prepared in anhydrous tetrahydrofuran (THF) under red light and stored at −80°C until use.
2.2 Expression of Ferret CMO2 in Sf9 cells
The complete ferret CMO2 coding sequence (Genbank™ AY527150.1) was subcloned from pRcHA-fCMO2 plasmid into the baculovirus expression vector pFastBac1 (Invitrogen) by PCR using the BamHI/SpeI site as previously described [18]. Briefly, Spodoptera frugiperda 9 (Sf9) cells were tansfected with the ferret CMO2 bacmid DNA, and the recombinant ferret CMO2 viral titer was amplified by propagation in Sf9 cells. Flasks (225 cm2) were seeded and infected at a multiplicity of infection (MOI) of 10. Four days post-infection, cell pellets were collected, centrifuged, washed 1X with cold PBS, and stored at −80°C until further use. Expression of ferret CMO2 protein in Sf9 cells was confirmed by both Coomassie Blue staining and Western Blotting analysis with a purified polyclonal antibody against ferret CMO2 [18].
2.3 Enzymatic Kinetic Assay
All procedures of enzyme preparation were conducted on ice. The Sf9 cell pellets containing either uninfected or infected recombinant ferret CMO2 baculovirus were suspended in 0.5 ml of lysis buffer (20 mM Tris-HCl; pH 8.0, 150 mM KCl, 0.1% Tween 20) and homogenized in a Potter-Elvehjem homogenizer for 60s. The homogenates were clarified by centrifugation at 10,000 × g for 30 minutes at 4°C. Supernatants were collected and either used immediately for enzymatic assays or stored at −80°C until further use. The substrate aliquots of carotenoids in anhydrous THF (β-cryptoxanthin, lutein, zeaxanthin and 3-OH-β-apo-10’-carotenal) were dried by N2 under red light and subsequently prepared in 4% Tween 40 in acetone, which is again evaporated by N2. The dried substrates were solubilized in buffer (20 mM Tris-HCl; pH 8.5, 150 mM KCl) and sonicated to obtain a clear micellar solution. All enzymatic assays were performed in a final volume of 1 ml containing assay buffer (20 mM Tris-HCl; pH 8.5, 150 mM KCl, 10 µM FeSO4, 3 mM NAD+, 0.3 mM DTT) and ~ 2 mg of enzyme supernatant (or various concentrations as indicated). Mixtures were pre-incubated for 5 min. at 37°C, and the cleavage reaction was initiated by adding 100 µl of substrate (β-cryptoxanthin, lutein or zeaxanthin at various concentrations). After incubation for 30 min. (or various time points as indicated) at 37°C in the dark with gentle shaking, reactions were terminated by addition of 1.5 ml absolute ethanol. Incubation mixtures were extracted 2X with 5 ml hexane:methyl-tert butyl ether (1:1, v/v). The combined extracts were dried by N2 under red light and dissolved in 200 µl ethanol:methyl-tert butyl ether (1:1, v/v) and analyzed by HPLC or LC-MS as described herein.
2.4 HPLC Analysis
A gradient reverse phase HPLC system was used for quantitative analysis of carotenoids and their polar metabolites. Briefly, the gradient reverse phase HPLC system consists of a Waters 2695 separations module and a Waters 2998 photodiode array detector. The enzymatic cleavage products of zeaxanthin, lutein and β-cryptoxanthin were analyzed on a reverse phase C18 column (4.6 × 250 mm, 5 µM) (Vydac 201TP54, Grace Discovery Sciences, Inc.) fitted with a Pecosphere C18 guard column (PerkinElmer, CT.) with a flow rate of 1.00 ml/min. The gradient procedure is as follows: 1) 50% solvent A (100% Water, 50mM ammonium acetate) and 50% solvent B (100% Acetonitrile) for 4 minutes followed by a 6 minute linear gradient to 20% solvent A and 80% solvent B; 2) a 9-minute hold followed by a 11-min linear gradient to 90% Solvent B and 10% Solvent C (Acetonitrile, Tetrahydrofuran, H2O, 50 mM ammonium acetate, 50:44:6, v/v/v); 3) a 3-min hold followed by an 11-min linear gradient to100% solvent C; 4) a 4-min hold followed by a 10 minute linear gradient to 50% solvent A and 50% solvent B; and 5) a 12-min hold on 50% solvent A and 50% solvent B before the next injection. The Waters 2998 programmable photodiode array detector was set at 450 nm and 296 nm for carotenoid metabolite and related volatile analysis. Carotenoid metabolites were identified on the basis of relative retention times (RT) and by comparison of spectra with those of pure standards.
2.5 LC-MS Analysis
The LC system consisted of an Agilent 1100 quaternary pump and UV-Vis diode array detector (Agilent Technologies, Palo Alto, CA). The column was a Vydac C18 201TP54 column (4.6 × 250 mm, 5 µM) (Grace Discovery Sciences, Inc.). HPLC-MS separations were monitored at 450 nm. Mass spectra data was obtained with an Agilent 1100 MSD equipped with an atmospheric pressure chemical ionization (APCI) ion source operating in positive ion mode. The quadrupole was scanned m/z 100 – 600. The capillary was 2500 V and the temperature of the drying gas (N2) was 350 °C at a flow rate of 9.0 L/min. The corona discharge voltage was optimized resulting in a current of 6.0 µA. The chromatographic conditions were similar to those used for HPLC analysis.
2.6 GC-MS Analysis
Cleavage assays were extracted and 50 µl was subjected to HPLC analysis as indicated above. HPLC flow-through containing peaks identified as putative volatile cleavage products were collected on ice and extracted twice with hexane:methyl-tert butyl ether (1:1, v/v). The combined extracts were dried by N2 under red light. The residue was reconstituted in diethyl ether, dried over anhydrous sodium sulfate, concentrated to dryness by N2, and dissolved in hexane for injection. GC-MS analysis were performed with an Agilent 5973N Mass Selective Detector coupled with an Agilent 6890 Series GC Detector. The oven was kept at 40 °C for 3 min and then increased to 250 °C at 5 °C min−1 with a helium carrier gas flow of 1.5 mL/min. One microliter of each sample was injected on a Supleco SAC-5 (Sigma-Aldrich, St. Louis, MO) capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Mass spectra were recorded in electron impact (EI) ionization mode at 70 eV. Identifications were carried out by comparison of EI mass data with published data or with data from authentic standards.
2.7 Incubation of 3-OH-β-apo-10’-carotenal with the S9 Fraction of Ferret Liver
Ferret liver (~1g) was homogenized with 5 ml of cold Tris Buffer A (20 mM Tris-HCl, 150 mM KCl, 0.5 mM DTT, pH 8.0) using an Ultra Turrax T8 Homogenizer (IKA, Germany). The homogenate was centrifuged at 9000 × g at 4 °C for 30 min and the supernatant is collected. The supernatant represented a mixture of microsomes and cytosol, containing aldehyde dehydrogenase [18, 35]. Reactions were carried out in a final volume of 1 ml of Buffer A containing 4 mg of S9 liver homogenates with or without added cofactors (3 mM NAD+). The mixtures were pre-incubated for 5 min at 37 °C before addition of substrates. Incubations were initiated by addition of 20 µl of 3-OH-β-apo-10’-carotenal as the substrate at the indicated concentrations. After 1 hr of shaking in a 37 °C water bath under red light, the incubations were terminated by addition of 1.5 ml of ethanolic KOH (20 mM KOH). Carotenoid metabolites were extracted twice with 5 ml hexane, combined and dried by N2. The remaining aqueous fraction was acidified by addition of 120 µl of 4 M HCL and extracted twice with 5 ml hexane and combined with the previous extraction. The dried fraction was dissolved in 100 µl ethanol:methyl-tert butyl ether (1:1, v/v) and subjected to HPLC and LC/MS analysis as described.
2.8 Solid phase extraction (SPE) of apo-carotenoic acid derivatives
To facilitate the positive identification of oxidative apo-carotenoic acid derivatives, acidic extracts were applied to an aminopropyl SPE column [36]. Briefly, 3-hydroxy-β-apo-10’-carotenal was incubated with ferret liver S9, extracted, and dried by N2 as before. The dried extracts were reconstituted in chloroform and applied to pre-conditioned aminopropyl SPE columns (Strata NH2, Phenomenex, USA) and placed in the Vac Elut apparatus and washed twice with hexane. A chloroform:isopropanol (2:1, v/v) mixture was next applied to the column, eluting the neutral lipid layer, which was discarded. 3-OH-β-apo-10’-carotenoic acid was eluted with diethyl ether (containing 5% acetic acid), collected and dried under N2, reconstituted in 100 µl ethanol:methyl-tert butyl ether (1:1, v/v) and subjected to HPLC and LC/MS analysis as described.
3. RESULTS
3.1 Cleavage of Zeaxanthin, Lutein, β-Cryptoxanthin and using Recombinant Ferret CMO2 and Identification of Cleavage Products
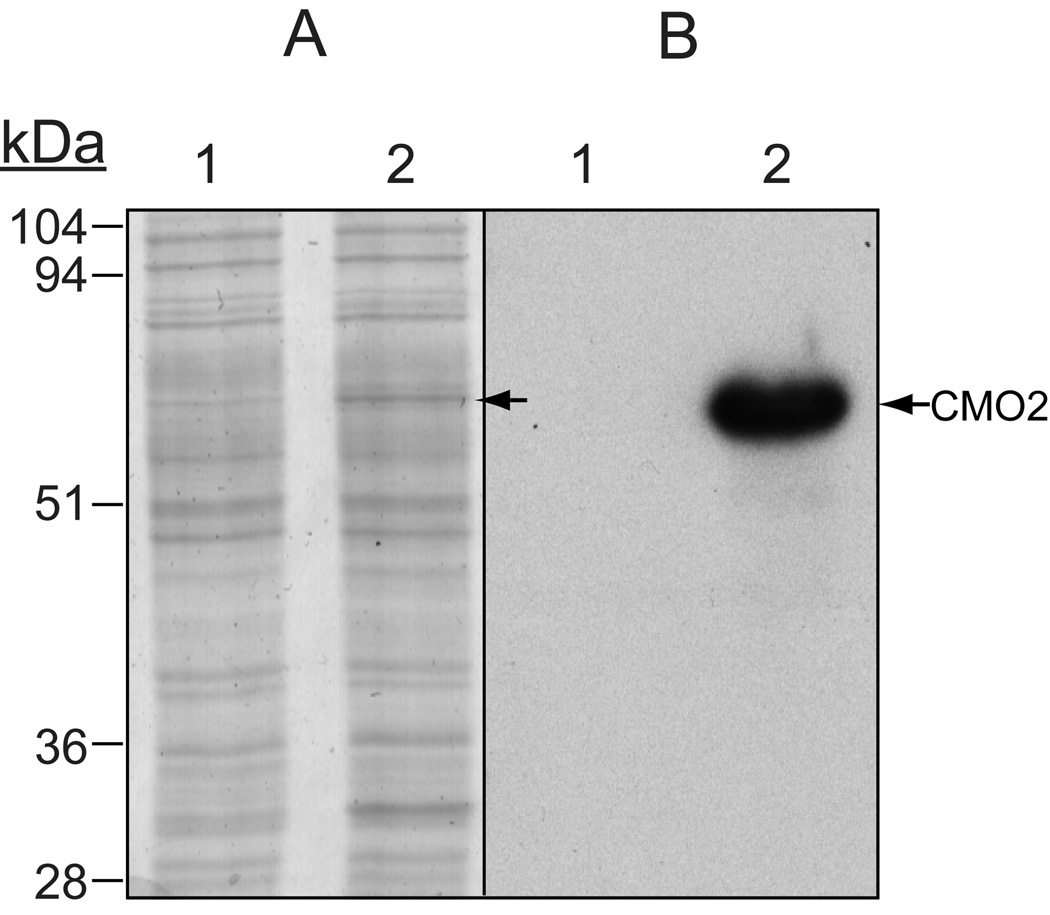
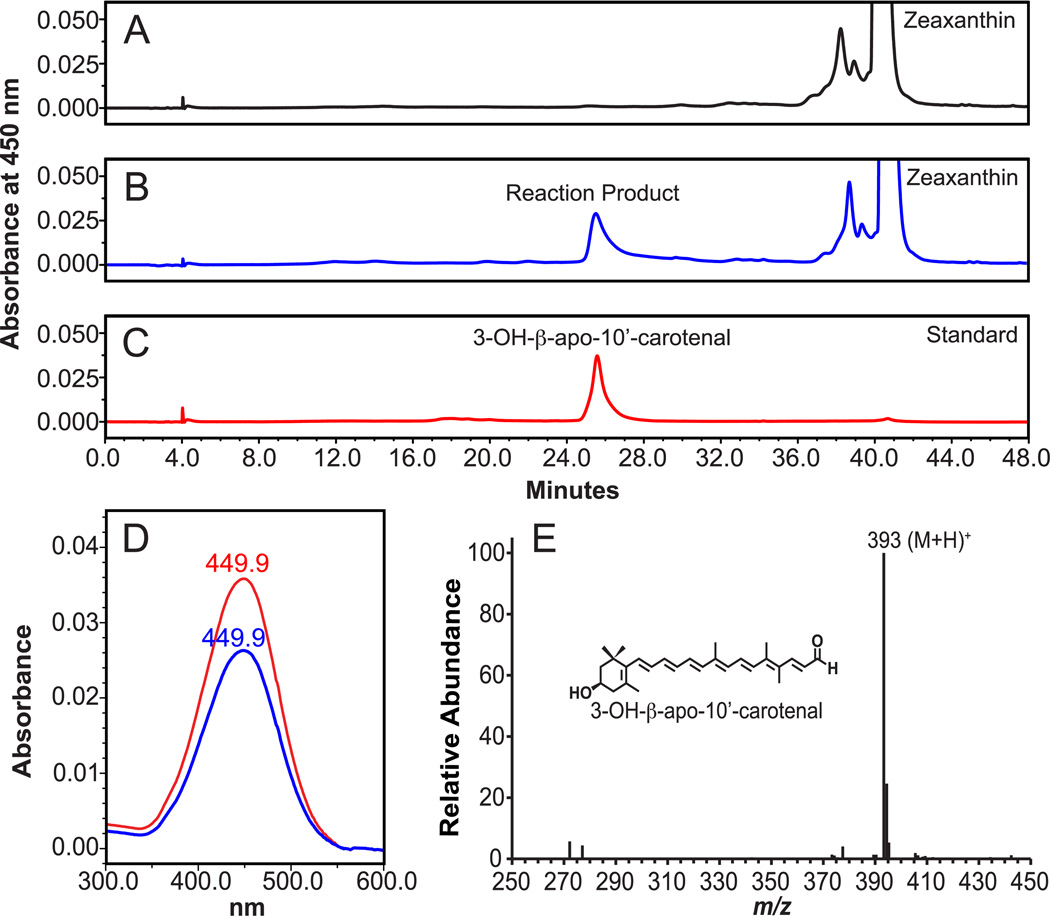
To confirm expression of the recombinant ferret CMO2 in the baculovirus system, we demonstrated, using both Coomassie Blue staining and immunobloting with a polyclonal-CMO2 antibody (Figure 1), expression of a protein of approximately 60 kDa, which is the predicted molecular mass of ferret CMO2 protein [18]. To demonstrate enzymatic cleavage activity, insect cell homogenates infected with ferret CMO2 baculovirus were incubated with zeaxanthin, lutein or β-cryptoxanthin at varying concentrations. For zeaxanthin, HPLC separation with monitoring at 450 nm revealed the production of a new peak in the incubations containing CMO2-infected Sf9 cell lysates. The RT (25.5 min.) and absorption spectrum (UVmax 449.9 nm) of the new peak matched the RT (25.5 min.) and absorption spectrum (UVmax 449.9 nm) of the authentic 3-OH-β-apo-10’-carotenal standard (Figure 2). To identify corresponding cleavage products, samples were subjected to LC-MS analyses, and since the system was operated in APCI+ mode, quasimolecular ions generally appeared as (M+H)+ signals. LC-MS analysis of the new peak identified a quasimolecular ion of m/z 393 (M+H)+ (Figure 2), which corresponds to the quasimolecular ion of the authentic 3-OH-β-apo-10’-carotenal standard, confirming the formation of 3-OH-β-apo-10’-carotenal.
Fig 1.
Expression of ferret CMO2 in Sf9 insect cells. Ferret CMO2 was expressed in Sf9 insect cells using a baculovirus system as described under “Experimental Procedures.” The cell lysates from uninfected (lane 1) and ferret CMO2 baculovirus-infected (lane 2) insect cells were boiled in reducing sample buffer and subjected to 10% SDS-PAGE. The protein expression was detected by Coomassie Blue stain (A) and by detection with a CMO2-specific polyclonal antibody after transfer to a polyvinylidene difluoride membrane (B).
Fig 2.
Identification of cleavage products from zeaxanthin by HPLC and LC-MS analysis. Zeaxanthin (20 µM) was incubated with the homogenates from either uninfected (A) or ferret CMO2-baculovirus infected (B) insect cells for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Peaks corresponding to the authentic 3-OH-β-apo-10’-carotenal standard (C) were detected at 450 nm only in the incubation mixture with the homogenates of ferret CMO2-baculovirus infected cells (B), but not in that of the uninfected cells (A). D, spectral analysis of the cleavage product (blue line) of zeaxanthin vs. 3-OH-β-apo-10’-carotenal standard (red line). Both the RT and absorption spectrum of the product matched exactly with that of the 3-OH-β-apo-10’-carotenal standard. LC-MS analysis (E) of the cleavage product showed a base peak of m/z 393, which corresponds to the protonated molecule (M+H)+ of the authentic 3-OH-β-apo-10’-carotenal standard.
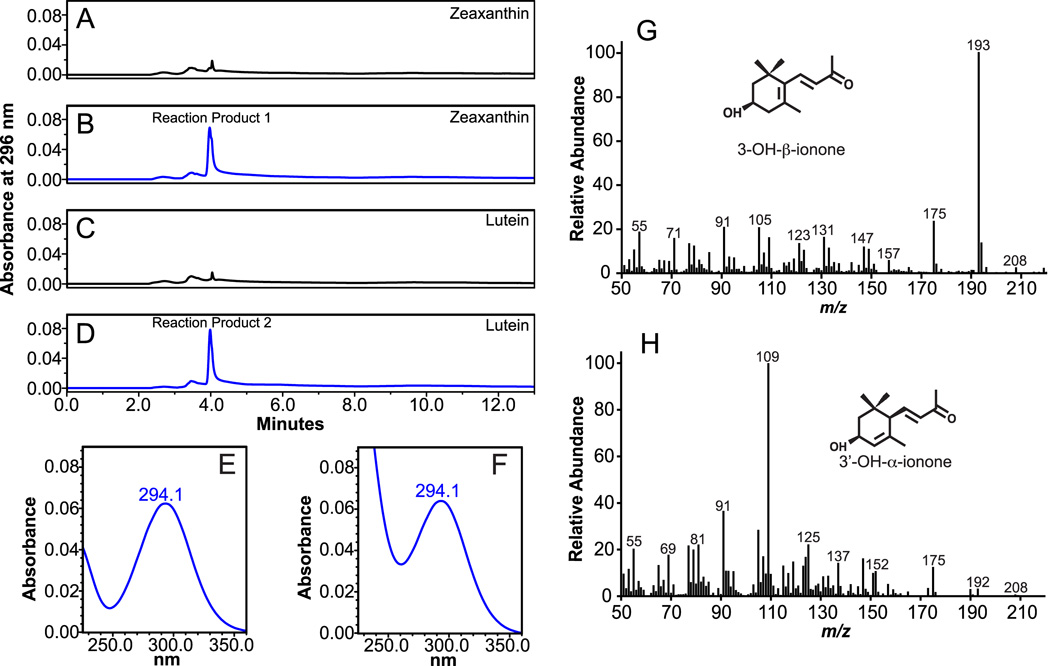
In addition to formation of the 3-OH-β-apo-10’-carotenal product, cleavage of zeaxanthin at 9’,10’ (9,10) double bond results in the formation of the C13 compound 3-OH-β-ionone. HPLC analysis with monitoring at 296 nm revealed the presence of a new peak with a RT of 3.9 min. (Figure 3B) and UVmax of 294.1 nm (Figure 3E), which is similar to published chromatographic data of 3-OH-β-ionone [37]. Due to the absence of a 3-OH-β-ionone authentic standard, the identity of the new peak was investigated by GC-MS analysis after HPLC separation. The collected peak was identified by the presence of the major ions of m/z 193 and m/z 175, with a parent molecular ion of m/z 208 (Figure 3G), which are indicative of 3-OH-β-ionone [38, 39]. Taken together, the analyses clearly demonstrate cleavage of zeaxanthin at the 9’,10’ (9,10) double bond by ferret CMO2, resulting in the formation of 3-OH-β-apo-10’-carotenal and 3-OH-β-ionone (Figure 11A).
Fig 3.
Identification of volatile cleavage products from zeaxanthin and lutein by HPLC and GC-MS analysis. Zeaxanthin (20 µM) and Lutein (20 µM) were incubated with homogenates from either uninfected (A and C) or ferret CMO2-baculovirus infected (B and D) insect cell lysates for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Unknown reaction products 1 and 2 were detected at 296 nm only in the incubation mixtures with ferret CMO2-baculovirus infected cells (B and D) but not in that of the uninfected cells (A and C). Spectral analysis of reaction products 1 (E) and 2 (F) indicated the presence of ionone compounds. GC-MS analysis of reactions product 1 (B) from zeaxanthin cleavage indicated the presence of 3-OH-β-ionone (G) and GC-MS analysis of reaction product 2 (C) from lutein cleavage indicated the presence of both 3-OH-bionone (G) and 3’-OH-α-ionone (H). The EI mass spectra were matched to previously published spectra [38, 39].
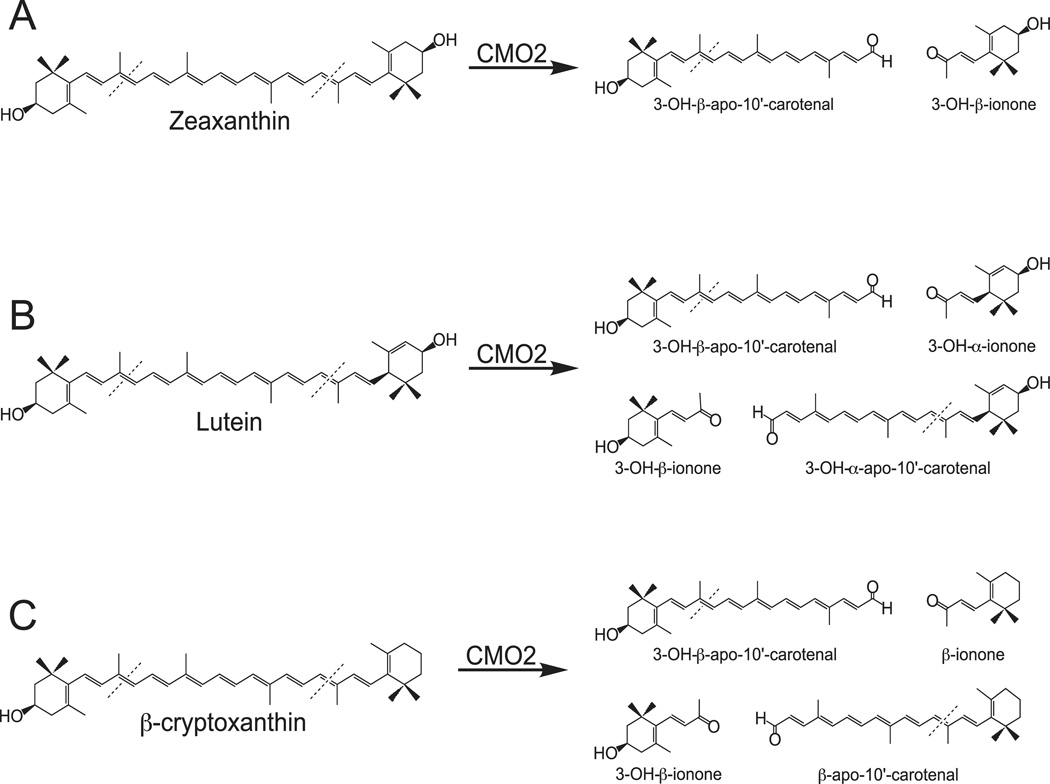
Fig 11.
Metabolism of Hydroxy-Carotenoids by Ferret Carotene-9’,10’-Oygenase (CMO2). Enzymatic cleavage of zeaxanthin (A), lutein (B) and β-cryptoxanthin (C) by ferret CMO2.
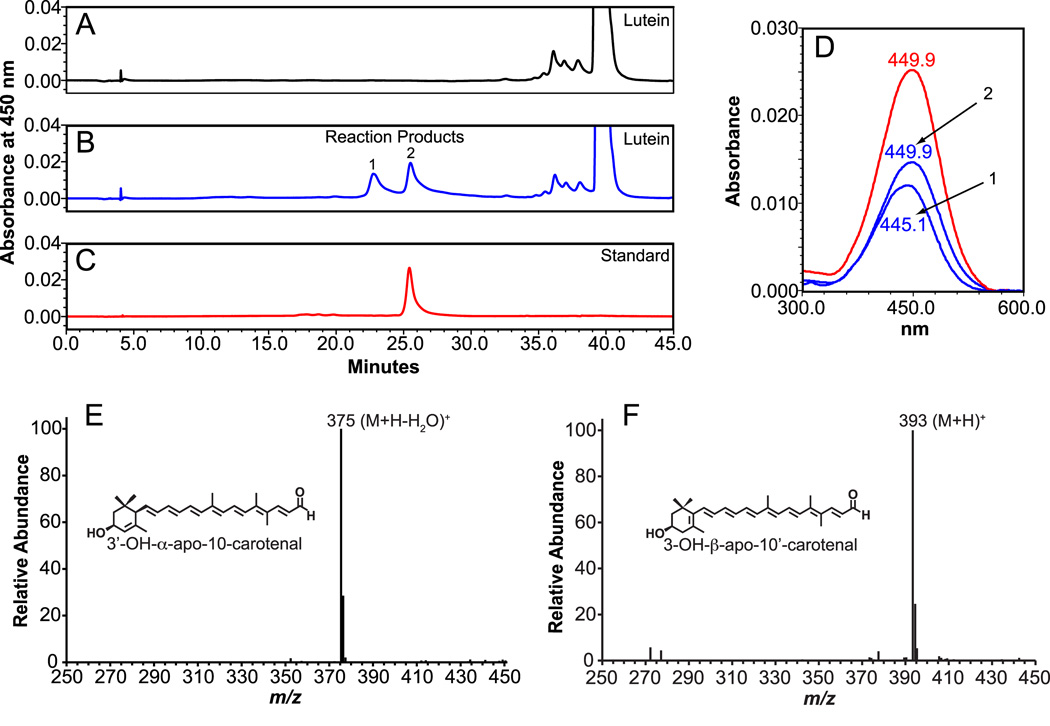
Lutein and zeaxanthin are constitutional isomers, differing in orientation of the ionone ring system. These differences may have an impact on functional roles [40]. Therefore, we investigated cleavage activity of ferret CMO2 towards lutein. HPLC separation with monitoring at 450 nm revealed the formation of two new peaks when incubated with ferret CMO2 (Figure 4). Peak 1 was shifted 2.5 min. earlier in RT (23.0 min.) and had an absorption spectrum 4.8 nm shorter (UVmax 445.1 nm) than the 3-OH-β-apo-10’-carotenal standard (Figure 4). The RT (25.5 min.) and absorption spectra (UVmax 449.9 nm) of peak 2 matched the RT and absorption spectra of the authentic 3-OH-β-apo-10’-carotenal standard. Lutein possesses an allylic hydroxyl group at the C-3’ position of the e-ring, and due to the shift in the C-5’ - C-4’ double bond, the loss of water from the protonated parent ion in APCI+ mode gives a more stable allylic radical cation [41]. Thus, APCI+-MS analysis allows differentiation of cleavage at either the 9,10 or 9’,10’ carbon-carbon double bond. Peak 1 possessed a base peak of m/z 375, corresponding to the (M+H−H2O)+ ion, confirming the presence of the 3’-OH-α-apo-10-carotenal product. Peak 2 was confirmed by the presence of a quasimolecular ion at m/z 393 (M+H)+, which corresponds to the quasimolecular ion of the 3-OH-β-apo-10’-carotenal standard.
Fig 4.
Identification of cleavage products from lutein by HPLC and LC-MS analysis. Lutein (20 µM) was incubated with the homogenates from either uninfected (A) or ferret CMO2-baculovirus infected (B) insect cells for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Two reaction products (1 and 2) were detected at 450 nm only in the incubation mixture with the homogenates of ferret CMO2-baculovirus infected cells (B), but not in that of the uninfected cells (A). D, spectral analysis of the cleavage products (blue line, 1 and 2) of lutein vs. 3-OH-β-apo-10’-carotenal standard (red line). Both the RT and absorption spectrum of the peak 2 matched exactly with that of the 3-OH-β-apo-10’-carotenal standard (C). LC-MS analysis (E) of peak 1 displayed a base peak of m/z 375, corresponding to the (M+H−H2O)+ ion of the 3’-OH-α-apo-10-carotenal product, while peak 2 (F) displayed a base peak of m/z 393, which corresponds to the (M+H)+ ion of the authentic 3-OH-β-apo-10’-carotenal standard.
Cleavage of lutein at the 9,10 and 9’,10’ double is expected to produce both 3-OH-β-ionone and 3’-OH-α-ionone. HPLC separation with monitoring at 296 nm revealed the presence of a new peak with a RT of 3.9 min. (Figure 3D) and a UVmax of 294.1 nm (Figure 3F), similar to that observed with zeaxanthin yet displaying a broader spectrum indicating additional compounds (Figure 3F). Our HPLC program did not allow separation of 3-OH-β-ionone and 3’-OH-α-ionone, thus, the peak was collected, extracted and subjected to GC-MS. The presence of 3-OH-β-ionone was confirmed by the presence of major ions at m/z 193 and m/z 175 (Figure 3G) while 3’-OH-α-ionone was confirmed by the presence of major ions at m/z 109 and m/z 175, with a parent molecular ion at m/z 208 (Figure 3H) [38]. Taken together, these analyses demonstrate cleavage of lutein by ferret CMO2 at both the 9,10 and 9’,10’ double bond, forming 3-OH-β-apo-10’-carotenal, 3’-OH-α-ionone, 3’-OH-α-10-carotenal, and 3-OH-β-ionone (Figure 11B).
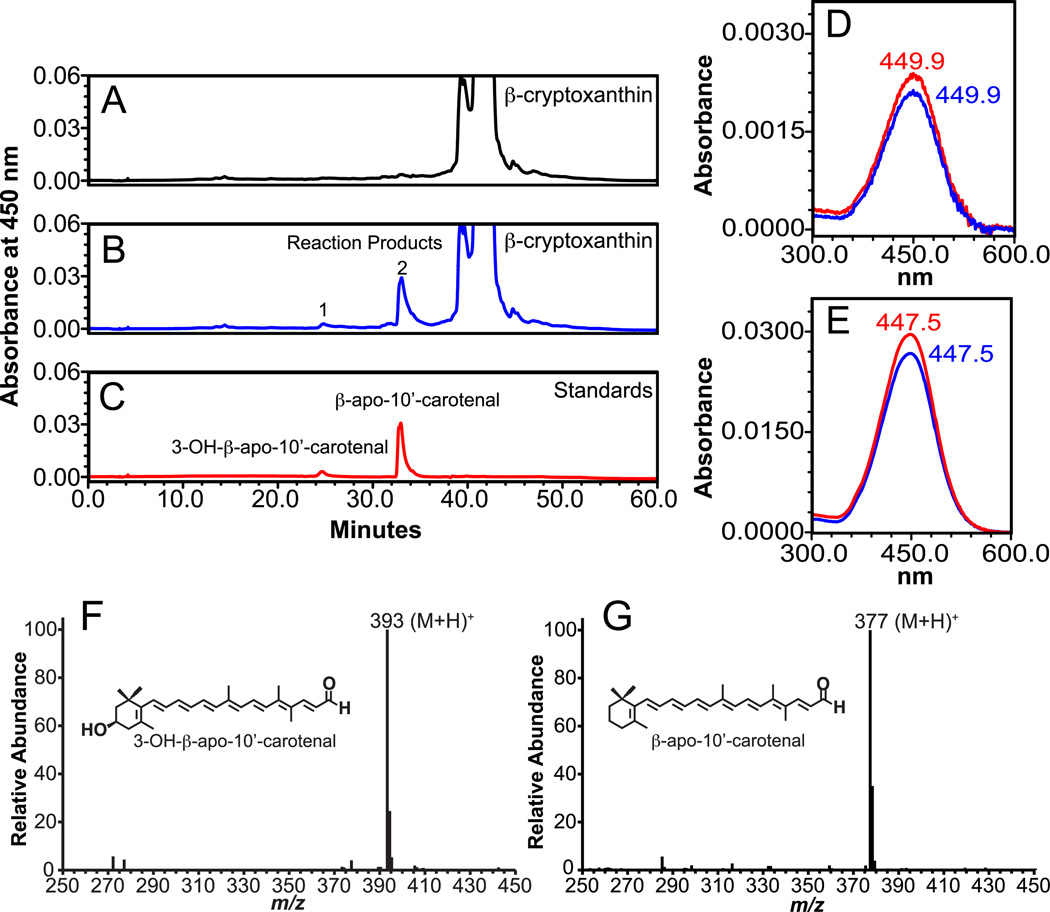
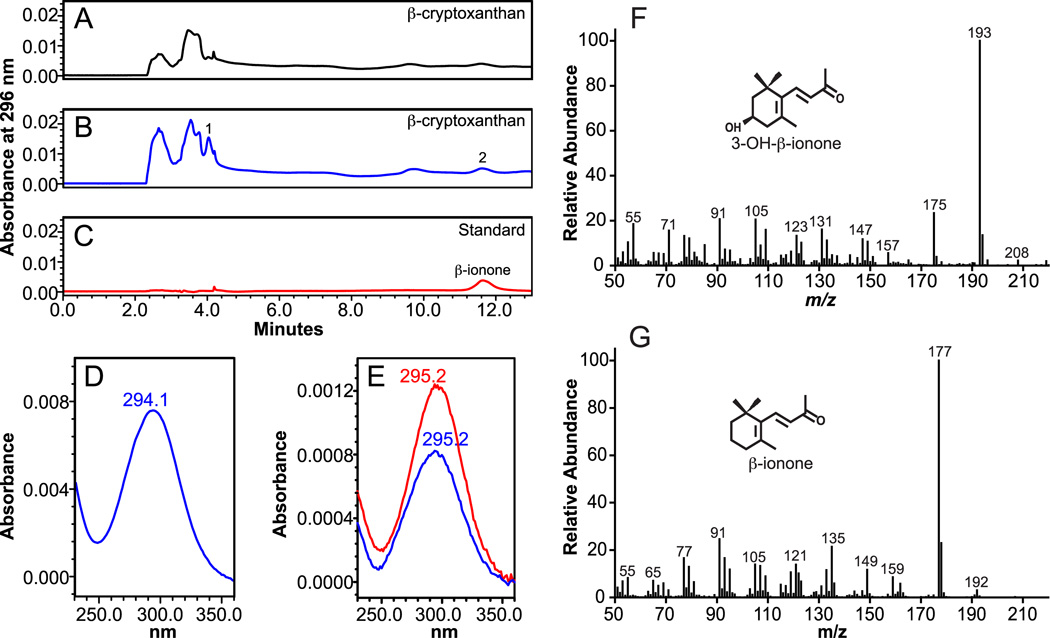
For β-cryptoxanthin, HPLC separation revealed the production of four new peaks in the incubations containing CMO2-infected Sf9 cell lysates. At 450 nm, the RT (25.5 and 33.4 min) and absorption spectra (UVmax 449.9 nm and 447.5 nm) of peaks 1 and 2 matched the RT and absorption spectra of the 3-OH-β-apo-10’-carotenal and β-apo-10’-carotenal authentic standards, respectively (Figure 5). The identity of peaks 1 and 2 were further verified by their quasimolecular ions at m/z 393 (M+H)+ and 377 (M+H)+, respectively (Figure 4), which correspond to the quasimolecular ions of the authentic 3-OH-β-apo-10’-carotenal and β-apo-10’-carotenal standards, respectively. At 296 nm, the RT (3.9 min.) and absorption spectra (UVmax 294.1 nm) of peak 1 was similar to that of the identified 3-OH-β-ionone. The RT (11.9 min.) and absorption spectra (UVmax 295.2 nm) of peak 2 matched the RT and absorption spectra of the authentic β-ionone standard (Figure 6). GC-MS analysis revealed the presence of a major ion at m/z 177 indicating the presence of β-ionone [39], while the presence of major ions of m/z 193 and m/z 175 confirmed the presence of 3-OH-β-ionone [39]. The above analyses demonstrate that ferret CMO2 cleaves β-cryptoxanthin at the 9,10 and 9’,10’ double bond forming 3-OH-β-apo-10’-carotenal, β-ionone, β-apo-10-carotenal, and 3-OH-β-ionone (Figure 11C). The formation of β-apo-10’-carotenal and 3-OH-β-apo-10’-carotenal was significantly decreased by the deletion of Fe2+ from the incubation mixture (78%, 71%, and 67% lower from three independent experiments, compared with that of the complete reaction), indicating that iron was an essential co-factor for the reaction, as previously described [18]. Only trace amounts of other peaks were detected in two control reactions done with uninfected cell homogenates or without cell homogenates. These trace peaks likely represent non-enzymatic cleavage of carotenoids, as previously described [7, 18].
Fig 5.
Identification of cleavage products from β-cryptoxanthin by HPLC and LC-MS analysis. β-Cryptoxanthin (100 µM) was incubated with the homogenates from either uninfected (A) or ferret CMO2-baculovirus infected (B) insect cells for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Two reaction products (1 and 2) were detected at 450 nm in the incubation mixture containing ferret CMO2-baculovirus infected cell homogenate (B) but not the uninfected SF9 cell homogenate (A). D and E, spectral analysis of the cleavage products (blue line, 1 and 2) of β-cryptoxanthin vs. 3-OH-β-apo-10’-carotenal standard (red line, D) and β-apo-10’-carotenal standard (red line, E). Both the RT and absorption spectrum of reaction product 1 and 2 matched exactly with that of the 3-OH-β-apo-10’-carotenal and β-apo-10’-carotenal standard (C), respectively. LC-MS analysis (F) of peak 1 displayed a base peak of m/z 393, corresponding to (M+H)+ 3-OH-β-apo-10-carotenal product, while peak 2 (G) displayed a base peak of m/z 377, which corresponds to the (M+H)+ of the authentic β-apo-10’-carotenal standard.
Fig 6.
Identification of volatile cleavage products from β-cryptoxanthin by HPLC and GC-MS analysis. β-Cryptoxanthin (100 µM) was incubated with homogenates from either uninfected (A) or ferret CMO2-baculovirus infected (B) insect cell lysates for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Unknown reaction products 1 and 2 were detected at 296 nm only in the incubation mixtures with ferret CMO2-baculovirus infected cells (B) but not in that of the uninfected cells (A). Spectral analysis of reaction products 1 (D) and 2 (E), which matched in retention time and absorption spectra of the β-ionone standard, indicated the presence of ionone compounds. GC-MS analysis of reactions product 1 from β-cryptoxanthin cleavage indicated the presence of 3-OH-β-ionone (F) and analysis of reaction product 2 indicated the presence of β-ionone (G). The EI mass spectra were matched to spectra found in literature [38, 39].
3.2 In Vitro Kinetic Analysis
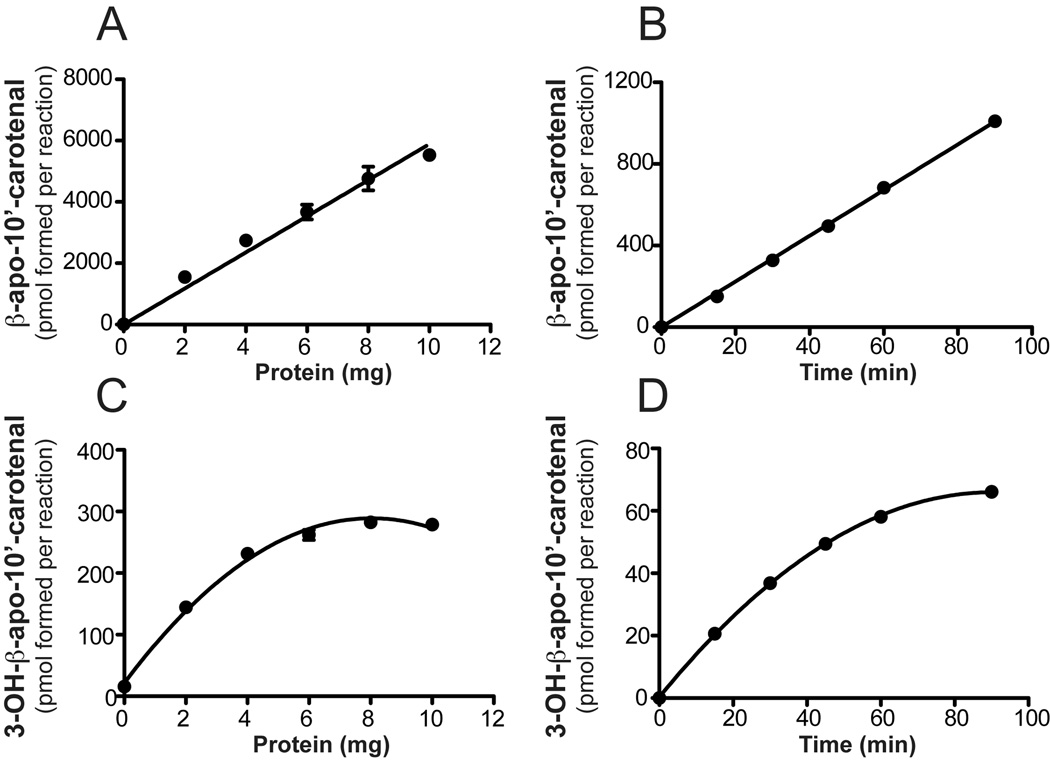
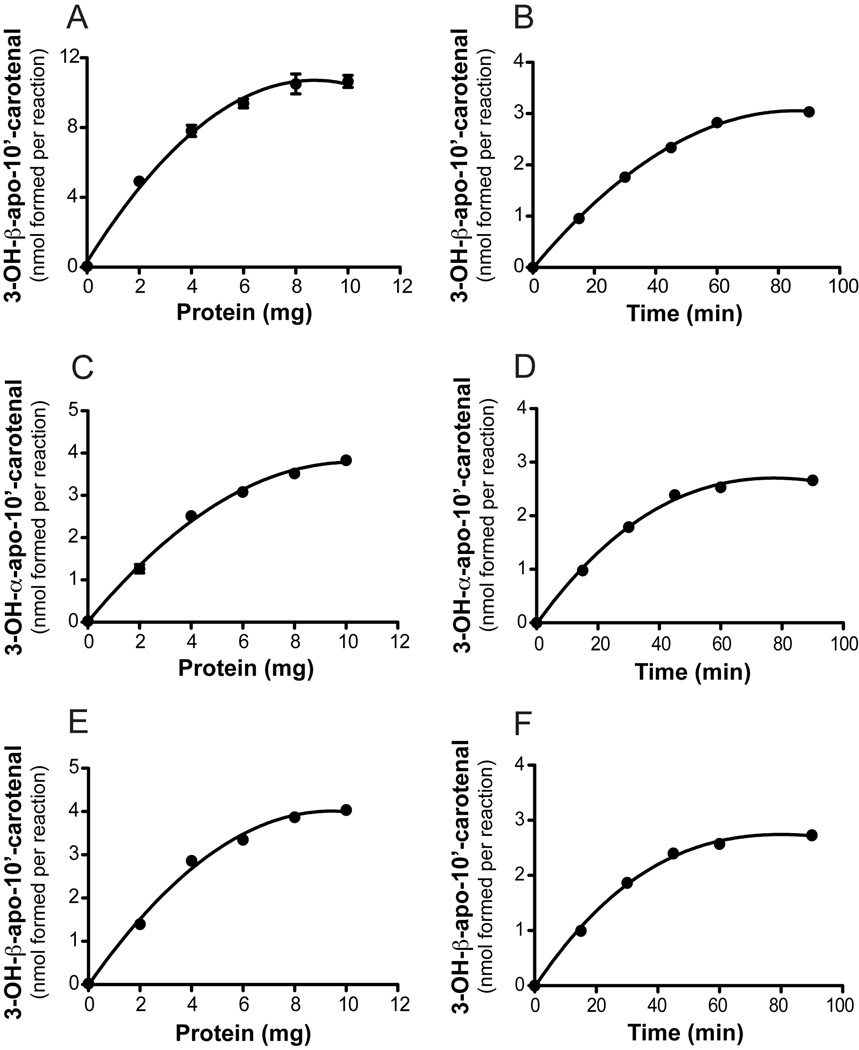
To estimate the substrate specificity and kinetic constants for recombinant ferret CMO2, we performed in vitro assays with zeaxanthin, lutein and β-cryptoxanthin as substrates. Using β-cryptoxanthin as the substrate (~50 µM), the production of β-apo-10’-carotenal was linear up 10 mg/ml (Figure 7) while formation of 3-OH-β-apo-10’-carotenal was linear up to 2 mg/ml with only a slight increase in formation up to 10 mg/ml. The production of β-apo-10’-carotenal from β-cryptoxanthin was linear up to 90 minutes while 3-OH-β-apo-10’-carotenal formation was linear up to 30 min (Figure 7). The production of 3-OH-β-apo-10’-carotenal from zeaxanthin (~50 µM) was linear up to 4 mg/ml with only a slight increase up to 10 mg/ml (Figure 8). The production of 3-OH-β-apo-10’-carotenal and 3’-OH-α-apo-10’-carotenal from lutein (~50 µM) was linear up to 4 mg/ml with only a slight increase up to 10 mg/ml (Figure 8). Using 2 mg/ml ferret CMO2 lysate, formation of 3-OH-β-apo-10’-carotenal and 3’-OH-α-apo-10-carotenal was linear up to 30 min. using zeaxanthin and lutein as substrates (Figure 8), respectively. To ensure less than 10% substrate conversion for all substrates investigated, incubations were carried out with 2 mg/ml ferret CMO2 lysate over a 30 min. period.
Fig 7.
Protein- and time-dependent cleavage of β-cryptoxanthin by ferret carotene-9’, 10’-oxygenase (CMO2). Reaction velocity as a function of protein concentration (A and C) and time (B and D) is plotted for both β-apo-10’-carotenal and 3-OH-β-apo-10’-carotenal formation. For protein-dependent cleavage, β-cryptoxanthin (50 µM) was incubated with various protein concentrations of cell homogenates expressing ferret CMO2 at 37 °C for 60 min. For time-dependent formation, β-cryptoxanthin (50 µM) was incubated with ~2 mg of homogenate expressing ferret CMO2 at 37 °C for various time points. Data are the average of two independent experiments performed in duplicate.
Fig 8.
Protein- and time-dependent cleavage of zeaxanthin and lutein by ferret carotene-9’, 10’-oxygenase (CMO2). Reaction velocity as a function of protein concentration (A) and time (B) is plotted for 3-OH-β-apo-10’-carotenal formation from zeaxanthin cleavage by CMO2. Reaction velocity as a function of protein and time is plotted for 3’-OH-α-apo-10-carotenal (C and D) and 3-OH-β-apo-10’-cartenal (E and F) formation from lutein cleavage by CMO2. For protein-dependent cleavage, zeaxanthin (50 µM) and lutein (50 µM) were incubated with various protein concentrations of cell homogenates expressing ferret CMO2 at 37 °C for 60 min. For time-dependent formation, zeaxanthin (50 µM) and lutein (50 µM) were incubated with ~2 mg of homogenate expressing ferret CMO2 at 37 °C for various time points. Data are the average of two independent experiments performed in duplicate.
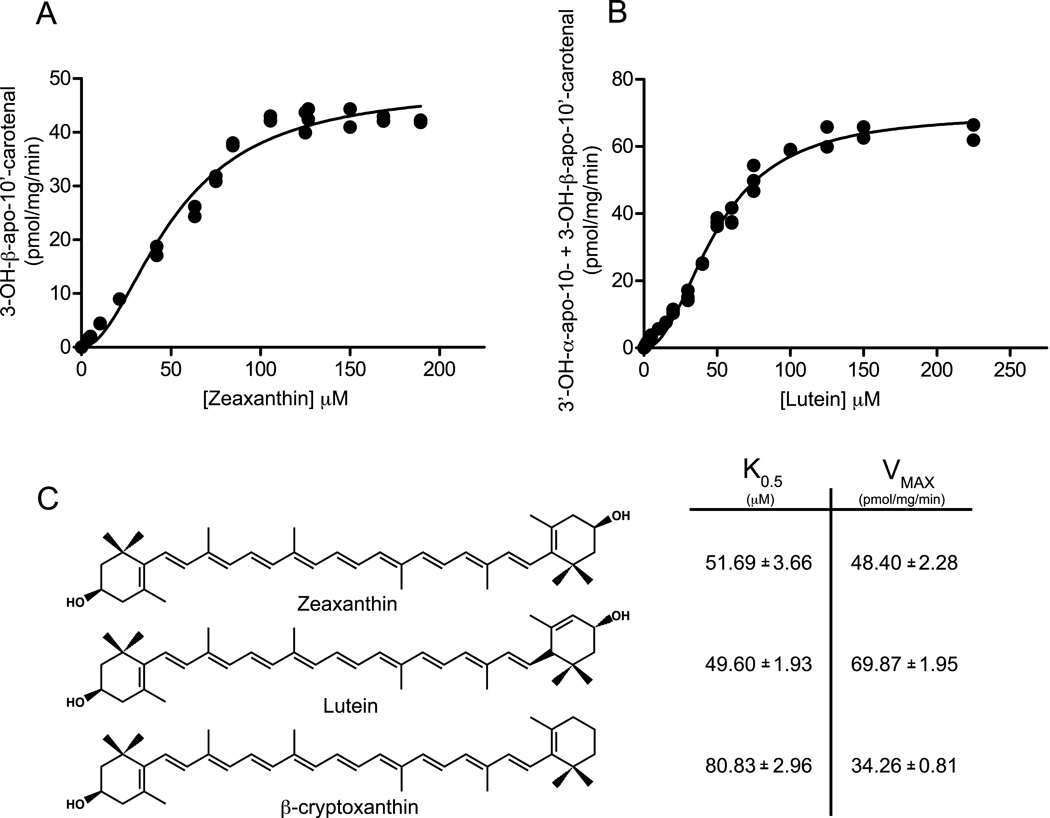
Kinetic constants for recombinant ferret CMO2 were estimated by varying the concentrations of carotenoid substrates. Reaction curves displayed a sigmoidal shape for all substrates, thus kinetic parameters were estimated from a positive cooperativity allosteric kinetic model (GraphPad Prism, version 5.02, San Diego, CA, USA). All kinetic constants are identified as apparent K0.5 and apparent Vmax. Production of 3-OH-β-apo-10’-carotenal from zeaxanthin (0 – 200 µM) was fit to an allosteric enzyme kinetic model and apparent K0.5 and Vmax values of 51.49 ± 3.66 µM and 48.40 ± 2.28 µM pmol of 3-OH-β-apo-10’-carotenal mg−1 min−1 were estimated (Figure 9A). Using Lutein as the substrate (0 – 225 µM), formation of 3-OH-β-apo-10’-carotenal had estimated apparent K0.5 and Vmax values of 47.97 ± 2.40 µM and 31.91 ± 1.14 pmol mg−1 min−1 while formation of 3-OH-β-apo-10’-carotenal had estimated apparent K0.5 and Vmax values of 51.10 ± 2.10 µM and 38.0 ± 1.13 pmol mg−1 min−1. The combined kinetics had estimated apparent K0.5 and Vmax values of 49.60 ± 1.93 µM and 69.87 ± 1.95 pmol of 3-OH-β-apo-10’-carotenal and 3-OH-α-apo-10-carotenal mg−1 min−1 (Figure 9B). Using β-cryptoxanthin as the substrate (0 – 450 µM), production of β-apo-10’-carotenal had estimated apparent K0.5 and Vmax values of 79.29 ± 2.87 µM and 31.45 ± 0.74 pmol mg−1 min−1 while production of 3-OH-β-apo-10’-carotenal had estimated apparent K0.5 and Vmax values of 110.0 ± 7.96 µM and 2.92 ± 0.13 pmol mg−1 min−1. The combined kinetics had estimated apparent K0.5 and Vmax values of 80.83 ± 2.96 µM and 34.26 ± 0.8117 pmol of β-apo-10’-carotenal and 3-OH-β-apo-10’-carotenal mg−1 min−1 (Figure 9C). Hill coefficients of h = 1.95, h = 2.13 and h = 2.44 were estimated for zeaxanthin, lutein and β-cryptoxanthin, respectively.
Fig 9.
In vitro kinetic analysis of recombinant ferret CMO2 with carotenoids as substrates. Reaction velocity (pmol product formed/mg/min) as a function of substrate concentration (µM) is plotted for a 30-min reaction with 2 mg of crude lysates of recombinant ferret CMO2 containing 0 – 200 µM zeaxanthin (A) and 0 – 225 µM lutein (B). Structures and estimated relative kinetic constants for zeaxanthin, lutein and β-cryptoxanthin are compared (C). Product quantification was performed by reverse-phase HPLC analysis as described under “Experimental Procedures.” These data are the average of four independent experiments performed in duplicate.
3.2 Cleavage of 3-OH-β-apo-10’-carotenal using Recombinant Ferret CMO2
Initial velocity determination revealed a discord between protein- and time-dependent formation of β-apo-10’-carotenal and 3-OH-β-apo-10’-carotenal from β-cryptoxanthin (Figure 7), suggesting potential cleavage of 3-OH-β-apo-10’-carotenal by CMO2. Thus, cleavage of 3-OH-β-apo-10’-carotenal by CMO2 was investigated. 3-OH-β-apo-10’-carotenal (5 µM) was incubated with 2 mg ferret CMO2 lysate, uninfected Sf9 lysates or buffer alone for 30 min. Cleavage activity was quantified as percent parent remaining and normalized to buffer alone (100%). Incubation of 3-OH-β-apo-10’-carotenal with uninfected Sf9 insect cell lysate resulted in 45% decrease in the parent compound, indicating that the 3-OH-apo-10’-carotenal metabolite is highly unstable. However, when 3-OH-apo-10’-carotenal was incubated with CMO2 lysate, there was a 67% decrease in the parent compound, accompanied by appearance of 3-OH-β-ionone (data not shown), indicating the further cleavage of 3-OH-β-apo-10’-carotenal by CMO2 at the 9,10 double bond. However, we did not detect the expected apo-10,10’-carotenedialdehyde product in addition to 3-OH-β-ionone.
3.4 In Vitro Oxidation of 3-OH-β-apo-10’-carotenal by Ferret Hepatic Homogenates
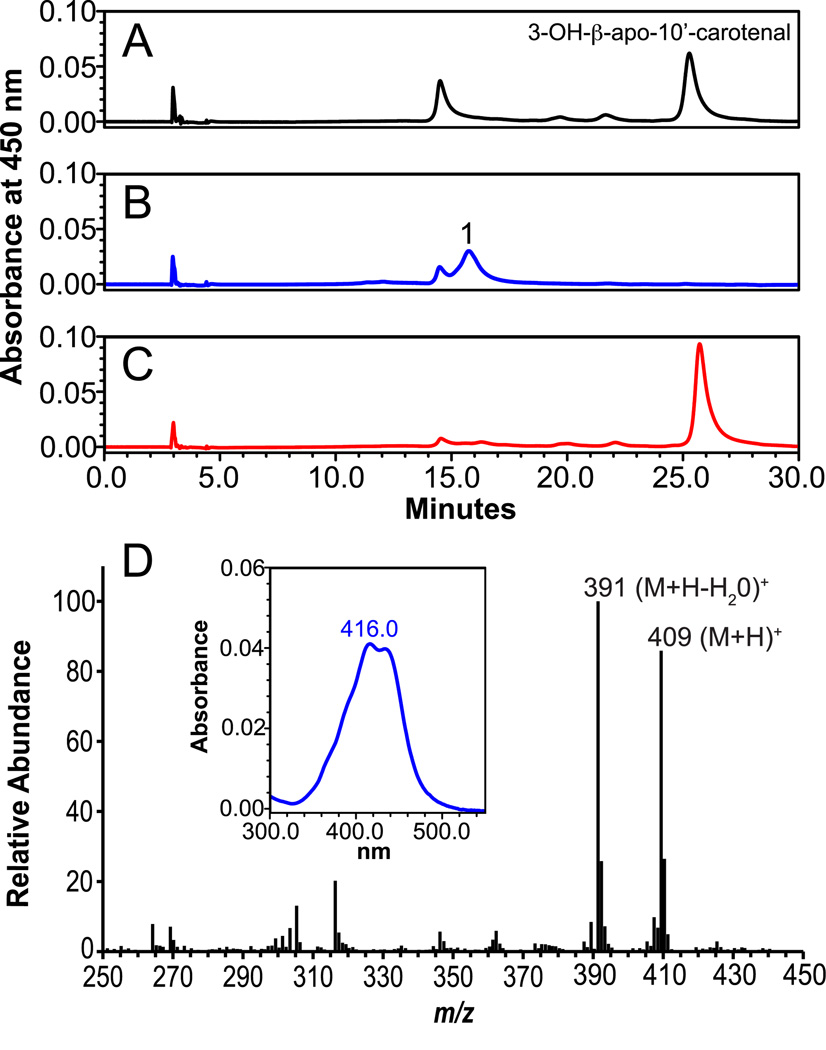
Acid derivatives of apocarotenoid products have been identified as biologically active metabolites, serving as intermediates in retinoic acid formation and possessing unique biological activities [42]. Therefore, we sought to determine the in vitro conversion of 3-OH-β-apo-10’-carotenal to 3-OH-β-apo-10’-carotenoic acid. When NAD+ (3 mM) was present in the reaction mixture, incubation of 3-OH-β-apo-10’-carotenal (2 or 10 µM) with ferret liver S9 homogenate resulted in the formation of 3-OH-β-apo-10’-carotenoic acid (Figure 10). The new peak had a retention time of 16 min and UVMax of 416 nm (Figure 10D, Inset). The new peak was retained when subjected to aminopropyl SPE (data not shown), and LC-MS analysis identified quasimolecular ions at m/z 409 (M + H)+ and 391 (M+H−H2O)+ (Figure 10D). In the presence of the aldehyde dehydrogenase inhibitor citral (1 mM), which blocks conversion of retinal to retinoic acid [35], 3-OH-β-apo-10’-carotenoic acid formation was completely inhibited (Figure 10C), indicating enzymatic conversion of 3-OH-β-apo-10’-carotenal into 3-OH-β-apo-10’-carotenoic acid.
Fig 10.
3-OH-β-apo-10’-carotenoic acid is generated from incubation of 3-OH-β-apo-10’-carotenal with ferret liver lysates and NAD+. Incubation of 3-OH-β-apo-10’-carotenal (10 µM) with ferret liver lysate (1 mg) and NAD+ (3 mM) resulted in the formation of new peak (B, peak 1), which was not present when incubated without NAD+ (A). In the presence of the aldehyde dehydrogenase inhibitor citral (1 mM), formation was inhibited (C). Inset: spectral anaylsis of oxidation product with UVmax of 416. MS analysis (D) identified base peaks of m/z 409 and m/z 391, corresponding to the protonated ion of the parent compound and loss of water, indicating the presence of 3-OH-β-apo-10’-carotenoic acid.
4. DISCUSSION
The present study is the first documentation of excentric enzymatic cleavage of xanthophyll carotenoids by a vertebrate carotenoid cleavage oxygenase, adding to the accumulating evidence that CMO2 plays a key role in carotenoid metabolism, especially of non-provitamin A carotenoids. We demonstrate that ferret CMO2 catalyzes the excentric cleavage of hydroxy carotenoids, including the non-provitamin A zeaxanthin and lutein, and provitamin A β-cryptoxanthin, at both the 9,10 and 9’,10’ double bond producing both volatile and non-volatile apocarotenoid cleavage products (Figure 11). Importantly, we showed the xanthophylls zeaxanthin and lutein are preferentially cleaved over the mono-hydroxy β-cryptoxanthin. This provides strong biochemical evidence supporting the recent genetic evidence that accumulation of the xanthophylls lutein and zeaxathin in adipose tissue and skin are due to mutations in the CMO2 gene [30, 32]. Considering the possible beneficial effects of lutein, zeaxanthin and β-cryptoxanthin in human health [1–3], enzymatic cleavage of xanthophylls by CMO2 represents a new avenue of research regarding vertebrate carotenoid metabolism and biological function.
While previous studies have shown a key role for CMO1 in catalyzing the symmetric cleavage of provitamin A carotenoids to vitamin A [11, 23, 43], similar evidence for a definitive role of CMO2 in carotenoid metabolism has been lacking. The presence of at least one unsubstituted β-ionone ring has been proposed to be sufficient for cleavage of carotenoids by CMO1 [12, 14], and indeed, low or no cleavage activity was detected when all-trans lycopene or zeaxanthin was investigated as a substrate [14]. In contrast, previous investigations of vertebrate CMO2 substrate recognition demonstrated cleavage of both β-carotene and cis-isomers of lycopene, respectively [18, 19], suggesting both the central polyene chain backbone and the ionone-ring structures are important in substrate specificity. In the present study, we demonstrate that the xanthophylls zeaxanthin and lutein are preferentially cleaved over the mono-hydroxy β-cryptoxanthin, indicating an important role of substitution of the ionone ring structures in CMO2 cleavage (Figure 11). There was little difference in cleavage activity between the β- and ε-ionone ring of lutein indicating little effect of ring orientation on cleavage activity. However, symmetry of substitution of the ionone ring in β-cryptoxanthin had a major influence on cleavage activity, i.e. 10-fold greater formation of β-apo-10’-carotenal compared to 3-OH-β-apo-10’-carotenal formation. These results suggest that symmetry of substitution and not simply substitution alone is important in CMO2 substrate recognition.
In the present study, ferret CMO2 cleaved zeaxanthin into 3-OH-β-apo-10’carotenal and 3-OH-β-ionone, indicating cleavage at either the 9’10’ or 9,10 carbon-carbon double bond. However, lutein was converted into 3-OH-β-apo-10’carotenal, 3’-OH-α-apo-10-carotenal, 3-OH-β-ionone, and 3’-OH-α-ionone while β-cryptoxanthin was converted into β-apo-10’-carotenal, 3-OH-β-apo-10-carotenal, β-ionone, and 3-OH-β-ionone indicating cleavage at both the 9,10 and 9’,10’ carbon-carbon double bond. This is supported by our further observation of CMO2 cleavage activity towards 3-OH-β-apo-10’-carotenal, forming 3-OH-β-ionone. Members of the plant carotenoid cleavage dioxygenase 1 (CCD1) family of CCOs display a similar pattern of carotenoid cleavage, cleaving multiple substrates at both the 9,10 and 9’,10’ double bond [44]. However, the main products of CCD1 cleavage are C14 dialdehydes and C13 volatile compounds, indicating both C40 and C27 compounds can serve as substrates for CCD1 orthologues. However, we did not detect any C14 dialdehydes in our cleavage reactions, which could be due to further degradation, nor did we detect any cleavage products when β-apo-8’-carotenal as the substrate (data not shown). Our data demonstrates that cleavage by CMO2 at either the 9,10 or 9’,10’ double bond is mutually exclusive. Whether CMO2 can cleave additional substrates clearly needs further investigation.
Our kinetic analyses indicate that ferret CMO2 cleavage activity was higher toward zeaxanthin and lutein than β-cryptoxanthin in vitro. While the estimated apparent K0.5 concentrations obtained in the current study are higher than concentrations achieved in vivo (e.g., 1 – 2 µM for lutein and zeaxathin [45, 46]), studies investigating recombinant CMO1 cleavage activity towards β-carotene estimated Km values ranging from 6 – 31 µM [47], which is significantly higher than in vivo β-carotene concentrations [48]. These studies have contributed greatly to the understanding of the mechanisms and substrate specificity of CMO1. In the current study when zeaxanthin, lutein and β-cryptoxanthin where used as substrates, however, substrate-dependent cleavage did not follow Michaelis-Menten kinetics. Comparison of kinetic models indicated that an allosteric model of positive cooperativity was a more appropriate fit of enzymatic activity. Human CMO1 was shown to be a tetrameric enzyme in vitro [12] yet displayed Michaeli-Menten kinetics. However, one of the limitations of the current study, especially in regards to estimating kinetic parameters, is the use of crude enzyme lysates. Crude enzyme lysates can give the impression of allosteric kinetics due to the low percentage of enzyme present, especially at lower substrate concentrations [49]. This combination decreases the interaction between substrate and enzyme, and thus, a lower rate is observed (an especially pertinent observation with carotenoids, which must be micellized for solubilization). In the current study, protein and time-dependency were markedly different between 3-OH-β-apo-10’-carotenal and β-apo-10’-carotenal formation (Figure 7). The increased protein concentration and incubation time led to an increase in 3-OH-β-apo-10’-carotenal cleavage, necessitating the use of shorter incubations and decreased protein concentrations for kinetic estimations. This becomes an issue when the substrate is substantially diluted. The lag in activity displayed in the kinetic curves could in fact be due to the substrate and protein dilution. Therefore, investigations using purified enzyme need to be carried out to further clarify the kinetics of CMO2. However, the procurement of purified CMO2 remains a significant challenge [18, 19].
In the present study, we demonstrate in vitro oxidation of 3-OH-β-apo-10’-carotenal to the 3-OH-β-apo-10’-carotenoic acid product. Feeding of apocarotenals results in the formation of apo-carotenoic acids in vivo [7, 20, 35], suggesting formation of acid products as the first step in further metabolic transformation of apocarotenoid products. Apocarotenoids are important bioactive mediators in plants and animals. In plants, they play key roles in reproduction, defense, and architecture [50]. 3-OH-β-Apo-10’-carotenoic acid has been identified in Boronia megastigma (Nees) during flower development, indicating cleavage of xanthophylls and oxidation of cleavage products [51, 52]. In animals, a number of studies have identified a broad array of apocarotenoids and apolycopenoids with potential biological activities [7, 18, 20, 53, 54]. Non-volatile apo-carotenoids and apo-lycopenoids, such as apo-10’, apo-12’-, and apo-14’-, can inhibit cell growth [26, 55–57], stimulate differentiation [58], transactivate nuclear receptors [26] or antagonize nuclear receptor activation [59] and induce expression of phase II enzymes via activation of the Nrf2 transcription factor [27]. Recently, we observed that 3-OH-β-apo-10’-carotenal exerts a dose-dependent effect on both growth inhibitory effect and induction of cell death in HepG2 liver cancer cells, BEAS-2B immortalized bronchial epithelial cells and A549 lung cancer cell lines (Mein et al., unpublished data). These ongoing studies implicate a role for 3-OH-β-apo-10’-carotenal in the regulation of cell proliferation and apoptosis. The volatile apo-carotenoid β-ionone has also been shown to inhibit cell proliferation and induce apoptosis both in vitro [60–63] and in vivo [64] and induce expression of phase I and phase II enzymes in rats and mice [65–67]. In previous studies, β-cryptoxanthin dose-dependently increased RARE-dependent promoter activity in cells co-transfected with RAR expression vector [68] and was shown to bind and activate RAR receptors using a yeast two-hybrid system [69]. Whether the activity of β-cryptoxanthin in these systems could be due to its conversion to apo-carotenoic acid metabolites is unclear. We clearly demonstrate excentric cleavage of β-cryptoxanthin, forming both apo-carotenoids and 3-OH-apo-carotenoids, which can be further oxidized to 3-OH-apo-carotenoic acids. While the activity of β-apo-10’-carotenoids has been investigated, research regarding the biological activity of 3-OH-apo-carotenoid products is clearly needed.
Cleavage of lutein and zeaxanthin by CMO2 raises important questions as to the role of zeaxanthin and lutein in the eye. Several carotenoids, including lutein, zeaxanthin, β-carotene, α-carotene, and lycopene, have been identified in the ciliary body and human retinal pigment epithelia (RPE) of the eye [34]. Both CMO1 and CMO2 are strongly expressed in the RPE [70, 71], and CMO1 actively converts β-carotene and β-cryptoxanthin into all-trans-retinal [72, 73], which is isomerized to the visual pigment chromophore 11-cis-retinal by RPE65 [74]. In flies, an 11-cis-configuration of 3-OH-retinal is essential for visual pigment biogenesis [75]. Although no apocarotenoids resulting from excentric cleavage by CMO2 have been identified in the RPE [76], 3-OH-β-apo-14’-carotenal and 3-OH-β-ionone have been identified in cadaver retinas [77]. In addition, several non-dietary lutein and zeaxanthin metabolites, including 3’-epilutein, 3-OH-β,ε-caroten-3’-one and meso-zeaxanthin, have also been identified in these regions [34]. The physiologic relevance of these metabolites is unknown, yet they are most likely formed by oxidation-reduction and isomerization reactions of lutein and zeaxanthin. Recently, β-carotene, lutein and zeaxanthin cleavage products demonstrated activity in cultured RPE cells, indicating proinflammatory effects of carotenoid-derived oxidation products [78]. However, the susceptibility of lutein and zeaxanthin to degradation in the RPE may be limited by protein binding [79, 80]. Nonetheless, the identification of 3-OH-apo-carotenoids in vivo remains a significant challenge. We did not detect any 3-OH-apo-carotenoids in mice or ferrets supplemented with either lutein or β-cryptoxanthin (unpublished data). This could be due to the detection limitations of our analytical systems, difficulty of extraction from complex biological matrices or stability of metabolites. Clearly, the development and utilization of more sensitive techniques for the identification and detection of apo-carotenoids in vivo [29] will greatly enhance research in this area.
Taken together, the broad substrate specificity of CMO2 demonstrated herein may help us understand the potential biologic role of CMO2 in carotenoid metabolism and function. Recent experimental data suggests that carotenoid metabolites may have more important biological roles than their parent compounds [8, 42]. However, excessive accumulation of excentric carotenoid cleavage products has also demonstrated potential harmful effects [9, 81], especially when coupled with a highly oxidative environment (e.g. the lungs of a cigarette smoker or liver of an excessive alcohol drinker) [82–84]. Therefore, depending on the dose, these metabolites may have specific actions on several important cellular signaling pathways and molecular targets. In considering the efficacy and complex biological functions of carotenoids in human chronic disease prevention, future investigations of carotenoids must take into account the regulatory mechanisms and carotenoid cleavage activity of CMO2 and further metabolic transformation of carotenoid metabolites.
Research Highlights
-
▶
Vertebrate CMO2 enzymatically cleaves xanthophylls at the 9,10 and 9’,10’ double bond.
-
▶
Lutein and zeaxanthin are preferentially cleaved over β-cryptoxanthin.
-
▶
3-OH-β-apo-10’-carotenal is oxidized to 3-OH-β-apo-10’-carotenoic acid.
ACKNOWLEDGEMENTS
We thank Kang-Quan Hu for technical support and members of the Nutrition and Cancer Biology Laboratory for helpful discussions. The work was supported by NIH grant R01CA104932 and by the US Department of Agriculture, under agreement NO 1950-51000-064S. J.R.M was supported by training grant T32 DK062032 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the National Institutes of Health or the U.S. Department of Agriculture.
ABBREVIATIONS
- CMO1
β,β-carotene-15,15’-monooxygenase
- CMO2
carotene-9’,10’-monooxygenase
- HPLC
high performance liquid chromatography
- LC-MS
liquid chromatography – mass spectrometry
- GC-MS
gas chromatography – mass spectrometry
- NAD+
β-nicotinamide adenine dinucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Johnson EJ, Krinsky NI. In: Carotenoids Volume 5: Nutrition and Health. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser Verlag; 2009. pp. 287–300. [Google Scholar]
- 2.Schalch W, J.T Landrum, R.A Bone. In: Carotenoids Volume 5: Nutrition and Health. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser Verlag; 2009. pp. 301–334. [Google Scholar]
- 3.Rock CL. In: Carotenoids Volume 5: Nutrition and Health. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser Verlag; 2009. pp. 269–286. [Google Scholar]
- 4.Bendich A, Nutr J. 1989;119:135–136. doi: 10.1093/jn/119.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Analytical chemistry. 1997;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- 6.Tang GW, Wang XD, Russell RM, Krinsky NI. Biochemistry. 1991;30:9829–9834. doi: 10.1021/bi00105a003. [DOI] [PubMed] [Google Scholar]
- 7.Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM. Arch Biochem Biophys. 1991;285:8–16. doi: 10.1016/0003-9861(91)90322-a. [DOI] [PubMed] [Google Scholar]
- 8.Mein JR, Lian F, Wang XD. Nutr Rev. 2008;66:667–683. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XD. In: Carotenoids in Health and Disease. Krinsky NI, Mayne ST, Sies H, editors. New York: Marcel Dekker; 2004. pp. 313–335. [Google Scholar]
- 10.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Science (New York, 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 11.von Lintig J, Vogt K. The Journal of biological chemistry. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 12.Lindqvist A, Andersson S. The Journal of biological chemistry. 2002;277:23942–23948. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 13.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. The Journal of biological chemistry. 2001;276:32160–32168. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- 14.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr The Journal of biological chemistry. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 15.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. The Biochemical journal. 2001;354:521–529. doi: 10.1042/0264-6021:3540521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, Kerrigan M, Campochiaro M, Campochiaro P, Palczewski K, Zack DJ. Genomics. 2001;72:193–202. doi: 10.1006/geno.2000.6476. [DOI] [PubMed] [Google Scholar]
- 17.J Glover. Vitamins and hormones. 1960;18:371–386. doi: 10.1016/s0083-6729(08)60869-9. [DOI] [PubMed] [Google Scholar]
- 18.Hu K, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The Journal of biological chemistry. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. The Journal of biological chemistry. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 20.Sharma RV, Mathur SN, Dmitrovskii AA, Das RC, Ganguly J. Biochim Biophys Acta. 1976;486:183–194. doi: 10.1016/0005-2760(77)90083-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. The Journal of biological chemistry. 1996;271:26490–26498. [PubMed] [Google Scholar]
- 22.Napoli JL, Race KR. The Journal of biological chemistry. 1988;263:17372–17377. [PubMed] [Google Scholar]
- 23.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. The Journal of biological chemistry. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 24.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, Macgibbon AH, Spelman RJ, Lehnert K, Snell RG. Genetics. 2009;182:923–926. doi: 10.1534/genetics.109.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian R, Pitchford WS, Morris CA, Cullen NG, Bottema CD. Animal genetics. 2009;41:253–259. doi: 10.1111/j.1365-2052.2009.01990.x. [DOI] [PubMed] [Google Scholar]
- 26.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 27.Lian F, Wang XD. International journal of cancer. 2008;123:1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mein JR, Wang XD. In: Carotenoids: Physical, Chemical, and Biological Functions and Properties. Landrum JT, editor. CRC Press; 2010. pp. 417–435. [Google Scholar]
- 29.Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. J Agric Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vage DI, Boman IA. BMC genetics. 2010;11:10. doi: 10.1186/1471-2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill F. Nature. 1962;194:865–866. doi: 10.1038/194865a0. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. PLoS genetics. 2008;4:e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castaneda MP, Hirschler EM, Sams AR. Poultry science. 2005;84:143–147. doi: 10.1093/ps/84.1.143. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Experimental eye research. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 35.Wang XD, Krinsky NI, Tang GW, Russell RM. Arch Biochem Biophys. 1992;293:298–304. doi: 10.1016/0003-9861(92)90399-h. [DOI] [PubMed] [Google Scholar]
- 36.Tang GW, Russell RM. Journal of lipid research. 1990;31:175–182. [PubMed] [Google Scholar]
- 37.Scherzinger D, Al-Babili S. Molecular microbiology. 2008;69:231–244. doi: 10.1111/j.1365-2958.2008.06282.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Limones C, Schnabele K, Blanco-Portales R, Luz Bellido M, Caballero JL, Schwab W, Munoz-Blanco J. J Agric Food Chem. 2008;56:9277–9285. doi: 10.1021/jf801096t. [DOI] [PubMed] [Google Scholar]
- 39.Vogel JT, Tan BC, McCarty DR, Klee HJ. The Journal of biological chemistry. 2008;283:11364–11373. doi: 10.1074/jbc.M710106200. [DOI] [PubMed] [Google Scholar]
- 40.Landrum JT, Chatfield DC, Mebel AM, Alvarez-Calderon F, Fernandez MV. Arch Biochem Biophys. 2010;493:169–174. doi: 10.1016/j.abb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Dachtler M, Glaser T, Kohler K, Albert K. Analytical chemistry. 2001;73:667–674. doi: 10.1021/ac000635g. [DOI] [PubMed] [Google Scholar]
- 42.Wang XD. In: Carotenoids Volume 5: Nutrition and Health. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser Verlag; 2009. pp. 383–408. [Google Scholar]
- 43.von Lintig J, Wyss A. Arch Biochem Biophys. 2001;385:47–52. doi: 10.1006/abbi.2000.2096. [DOI] [PubMed] [Google Scholar]
- 44.Kloer DP, Schulz GE. Cell Mol Life Sci. 2006;63:2291–2303. doi: 10.1007/s00018-006-6176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bone RA, Landrum JT. Arch Biochem Biophys. 2010 doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 46.Bone RA, Landrum JT, Guerra LH, Ruiz CA. J Nutr. 2003;133:992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- 47.Lietz G, Lange J, Rimbach G. Arch Biochem Biophys. 2010 doi: 10.1016/j.abb.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 48.I.o.M. National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C.: National Academy Press; 2000. [Google Scholar]
- 49.A.a.G. Rogers Y. In: Plant Metabolic Networks. Schwender J, editor. New York: Springer; 2009. pp. 98–100. [Google Scholar]
- 50.Bouvier F, Isner JC, Dogbo O, Camara B. Trends in plant science. 2005;10:187–194. doi: 10.1016/j.tplants.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Cooper CM, Davies NW, Menary RC. J Agric Food Chem. 2003;51:2384–2389. doi: 10.1021/jf026007c. [DOI] [PubMed] [Google Scholar]
- 52.Cooper CM, Davies NW, Menary RC. J Agric Food Chem. 2009;57:1513–1520. doi: 10.1021/jf802610p. [DOI] [PubMed] [Google Scholar]
- 53.al-Hasani SM, Parrish DB. J Nutr. 1972;102:1437–1440. doi: 10.1093/jn/102.11.1437. [DOI] [PubMed] [Google Scholar]
- 54.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr J Nutr. 2003;133:4189–4195. doi: 10.1093/jn/133.12.4189. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki T, Matsui M, Murayama A. Journal of nutritional science and vitaminology. 1995;41:575–585. doi: 10.3177/jnsv.41.575. [DOI] [PubMed] [Google Scholar]
- 56.Tibaduiza EC, Fleet JC, Russell RM, Krinsky NI. J Nutr. 2002;132:1368–1375. doi: 10.1093/jn/132.6.1368. [DOI] [PubMed] [Google Scholar]
- 57.Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD. J Nutr. 2004;134:667–673. doi: 10.1093/jn/134.3.667. [DOI] [PubMed] [Google Scholar]
- 58.Winum JY, Kamal M, Defacque H, Commes T, Chavis C, Lucas M, Marti J, Montero JL. Farmaco. 1997;52:39–42. [PubMed] [Google Scholar]
- 59.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Molecular endocrinology (Baltimore, Md. 2007;21:77–88. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]
- 60.Duncan RE, Lau D, El-Sohemy, M.C. A. Archer, Biochemical pharmacology. 2004;68:1739–1747. doi: 10.1016/j.bcp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Li B, Ma R, Chen B. Wei sheng yan jiu = Journal of hygiene research. 2004;33:151–153. 157. [PubMed] [Google Scholar]
- 62.Liu JR, Chen BQ, Yang BF, Dong HW, Sun CH, Wang Q, Song G, Song YQ. World J Gastroenterol. 2004;10:348–351. doi: 10.3748/wjg.v10.i3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mo H, Elson CE. J Nutr. 1999;129:804–813. doi: 10.1093/jn/129.4.804. [DOI] [PubMed] [Google Scholar]
- 64.Liu JR, Sun XR, Dong HW, Sun CH, Sun WG, Chen BQ, Song YQ, Yang BF. International journal of cancer. 2008;122:2689–2698. doi: 10.1002/ijc.23453. [DOI] [PubMed] [Google Scholar]
- 65.Aoki K, Takimoto M, Ota H, Yoshida T. Pharmacology & toxicology. 2000;87:26–32. doi: 10.1111/j.0901-9928.2000.870105.x. [DOI] [PubMed] [Google Scholar]
- 66.Jeong TC, Kim HJ, Yun CH, Lee SS, Yang KH, Han SS, Roh JK. Biochem Biophys Res Commun. 1995;216:198–202. doi: 10.1006/bbrc.1995.2610. [DOI] [PubMed] [Google Scholar]
- 67.Liu JR, Dong HW, Sun XR, Wang Q, Sun WG, Parry JW, Liu Q, Han XH, Sun CH, Chen BQ, Yang BF. Nutr Cancer. 2010;62:58–65. doi: 10.1080/01635580903191510. [DOI] [PubMed] [Google Scholar]
- 68.Lian F, Hu KQ, Russell RM, Wang XD. International journal of cancer. 2006;119:2084–2089. doi: 10.1002/ijc.22111. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, Kagechika H, Inoue M. Biochemical pharmacology. 2007;74:256–264. doi: 10.1016/j.bcp.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Lindqvist A, Andersson S. J Histochem Cytochem. 2004;52:491–499. doi: 10.1177/002215540405200407. [DOI] [PubMed] [Google Scholar]
- 71.Lindqvist A, He YG, Andersson S. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 72.Bhatti RA, Yu S, Boulanger A, Fariss RN, Guo Y, Bernstein SL, Gentleman S, Redmond TM. Investigative ophthalmology & visual science. 2003;44:44–49. doi: 10.1167/iovs.02-0167. [DOI] [PubMed] [Google Scholar]
- 73.Chichili GR, Nohr D, Schaffer M, von Lintig J, Biesalski HK. Investigative ophthalmology & visual science. 2005;46:3562–3569. doi: 10.1167/iovs.05-0089. [DOI] [PubMed] [Google Scholar]
- 74.Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. The Journal of biological chemistry. 2006;281:2835–2840. doi: 10.1074/jbc.M508903200. [DOI] [PubMed] [Google Scholar]
- 75.Voolstra O, Oberhauser V, Sumser E, Meyer NE, Maguire ME, Huber A, von Lintig J. The Journal of biological chemistry. 2010;285:2130–2139. doi: 10.1074/jbc.M109.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Investigative ophthalmology & visual science. 2002;43:3383–3392. [PubMed] [Google Scholar]
- 77.Prasain JK, Moore R, Hurst JS, Barnes S, van Kuijk FJ. J Mass Spectrom. 2005;40:916–923. doi: 10.1002/jms.868. [DOI] [PubMed] [Google Scholar]
- 78.Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Experimental eye research. 2008;86:70–80. doi: 10.1016/j.exer.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhosale P, Bernstein PS. Arch Biochem Biophys. 2007;458:121–127. doi: 10.1016/j.abb.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Survey of ophthalmology. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Siems W, Wiswedel I, Salerno C, Crifo C, Augustin W, Schild L, Langhans CD, Sommerburg O. The Journal of Nutritional Biochemistry. 2005;16:385–397. doi: 10.1016/j.jnutbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Liu C, Russell RM, Wang XD. J Nutr. 2003;133:173–179. doi: 10.1093/jn/133.1.173. [DOI] [PubMed] [Google Scholar]
- 83.Veeramachaneni S, Ausman LM, Choi SW, Russell RM, Wang XD. J Nutr. 2008;138:1329–1335. doi: 10.1093/jn/138.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]