Abstract
Summary
Background
Synaptic transmission can occur in a binary or graded fashion depending on whether transmitter release is triggered by action potentials or by gradual changes in membrane potential. Molecular differences of these two types of fusion events and their differential regulation in a physiological context have yet to be addressed. Complexin is a conserved SNARE-binding protein that has been proposed to regulate both spontaneous and stimulus-evoked synaptic vesicle (SV) fusion.
Results
Here, we examine complexin function at a graded synapse in C. elegans. Null complexin (cpx-1) mutants are viable although nervous system function is significantly impaired. Loss of CPX-1 results in a 3-fold increase in the rate of tonic synaptic transmission at the neuromuscular junction while stimulus-evoked SV fusion is decreased 10-fold. A truncated CPX-1 missing its C-terminal domain can rescue stimulus-evoked synaptic vesicle exocytosis but fails to suppress tonic activity, demonstrating that these two modes of exocytosis can be distinguished at the molecular level. A CPX-1 variant with impaired SNARE-binding also rescues evoked but not tonic neurotransmitter release. Finally, tonic but not evoked release can be rescued in a syntaxin point mutant by removing CPX-1. Rescue of either form of exocytosis partially restores locomotory behavior indicating that both types of synaptic transmission are relevant.
Conclusion
These observations suggest a dual role for CPX-1: suppressing SV exocytosis driven by low levels of endogenous neural activity while promoting synchronous fusion of SVs driven by a depolarizing stimulus. Thus, patterns of synaptic activity regulate complexin's inhibitory and permissive roles at a graded synapse.
Introduction
Neurotransmitter release is predominantly driven by action potentials in the vertebrate nervous system while graded neurotransmission is typically observed at sensory synapses and at dendrodendritic connections [1-4]. Invertebrate nervous systems display both impulse-driven and graded synaptic transmission, sometimes at the same synapse [5-7]. It is widely thought that the underlying mechanism of synaptic vesicle (SV) exocytosis is identical in these two forms of signaling although the molecular differences have yet to be explored. Synaptic transmission is initiated by the fusion of a synaptic vesicle with the presynaptic membrane, a highly regulated process involving a host of specialized presynaptic proteins [8-10]. SV fusion is catalyzed by the assembly of a four-helix bundle containing three major SNARE proteins: Synaptobrevin, SNAP-25, and Syntaxin. The detailed molecular chain of events progressing from SNARE assembly to SV fusion is not fully understood, but several SNARE binding proteins have been proposed to regulate this sequence [10, 11]. In particular, the protein complexin has garnered much interest because of its ability to bind to the assembled ternary SNARE complex with high affinity. Complexin is a 15-18 kDa cytoplasmic protein which contains a central α-helical region that binds within the groove between Synaptobrevin and Syntaxin in the assembled SNARE complex [12-16]. How the binding of complexin to the SNARE complex regulates the probability of SV fusion is controversial.
In vitro studies using SNARE-mediated fusion of proteoliposomes or cell-cell fusion have suggested that complexin either inhibits [17-19] or stimulates [20, 21] fusion. In several synaptic preparations, complexin function was explored in the context of two types of SV exocytosis events: action potential-triggered fusion initiated by a depolarizing stimulus (evoked release) and stochastic fusion events monitored in the absence of activity (spontaneous release). Neuronal cultures and brain slices derived from complexin knockout mice exhibited a reduction in evoked neurotransmitter release as well as decreased spontaneous fusion events [22-25]. In contrast, complexin knockdown by RNA interference led to elevated spontaneous release in conjunction with decreased evoked release [26]. Genetic deletion of complexin in Drosophila melanogaster also increased tonic fusion at the neuromuscular junction (NMJ) while simultaneously reducing evoked release [27, 28]. Cross-species rescue experiments and chimeric variants of complexin demonstrated that mouse and fly complexins share domains which can both enhance and inhibit exocytosis, indicating that complexin is a multifunctional protein and possibly has multiple duties at the synapse [25].
In this study, we investigated the consequences of removing the complexin ortholog CPX-1 on neurotransmission in C. elegans. We provide evidence that CPX-1 inhibits the continuous release of neurotransmitter driven by low levels of endogenous neural activity (tonic release). In contrast, CPX-1 is required for SV fusion evoked by a depolarizing stimulus electrode. Both these modes of synaptic transmission are shown to be behaviorally relevant. Each mode of exocytosis could be independently rescued, suggesting that complexin operates through distinct mechanisms during synaptic transmission. Thus, CPX-1 can function to regulate synaptic transmission in an activity-dependent manner. A detailed understanding of complexin's role may help delineate the molecular mechanisms underlying SV exocytosis.
Results
Mutants lacking CPX-1 have motor system defects
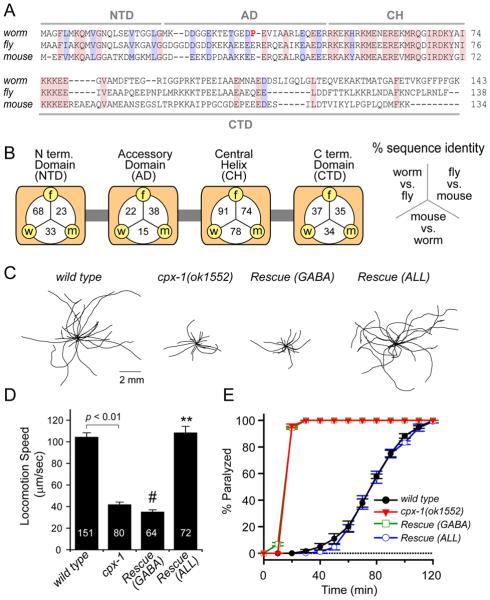
The C. elegans genome contains two complexin homologs: cpx-1 and cpx-2. Of these two genes, cpx-1 shares a higher degree of homology with the mouse (32% vs 19% identity) and fly (44% vs 28% identity) complexins (Figure 1A). The protein structure and function of mouse complexin suggests that it possesses four domains, with the central helix (CH) domain displaying the highest degree of conservation. The worm complexin protein CPX-1 contains a CH domain with 78% identity to the mouse Cplx1 and 91% identity to the Drosophila complexin, dmCplx (Figure 1B). In order to study the role of complexin in the worm nervous system, we utilized the mutant cpx-1(ok1552), which carries a 1.3 kb deletion of the cpx-1 gene removing one of its two exons. cpx-1(ok1552) animals are viable but have significant defects in locomotion, while heterozygous animals are indistinguishable from wild type. We also examined cpx-2 mutants and based on expression data and behavioral studies, we found that CPX-2 does not function redundantly with CPX-1 (Figure S1). We thus focused on CPX-1 as the major complexin in C. elegans.
Figure 1. cpx-1 mutants display motor system defects.
A. Protein sequence alignment for C. elegans CPX-1 (worm), D. melanogasterdmCplx Genbank AAF69518.1 (fly), and mouse Cplx1 (mouse) using a ClustalW algorithm. The N-terminal domain (NTD), accessory domain (AD), central helix (CH) and C-terminal domain (CTD) are indicated in gray. Identically-conserved residues across all three species are shaded in red while similar residues are shaded in blue. B. Percent sequence identity for each domain is indicated for each pair-wise comparison: worm (w), fly (f), and mouse (m). C. Representative trajectories for 20 animals for wild type, cpx-1(ok1552), cpx-1 expressing a rescuing cDNA under a GABA promoter, and cpx-1 expressing a rescuing cDNA in all neurons. D. Summary of average speed for the four strains. The number of animals measured for each average is indicated within the bar. E. Paralysis time course on 1.0 mM aldicarb for wild type (black circles), cpx-1 (red triangles), rescue in GABA neurons (green open squares), and rescue in all neurons (blue open circles). Data are mean ± SEM. # = significantly different from wild type (p<0.01) but not significantly different from cpx-1. ** = significantly different from cpx-1 (p<0.01) but not significantly different from wild type. Significance determined by Tukey-Kramer Method.
We quantified the decrease in locomotion of cpx-1 mutants by recording the center-of-mass speed of individual animals moving on an agar plate (Figure 1C). Compared to wild-type controls, cpx-1 mutants moved at significantly slower speeds (40 ± 2.2% of wild type), consistent with a nervous system defect (Figure 1D). We cloned the full-length cDNA encoding CPX-1 and expressed a CPX-1::GFP fusion protein under a neuronal promoter (snb-1 Synaptobrevin) in cpx-1 mutants. CPX-1::GFP restored locomotion (104 ± 5.7% of wild type) confirming that the locomotion defect was specific to loss of complexin in the nervous system (Figure 1D).
We also examined the sensitivity of cpx-1 mutants to aldicarb, a cholinesterase inhibitor. Acetylcholine (ACh) levels in the neuromuscular junction (NMJ) are rapidly reduced through the enzymatic action of acetylcholinesterases, and inhibition of these enzymes results in a buildup of ACh followed by paralysis of the animal [29]. Animals with defects in ACh secretion are resistant to aldicarb whereas animals secreting higher than normal levels of ACh are hypersensitive and quickly paralyze upon exposure to aldicarb [30-32]. We examined the time course of paralysis on 1.0 mM aldicarb for wild type and cpx-1 mutant animals (Figure 1E). cpx-1 mutants paralyzed within 20 minutes of aldicarb exposure while wild-type animals required two hours to paralyze. Neuronal expression of CPX-1::GFP completely restored normal aldicarb sensitivity, suggesting that CPX-1 normally inhibits the secretion of ACh. Alternatively, if CPX-1 specifically acts in GABA motor neurons to promote release, decreased GABA release in the cpx-1 mutant could also account for its hypersensitivity to aldicarb [33]. To test this hypothesis, we attempted to rescue cpx-1 mutants with CPX-1::GFP under a GABA-specific promoter (unc-25 GAD). Neither locomotion nor aldicarb sensitivity were altered (Figure 1D&E) suggesting that defects in GABA neuron function in cpx-1 mutants cannot account for the observed behavioral phenotypes. Consistent with a role for CPX-1 in ACh release, we found that rescue of CPX-1::GFP under a cholinergic promoter (unc-17 VAChT) nearly completely rescued wild-type aldicarb sensitivity (Figure S2A).
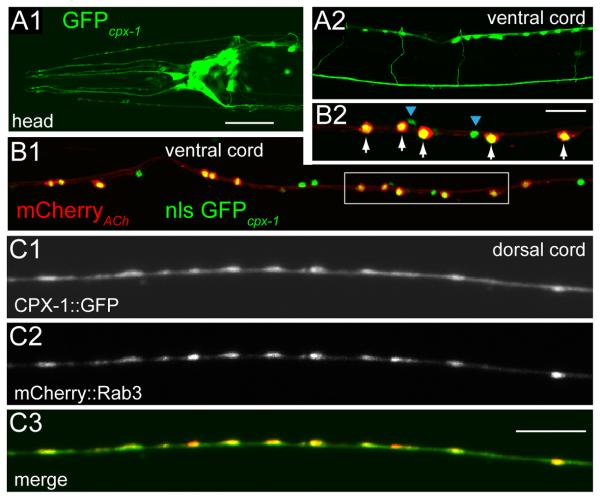
CPX-1 is a synaptic protein broadly expressed in the nervous system
To examine the expression of cpx-1 in C. elegans, we drove expression of GFP under the control of the cpx-1 promoter (3 kb upstream region). Expression was observed in a large number of neurons in the head, ventral cord, and tail, including all cholinergic and GABAergic motor neurons in the ventral cord (Figure 2A&B). In contrast, cpx-2 is expressed in only a small number of neurons having little or no overlap with cpx-1 expression (Figure S1B). To examine the subcellular localization of CPX-1, CPX-1::GFP was imaged in the dorsal nerve cord under the control of a modified unc-129 promoter which expresses in a subset of DA and DB cholinergic motor neurons [34]. CPX-1::GFP was diffusely expressed throughout the axon with some enrichment at presynaptic terminals based on colocalization with an SV marker mCherry::Rab-3 (Figure 2C).
Figure 2. CPX-1 is expressed throughout the nervous system and localizes to synapses.
A1. Fluorescent image of the head region expressing GFP under the cpx-1 promoter. A2. Expression of GFP under the cpx-1 promoter in the body region. Note the cell bodies in the ventral nerve cord at the top with commissural fibers extending to the dorsal nerve cord. B1. Expression of mCherry under a cholinergic promoter (unc-17 VAChT) and a nuclear-localized GFP under the cpx-1 promoter in the ventral nerve cord. B2. Expanded view of the inset in B1 showing individual cholinergic (white arrows) and GABAergic (blue arrowheads) motor neurons expressing cpx-1. Fluorescent images of the dorsal nerve cord expressing a CPX-1::GFP fusion protein (C1) and mCherry::Rab3 (C2). C3. The two fluorescent images merged and color coded. Scale bars are 25 μm in A1/2, 30 μm in B1, 10 μm in B2, 5 μm in C1-C3.
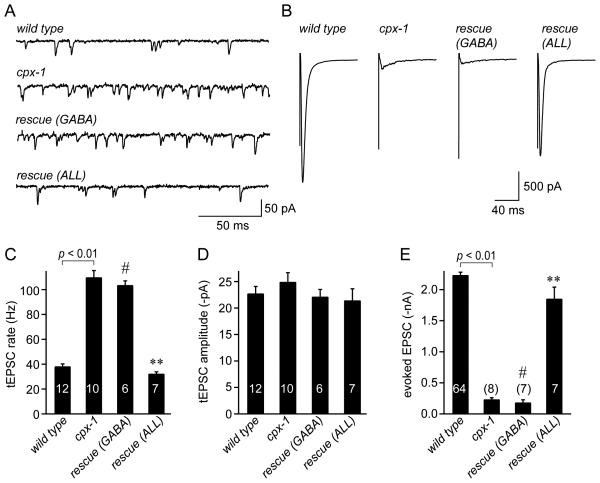
Tonic activity is elevated and evoked release is decreased in the absence of complexin
The behavioral assays and expression pattern indicate that CPX-1 plays a major role in the nervous system. To examine the effects of removing CPX-1 on synaptic transmission, we recorded synaptic activity at the NMJ in dissected animals. The worm NMJ is a graded synapse and basal activity in the nervous system of the dissected animal continuously drives neurotransmitter release as measured by whole-cell patch-clamp recordings from body wall muscle [7]. This tonic excitatory postsynaptic current (tEPSC) rate depended on external calcium since over 90% of these fusion events were eliminated in zero calcium external solution (Figure S3). cpx-1 mutants display a large increase (290% of wild type) in tonic EPSC rate (tEPSC rate, Figure 3A&C), with no change in the tEPSC amplitude (Figure 3D). Even in low calcium, spontaneous fusion was significantly increased in cpx-1 mutants (Figure S3). Surprisingly, EPSCs evoked by a depolarizing stimulus electrode (eEPSCs) were decreased by about 90% in cpx-1 mutants (Figure 3B&E), suggesting a requirement for CPX-1 during SV fusion evoked by high levels of activity. Total synaptic charge was decreased to a similar degree indicating that the eEPSC peak was not decreased simply due to asynchrony of the evoked fusion events in the cpx-1 mutant (data not shown). Consistent with the behavioral assays, CPX-1 expression in GABA neurons failed to rescue either the tEPSC rate or eEPSC whereas expression in all neurons rescued both modes of exocytosis. Thus, SV exocytosis is suppressed by CPX-1 during low levels of activity while high levels of activity require CPX-1 to promote synchronous exocytosis.
Figure 3. Tonic release increases while evoked release decreases at the NMJ in the absence of complexin.
Examples of tonic (A) and evoked (B) EPSCs for wild type, cpx-1(ok1552), rescue in GABA neurons, and rescue in all neurons. Average tonic EPSC rate (C), tEPSC amplitude (D), and evoked EPSC peak (E) for each of the four strains shown above. See Methods for details of recordings. The tEPSC rate increased in cpx-1 mutants and remained elevated in the GABA rescue transgenics. Rescue in all neurons decreased the tonic release rate slightly below wild type. The evoked EPSC decreased in cpx-1 mutants and rescue in GABA neurons failed to restore the EPSC while rescue in all neurons restored the EPSC. Data are mean ± SEM. # = significantly different from wild type (p<0.01) but not significantly different from cpx-1. ** = significantly different from cpx-1 (p<0.01) but not significantly different from wild type. Significance determined by Tukey-Kramer Method.
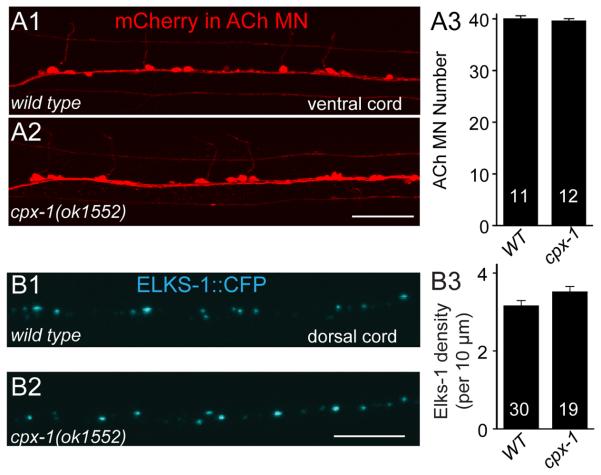
Synaptic alterations of cpx-1 mutants are not the result of changes in nervous system development
Complexin has been proposed to play a role in synapse development in mouse and fly [27, 35] so it is important to examine whether the nervous system has been altered in cpx-1 mutants. In particular, developmental changes in the motor neurons and NMJs may explain the behavioral and electrophysiological effects described above. The number of cholinergic motor neurons in the ventral cord was identical between wild type and cpx-1 mutant animals (Figure 4A). Furthermore, we counted the number of active zones per unit length of axon by imaging the fluorescently-tagged active zone (AZ) protein ELKS-1::CFP in wild type and cpx-1 mutant animals and found nearly identical AZ densities in the two strains (Figure 4B). Thus neither the number of motor neurons nor synapse number is altered appreciably in the absence of cpx-1.
Figure 4. No gross changes in motor neuron number or synapse density were observed in cpx-1 mutants.
A. The ventral nerve cord was imaged in animals expressing mCherry under a cholinergic promoter in wild type (A1) and cpx-1(ok1552) mutant (A2) backgrounds. There was no significant difference in the number of ventral cord motor neurons in both genotypes as summarized in A3. B. The dorsal nerve cord was imaged in animals expressing the active zone protein ELKS-1::CFP in a subset of DA/DB motor neurons in wild-type (B1) and cpx-1 mutant (B2) backgrounds. Loss of CPX-1 did not significantly alter the NMJ active zone density as summarized in B3. Data are mean ± SEM. Number of animals analyzed is indicated within each bar. Scale bars are 5 μm in A and B.
Over-expression of CPX-1 does not affect the NMJ
The experiments described above establish that loss of CPX-1 at the synapse directly leads to dysregulation of tonic and stimulus-evoked neurotransmission. If complexin functions as a fusion clamp by binding the trans-SNARE complex, then it is possible that over-expressing CPX-1 will inhibit synaptic transmission and alter behavior by driving the formation of excess CPX-1/trans-SNARE complexes [22, 25, 36] . We examined the aldicarb sensitivity of cpx-1 mutant animals expressing different amounts of a fluorescently-tagged rescuing complexin (CPX-1::pG). We found that rescue was indistinguishable over a five-fold range of protein expression as measured by axonal fluorescence levels (Figure S5A&D). There was also no effect on aldicarb sensitivity when CPX-1 was over-expressed in subsets of motor neurons or in synaptic mutants with elevated rates of transmitter release (Figure S5B&C). Finally, we observed no change in locomotory behavior in these over-expression strains (data not shown). Thus CPX-1 does not appear to be a limiting factor in the regulation of synaptic transmission.
Effects of CPX-1 domain deletions on behavior
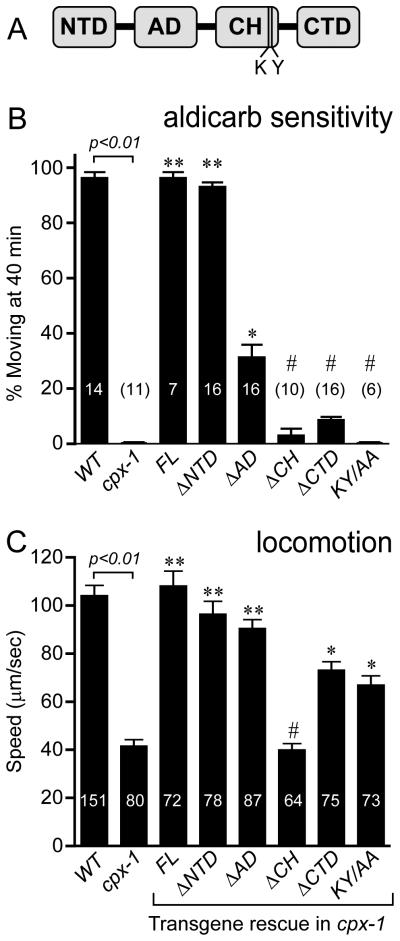
Having established that over-expressing CPX-1 does not alter aldicarb sensitivity or locomotion, we analyzed the ability of four domain-deletion mutants and a double point mutant to rescue the aldicarb hypersensitivity and locomotion defects of cpx-1 mutants. The rescue constructs were fused to GFP in order to assess relative protein expression levels in the axon. For the strains presented here, all mutant CPX-1 variants were expressed at the synapse with a comparable abundance to the full-length rescue construct (Figure S5D). In agreement with mammalian complexin studies, the central helix was required for CPX-1 function in that its deletion (ΔCH) completely eliminated rescue of both aldicarb sensitivity and locomotion (Figure 5). A double point mutation near the end of the central helix (K71A, Y72A) significantly decreases SNARE binding in this highly conserved region of complexin in both mouse and fly [25, 36]. As expected from the ΔCH rescue, the KY/AA mutation eliminates rescue of aldicarb sensitivity (Figure 5B). However, we observed a significant recovery of locomotory function in the KY/AA mutant, suggesting that some aspect of CPX-1 function persists in this SNARE-binding mutant (Figure 5C).
Figure 5. Effects of removing complexin protein domains on behavior.
A. Cartoon depicting the four protein domains of CPX-1: N-terminal Domain (NTD), Accessory Domain (AD), Central Helix (CH), and C-terminal Domain (CTD). B. Average percentage of animals paralyzed on 1.0 mM aldicarb after 40 minutes for wild type (WT), cpx-1, full-length rescue CPX-1 (FL), rescue with each of the domains individually deleted as indicated, and a double point mutant K71A Y72A (KY/AA) all in cpx-1(ok1552). Number of aldicarb assays for each strain is indicated either within or above the bar. C. Average speed for the same strains. Number of animals analyzed for each strain is indicated within or above the bar. Data are mean ± SEM. # = significantly different from wild type (p<0.01) but not significantly different from cpx-1. ** = significantly different from cpx-1 (p<0.01) but not significantly different from wild type. * = significantly different from both cpx-1 and wild type (p<0.01). Significance determined by Tukey-Kramer Method. Details of the domain deletions are described in the Methods section.
Interestingly, loss of the N-terminal domain (ΔNTD) had no effect on rescue whereas loss of the C-terminal domain (ΔCTD) impaired rescue in both behavioral assays (Figure 5). Deletion of the accessory domain (ΔAD) impaired rescue of aldicarb sensitivity to some extent while locomotion was almost fully restored (Figure 5). Note that we refer to this region of complexin as the accessory domain rather than helix since the C. elegans ortholog contains a proline residue within this region (see Figure 1) so a purely alpha helical conformation would not be expected in this region of CPX-1.
Effects of CPX-1 domain deletions on synaptic transmission
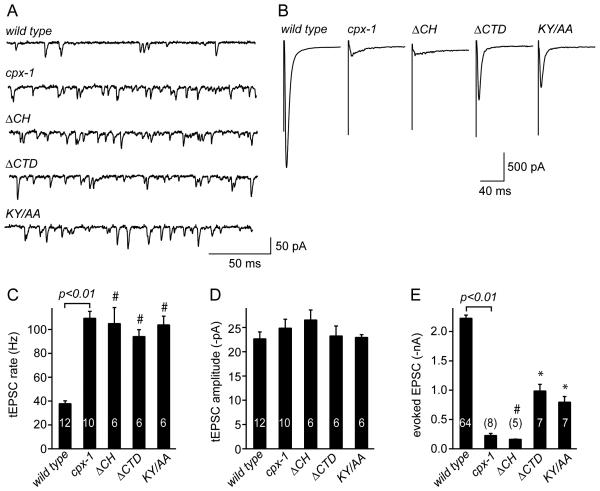
We directly tested the contributions of the CH and CTD domains to the regulation of synaptic transmission by monitoring SV fusion at the NMJ in these domain-deletion rescue animals. Rescue of cpx-1 with the SNARE-binding mutant KY/AA failed to rescue the tonic rate of fusion (Figure 6A&C). Likewise, a truncated CPX-1 variant lacking its CTD also failed to inhibit tonic neurotransmission, suggesting that both the SNARE-binding region and the C terminus are important for complexin's inhibitory function (Figure 6A&C). In contrast, the eEPSC was partially restored in both ΔCTD and KY/AA rescue animals, indicating that these regions contribute to but are not essential to CPX-1 function during stimulus-evoked SV fusion (Figure 6B&E). In both cases, restoration of evoked neurotransmitter release correlated with rescue of locomotion, demonstrating the behavioral relevance of evoked release. In all cases, no effect on the tEPSC amplitude was observed (Figure 6D). Thus, the eEPSC can be rescued independently of the tEPSC, further supporting the notion that complexin separately regulates these two modes of exocytosis.
Figure 6. Central Helix-SNARE interactions and the C-terminal domain of CPX-1 are required for its inhibition of tonic but not evoked SV fusion.
Representative traces for tonic (A) and evoked (B) EPSCs are shown for wild type, cpx-1(ok1552), rescue with a central helix deletion (ΔCH), a C-terminal domain deletion (ΔCTD), and rescue with a double point mutant in the central helix predicted to decrease SNARE binding (KY/AA) all in cpx-1(ok1552). Average values for the frequency (C) and amplitude (D) of tEPSCs and peak eEPSC amplitude (E) are shown for each strain. Data are mean ± SEM. # = significantly different from wild type (p<0.01) but not significantly different from cpx-1. * = significantly different from both cpx-1 and wild type (p<0.01). Significance determined by Tukey-Kramer Method.
Loss of complexin rescues a Syntaxin mutant
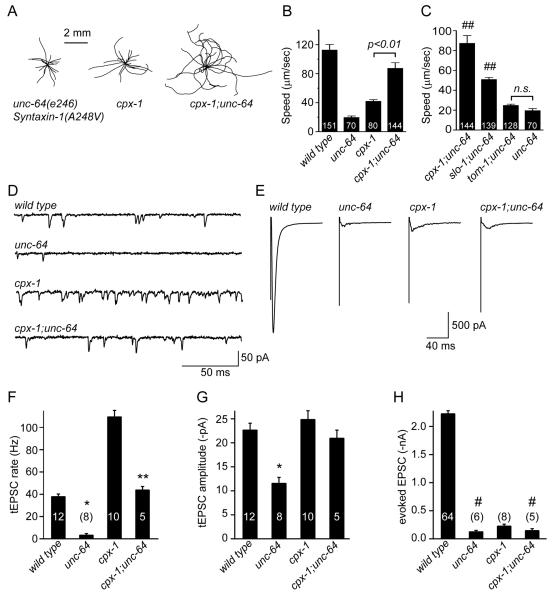
The elevated rate of tonic neurotransmission observed in cpx-1 mutants is distinct among known synaptic mutants in C. elegans. Two hypersecretion mutants have previously been characterized at the worm neuromuscular junction: slo-1 BK channel and tom-1 Tomosyn. Interestingly, evoked SV fusion is significantly increased in both of these mutants while tonic SV fusion is relatively unaltered [37-39], further supporting the notion that these two modes of exocytosis can be distinguished at the molecular level. Because some SNARE mutations that disrupt assembly of the 4-helix bundle are thought to slow the forward rate of fusion [26, 40], we wondered if removing complexin's inhibitory function would counteract a SNARE mutation and restore fusion. To test this hypothesis, we utilized a mutant Syntaxin 1 where a membrane-proximal conserved SNARE residue (alanine 248) is substituted with valine: unc-64 Syx(A248V)[41]. This mutation is not in the region of Syntaxin 1 which interacts with complexin so initial trans-SNARE assembly and complexin binding would not be predicted to be altered. However, the unc-64 Syx(A248V) nearly eliminates both tonic and stimulus-evoked SV fusion, suggesting that a late stage in SNARE assembly is impaired [39].
Intriguingly, while both unc-64 Syx(A248V) and cpx-1 moved at much lower speeds than wild-type animals (17 ± 1.9% and 37 ± 2.1% of wild type respectively), the cpx-1;unc-64Syx(A248V) double mutant displayed near wild-type locomotion (78 ± 6.9%, Figure 7A&B). Thus, loss of CPX-1 appears to restore motor system function in the Syntaxin mutant. In addition to slower locomotion, unc-64 Syx(A248V) mutants sometimes fail to initiate movement and remain quiescent throughout the locomotion assay (17.1 ± 4% quiescent versus 2.7 ± 1.6% for wild type – see Figure S6A). The cpx-1;unc-64Syx(A248V) double mutant failed to reduce the elevated quiescence, demonstrating that some locomotor phenotypes are not restored in the absence of complexin.
Figure 7. Loss of CPX-1 restores tonic fusion in a Syntaxin 1 mutant.
A. Representative locomotion trajectories for a hypomorphic allele of Syntaxin 1 unc-64(e246) corresponding to A248V as well as cpx-1 and the cpx-1 Syntaxin(A248V) double mutant (cpx-1;unc-64). B. Summary of average speed for wild-type (WT) animals in addition to the three strains described above. C. Summary of average speed for Syntaxin 1(A238V) unc-64 mutants and three double mutants where unc-64 is paired with cpx-1 (cpx-1;unc-64), slo-1 BK Channel (slo-1;unc-64), and tom-1 Tomosyn (tom-1;unc-64). Number of animals analyzed for each strain is indicated within the bar. ## = significantly different from unc-64 (p<0.01). Representative traces for tonic (D) and evoked (E) EPSCs are shown for all four strains. Average values for the frequency (F) and amplitude (G) of tEPSCs and peak eEPSC amplitude (H) are shown for each strain. Data are mean ± SEM. # = significantly different from wild type (p<0.01) but not significantly different from cpx-1. ** = significantly different from cpx-1 (p<0.01) but not significantly different from wild type. * = significantly different from both cpx-1 and wild type (p<0.01). Significance determined by Tukey-Kramer Method.
While it is possible that mutants with enhanced synaptic transmission may generally rescue the Syntaxin mutant, loss of complexin had a significantly larger impact on the unc-64 Syx(A248V) mutant than the hypersecretion mutants slo-1 BK channel and tom-1 Tomosyn. Although evoked SV exocytosis is increased in each mutant [37-39], neither could restore locomotion to the same extent as loss of CPX-1 (Figure 7C). Finally, rescue of locomotion in cpx-1 might arise simply from an impairment of SV fusion that protects a pool of vesicles from depletion. We examined three mutants that are defective in distinct aspects of SV exocytosis: snb-1 Synaptobrevin, unc-2 Ca2+ voltage-dependent channel, snt-1 Synaptotagmin, and found that locomotion was not significantly rescued (Figure S6B).
We next examined the effects of removing CPX-1 in the A248V Syntaxin mutant on SV fusion. While unc-64 Syx(A248V) almost eliminated tEPSC fusion events, the cpx-1;unc-64Syx(A248V) double mutant displayed wild-type rates (Figure 7D&F). In contrast, the eEPSC amplitude was not restored in the cpx-1;unc-64Syx(A248V) double, highlighting the distinct role of CPX-1 in supporting this mode of exocytosis (Figure 7E&H). The average tEPSC amplitude was decreased by about 43% in the unc-64Syx(A248) mutant and restored in the cpx-1;unc-64 Syx(A248V) double mutant (Figure 7G). While smaller tonic fusion events might cause an apparent decrease in tEPSC frequency if a significant number fall below detection threshold, a 43% decrease in tEPSC amplitude is expected to cause only a 0.2% decrease in tEPSC frequency under our recording conditions (Figure S6C&D). Thus, nearly all of the observed decrease in tEPSC rate (and its restoration in the double mutant) represents a true change in fusion event frequency. These observations suggest that most of the tonic exocytosis defect observed in the unc-64 Syx(A248V) mutant was specifically alleviated by removal of CPX-1 while evoked EPSCs were largely unaffected. Furthermore, restoration of tonic neurotransmission was accompanied by an increase in locomotion, demonstrating that this mode of exocytosis is relevant to locomotory behavior.
Discussion
The results of this study lead us to several conclusions about the role of complexin in C. elegans. First, animals develop normally in the absence of CPX-1. Second, CPX-1 has both negative and positive functions in neurotransmission: it inhibits fusion of SVs driven by endogenous activity while simultaneously promoting synchronous neurotransmitter release evoked by a strong depolarizing stimulus. Third, the positive and negative functions can be separated within the structure of complexin. The N-terminal domain does not appear to play a major role in CPX-1 function while the SNARE-interacting central helix is essential for both regulatory functions of CPX-1. The C-terminal domain and tight SNARE binding are required only for complexin's inhibitory role. Fourth, complexin's function in the final stages of fusion is underscored by its genetic interactions with syntaxin. Specifically, a syntaxin `zippering' mutant can be rescued by removing complexin. These data suggest that complexin bound to the trans-SNARE complex raises the energy barrier for SV fusion. The opposing effects of complexin removal on tonic and evoked release suggest that these are distinct exocytic events that can be separated at the molecular level (see model in Figure S7A).
Separating functions of CPX-1 domains
N-terminal Domain (NTD)
There is evidence from murine culture studies that the NTD facilitates evoked SV fusion and a few residues within the NTD are identical across several phyla (68% identity between worm and fly), suggesting a conserved function of this domain [24, 25]. The fly complexin NTD, however, does not appear to affect synaptic transmission when expressed in mouse neurons [25]. Similar to dmCplx, we observed complete rescue of function with a version of CPX-1 missing the entire NTD. Thus, this domain does not play a major role in complexin function in C. elegans.
Accessory Domain (AD)
Prior studies on the AD have suggested an inhibitory role in fusion [22, 25, 42, 43]. The nematode CPX-1 AD contains a proline and therefore will probably not adopt a purely helical structure. Regardless, aldicarb sensitivity cannot be fully rescued in the absence of this domain so this region contributes to the suppression of ACh release, in agreement with fly and mouse complexin.
Central Helix (CH)
The physical interaction of complexin with the SNARE complex is mediated by residues of the CH and residues lining the groove between Syntaxin and Synaptobrevin. The CH-deleted CPX-1 failed to rescue in all of our behavioral and electrophysiological assays suggesting an essential requirement for complexin function in the worm, a conclusion that holds for all studies of complexin function. SNARE binding is reduced by mutating two key residues (K71A Y72A) [22, 36]. This mutation eliminated the facilitatory and inhibitory functions of mouse Cplx1 as well as the inhibitory function of fly dmCplx in mouse cultured neurons [22, 25, 26]. In contrast, we found that the defective CPX-1(KY/AA) can still support evoked fusion to a significant degree at the C. elegans NMJ, suggesting that tight SNARE binding is not essential for promoting neurotransmission.
C-terminal Domain (CTD)
Little is known about the structure or function of the C-terminal domain of complexin. An in vitro study suggests that this region of complexin contributes to its function by binding to membranes and stimulating liposome fusion [44]. However, in mouse autaptic cultures, a truncated Cplx1 missing its entire CTD was able to rescue synaptic transmission in the Cplx1/2 double knock out [22]. In contrast, the fly dmCplx CTD is required for suppression of spontaneous and evoked SV fusion at the fly NMJ [28] and in mouse autaptic cultures [25]. We find that without its CTD, CPX-1 can no longer suppress tonic release, establishing a requirement for the CTD in the fusion clamp activity of complexin. In contrast to tonic release, the CTD deletion mutant retains 50% normal evoked responses, demonstrating that this domain is not essential for the facilitation of evoked SV fusion by complexin.
A distinct role of complexin during stimulus-evoked release
Decreased evoked release has been observed in mouse [14, 22, 26], fly [27], and worm synapses (this study) lacking complexin. This deficit could have simply reflected the depletion of a readily releasable pool of SVs in the wake of an elevated spontaneous fusion rate. Indeed, Hobson et al report a substantial decrease in SV number in cpx-1 mutant worms suggesting that vesicle pool depletion contributes to this defect in exocytosis [45]. However, our data reveal a separate role for complexin in supporting evoked fusion. We show here that evoked release can be restored to a significant degree even when tonic release remains elevated (KY/AA and CTD deletion rescue). The central helix is required for rescue of evoked release indicating that perhaps the central helix has additional functions during evoked fusion. Perhaps complexin interacts with another target at the synapse to promote fusion when calcium levels are elevated. Further studies will be needed to address this possibility.
Possible roles for complexin in the regulation of synaptic transmission
By demonstrating opposing roles for complexin in the control of tonic release versus evoked responses, we have shown that both forms of synaptic transmission are distinct and differentially regulated. If the level of synaptic activity determines whether complexin will inhibit or support SV fusion, complexin can serve as a temporal filter, suppressing low frequency activity while boosting higher frequencies (Fig S7B). The tonic and phasic outputs seem to play different roles in the regulation of behavior. Tonic release in the absence of evoked release is sufficient for basal locomotory speed but not normal levels of foraging behavior. Thus, complexin can act as a master switch for temporal coding mechanisms at the synapse, gating the balance of tonic and phasic output.
Experimental Procedures
Details of the behavioral assays, electrophysiology, and fluorescence imaging as well as a list of all strains used in this study can be found in Supplemental Experimental Procedures.
Complexin Mutants and Rescue Constructs
C. elegans full-length complexin CPX-1 is 143 amino acids encoded by a 1.2 kb gene harboring a single 764 base intron. For the structural variants of CPX-1 used in this study, we used the following residue positions to define each domain. The first 30 amino acids are removed in the N-terminal deletion CPX. Residues 31 through 50 are removed in the accessory domain deletion. The central helix deletion corresponds to residues 51 through 79. The C-terminal domain deletion removes the final 50 residues of CPX-1 and includes a 12 residue linker between CPX-1 and paGFP.
Statistical Analysis
For datasets requiring a single pair-wise comparison, we used Student's t-test to compute significance. For all other comparisons, we used the Tukey-Kramer method for multiple comparisons. This test assumes independent, normal distributions with equal variance. In cases where sample variance was significantly different (calculated using Levene's test), we employed the Newman-Keuls method to test significance. Significance was defined by the criterion p < 0.01.
Supplementary Material
Acknowledgements
We thank the C. elegans stock center and S. Mitani for strains. We thank Miriam Goodman's lab for use of their worm tracking software, Cori Bargmann, Josh Kaplan, Jihong Bai, Peter Juo, Anant Menon, Tim Ryan and members of the Ryan lab for advice and critically reading the manuscript, and Kashyap Rajagopal for assisting in developing the locomotion analysis. This work was supported by awards from the Rita Allen Foundation (188423) and the March of Dimes (Basil O'Connor Award 5-FY09-133) and by National Institutes of Health Grant GM095674 (J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katz B, Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967;167:8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt RO, Dev P, Smith BH. Electrotonic processing of information by brain cells. Science. 1976;193:114–120. doi: 10.1126/science.180598. [DOI] [PubMed] [Google Scholar]
- 3.Heidelberger R. Mechanisms of tonic, graded release: lessons from the vertebrate photoreceptor. J Physiol. 2007;585:663–667. doi: 10.1113/jphysiol.2007.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Manor Y, Nadim F, Abbott LF, Marder E. Temporal dynamics of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci. 1997;17:5610–5621. doi: 10.1523/JNEUROSCI.17-14-05610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows M. Graded synaptic interactions between local premotor interneurons of the locust. J Neurophysiol. 1979;42:1108–1123. doi: 10.1152/jn.1979.42.4.1108. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Hollopeter G, Jorgensen EM. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc Natl Acad Sci U S A. 2009;106:10823–10828. doi: 10.1073/pnas.0903570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 9.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 10.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melia TJ., Jr. Putting the clamps on membrane fusion: how complexin sets the stage for calcium-mediated exocytosis. FEBS Lett. 2007;581:2131–2139. doi: 10.1016/j.febslet.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 13.McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 14.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 15.Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray structure of a neuronal complexin-SNARE complex from squid. J Biol Chem. 2002;277:26517–26523. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- 16.Pobbati AV, Razeto A, Boddener M, Becker S, Fasshauer D. Structural basis for the inhibitory role of tomosyn in exocytosis. J Biol Chem. 2004;279:47192–47200. doi: 10.1074/jbc.M408767200. [DOI] [PubMed] [Google Scholar]
- 17.Chicka MC, Chapman ER. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry. 2009;48:657–659. doi: 10.1021/bi801962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 19.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 20.Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM, Sollner TH. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc Natl Acad Sci U S A. 2009;106:2001–2006. doi: 10.1073/pnas.0812813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon TY, Lu X, Diao J, Lee SM, Ha T, Shin YK. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2008;15:707–713. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, Brose N, Rosenmund C. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat Struct Mol Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci U S A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010 doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, Rosenmund C. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 28.Cho RW, Song Y, Littleton JT. Comparative analysis of Drosophila and mammalian complexins as fusion clamps and facilitators of neurotransmitter release. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rand JB, Russell RL. Molecular basis of drug-resistance mutations in C. elegans. Psychopharmacol Bull. 1985;21:623–630. [PubMed] [Google Scholar]
- 30.Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci U S A. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dittman JS, Kaplan JM. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J Neurosci. 2008;28:7104–7112. doi: 10.1523/JNEUROSCI.0378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 33.Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, Ch'ng Q, Tavazoie M, Kaplan JM. An RNAi screen identifies genes that regulate GABA synapses. Neuron. 2008;58:346–361. doi: 10.1016/j.neuron.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Sieburth D, Ch'ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, de la Iglesia N, Shen J, Bonni A. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326:575–578. doi: 10.1126/science.1177087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Gracheva EO, Burdina AO, Holgado AM, Berthelot-Grosjean M, Ackley BD, Hadwiger G, Nonet ML, Weimer RM, Richmond JE. Tomosyn inhibits synaptic vesicle priming in Caenorhabditis elegans. PLoS Biol. 2006;4:e261. doi: 10.1371/journal.pbio.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen JM, Madison JM, Dybbs M, Kaplan JM. Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron. 2006;51:303–315. doi: 10.1016/j.neuron.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZW, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 40.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 41.Saifee O, Wei L, Nonet ML. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell. 1998;9:1235–1252. doi: 10.1091/mbc.9.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu B, Song S, Shin YK. Accessory alpha-helix of complexin I can displace VAMP2 locally in the complexin-SNARE quaternary complex. J Mol Biol. 2010;396:602–609. doi: 10.1016/j.jmb.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seiler F, Malsam J, Krause JM, Sollner TH. A role of complexin-lipid interactions in membrane fusion. FEBS Lett. 2009;583:2343–2348. doi: 10.1016/j.febslet.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin inhibits spontaneous fusion but stimulates evoked release in C. elegans. Current Biology. 2011 doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.