Abstract
Objectives
Previously we reported that annexin 2 (anxa2) plays an important role in hematopoietic stem cell (HSC) localization to the endosteal/osteoblastic marrow niche. The study explored the role that annexin 2 plays in presenting stromal derived factor-1 (SDF-1 or CXCL12) to HSCs.
Materials and Methods
Competitive long-term bone marrow transplant (CLT-BMT) assays were used to determine if HSC engraftment is altered in annexin 2-deficient animals. Colony-forming cell assays, CXCL12 Elisa, and real-time RT-PCR analyses were employed to determine stem or progenitor cell mobilization by G-CSF. Immunohistochemistry, immunoprecipitation, binding assays, and chemotactic assays were employed to determine if annexin 2 is associated with CXCL12. Degradation assays were also used to determine if annexin 2 and CXCL12 protect each other from proteolytic degradation.
Results
Anxa2−/− animals have fewer HSC in their marrows, and the HSCs in anxa2−/− animals express less CXCR4 and CXCR7 suggesting a cell intrinsic defect. Transplantation studies of wild-type marrow into anxa2−/− animals demonstrated a cell extrinsic defect in the anxa2−/− animals. CXCL12 binds directly to annexin 2, and this interaction facilitates the presentation of CXCL12 to HSCs. Yet the binding of CXCL12 to annexin 2 does not protect CXCL12 from proteolytic cleavage following stem or progenitor cell mobilization by G-CSF.
Conclusions
These results suggest that annexin 2 serves as an anchor for CXCL12 to help in the localization of HSCs to the niche.
Keywords: Annexin 2, CXCL12, osteoblasts, HSCs, localization, homing, mobilization
INTRODUCTION
In adult mammals nearly all tissues maintain a population of stem cells that sustains normal tissue homeostasis and are able to respond to injury. Under basal conditions, the slowly cycling stem cells are supported and regulated by their microenvironment, commonly referred to as the stem cell niche. Hematopoietic stem cell (HSCs) activities are now known to reside almost exclusively in bone marrow niches in post-fetal life. Within the niche, HSCs are believed to receive both quiescence and growth stimulating signals originating from diverse sources, including fibroblasts, endothelial, reticular cells, adipocytes, and osteoblasts1-5.
As early as the 1970’s, investigation suggested that events localized to endosteal surfaces, suggesting osteoblasts play a central role in hematopoiesis 6,7. Since then it has been shown that osteoblasts are crucial components of the normal HSC niche 3-5,8,9, where HSCs reside in close proximity to endosteal bone surfaces rather than being randomly distributed in the marrow cavity 10,11. Moreover, HSCs lodge near endosteal surfaces during bone marrow transplantation, further suggesting a role for osteoblasts as niche regulators 12,13. In addition, significant progress has been made in determining the role that niche-derived cytokines and adhesion molecules play in HSC activities including VCAM-1, ICAM-1, CD44, CD164, osteopontin, N-cadherin, calcium, Wnt signaling pathways, Notch-1/Jagged-1 interactions, and Ang-1/Tie2-mediated events 2,14-17. Many of these signals are regulated by osteoblasts.
Recently, it was determined that cell-to-cell contact is critical for the survival of HSCs on osteoblasts 18. In addition, it was established that osteoblasts express stromal-derived factor-1 (SDF-1 or CXCL12)15, a key regulator of stem cell migration, homing, retention, and mobilization 19-21. We identified that annexin 2 (anxa2) regulates HSC binding to osteoblasts and homing to the bone marrow microenvironment22. Based upon these set of observations, it was hypothesized that annexin 2 may regulate the presentation of CXCL12 to HSCs.
This study explored the role that annexin 2 plays in presenting CXCL12 to HSCs. It is demonstrated that HSC number, engraftment, and the expression of the two CXCL12 receptors (CXCR4 and CXCR7) are compromised in the marrow of annexin 2-deficient (anxa2−/−) animals. Moreover, the loss of annexin 2 is associated with decreased levels of CXCL12 in marrow. Critically, it was shown that CXCL12 and annexin 2 directly bind to one and other, and this interaction facilitates hematopoietic progenitor cell migration in response to CXCL12. Yet the binding of CXCL12 to annexin 2 does not prevent degredation of CXCL12 under conditions that stimulate HSC mobilization. Together these results demonstrate that annexin 2 expressed by osteoblasts is critical for the HSC localization, homing, retention, and mobilization of HSCs from the marrow and provides an important link with CXCL12 function in the marrow.
MATERIALS AND METHODS
Anxa2−/− animals
Ling et al. generated the annexin 2-deficient (anxa2−/−) animals employed in our study 23. Dr. K. A. Hajjar (Weill Medical College of Cornell University, New York, NY) graciously provided a pair of the homozygous anxa2−/− animals for breeding.
HSC isolation
Bone marrow cells were flushed from femurs and tibias with Hanks buffered salt solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum HBSS (Invitrogen, Carlsbad, CA). Cells were triturated and filtered through nylon screens (40 μm; BD Falcon, Bedford, MA) to obtain single-cell suspensions. Cells were incubated first with a biotinylated anti-lineage (CD5, CD45R [B220], CD11b, Gr-1 [Ly-6G/C], and Ter-119) antibody cocktail (Miltenyi Biotec, Auburn, CA) and an antibody cocktail of anti-CD150PE (Clone TC15-12F12.2, BioLengend, San Diego, CA), CD48FITC (Clone BCM-1, BD Pharmingen, San Diego, CA), CD41FITC (Clone MWReg30, BD Pharmingen), c-KitPE/Cy7 (Clone 2B8, eBioscience, San Diego, CA), and Sca1APC (clone D7, eBioscience) for 20 min on ice, then rinsed, and stained with anti-BIO micro beads (Miltenyi Biotec) and streptavidin-APC/Cy7 conjugated secondary antibody (BD Pharmingen) for another 20 min. Lin− cells were then enriched by magnetic separation (AutoMACS, Miltenyi Biotec), and then resuspended in 2 mg/ml 7-AAD (eBioscience) to discriminate live from dead cells. Only live (7-AAD) cells were included in analyses and sorts. HSCs isolated on the basis of the signaling lymphocyte activation molecule (SLAM) family of receptors (SLAM HSCs) were sorted on a FACS Vantage dual laser flow cytometer (Becton Dickinson, San Jose, CA) by gating on cells that are CD150+Lin−CD48−CD4−Sca1+c-Kit+. In some cases, Lin−Sca1+c-Kit+ (LSK) cells were obtained using an antibody cocktail of anti-Sca1APC, c-KitPE/Cy7, and anti-Biotin FITC antibody/biotinylated anti-lineage antibody cocktail.
Competitive long-term bone marrow transplant (CLT-BMT) Assays
CLT-BMT assays were used to determine if HSC engraftment is altered in annexin 2-deficient animals. Recipient animals were exposed to 570 cGy twice in three hours in a gamma cell 40 cesium source. SLAM HSCs were obtained from C57BL/Ka-CD45.1 animals, and then 25 HSCs were transplanted intravenously (i.v.) into anxa2+/+ (CD45.2) or anxa2−/− (CD45.2) animals with a radioprotective dose (RPD) of cells (2×105 cells) from recipient animal strains. Starting at 4 weeks after transplantation and continuing for at least 16 weeks, the CD45.1 phenotypes were determined in peripheral blood including circulating granulocytes (anti-Gr1 [Ly-6G], clone RB6-8C5, eBioscience), monocytes (anti-Mac1 [CD11b], clone M1/70, eBioscience), B cells (anti-B220 [CD45R], clone RA3-6B2, eBioscience), and T cells (anti-CD3 [CD3e], clone 145-2C11, eBioscience).
Isolation of primary murine calvarial cells
Primary calvarial cells were isolated as previously described 24. Briefly, calvariae of mice (1-4 day old) were dissected, isolated from periosteum, and subjected to sequential digestions of 20, 40, and 90 min in collagenase A (2 mg/ml; Roche Molecular Biochemicals, Indianapolis, IN) with 0.25% trypsin (Invitrogen). Cells from the third digestion were washed, and cultured in α-MEM (Invitrogen) with 10% FBS, 1% penicillin, and streptomycin.
RNA analysis and real-time RT-PCR
Total RNA was harvested from cells using with RNeasy Mini or Micro Kit (Qiagen, Valencia, CA). First-strand cDNA synthesis and real-time RT-PCR were performed following the manufacturers directions (Applied Biosystems, Foster City, CA). MessageBooster™ cDNA synthesis kit was utilized when using mRNA isolated from HSCs (Epicentre Biotechnologies, Madison, WI). Taqman predeveloped assay reagents were used for detection of annexin 2 (anxa2), CXCL12, CXCR4, CXCR7, Cyclin D1, Cyclin A1, and β–actin (FAM/MGB probes, Applied Biosystems). A universal mouse reference RNA (Stratagene) was used to generate a relative standard curve. Real-time detection of RT-PCR products was performed using an ABI PRISM 7700 sequence detector (Applied Biosystems) (School of Dentistry’s Molecular Biology Core Facility). mRNA expression was calculated based on a relative standard curve and normalized to β–actin.
Binding assays
Purified bovine lung annexin 2 tetramer (Biodesign International, Saco, ME), CXCL12 (R&D systems, Minneapolis, MN), parathyroid hormone (PTH; Bachem Bioscience Inc., King of Prussia, PA) or BSA (Sigma-Aldrich, St. Louis, MO) was coated onto ELISA plates for 12h at 4°C. Subsequently the plates were washed and blocked with 1% BSA for 1h at room temperature, and then incubated with CXCL12 for 2 h at room temperature. PTH, fibronectin (FN, Sigma-Aldrich), collagen type 1a (COL 1a, Sigma-Aldrich) or anti-annexin 2 antibody (BD Pharmingen, San Diego, CA) was also included in selected assays to evaluate if CXCL12 to annexin 2 is specific binding. After washing, bound CXCL12 was detected with a biotin labeled mAb against CXCL12 (R&D Systems, clone 79014) and strepavidin HRP. In other assays, biotinylated CXCL12 was mixed with BSA or annexin 2 and immunoprecepitation (IP) was performed with an antibody to annexin 2 (BD Pharmingen) that was collected on protein G sepharose beads (Sigma-Aldrich). CXCL12 detection was performed with strepavidin HRP and chemiluminesence.
Chemotactic assays
Whole bone marrow cells recovered from anxa2+/+ or anxa2−/− animals were resuspended in serum-free α-MEM containing antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, Invitrogen). Lin−Sca1+c-Kit+ (LSK) bone marrow cells were isolated by a FACS Vantage dual laser flow-cytometer (Becton Dickinson) and equilibrated for 10 min at 37°C. 5,000 LSK cells or 1×106 whole bone marrow cells were seeded onto the top well of dual chamber, 5-μm TranswellR microporous membranes in 24-well plates (Costar Corp, Cambridge, MA) containing 650 μl serum-free α-MEM containing 0.5% BSA. CXCL12 (200ng/ml) and/or purified bovine lung annexin 2 tetramer (200ng/ml) were placed in the bottom well of the chamber as chemotaxis targets. The plates were incubated at 37°C in 95% humidity and 5% CO2. The number of cells that had migrated into the lower chambers was determined by manual counting on a hemocytometer. To determine the effects on progenitor cells, whole bone marrow was plated into the top chamber. Migrated cells into the bottom chambers were separately collected and 2×105 cells were cultured in methylcellulose medium for 2 weeks for hematopoetic progenitor colony formation.
Evaluation of Erk Pathway
Bone marrow cells from wild-type (anxa2+/+) animals were treated with annexin 2, CXCL12 or the combination of annexin 2 and CXCL12 for 1h at 37 °C. The cells were incubated with the antibodies to phosphorylated p44/42 MAP kinase (Erk1/2) or p44/42 MAP kinase (Erk1/2) (Cell Signaling Technology, Danvers, MA) for 20 min at 4°C, and were subsequently stained with anti-Sca1APC, anti-c-KitPE/Cy7, and biotinylated-anti-lineage (CD5, CD45R [B220], CD11b, Gr-1 [Ly-6G/C], and Ter-119) antibody cocktail for another 20 min at 4°C. p-Erk or Erk activation was analyzed on a FACS Vantage dual laser flow-cytometer (Becton Dickinson) by gating on Lin−Sca1+c-Kit+ populations.
CXCL12 Degradation assays
To determine if annexin 2 and CXCL12 protect each other from proteolytic degradation, purified bovine lung annexin 2 tetramer (200 ng/ml; Biodesign International) was incubated with 200 ng/ml biotinylated CXCL12 (bCXCL12; R&D systems) for 2.5 h at 4°C, and then incubated with 10 μg/ml of elastase (Genway Biothech, San Diego, CA) or rhCD26/dipeptidyl peptidase IV (DPPIV; 35 μU/mg of protein; EMD Chemicals, Gibbstown, NJ) for 2h at 37°C. The proteins were run on 20% Tricine gels, and the remaining annexin 2 and bCXCL12 were detected using biotinylated antibodies and strepavidin-horseradish peroxidase (HRP) with enhanced chemiluminescence (ECL).
Immunohistochemistry
Murine bones were harvested and fixed in 10% neutral buffered formalin overnight, decalcified in 10% EDTA pH 7.5 for 10 days at 4°C. Paraffin-embedded slides (5 to 8 μm) were prepared and stained with antibodies to annexin 2, CXCL12 or an IgG matched isotype control in conjunction with a HRP-AEC staining system kit using anti-mouse biotinylated antibodies following the manufactures protocols (R&D Systems), and counter stained with hematoxylin (Sigma-Aldrich). In some cases, frozen sections were stained with antibodies to annexin 2 or CXCL12. Images were acquired on a Zeiss LSM510 microscope. The percentage of the field stained by immunohistochemistry was quantified using an Image Pro Plus v.5.1 image analysis system.
BrdU Injections
Anxa2+/+ and anxa2−/− animals were administered vehicle (0.9% saline) or a single dose (180 μg) of bromodeoxyuridine (BrdU; Sigma-Aldrich) by intraperitoneal injection and then fed continuously with water containing 800 μg/ml BrdU and 5% glucose for 48 h. HSCs were stained using the BrdU labeling kit (BD Pharmingen). Percent BrdU positive of HSCs from anxa2+/+ and anxa2−/− animals was measured from at least three individual experiments and results are expressed as mean SD.
In Vivo G-CSF administration
Anxa2+/+ and anxa2−/− animals were administered granulocyte-colony stimulating factor (G-CSF) (Neupogen; Amgen, Thousand Oaks, CA) or vehicle (0.9% saline) by intraperitoneal injection at 250μg/kg (100 μl) per day for 5 days. Four hours after the final injection of G-CSF, bone marrow cells, peripheral blood cells, marrow extracellular fluids, and the bone samples were collected.
Colony-forming cell assays
Bone marrow and peripheral blood cells were collected from anxa2+/+ and anxa2−/− animals (n = 5). Test cells (2 × 105 cells) were plated onto 35-mm tissue culture dish in a methylcellulose medium with recombinant cytokines for colony assays of murine cells (Methocult M3434; Stem Cell Technologies, Vancouver, BC, Canada). Cultures were plated in triplicate and placed in a humidified chamber with 6% CO2 at 37°C. The burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte, monocyte, and macrophage (CFU-GM), colony-forming unit-granulocyte, erythroid, monocyte, macrophage, and megakaryocyte (CFU-GEMM), and the total progenitor colony numbers (CFU-C) were counted at 14 day after plating.
CXCL12 ELISA
The bone marrow extracellular fluids were obtained by flushing each femur with 500 μl ice-cold PBS and the supernatant was harvested by centrifugation at 400g for 5 minutes. CXCL12 levels in the marrow fluids were analyzed by antibody sandwich ELISA (sensitivity 31.25 pg/ml, detection range 62.5 to 5000 pg/ml CXCL12; R&D Systems). MC3T3-E1 conditioned medium was used as positive controls for CXCL12. CXCL12 levels were normalized by total protein (BioRad, Hercules, CA).
Statistical analyses
Results are presented as mean ± SEM. Significance of the difference between two measurements was determined by unpaired Student’s t test, and multiple comparisons were evaluated by the Newman-Keuls multiple comparison test. Values of P <0.05 were considered significant.
RESULTS
Annexin 2 deficiency alters HSCs activities
Recently we reported that annexin 2 regulates the homing and binding of HSCs to the bone marrow microenvironment15. To further explore the role that annexin 2 plays in regulating HSC functions, it was first determined if HSCs and annexin 2 co-localize in marrow. As shown in Figure 1A, the majority of HSCs (CD150+Lin−CD48−CD41− phenotype) that were located in the endosteal regions were associated with annexin 2 protein.
Figure 1. Annexin 2 deficiency alters HSCs activities.
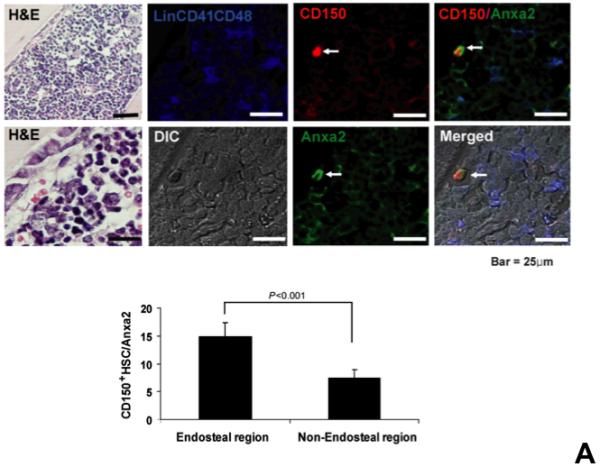
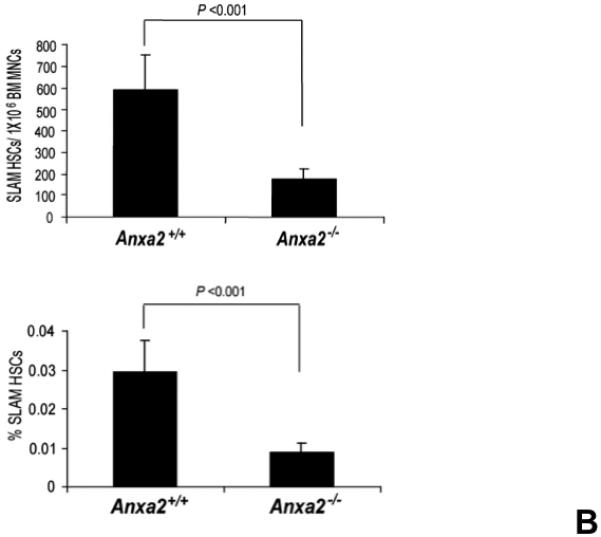
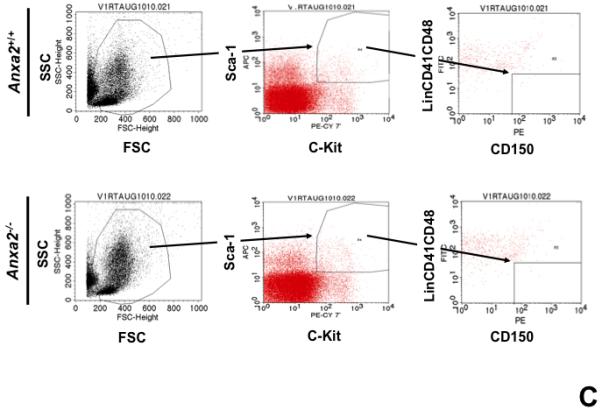
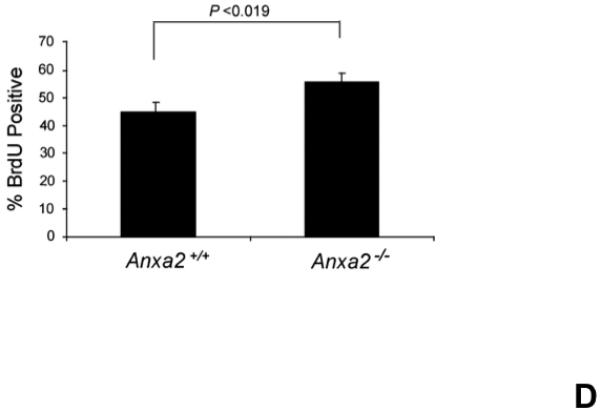
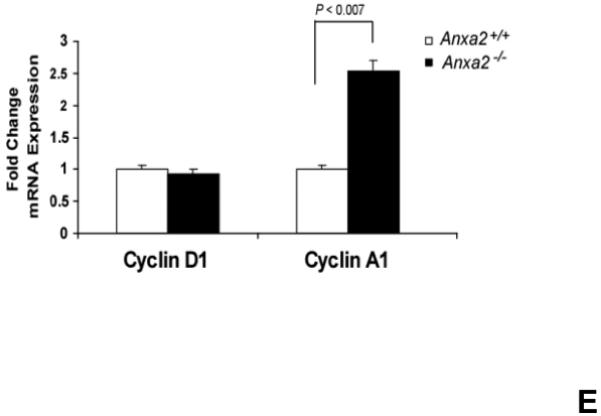
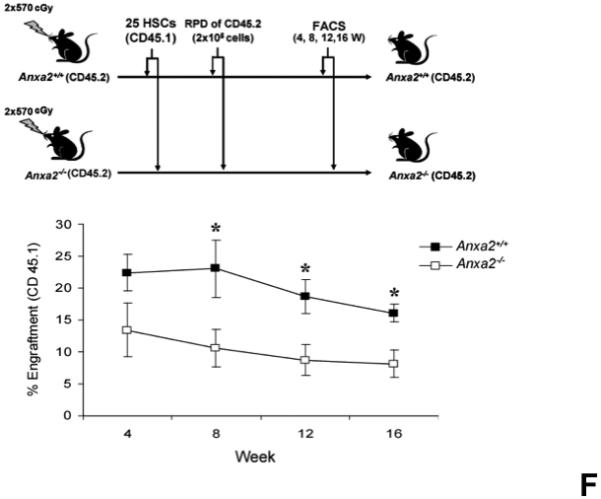
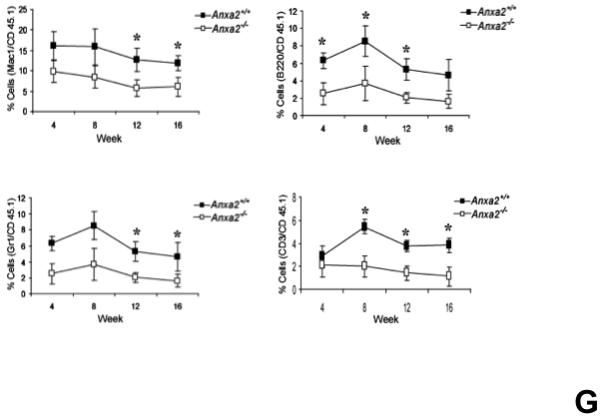
(A) Co-localization of HSCs with annexin 2 in marrow wase determined by H&E stain and immunofluorecent staining. Mouse femurs were stained with H&E (original magnification at 20x and 60x). Lin+CD48+CD41+ cells (blue) were detected by the cocktail antibodies to lineages. Differential interference contrast (DIC) image showed bone structure. HSCs were detected by the antibody to CD150+ (red) without blue coloring (CD150+Lin−CD48−CD41−). Annexin 2 positive cells were detected by anti-annexin 2 antibody (green). Co-localization of HSCs with annexin 2 (yellow) was showed on endosteal surfaces. Merged image showed with DIC and co-localization of HSCs with annexin 2. Original magnification at 60x. Bar = 25 microns. The numbers of co-localization of HSCs with annexin 2 (CD150+HSC/anxa2) were quantified on the serial sections of the 10 long bones from normal wild-type (anxa2+/+) animals. Endosteal regions and non-endosteal region were defined as 12 cell diameters from bone surfaces. (B) The absolute number and percent of HSCs present in the marrow of anxa2−/− vs anxa2+/+ animals were determined by FACS analysis. (C) Histograms of HSCs using SLAM families by FACS analysis. (D) Cell cycle analysis of SLAM HSCs stained with BrdU staining was determined by FACS analysis. (E) mRNA expression of cyclin D1 and cyclin A1 were examined by real-time RT-PCR and normalized to β–actin to determine the proliferating status on HSCs. (F) The percentages of HSC (CD45.1) engraftment in anxa2+/+ (CD45.2) or anxa2−/− (CD 45.2) animals were determined by competitive long-term bone marrow transplant assays at 4 to 16 weeks post-transplant. (G) The repopulating lineages (MAC-1, B220, Gr-1 or CD3 cells) in peripheral blood of anxa2+/+ (CD45.2) or anxa2−/− (CD 45.2) animals were also determined at 4 to 16 weeks post-transplant. Data are presented as the mean ± standard error of the mean. *P< 0.001 significant differences from anxa2 +/+ control groups.
As HSCs and the expression of annexin 2 are located in the same region in marrow, it was next determined what the loss of annexin 2 expression would be HSC numbers in marrow. For these studies, the HSC numbers in marrow of anxa2−/− vs anxa2+/+ animals were determined by FACS analysis. Fewer HSCs were observed in the marrows of anxa2−/− compared with wild-type anxa2+/+ animals (Figure 1B, C). One possibility as to why fewer HSCs found in the marrows of anxa2−/− animals, and annexin 2 could regulate HSC proliferation so that HSCs from the anxa2−/− animals may progress through the cell cycle faster than wild-type HSCs. Therefore, BrdU staining was performed on HSCs obtained from both anxa2+/+and anxa2−/− animals to determine if annexin 2 regulates stem cell renewal. More BrdU positive HSCs in marrow of anxa2−/− vs anxa2+/+ animals were identified (Figure 1D). In addition, an increase of mRNA expression of cyclin A1 was observed in the fast proliferating HSCs in the anxa2−/− animals (Figure 1E).
A second possibility as to why anxa2−/− animals have fewer HSCs in their marrows is that annexin 2 may be required for HSCs to home to the marrow. Therefore, competitive engraftment studies were performed in which twenty-five HSCs (CD150+Lin−CD48−CD4−Sca1+cKit+ cells) derived from C57BL/Ka-CD45.1 wild-type mice were transplanted into anxa2+/+ (CD45.2) or anxa2−/− (CD45.2) animals. The level of engraftment was determined as a percentage of CD45.1 cells in the peripheral blood over time. Significantly lower levels of engraftment were observed when the anxa2−/− animals were transplanted with wild-type marrow cells compared with wild-type animals receiving the same cells as transplants (Figure 1F). Moreover, the engraftment defect encompassed all lineages evaluated including granulocytes, monocytes, B-cells, and T-cells (Figure 1G). These data suggest that the loss of annexin 2 changes the ability of HSCs to home to the marrow.
Annexin 2 is associated with CXCL12 expression In Vivo and In Vitro
Since both annexin 2 and CXCL12 are required for HSC engraftment and both annexin 2 and CXCL12 are expressed at endosteal surfaces, studies were designed to determine if a relationship exists between the expression of annexin 2 and CXCL1215,22. Accordingly, long bones of anxa2+/+ and anxa2−/− animals were stained for both annexin 2 and CXCL12. As expected, annexin 2 expression was not observed in the anxa2−/− animals, whereas strong staining for annexin 2 was observed in the epiphysis region of wild-type (anxa2+/+) animals (Figure 2A). Likewise, CXCL12 expression was robustly expressed in marrows of anxa2+/+ animals, but little expression was observed in the marrows of anxa2−/− animals (Figure 2A). In previous studies it was noted that CXCL12 expression was primarily observed early in osteoblastic differentiation15. Therefore primary osteoblasts were cultured from anxa2+/+ and anxa2−/− animals and stained for annexin 2 and CXCL12. As expected, osteoblasts derived from anxa2−/− animals did not express annexin 2, whereas strong expression of annexin 2 was observed for osteoblasts obtained from wild-type animals (Figure 2B). Osteoblasts derived from the anxa2+/+ animals stained as intensely for CXCL12 as compared with osteoblasts isolated from anxa2−/− animals (Figure 2B) and the levels of CXCL12 mRNA expression were correlated with protein expression (Figure 2C).
Figure 2. Reduced expression of CXCL12 in the marrows of annexin 2-deficient animals.
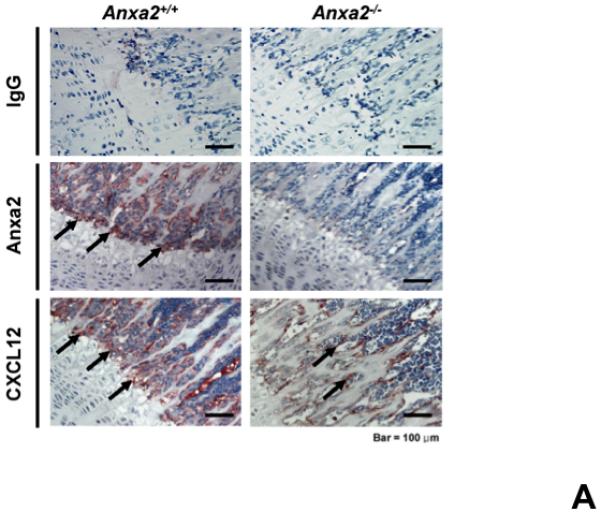
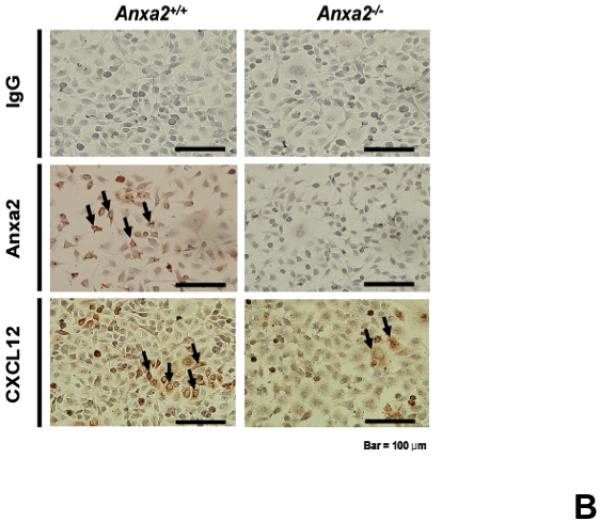
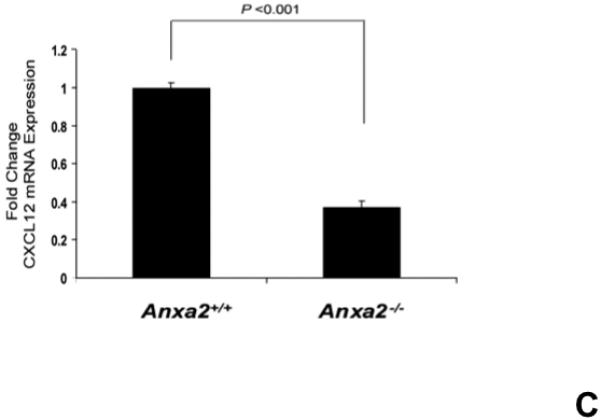
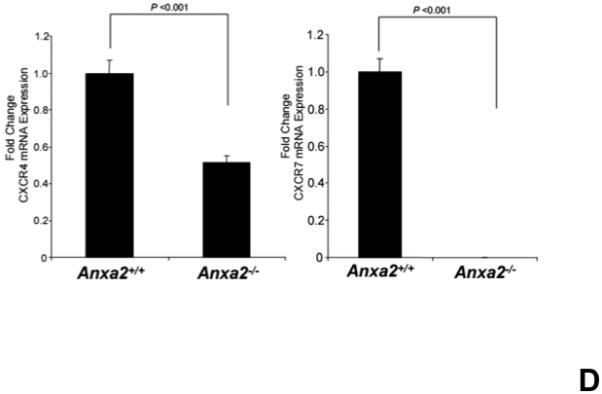
(A) Immunohistochemistry for annexin 2 and CXCL12 were performed on the long bones of anxa2 +/+ and anxa2−/− animals using monoclonal antibodies (mAbs) or isotype-matched immunoglobulin G (IgG) controls. Original magnification at 20x. Bar = 100 microns. (B) Immunohistochemistry for annexin 2 and CXCL12 were performed on the primary murine osteoblasts derived from anxa2 +/+ and anxa2−/− animals using the monoclonal antibodies (mAbs) or isotype-matched immunoglobulin G (IgG) controls. Original magnification at 40x. Bar = 100 microns. (C) The CXCL12 mRNA expression was examined by real-time RT-PCR for quantification of CXCL12 expression on the primary murine osteoblasts in Figure 2B. (D) CXCR4 and CXCR7 mRNA expression by HSCs isolated from anxa2 +/+ or anxa2−/− animals were examined by real-time RT-PCR. Data are presented as the mean ± standard error of the mean. *P< 0.001 significant differences from anxa2 +/+ control groups.
The previous results suggest that the loss of annexin 2 expression is associated with a reduction in the expression of CXCL12 by components of HSC niche. Since CXCL12 is critical for homing and engraftment of HSCs, the CXCL12 receptors CXCR4 and CXCR7 expressed by HSCs were examined. The expression of mRNA for CXCR4 and CXCR7 from HSCs were significantly reduced in annexin 2-deficient animals (anxa2−/−) compared to wild-type animals (Figure 2D). The combination of the receptor levels and staining data suggests that annexin 2 may regulate the presentation of CXCL12 to HSCs, and HSCs express fewer CXCL12 receptors in the absence of annexin 2.
Annexin 2 binds CXCL12
CXCL12 is known to bind to several extracellular matrix molecules 25,26. It was next hypothesized that CXCL12 may bind to annexin 2, as both annexin 2 and CXCL12 are expressed at endosteal marrow surfaces and CXCL12 levels are reduced in the anxa2−/− animals. Therefore annexin 2-CXCL12 in vitro binding assays were performed to test this possibility (Figure 3A). When non-specific binding to the assay plates was blocked with bovine serum albuman (BSA), little or no CXCL12 bound to the plates. When annexin 2 was first added to the plates and non-specific binding was blocked with BSA, CXCL12 bound to annexin 2 (Figure 3A). Further competition binding assays were performed using saturating concentrations of parathyroid hormone (PTH), fibronectin (FN), collagen type 1a (COL1a) or anti-annexin 2 antibody to determine if CXCL12 binding to annexin 2 is specific. Here PTH was chosen as an example of a systemic hormone, FN and COL1a served as models of bone matrix proteins. PTH, FN or COL1a was not able to compete with CXCL12 for annexin 2 binding (Figure 3A). Moreover, PTH did not directly bind CXCL12 (Figure 3A). However, anti-annexin 2 antibody prevented the binding of CXCL12 to annexin 2 (Figure 3A).
Figure 3. CXCL12 binds to annexin 2.
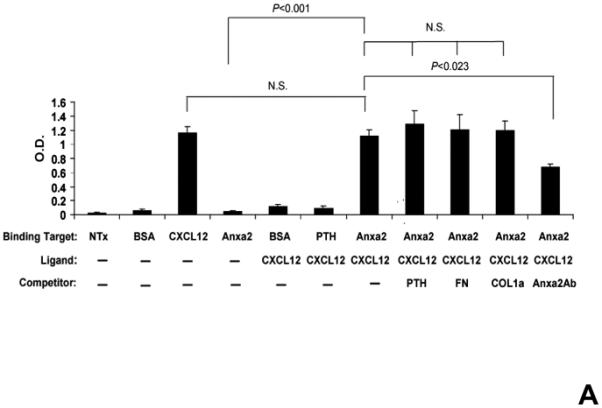
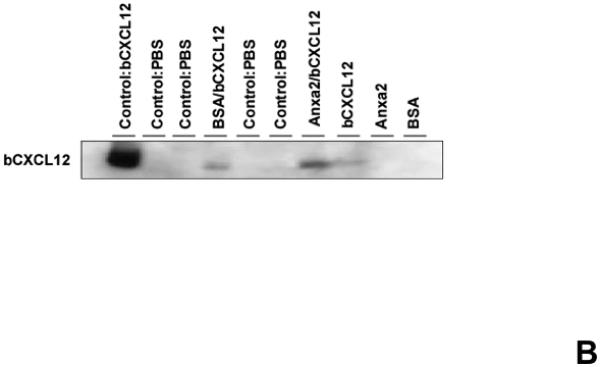
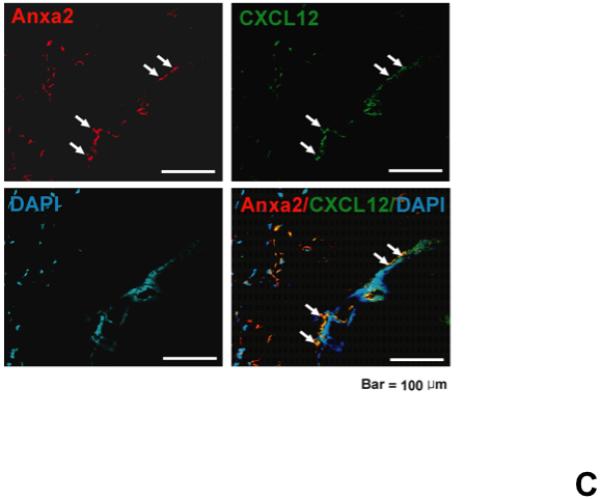
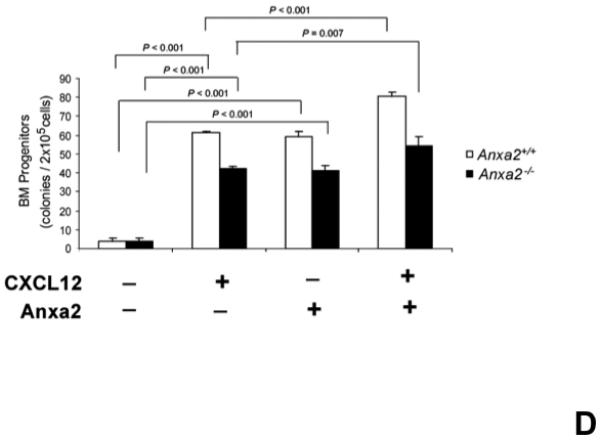
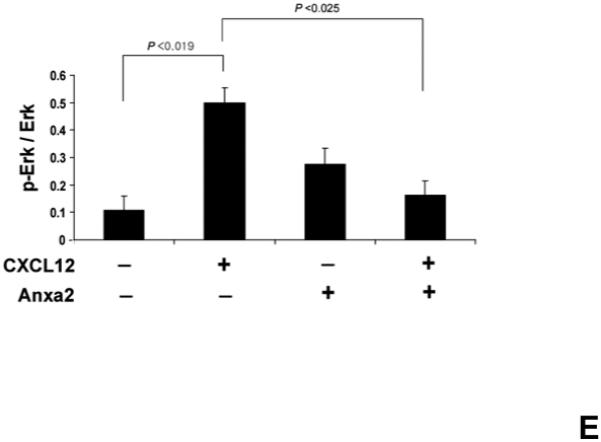
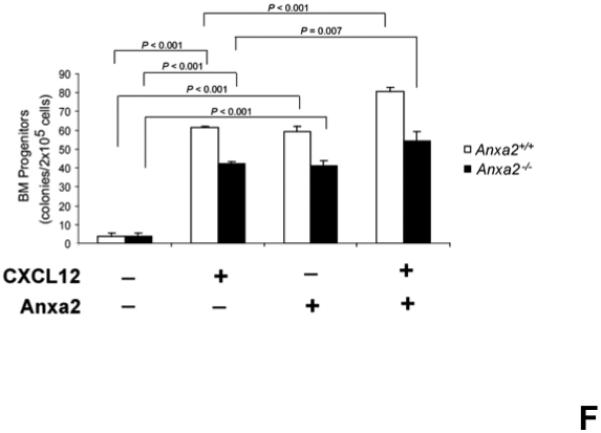
(A) In vitro binding assays between CXCL12 and annexin 2 were performed to determine binding specificity. Annexin 2 was used as a binding target for CXCL12 and non-specific binding was blocked with bovine serum albuman (BSA). Parathyroid hormone (PTH), fibronectin (FN), collagen 1a (COL1a) or anti-annexin 2 antibody was used as competitors. (B) Biotinylated CXCL12 (bCXCL12) was incubated alone or together with annexin 2. Immune precipitation (IP) was performed with an antibody to annexin 2 and the proteins were collected on protein G sepharose beads. bCXCL12 detection accomplished by Western blot using strepavidin-HRP. Control-proteins were run without IP. (C) Co-localization of CXCL12 with annexin 2 was determined by immunofluorecent staining on the frozen sections of the long bones in the wild-type (anxa2 +/+) animals. Annexin 2 positive cells were detected by anti-annexin 2 antibody (red). CXCL12 positive cells were detected by anti-CXCL12 antibody (green). DAPI stained nuclei (blue). Co-localization of CXCL12 with annexin 2 (yellow-orange) was showed on the endosteal surfaces. Original magnification at 60x. Bar = 100 microns. (D) Migration assays of LSK cells from anxa2+/+ and anxa2−/− animals were performed using dual chambers to determine the hematopoietic progenitor cell migration in response to CXCL12-annexin 2 interactions. (E) Erk and Erk phosphorylation in LSK cells were evaluated by FACS analysis to determine the signaling in response to CXCL12-annexin 2 interactions. (F) Hematopoietic progenitor colony-forming cell assays performed on 2×105 cells that migrated to CXCL12, annexin 2 or the combination. Data are presented as the mean ± standard error of the mean. *P<0.05 as determined by Student-T test.
To validate that CXCL12 binds directly with annexin 2, biotinalyated-CXCL12 and annexin 2 were incubated together, and immunoprecipitation was performed using an anti-annexin 2 antibodies. Subsequently, the proteins separated on SDS-PAGE gels and the resulting Western blots were probed with strepavidin HRP to detect the biotinylated CXCL12. CXCL12 was detected when CXCL12 was incubated with annexin 2 and immuneprecipitation of annexin 2 was performed (Figure 3B). No CXCL12 was detected in the immuneprecipitations when CXCL12 was incubated with BSA alone or when annexin 2 alone was included in the assay (Figure 3B). These data demonstrate that CXCL12 binds to annexin 2. To determine if annexin 2 binds CXCL12 in vivo, co-localization studies were performed. Annexin 2 and CXCL12 were widely expressed by marrow osteoblasts. Co-localization studies demonstrated that the expression of annexin 2 and CXCL12 overlaps on the marrow endosteum (Figure 3C).
Functional consequences of CXCL12 binding to annexin 2
To determine if the binding of CXCL12 to annexin 2 has functional consequences the ability of CXCL12-annexin 2 interactions to alter HSC migration was determined. For these studies, Lin−Sca1+cKit+ (LSK) cells were isolated from anxa2+/+ or anxa2−/− animals and placed in the upper chamber of a dual chamber 5-μm TranswellR microporous membranes in 24-well plates. CXCL12, annexin 2 or the combination was added into the lower chamber. Without CXCL12, few anxa2+/+ LSK cells migrated into the bottom chamber (Figure 3D). Both CXCL12 and annexin 2 stimulated the migration of the LSK cells. A synergistic migration response was seen when CXCL12 bound to annexin 2 was used as the chemoattractant for the LSK cells compared to either control group alone (Figure 3D). Interestingly, the migration of LSK cells obtained from anxa2−/− animals followed a similar pattern of migration, as did LSK cells isolated from wild-type animals, except that the response to either of the stimuli was muted.
To explore if CXCL12 bound to annexin 2 is able to activate signaling cascades, we next examined the effects of annexin 2, CXCL12 or the combination of annexin 2 and CXCL12 on Erk signaling. Marrow cells from the wild-type (anxa2+/+) animals were treated with CXCL12, annexin 2 or the combination, and Erk and phosphorylated Erk levels in LSK cell populations were evaluated by FACS analysis. The data demonstrate that CXCL12 induces Erk phosphorylation, whereas signaling of CXCL12 bound to annexin 2 was inhibited (Figure. 3E). These data suggested that once HSCs have localized by CXCL12 to the stem cell niche, annexin 2 regulates CXCL12 signaling.
Migration studies were also performed using whole bone marrow cells. In this case however, hematopoietic colony assays were performed on the cells that had migrated in response to CXCL12, annexin 2 or the combination. As expected, significantly more hematopoietic progenitors were found in the marrows of the anxa2+/+ animals relative to the numbers found in the marrows of the anxa2−/− animals (Figure 3F). The migrated cells from both animals were responsed to either CXCL12 or annexin 2 for the hematopoietic progenitors (Figure 3F). The numbers of hematopoietic progenitors were enhanced when CXCL12 bound to annexin 2 served as the chemoattractant (Figure 3F). These data demonstrate that CXCL12 bound to annexin 2 produces a synergistic effect on the migratory response of hematopoietc stem/progenitor cells.
CXCL12 bound to annexin 2 is not protected from proteolysis
Previously it was demonstrated that CXCL12 bound to syndecin IV protects CXCL12 from proteolysis 25,26. To determine if annexin 2 also protects CXCL12 from degradation, biotinylated CXCL12 (bCXCL12) was incubated with annexin 2, and then incubated with elastase or CD26/DPPIV for 12h at 37°C. After incubation, the proteins were separated on 20% Tricine gels and Western blots performed. The data demonstrates that the binding of CXCL12 to annexin 2 was not protected from enzymatic degradation (Figure 4A). Likewise, annexin 2 was not protected from proteolysis when bound to CXCL12 (Figure 4B).
Figure 4. CXCL12 bound to annexin 2 is not protected from proteolytic degradation.
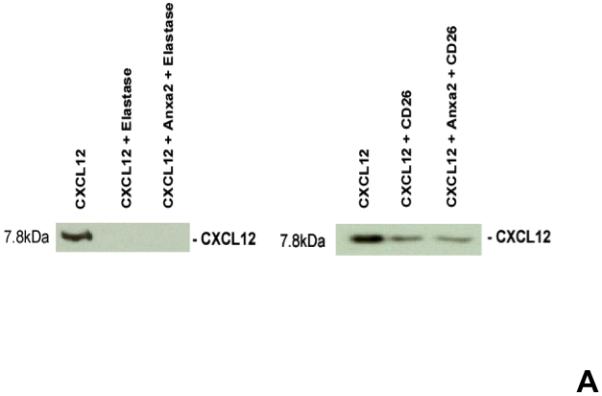
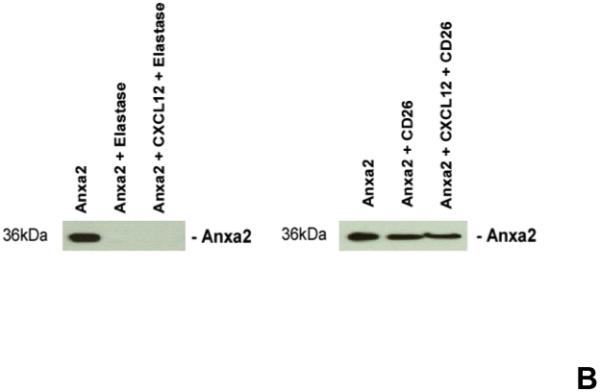
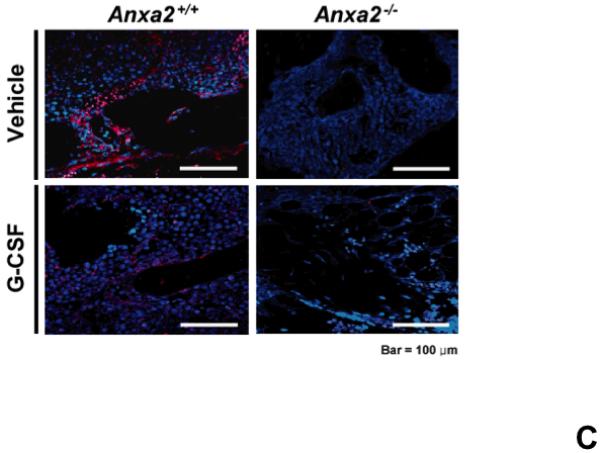
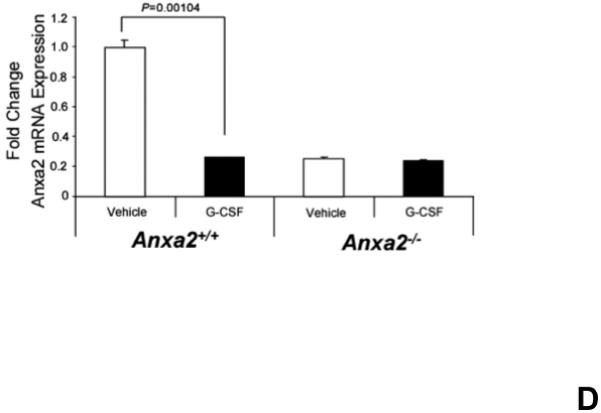
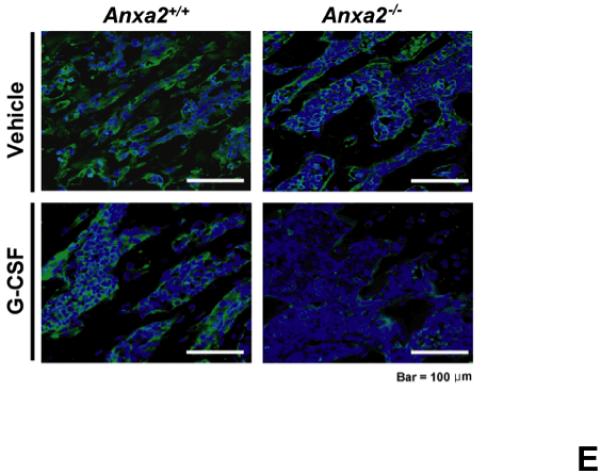
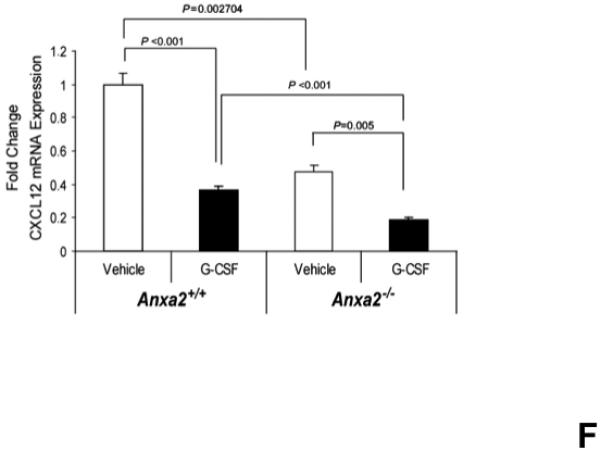
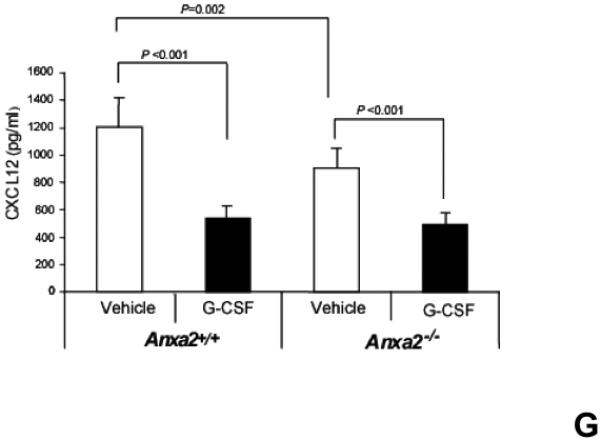
(A) To determine if annexin 2 is able to prevent degradation of CXCL12, biotinylated CXCL12 (bCXCL12) was incubated with annexin 2 for 2.5h at 4°C, and then incubated with elastase or CD26/dipeptidyl peptidase IV. Degradation of bCXCL12 was determined by Western blots. (B) Identical studies were performed as reported for (A), however in this case the Western blots were stained for annexin 2. (C-G) To validate that annexin 2 does not regulate CXCL12 degradation, anxa2+/+ and anxa2−/− animals were administered G-CSF or vehicle (0.9% saline) by intraperitoneal injection at 250μg/kg (100 μl) per day for 5 days. The bones were harvested and processed for (C) immunohistochemistry (original magnification at 40x, Bar = 100 microns) or (D) Real time RT-PCR was performed to determine annexin 2 levels. (E) Representitive immunohistochemistry for CXCL12 was performed (original magnification at 40x. Bar = 100 microns). (F) CXCL12 mRNA was examined by real time RT-PCR. (G) CXCL12 expression in the marrow extracellular fluids was evalulated by Elisa. Data are presented as the mean ± standard error of the mean. *P<0.05 as determined by Student-T test.
To determine if annexin 2 regulates HSC mobilization, anxa2 +/+ and anxa2−/− animals were administered G-CSF (250μg/kg) or vehicle (0.9% saline) for 5 days. As predicted, in response to G-CSF treatments, both annexin 2 (Figure 4C, D) and CXCL12 levels (Figures 4E, F, G) were decreased in the animals marrow. Importantly, more hematopoetic progenitor cells were mobilized out of the marrows of anxa2−/− animals compared to the wild-type animals (Table 1, and Supplemental Table 1). Taken together, these data provide direct evidence that while annexin 2 participates in the presentation of CXCL12 to HSCs, and annexin 2 participates in regulating HSCs/HPCs mobilization.
Table 1. Mobilization of hematopoietic progenitor cells in response to G-CSF.
To determine if CXCL12 binding to annexin 2 regulates HSC mobilization, anxa2+/+ and anxa2−/− animals were administrated G-CSF by intraperitoneal injection over five days whereupon bone marrow and blood cells were collected from anxa2+/+ and anxa2−/− animals (n = 5). Cells (2 × 105 cells) were plated onto 6 well plates in a methylcellulose medium for the hematopoietic colony-forming cell assays. BFU-E, CFU-GM, CFU-GEMM, and the total progenitor colony numbers (CFU-C) were counted at 14 day after plating. The total progenitor colony numbers (CFU-C) were presented in Table 1 and BFU-E, CFU-GM and CFU-GEMM data is presented in Supplemental Table 1. The average of progenitor colonies in bone marrow (BM Vehicle), peripheral blood (PB Vehicle), bone marrow with G-CSF treatment (BM G-CSF), and peripheral blood with G-CSF treatment (PB G-CSF) were enumerated. Delta PB (Δ PB) was calculated by the average percent of progenitor colonies in PB Vehicle normalized to the numbers of progenitor colonies in PB G-CSF. Delta PB/BM (Δ PB/BM) was calculated by the average percent values in Delta PB (Δ PB) normalized to the numbers of progenitor colonies in BM vehicle. Data are presented as the mean ± standard error of the mean.
| BM Vehicle | BM G-CSF-- | PB Vehicle-- | PB G-CSF-- | PB | Δ PB/BM | |
|---|---|---|---|---|---|---|
| Anxa2 +/+ | 146 ± 10.0* | 103.6 ± 3.2* | 8.1 ± 2.2 | 17.5 ± 1.0 * | 55.0 ± 1.0 | 37.7 ± 0.7* |
| Anxa2 −/− | 99.6 ± 9.2 | 76.6 ± 7.7 | 8.3 ± 1.9 | 31.8 ± 0.9 | 55.1 ± 1.1 | 55.2 ± 1.1 |
P< 0.001 significant differences from anxa2+/+ wild-type control groups.
DISCUSSION
HSCs and osteoblasts are both located near endosteal surfaces in which suggested a functional relationship between these two cellular populations. Recently it was demonstrated that annexin 2, an osteoblast-expressed adhesion molecule, participates in localizing HSCs to endosteal surfaces22. Where adhesion of HSCs to osteoblasts derived from annexin 2-deficient animals (anxa2−/−) was significantly impaired compared to osteoblasts obtained from wild-type animals (anxa2+/+). In the current work the role that annexin 2 plays in regulating HSC engraftgment was studied. Using transplantion of wild-type HSCs into anxa2+/+ and anxa2−/− animals, it was observed that fewer HSCs were able to engraft into the marrow of anxa2−/− anmals. Since engraftment was impaired in anxa2−/− animals, it was determined if HSCs derived from annexin 2-deficient animals express normal levels of CXCR4 and CXCR7. Both of the CXCL12 receptor mRNA levels were reduced in HSCs derived from the anxa2−/− animals. These data suggest that annexin 2-deficient environments may also represent a cell autonomous defect, not restored with normal HSC functions. Importantly, osteoblastic cells of the HSC niche expressed lower levels of CXCL12 in the anxa2−/− animals. From these observations it was found that annexin 2 binds to CXCL12 (See model; Figure 5). Using immunohistochemistry, it was observed that annexin 2 present at endosteal surfaces co-localize with CXCL12. These findings suggest annexin 2 plays a role in presenting CXCL12 to HSCs, in addition to its role in localizing HSCs into the bone marrow niche.
Figure 5. Model of CXCL12 bound to annexin 2.
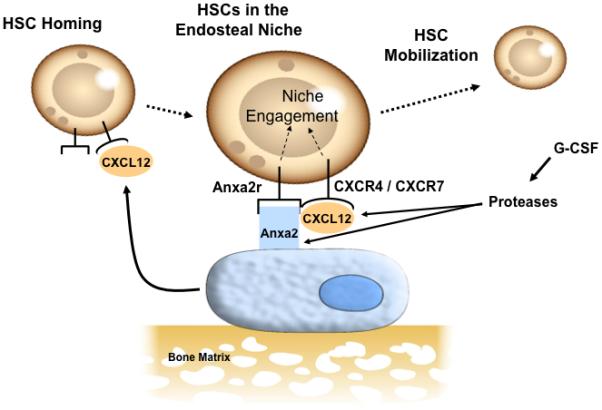
A model for how annexin 2 regulates CXCL12 presentation to HSCs. HSC homing and engraftment is dependent on CXCL12 and annexin 2 expression in marrow. The two CXCL12 receptors (CXCR4 and CXCR7) function to localize to the endosteal niche and annexin 2 and its receptor (anxa2r) serve as binding factors. That CXCL12 and annexin 2 directly bind to one and another, and this interaction facilitates HSCs migration in response to CXCL12. Yet the binding of CXCL12 to annexin 2 does not prevent degredation of CXCL12 under HSC mobilizating conditions, as both CXCL12 are degraded by enzymes activated by G-CSF during mobilization. Together these results demonstrate that annexin 2 expressed by osteoblasts is critical for the HSC localization, homing, and mobilization of HSCs from the marrow and provides an important link with CXCL12 function in the marrow.
Previous studies have shown that CXCL12 may be presented to cells as either a secreted form or associated with the extracellular matrix proteins 25-28. In fact, differential expression of proteoglycans on the luminal side of bone marrow endothelial cells has been shown to capture and present CXCL12 to HSCs and direct their migration through the endothelial barriers 29. These observations along with our data suggest that CXCL12 binds to a number of proteins in the marrow. Yet it is not clear, what the biologic consequences of these different interactions are. A possibility is that the different molecules interact with CXCL12 provide different signals to a number of cell types in the marrow. For example, the binding of CXCL12 to annexin 2 does not protect CXCL12 from degradation, whereas CXCL12 binding to syndecin IV protects CXCL12 from degradation. Proteolytic degradation of CXCL12 in the bone marrow is known to play a critical role regulating stem and progenitor cell mobilization into the peripheral circulation30-32. CXCL12 is cleaved by metalloproteinases, CD26/dipeptidyl peptidase IV, serine proteases, and leukocyte elastase33-38. Our data show that annexin 2 may localize CXCL12 in marrow but these interactions do not protect CXCL12 from degradation. Intrigingly, while CXCL12 bound to annexin 2 was not protected from degradation, CXCL12 bound to annexin 2 was a potent stimulator of stem/ progenitor cell migration. These findings suggest that different mechanisms have envolved over time to regulate CXCL12 presentation to target cells, and when CXCL12 bound to different molecules may have illicit different responses in target cells.
In the current work, we found that HSCs co-localize with annexin 2, and annexin 2 co-localizes with CXCL12. This raises the possibility that CXCL12/annexin 2 may serve as a marker for the HSC niche. Identification of the HSC niche has relied most heavily on co-localization studies of transplanted labled HSC cells39. In addition, histological analyses of the distribution of HSCs in the marrow against spindle-shaped N-cadherin+ osteoblastic or “SNO” cells possibly representing osteoblastic lining cells have been proposed as cells that also constitute the HSC niche2. This is in contrast to larger, more “oval-shaped, matrix-forming osteoblasts”2. Yet the findings that N-cadherin is a suitable marker for the HSC niche has generated considerable controversy 40-43, and more recent work suggests that osteoprogenitor cells may represent cells which constitute the HSC niche44. This observation would be consistent our previous findings that CXCL12 is expressed early during osteoblastic maturation15. Yet, clearly there is a need for the identification of markers for the HSC niche. Our observations that HSCs co-localize with CXCL12, and by inference annexin 2, suggests a novel set of tools that may be useful to define the location of the HSC niche.
In summary, recent works in mice 3,4,9 and in human systems 45,46 have demonstrated that HSCs are regulated by, and localized to, endosteal cells. One common feature of endosteal osteoblast-HSC cross-talk is the requirement for cell-cell contacts. The data presented here suggest that annexin 2 plays a role in the homing and retention of HSCs to the marrow. Likewise, CXCL12 has been shown to be a critical regulator of HSC homing, retension, and mobilization. That CXCL12 binds to annexin 2 suggests a functional relationship between the two molecules that heretofore has not been appreciated and may well extend beyond HSC-osteoblastic interactions, but could include osteoblastic-osteoclastic coupling and tumor metastasis. Further studies are required.
Supplementary Material
Supplemental Table 1: Mobilization of hematopoietic progenitor cells in response to G-CSF.
Anxa2+/+ and anxa2−/− animals were administrated G-CSF by intraperitoneal injection over five days whereupon bone marrow and blood cells were collected from anxa2+/+ and anxa2−/− animals (n = 5). Cells (2 × 105 cells) were plated onto 6 well plates in a methylcellulose medium for the hematopoietic colony-forming cell assays. BFU-E, CFU-GM, CFU-GEMM, and the total progenitor colony numbers (CFU-C) were counted at 14 day after plating. The total progenitor colony numbers (CFU-C) were presented in Table 1. The average of progenitor colonies in bone marrow (BM Vehicle), peripheral blood (PB Vehicle), bone marrow with G-CSF treatment (BM G-CSF), and peripheral blood with G-CSF treatment (PB G-CSF) were enumerated. Data are presented as the mean ± standard error of the mean. *P< 0.05 significant differences from anxa2+/+ wild-type control groups.
ACKNOWLEDGMENTS
We thank Dr. Katherine A. Hajjar (Weill Medical College of Cornell University, New York, NY) for the anxa2−/− mice. This work was directly supported by a Pediatric Oncology Research Fellowship (Y.S.). This study was supported by grants from the Department of Defense (PC073952) and National Institutes of health (DK082481, DE020721, 1RC1DE020721, CA141426 and CA093900). No financial interest/relationships to the topic of this article have been declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INEREST DISCLOSURE
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Reference List
- (1).Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- (2).Zhang JW, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- (3).Zhu J, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004 Jun;26(6):595–9. doi: 10.1002/bies.20052. BioEssays. 2004;26:595.-599. [DOI] [PubMed] [Google Scholar]
- (4).Arai F, Hirao A, Ohmura M, et al. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- (5).Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem cell niche. Blood. 2005;105(7):2631–9. doi: 10.1182/blood-2004-06-2480. 2631-2639. [DOI] [PubMed] [Google Scholar]
- (6).Patt H, Maloney M. Bone formation and resorption as a requirement for marrow development. Proc Soc Exp Biol Med. 1972;140:207. doi: 10.3181/00379727-140-36426. [DOI] [PubMed] [Google Scholar]
- (7).Lord BI, Hendry JH. The distribution of haemopoietic colony-forming units in the mouse femur, and its modification by x rays. British Journal of Radiology. 1972;45:110–115. doi: 10.1259/0007-1285-45-530-110. [DOI] [PubMed] [Google Scholar]
- (8).Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- (10).Gong J. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- (11).Islam A, Glomski C, Henderson ES. Bone lining (endosteal) cells and hematopoiesis: a light microscopic study of normal and pathologic human bone marrow in plastic-embedded sections. Anatomical Record. 1990;227:300–306. doi: 10.1002/ar.1092270304. [DOI] [PubMed] [Google Scholar]
- (12).Nilsson SK, Dooner MS, Tiarks CY, et al. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–4020. [PubMed] [Google Scholar]
- (13).Quesenberry PJ, Becker PS. Stem cell homing: rolling, crawling, and nesting. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15155–15157. doi: 10.1073/pnas.95.26.15155. [comment]. [Review] [54 refs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- (15).Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) Production By Osteoblasts In The Hematopoietic Microenvironment And A Possible Mechanisms For Stem Cell Homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- (16).Stier S, Ko Y, Forkert R, et al. Matrix glycoprotein osteopontin is a stem cell niche constituent that constrains the hematopoietic stem cell pool size. Blood. 2004;104:191A. [Google Scholar]
- (17).Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005:2004–2011. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- (18).Crean SM, Meneski JP, Hullinger TG, et al. N-linked sialyated sugar receptors support haematopoietic cell-osteoblast adhesions. Br J Haematol. 2004;124:534–546. doi: 10.1046/j.1365-2141.2003.04786.x. [DOI] [PubMed] [Google Scholar]
- (19).Aiuti A, Tavian M, Cipponi A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. European Journal of Immunology. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- (20).Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- (21).Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- (22).Jung Y, Wang J, Song J, et al. Annexin II expressed by osteoblasts & endothelial cells regulates stem cell adhesion, homing & engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ling Q, Jacovina AT, Deora A, et al. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. Journal of Clinical Investigation. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Koh-Paige AJ, Demiralp B, Neiva K, et al. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146:4584–4596. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- (25).Hamon M, Mbemba E, Charnaux N, et al. A syndecan-4/CXCR4 complex expressed on human primary lymphocytes and macrophages and HeLa cell line binds the CXC chemokine stromal cell-derived factor-1 (SDF-1) Glycobiology. 2004;14:311–323. doi: 10.1093/glycob/cwh038. [DOI] [PubMed] [Google Scholar]
- (26).Charnaux N, Brule S, Hamon M, et al. Syndecan-4 is a signaling molecule for stromal cell-derived factor-1 (SDF-1)/CXCL12. Febs Journal. 2005;272:1937–1951. doi: 10.1111/j.1742-4658.2005.04624.x. [DOI] [PubMed] [Google Scholar]
- (27).Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- (28).Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- (29).Mbemba E, Saffar L, Gattegno L. Role of N-glycans and SDF-1alpha on the coassociation of CD4 with CXCR4 at the plasma membrane of monocytic cells and blood lymphocytes. FEBS Lett. 2002;514:209–213. doi: 10.1016/s0014-5793(02)02366-9. [DOI] [PubMed] [Google Scholar]
- (30).Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103(1):110–9. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- (31).Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. Journal of Clinical Investigation. 2003;111:187. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- (33).Lambeir AM, Proost P, Durinx C, et al. Kinetic Investigation of Chemokine Truncation by CD26/Dipeptidyl Peptidase IV Reveals a Striking Selectivity within the Chemokine Family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- (34).McQuibban GA, Butler GS, Gong JH, et al. Matrix Metalloproteinase Activity Inactivates the CXC Chemokine Stromal Cell-derived Factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- (35).Valenzuela-Fernandez A, Planchenault T, Baleux F, et al. Leukocyte Elastase Negatively Regulates Stromal Cell-derived Factor-1 (SDF-1)/CXCR4 Binding and Functions by Amino-terminal Processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- (36).Villalba S, Salvucci O, Aoki Y, et al. Serum inactivation contributes to the failure of stromal-derived factor-1 to block HIV-I infection in vivo. J Leukoc Biol. 2003;74:880–888. doi: 10.1189/jlb.0403149. [DOI] [PubMed] [Google Scholar]
- (37).Delgado MB, Clark-Lewis I, Loetscher P, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. European Journal of Immunology. 2001;31(3):699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- (38).De La Luz Sierra M, Yang F, Narazaki M, et al. Differential processing of stromal-derived factor-1{alpha} and stromal-derived factor-1{beta} explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- (39).Wein F, Pietsch L, Saffrich R, et al. N-cadherin is expressed on human hematopoietic progenitor cells and mediates interaction with human mesenchymal stromal cells. Stem Cell Res. 2010;4:129–139. doi: 10.1016/j.scr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- (40).Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- (41).Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Li P, Zon LI. Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell Stem Cell. 2010;6:199–202. doi: 10.1016/j.stem.2010.02.007. [DOI] [PubMed] [Google Scholar]
- (43).Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010 doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Taichman RS, Caldwell J, Biesecker LG, Emerson SG. Human Osteoblasts Are A Constitutive Source of G-Csf in the Bone-Marrow. Blood. 1993;82:A234. [Google Scholar]
- (46).Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Mobilization of hematopoietic progenitor cells in response to G-CSF.
Anxa2+/+ and anxa2−/− animals were administrated G-CSF by intraperitoneal injection over five days whereupon bone marrow and blood cells were collected from anxa2+/+ and anxa2−/− animals (n = 5). Cells (2 × 105 cells) were plated onto 6 well plates in a methylcellulose medium for the hematopoietic colony-forming cell assays. BFU-E, CFU-GM, CFU-GEMM, and the total progenitor colony numbers (CFU-C) were counted at 14 day after plating. The total progenitor colony numbers (CFU-C) were presented in Table 1. The average of progenitor colonies in bone marrow (BM Vehicle), peripheral blood (PB Vehicle), bone marrow with G-CSF treatment (BM G-CSF), and peripheral blood with G-CSF treatment (PB G-CSF) were enumerated. Data are presented as the mean ± standard error of the mean. *P< 0.05 significant differences from anxa2+/+ wild-type control groups.


