Abstract
Objective
To characterize skin wrinkles and rigidity in recently menopausal women.
Design
Baseline assessment of participants prior to randomization to study drug.
Setting
Multicenter trial, university medical centers.
Patients
Recently menopausal participants enrolled in the Kronos Early Estrogen Prevention Study (KEEPS).
Interventions
Skin wrinkles were assessed at 11 locations on the face and neck using the Lemperle wrinkle scale. Skin rigidity was assessed at the forehead and cheek using a durometer.
Outcome
Skin wrinkles and rigidity were compared among race/ethnic groups. Skin wrinkles and rigidity were correlated with age, time since menopause, weight, and BMI.
Results
In early menopausal women, wrinkles, but not skin rigidity, vary significantly among races (p=0.0003), where Black women have the lowest wrinkle scores. In White women, chronological age was significantly correlated with worsening skin wrinkles, but not with rigidity(p<0.001). Skin rigidity correlated with increasing length of time since menopause, however only in the White subgroup (p<0.01). In the combined study group, increasing weight was associated with less skin wrinkling (p<0.05).
Conclusions
Skin characteristics of recently menopausal women are not well studied. Ethnic differences in skin characteristics are widely accepted, but poorly described. In recently menopausal women not using hormone therapy (HT), significant racial differences in skin wrinkling and rigidity exist. Continued study of the KEEPS population will provide evidence of the effects of HT on the skin aging process in early menopausal women.
Keywords: Skin, estrogen, hormone therapy, wrinkles, ridigity, durometer, menopause, race, ethnicity
Introduction
Menopause is associated with an abundance of physiological changes that dramatically affect the lives of women. Declines in serum estrogen concentration can be detected in the years preceding menopause, leading to decreased feedback to the pituitary and a gradual rise of FSH. Changes during the menopause have significant impact on many organ systems, including reproductive, circulatory, musculoskeletal, endocrine, neurologic, urinary, as well as integumentary systems. The skin is the largest nonreproductive target on which estrogen acts. Skin changes are one of the most readily recognizable signs of aging, where the effects of estrogen on senile skin were reported as early as 1949.(1) Since that time, a wide range of skin parameters have been shown to correlate with post menopausal skin such as atrophy(2), thickness(3–5), dryness(6), hydration(7), elasticity(8), collagen content(9, 10), lipid content of the epidermis(11), sebum(12, 13), and wound healing capacity.(14–17) Understanding of the pathophysiological mechanisms underlying these phenomena began with the identification of estrogen receptors in the skin in 1980(18).
Interestingly, many clinicians report a subjective difference in the skin quality of patients using hormone therapy (HT).(19) This observation prompted a small retrospective pilot study of 11 patients, where we found that long term users of HT have less severe wrinkling and skin rigidity.(20) We sought to test these hypotheses in an ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS), a large multicenter prospective randomized placebo-controlled trial of HT in early menopausal women.(21) An important difference in the KEEPS population is that participants are recently menopausal, as opposed to our pilot study, where subjects were at least 5 years from menopause.
Skin aging has not previously been characterized at this endocrinologically unique time point as a woman has just entered menopause. Furthermore, racial differences in skin aging are recognized, but not well described.(22–25) Multiple investigators have hypothesized that skin pigmentation correlates with susceptibility to skin aging, where darker skin is more protected and lighter skin is more susceptible to the damaging effects of the sun.(24–26) Surprisingly, published reports supporting these hypotheses are sparse. One of the few studies providing objective data on racial skin differences was a report by Querleux et al, who demonstrated ultrasonographic dissimilarities in the skin of African American women.(27)
We hypothesized that racial differences in skin aging would exist and that these might impact our evaluation of response to hormone therapy. In this study we sought to define racial differences in skin aging characteristics at baseline enrollment among the racial groups in the KEEPS cohort. Defining the racial differences in skin in newly menopausal women here will allow us to accurately assess the effects of HT on skin aging as the study progresses.
Methods
The KEEPS trial is a multicenter, double blind, randomized placebo-controlled trial designed to compare the effects of early initiation of oval vs. transdermal estrogen, each with cyclic progesterone, on cardiovascular end points. Subjects enrolled in the parent KEEPS trial were offered participation in this ancillary skin study to evaluate the effects of HT on skin aging at two of the nine participating sites (Yale University, New Haven, CT and Albert Einstein College of Medicine, Bronx, NY) under approved IRB protocols at each institution. Written informed consent was obtained at enrollment. Information on race/ethnicity was obtained by self-report. Eligibility criteria for the parent KEEPS trial has been previously reported.(28) In addition to the inclusion/exclusion criteria for the parent trial, participants taking androgens, long term topical or systemic steroids, or retinoids were excluded for the skin study, as were those reporting a history of facial plastic surgery, Botox injections, use of chemical peels, scleroderma, known collagen disorders, facial trauma, or dermabrasion.
Prior to randomization, skin wrinkles were assessed by a single observer at each anatomical location using a objective visual scoring system, the Lemperle Scale.(29) Skin wrinkles were assessed at 11 locations on the face and neck: horizontal forehead, glabellar frown, cheek folds, preauricular lines (ear), periorbital lines (eye), nasolabial folds, upper lip lines, corner of mouth lines, marionette lines, chin crease, and neck folds. (Fig 1) Using a pictorial assessment schema, wrinkle severity was graded on a scale of 0 (none) to 5 (severe) at each anatomical site. Summation of scores from individual sites provided the “total wrinkle score”. Skin rigidity was assessed at the forehead (midpoint of the forehead, 2 cm cephalad to the glabella) and at the cheek (2 cm inferior to the inferior orbital ridge, in the mid pupillary line) using the durometer. Total durometer score was computed by summation of face and forehead scores.
Figure 1.
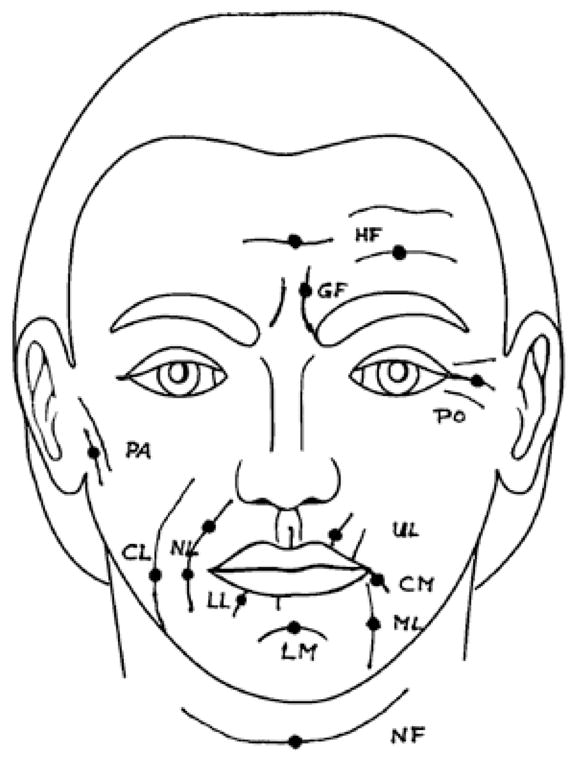
Schematic indicating facial locations of wrinkle assessment of the Lemperle Scale: horizontal forehead lines (HF), glabellar frown lines (GF), eye (PO, periorbital lines), ear (PA, preauricular lines), cheek lines (CL), nasolabial folds (NL), upper radial lip lines (UL), lower radial lip lines (LL), corner of the mouth lines (CM), marionette lines (ML), chin (LM, labiomental crease), and horizontal neck folds (NF).
Smoking and sun exposure were ascertained using an ordinal scale: frequency of sunscreen usage (0=never, 1=occasionally/seldom, 2=regularly/daily), Tanning bed (0=never, 1=occasionally/seldom, 2=regularly/daily), tanning bed lifetime usage (0=never, 1= 1–10 times, 2= >10 times), smoking frequency (0= never, 1= ≤1, 2= 2–5, 3= 6–10, 4= 11–15, 5= >15 cigarets per day). Any history of smoking was also assessed on a binary scale (yes or no). Smoking status/history was compared among races using Pearson’s Chi squared.
Average wrinkle scores and durometer measurements are reported as mean ± standard deviation (SD). Measurements were compared among groups using Kruskal-wallis test for nonparametric measures with Bonferroni correction for multiple comparisons. Participant characteristics were compared with skin aging assessment using pair-wise correlations. P-values are given in the text and tables.
Results
Demographics
Baseline skin assessment was completed in 106 participants from KEEPS. Mean age of these participants was 53.3 yrs (±2.7) and had an average duration of 1.8 yrs (±1.0) since menopause. Baseline demographics were also analyzed by racial group, which were broken in to Black (n=21), White (n=65), and Other (n=16). Race was not recorded for 3 participants. (Table 1) Past or current tobacco smoking, age and years since onset of menopause were comparable among racial groups; while height, weight, and BMI were significantly different. Inclusion criteria for the primary KEEPS trail required subjects to have not used HT for at least 6 months prior to enrollment. Only one participant (1 at AECOM and none at Yale) required a washout prior to randomization to study drug.
Table 1. Demographics by Race.
Baseline demographics of participants of the Ancillary Skin study of the KEEPS trial were similar among race/ethnic groups. Continuous variables were compared using Kruskal-wallis for nonparametric means (Mean±SD). Smoking status of participants was compared using Pearson’s Chi2.
| mean (±SD) | Total (n=106) | Black (n=21) | Other (n=16) | White (n=65) | P value |
|---|---|---|---|---|---|
| Age (yrs) | 53.3 (±2.7) | 53.6 (±2.5) | 52.8 (±1.9) | 53.3 (±3.0) | NS |
| Time since menopause (yrs) | 1.8 (±1.0) | 1.7 (±0.9) | 2.3 (±1.1) | 1.8 (±1.0) | NS |
| Weight (lbs) | 152.7 (±24.0) | 165.8 (±27.0) | 146.0 (±17.3) | 150.0 (±23.3) | P=0.047* |
| Ht (in) | 64.1 (±2.1) | 64.6 (±1.9) | 62.4 (±1.6) | 64.4 (±2.1) | p=0.002* |
| BMI | 26.1 (±4.2) | 28.0 (±4.7) | 26.5 (±3.6) | 25.4 (±4.0) | p=0.043* |
| H/o Smoking | 40/104 (38.5%) | 8/21 (38.1%) | 8/15 (53.3%) | 25/68 (36.8%) | NS |
| Current Smoker | 5/105 (4.8%) | 2/21 (9.5%) | 1/16 (6.3%) | 2/68 (2.9%) | NS |
p<0.05
Skin Wrinkles (Lemperle Scale)
Wrinkle scores at each anatomic location assessed by the Lemperle scale were generally low, with most approximating 1.5 on a scale of 0–5. Mean total wrinkle score in our baseline assessment was 17.4±7.4 with anatomical site specific differences noted; the lowest average wrinkle score was at the cheek (0.7±0.7) and the highest was at the neck (2.5±1.0). Statistically significant differences in skin wrinkles were detected among groups by race at the specific locations on the face: glabella frown, eye, ear, nasolabial fold, cheek, mouth, lip, marionette, chin, and total wrinkle score.(Table 2) In the Black subgroup, wrinkles scores were significantly lower than Non-Black participants at all facial locations except the neck (p<0.05). Consistent with this finding, total wrinkle scores were also lower in the Black subgroup (11.3±1.1) vs Non-Black group (19.0±0.8).
Table 2. Skin Wrinkles and Rigidity by Race.
Skin wrinkles were assessed at 11 locations on the face and neck. Average wrinkles scores at each location, as well as the total wrinkle score were determined for the entire study group and by race/ethnicity. Total wrinkle scores (Mean±SD) were significantly different among race/ethnic subgroups when compared using Kruskal-Wallis test for nonparametric means, but skin rigidity scores measured using the durometer were not.
| mean (±SD) | All (n=107) | Black (n=21) | Other (n=16) | White (n=69) | P value | |
|---|---|---|---|---|---|---|
| Wrinkles (Lemperle Scale) | Forehead | 1.2 (±0.9) | 0.8 (±1.0) | 1.3 (±0.8) | 1.3 (±0.9) | 0.07 |
| Glabellar frown | 1.5 (±1.1) | 0.8 (±0.8) | 0.9 (±0.8) | 1.8 (±1.1) | 0.0001* | |
| Eye | 1.7 (±1.0) | 0.9 (±0.7) | 1.5 (±0.7) | 2.0 (±1.0) | 0.0001* | |
| Ear | 1.3 (±0.8) | 0.6 (±0.5) | 1.2 (±0.9) | 1.5 (±0.8) | 0.0001* | |
| Nasolabial fold | 1.6 (±1.1) | 1.2 (±1.3) | 1.9 (±1.1) | 1.7 (±1.0) | 0.07 | |
| Cheek | 0.7 (±0.7) | 0.4 (±0.5) | 0.4 (±0.3) | 0.9 (±0.7) | 0.0009* | |
| Mouth | 1.7 (±1.1) | 1.0 (±1.0) | 1.0 (±0.7) | 2.0 (±1.0) | 0.0001* | |
| Lip | 1.1 (±0.9) | 0.3 (±0.4) | 0.8 (±0.7) | 1.4 (±0.8) | 0.0001* | |
| Marionette | 1.0 (±1.0) | 0.2 (±0.4) | 0.5 (±0.8) | 1.4 (±1.0) | 0.0001* | |
| Chin | 1.5 (±1.3) | 0.8 (±0.8) | 0.6 (±0.9) | 1.9 (±1.3) | 0.0001* | |
| Neck | 2.5 (±1.0) | 2.9 (±0.7) | 2.9 (±1.0) | 2.3 (±1.0) | 0.02 | |
| TOTAL SCORE | 17.4 (±7.4) | 11.3 (±5.2) | 15.4 (±5.2) | 19.8 (±7.2) | 0.0001* | |
| Rigidity | Forehead | 42.5 (±12.3) | 44.8 (±14.3) | 43.9 (±8.8) | 41.7 (±12.4) | NS |
| Face | 10.7 (±7.9) | 11.0 (±7.5) | 12.7 (±6.6) | 10.1 (±8.3) | NS | |
| TOTAL | 53.4 (±17.9) | 55.8 (±20.8) | 56.7 (±12.1) | 52.1 (±18.3) | NS |
p<0.0045
To assess for a relationship between wrinkle scores and demographic characteristics, a pairwise correlation test was used; a positive association of age with increasing skin wrinkle scores was found at the eye, ear, nasolabial fold, cheek, marionette, and total wrinkle score.(Table 3) The effects of age on skin wrinkles was most notable in the White subgroup, were statistically significant correlations with wrinkles were found at the forehead, eye, ear, nasolabial fold, cheek, mouth, marionette, and the total wrinkle score.(Table 3)
Table 3. Correlation of Age with Wrinkles and Skin Rigidity.
Chronological age was correlated with wrinkles and skin rigidity using a pair wise correlation. Age correlated with Skin Wrinkles only in the White subpopulation. Skin rigidity was not correlated with age, nor were any significant differences among races detected.
| Age | |||||
|---|---|---|---|---|---|
| Correlation Coefficient | All (n=107) | Black (n=21) | Other (n=16) | White (n=65) | |
| Wrinkles (Lemperle Scale) | Forehead | 0.17 | 0.07 | −0.17 | 0.27* |
| Glabellar frown | 0.08 | 0.08 | 0.01 | 0.10 | |
| Eye | 0.24* | 0.25 | 0.29 | 0.29* | |
| Ear | 0.31 | 0.39 | 0.40 | 0.37** | |
| Nasolabial fold | 0.22* | 0.19 | −0.18 | 0.35** | |
| Cheek | 0.20* | −0.07 | −0.21 | 0.31* | |
| Mouth | 0.19 | −0.07 | 0.53* | 0.25* | |
| Lip | 0.10 | 0.15 | 0.17 | 0.14 | |
| Marionette | 0.23* | 0.21 | 0.10 | 0.30* | |
| Chin | 0.18 | 0.23 | 0.01 | 0.22 | |
| Neck | 0.02 | 0.08 | −0.14 | 0.03 | |
| TOTAL SCORE | 0.30** | 0.24 | 0.14 | 0.40 | |
| Rigidity (Durometer) | Forehead | −0.07 | 0.09 | −0.01 | −0.13 |
| Face | −0.04 | −0.10 | −0.05 | −0.02 | |
| TOTAL | −0.07 | 0.02 | −0.04 | −0.10 | |
p<0.05,
p<0.005,
p<0.001
Overall, a close association with skin wrinkles and time since menopause (a measure of the length of time with reduced endogenous estrogen exposure) was not seen. No significance was detected in the combined study group.(Supplemental Table 1)
In general, a trend for lower wrinkle scores (fewer wrinkles) with increasing weight and body mass index (BMI, weight in kg/height m2) was observed. Weight was inversely correlated with wrinkles at the forehead, eye, nasolabial fold, lip, marionette, and total wrinkle score.(Supplemental Table 2) Significant differences among races between wrinkles and weight or BMI were not observed. Results for weight are shown in Supplemental Table 2, which were similar to results using BMI (data not shown). No relationship between wrinkle scores and height was appreciated.
Interestingly, waist circumference was found to correlate with nasolabial wrinkling, but only in the black and White subgroups. (Supplemental Table 3) The power to detect these differences may be more limited in the ethnic subgroup “Other” due to the smaller N. Conversely, a relationship between waist circumference and eye wrinkles was observed in the “Other” racial subgroup.
Severity of neck wrinkles was associated with a history of smoking (p=0.04), but not at any additional facial location. No correlation of wrinkles with current smoking was observed.
Skin Rigidity (durometer)
The durometer was first developed in the plastics industry to measure surface properties of various materials. In medicine, it has been validated in the scleroderma patient population as a way to measure skin hardness.(30–32) In this study, skin rigidity was assessed at the forehead and face where average scores were 42.5 ±12.3 and 10.7 ±7.9, respectively.(Table 2) Durometer measurements of skin rigidity demonstrated consistency between the forehead and face, which were closely correlated (0.55, p<0.01). Overall, durometer scores were not different among races/ethnicities (Table 2), nor was age associated with skin rigidity.(Table 3) When time since menopause in years (length of decreased estrogen exposure) was compared with skin rigidity, no significant relationship was detected. However, in the White subpopulation, forehead rigidity (0.29, p<0.05) and total durometer score (0.32, p<0.01) were associated with time since menopause.(Supplemental Table 1)
It is unknown if confounding factors contribute both to wrinkles and skin rigidity. To test this hypothesis, we compared wrinkles with rigid skin to assess for a relationship. Anatomical as well as racial differentials in the relationships between worsening skin wrinkles and increasing skin rigidity were observed. Wrinkle scores at the neck were associated with durometer scores at the forehead (0.25, p=0.01), face (0.25, p<0.01), and total durometer score (0.28, p<0.01) in the combined group. In contrast, an inverse relationship between skin rigidity and wrinkling was observed in the Black subpopulation, in whom durometer scores at both the forehead and face were inversely correlated with wrinkles at the mouth (−0.61, −0.55; p=0.01). Forehead skin rigidity was positively correlated with eye wrinkles in both the Hispanic (0.89, p=0.02) and White subpopulations (0.24, p=0.05). Statistically significant correlation between durometer scores at the forehead with total wrinkle score in the White subpopulation (0.24, p=0.05) with a trend observed at the face (0.21, p=0.08).
Weight and BMI were not associated with skin rigidity in this newly menopausal population.(Supplemental Table 2) No significant associations between skin rigidity and height, weight, BMI, history of smoking, or current smoking were detected in the group as a whole or within racial or ethnic subgroups.
Of the subject characteristics that were assessed, waist circumference demonstrated the strongest association with durometer measures of skin rigidity at the forehead (0.21, p<0.05), face (0.31, p<0.005), and total rigidity score (0.28, p<0.005). Associations between waist circumference and skin rigidity were most notable in the White subgroup.(Supplemental Table 3)
Discussion
Although the effects of estrogen on the skin are widely studied, characterization of the skin near the menopausal transition is lacking. Furthermore, data on racial differences in skin aging are sparse and conflicting.(33) Here we describe skin characteristics in recently menopausal women, and also delineate skin differences that exist among racial groups. Our finding that black women have significantly fewer wrinkles overall is the first to quantify such a racial difference in recently menopausal women.
Aging is most apparent by recognizable patterns of skin wrinkling. However, in early menopause, age appears to have the most dramatic association with skin wrinkles only in the White subgroup. Resistance to photoaging has been attributed to the protective effects of melanin.(24, 26) The observed relationship between advancing age and skin wrinkles in the White postmenopausal women in KEEPS is notable given the narrow age range of the participants enrolled in the parent study. Our findings of reduced wrinkling in Black postmenopausal women are consistent with prior data, which has shown that persons of color eventually succumb to the skin aging process, albeit at a slower rate.(24–26)
As was intended by the KEEPS study design, there was a narrow range of time since menopause in this study cohort. The working hypothesis for the KEEPS skin ancillary study is that HT will impact on the rate of skin aging; it is not surprising that baseline measures of skin wrinkling did not correlate with time since menopause given that subjects had only recently entered menopause. Increasing weight and BMI was observed to confer protection against skin wrinkling, an association that did not appear to be influenced by race. These data will provide an optimal reference point with which to compare the effects of HT on skin aging. Race, age and BMI should be considered when assessing skin aging.
Interestingly, large differences in skin rigidity were not detected in recently menopausal women. However, when differences were detected, rigidity did correlate with time since menopause in the White subpopulation of participants. As compared to skin wrinkles, skin rigidity appears more sensitive to hormonal status (ie time since menopause) rather than chronological age.
Of the 11 observed anatomical sites, only neck wrinkles were observed to relate to skin rigidity. This may imply a different physiology of the skin of the neck than the face, where wrinkling may be mostly affected by the same influences as rigidity, for instance hormonal status.
In summary, age at menopause and race appear to significantly affect skin wrinkling. Wrinkling correlates most closely with chronologic age and skin color rather than estrogen deprivation, at least in the short term. This is in contrast to skin rigidity that is closely correlated with time since menopause and therefore estrogen deprivation. Rigidity may be the earliest marker of estrogen loss on skin. Changes in rigidity may reflect the alterations in skin collagen and water content that occur rapidly in response to estrogen deprivation. Wrinkling is the end result of long term accumulated skin damage. Estrogen may rapidly reduce rigidity and prevent long term damage, while wrinkles may reflect existent damage that is not responsive to estrogen. We suggest that the changes in skin parallel the timing of estrogen response as in other tissues, where preexisting damage is not reversible with estrogen; in contrast estrogen may prevent or slow damage in healthy tissue. These findings provide the first quantification of skin wrinkles and rigidity in recently menopausal women and form an important reference point for studying the relative effects of HT on skin aging.
Supplementary Material
Increased time since menopause (years) was correlated with skin rigidity using a pair wise correlation, but only in the White subpopulation. Wrinkles were not correlated with time since menopause, nor were any significant differences among races detected.
Increased weight was correlated with fewer total skin wrinkles using pair wise correlations, where no clinically significant differences among races were detected. Weight was not correlated with skin rigidity.
Waist circumference was correlated with wrinkles and skin rigidity using a pair wise correlation. Increasing waist circumference was associated with worsening nasolabial wrinkles in the black and White subgroups. Durometer measures of skin rigidity were found to correlate most closely with waist circumference.
Acknowledgments
Support:
Kronos Longevity Research Institute
Reproductive Scientist Development Program NIH K12HD00849 and The Berlex Foundation (EFW)
NIH U54 HD052668 (HT)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.GOLDZIEHER J. The direct effect of steroids on the senile human skin. J Gerontol. 1949;4:104–12. doi: 10.1093/geronj/4.2.104. [DOI] [PubMed] [Google Scholar]
- 2.GOLDZIEHER J, RAWLS W, ROBERTS I, GOLDZIEHER M. Studies on aging: correlation of skin morphology with age and hormone excretion. J Gerontol. 1952;7:47–53. doi: 10.1093/geronj/7.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Rauramo L, Punnonen R. Effect of oral estrogen treatment with estriol succinate on the skin of castrated women. Z Haut Geschlechtskr. 1969;44:463–70. [PubMed] [Google Scholar]
- 4.Brincat M, Moniz C, Studd J, Darby A, Magos A, Emburey G, et al. Long-term effects of the menopause and sex hormones on skin thickness. Br J Obstet Gynaecol. 1985;92:256–9. doi: 10.1111/j.1471-0528.1985.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 5.Panyakhamlerd K, Chotnopparatpattara P, Taechakraichana N, Kukulprasong A, Chaikittisilpa S, Limpaphayom K. Skin thickness in different menopausal status. J Med Assoc Thai. 1999;82:352–6. [PubMed] [Google Scholar]
- 6.Dunn L, Damesyn M, Moore A, Reuben D, Greendale G. Does estrogen prevent skin aging? Results from the First National Health and Nutrition Examination Survey (NHANES I) Arch Dermatol. 1997;133:339–42. doi: 10.1001/archderm.133.3.339. [DOI] [PubMed] [Google Scholar]
- 7.Piérard-Franchimont C, Letawe C, Goffin V, Piérard G. Skin water-holding capacity and transdermal estrogen therapy for menopause: a pilot study. Maturitas. 1995;22:151–4. doi: 10.1016/0378-5122(95)00924-a. [DOI] [PubMed] [Google Scholar]
- 8.Piérard G, Letawe C, Dowlati A, Piérard-Franchimont C. Effect of hormone replacement therapy for menopause on the mechanical properties of skin. J Am Geriatr Soc. 1995;43:662–5. doi: 10.1111/j.1532-5415.1995.tb07202.x. [DOI] [PubMed] [Google Scholar]
- 9.Affinito P, Palomba S, Sorrentino C, Di Carlo C, Bifulco G, Arienzo M, et al. Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas. 1999;33:239–47. doi: 10.1016/s0378-5122(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 10.Brincat M, Moniz C, Studd J, Darby A, Magos A, Cooper D. Sex hormones and skin collagen content in postmenopausal women. Br Med J (Clin Res Ed) 1983;287:1337–8. doi: 10.1136/bmj.287.6402.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denda M, Koyama J, Hori J, Horii I, Takahashi M, Hara M, et al. Age- and sex-dependent change in stratum corneum sphingolipids. Arch Dermatol Res. 1993;285:415–7. doi: 10.1007/BF00372135. [DOI] [PubMed] [Google Scholar]
- 12.Guinot C, Malvy D, Ambroisine L, Latreille J, Mauger E, Guéhenneux S, et al. Effect of hormonal replacement therapy on skin biophysical properties of menopausal women. Skin Res Technol. 2005;11:201–4. doi: 10.1111/j.1600-0846.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 13.Callens A, Vaillant L, Lecomte P, Berson M, Gall Y, Lorette G. Does hormonal skin aging exist? A study of the influence of different hormone therapy regimens on the skin of postmenopausal women using non-invasive measurement techniques. Dermatology. 1996;193:289–94. doi: 10.1159/000246272. [DOI] [PubMed] [Google Scholar]
- 14.Ashcroft G, Greenwell-Wild T, Horan M, Wahl S, Ferguson M. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashcroft G, Ashworth J. Potential role of estrogens in wound healing. Am J Clin Dermatol. 2003;4:737–43. doi: 10.2165/00128071-200304110-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs E. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–55. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Merlo S, Frasca G, Canonico P, Sortino M. Differential involvement of estrogen receptor alpha and estrogen receptor beta in the healing promoting effect of estrogen in human keratinocytes. J Endocrinol. 2009;200:189–97. doi: 10.1677/JOE-08-0442. [DOI] [PubMed] [Google Scholar]
- 18.Punnonen R, Lövgren T, Kouvonen I. Demonstration of estrogen receptors in the skin. J Endocrinol Invest. 3:217–21. doi: 10.1007/BF03348266. [DOI] [PubMed] [Google Scholar]
- 19.Baumann L. A dermatologist's opinion on hormone therapy and skin aging. Fertil Steril. 2005;84:289–90. doi: 10.1016/j.fertnstert.2005.03.032. discussion 95. [DOI] [PubMed] [Google Scholar]
- 20.Wolff E, Narayan D, Taylor H. Long-term effects of hormone therapy on skin rigidity and wrinkles. Fertil Steril. 2005;84:285–8. doi: 10.1016/j.fertnstert.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 21.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2:228–39. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48:S139–42. doi: 10.1067/mjd.2003.273. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama-Nakagiri Y, Sugata K, Hachiya A, Osanai O, Ohuchi A, Kitahara T. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135–9. doi: 10.1016/j.jdermsci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Taylor S. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46:S41–62. doi: 10.1067/mjd.2002.120790. [DOI] [PubMed] [Google Scholar]
- 25.Wesley N, Maibach H. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003;4:843–60. doi: 10.2165/00128071-200304120-00004. [DOI] [PubMed] [Google Scholar]
- 26.Rawlings A. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79–93. doi: 10.1111/j.1467-2494.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 27.Querleux B, Baldeweck T, Diridollou S, de Rigal J, Huguet E, Leroy F, et al. Skin from various ethnic origins and aging: an in vivo cross-sectional multimodality imaging study. Skin Res Technol. 2009;15:306–13. doi: 10.1111/j.1600-0846.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 28.Harman S, Brinton E, Cedars M, Lobo R, Manson J, Merriam G, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 29.Lemperle G, Holmes R, Cohen S, Lemperle S. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735–50. doi: 10.1097/00006534-200111000-00048. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 30.Falanga V, Bucalo B. Use of a durometer to assess skin hardness. J Am Acad Dermatol. 1993;29:47–51. doi: 10.1016/0190-9622(93)70150-r. [DOI] [PubMed] [Google Scholar]
- 31.Kissin E, Schiller A, Gelbard R, Anderson J, Falanga V, Simms R, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55:603–9. doi: 10.1002/art.22093. [DOI] [PubMed] [Google Scholar]
- 32.Romanelli M, Falanga V. Use of a durometer to measure the degree of skin induration in lipodermatosclerosis. J Am Acad Dermatol. 1995;32:188–91. doi: 10.1016/0190-9622(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 33.Diridollou S, de Rigal J, Querleux B, Leroy F, Holloway Barbosa V. Comparative study of the hydration of the stratum corneum between four ethnic groups: influence of age. Int J Dermatol. 2007;46 (Suppl 1):11–4. doi: 10.1111/j.1365-4632.2007.03455.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased time since menopause (years) was correlated with skin rigidity using a pair wise correlation, but only in the White subpopulation. Wrinkles were not correlated with time since menopause, nor were any significant differences among races detected.
Increased weight was correlated with fewer total skin wrinkles using pair wise correlations, where no clinically significant differences among races were detected. Weight was not correlated with skin rigidity.
Waist circumference was correlated with wrinkles and skin rigidity using a pair wise correlation. Increasing waist circumference was associated with worsening nasolabial wrinkles in the black and White subgroups. Durometer measures of skin rigidity were found to correlate most closely with waist circumference.


