Summary
Epidemiological, neuropathological and functional neuroimaging evidence implicates global and regional derangements in brain metabolism and energetics in the pathogenesis of cognitive impairment. Nerve cell microcircuits are modified adaptively by excitatory and inhibitory synaptic activity and neurotrophic factors. Aging and Alzheimer’s disease (AD) cause perturbations in cellular energy metabolism, level of excitation/inhibition and neurotrophic factor release that overwhelm compensatory mechanisms and result in neuronal microcircuit and brain network dysfunction. A prolonged positive energy balance impairs the ability of neurons to respond adaptively to oxidative and metabolic stress. Experimental studies in animals demonstrate how derangements related to chronic positive energy balance, such as diabetes, set the stage for accelerated cognitive aging and AD. Therapeutic interventions to allay cognitive dysfunction that target energy metabolism and adaptive stress responses (such as neurotrophin signaling) have shown efficacy in animal models and preliminary studies in humans.
Brain energy metabolism and cognitive impairment
Several converging lines of evidence suggest a critical role for alterations in global and regional brain metabolism and energetics in the pathogenesis of cognitive impairment. Epidemiological evidence has implicated global disorders of metabolism (such as obesity and type II diabetes mellitus) in cognitive aging1 and Alzheimer’s disease (AD).2,3 Functional neuroimaging, including functional magnetic resonance imaging (fMRI) and 2-deoxy-2[(18)F]fluoro-D-glucose (FDG) positron emission tomography (PET) studies, have demonstrated regional metabolic changes correlating with cognitive impairment.4,5 Animal studies have established several links between these conditions, demonstrating mitochondrial and metabolic alterations in the brains of cognitively impaired animals6,7,8 and abnormal cognition and neuronal changes 9,10,11 in the brains of metabolically impaired animals. On the other hand, data suggest that manipulations that improve global energy metabolism (such as caloric restriction and exercise) may be effective in preventing12,13 or reversing14,15 cognitive impairment and attenuating the atrophy16,17 associated with brain aging and AD in humans and animals.18,19,20
A separate line of research implicates brain network dysfunction in cognitive impairment. Our complex cognitive functions and behavior are the emergent property of the brain’s hierarchical organization,21 which is based on nerve cells anatomically and functionally linked to form microcircuits, which in turn interconnect to constitute large-scale brain networks.22 The brain is organized in such a way that information processing takes place efficiently and economically in terms of metabolic costs.23 This suggests a fundamental link between brain energetics and network function, which is consistent with the fact that network dysfunction develops in the course of cognitive aging and AD, in parallel with metabolic derangements.
How could brain network dysfunction be linked to global or regional energetic derangements? Recent evidence has reinforced the old idea that neurodegenerative diseases, in particular AD, preferentially target specific networks.24,25,26 Within brain networks, a small number of nodes, referred to as connector hubs, have disproportionately numerous connections through which they integrate the functions of distant microcircuits.26 Connector hub nodes are vital for information flow over a whole network; their dysfunction from regional metabolic derangements secondary to neurodegenerative pathology may critically affect a network’s function22 resulting in phenotypic manifestations at the levels of cognition and behavior. Moreover, it has become evident that aging alters the way networks process information and handle cognitive tasks globally,23 in parallel with global changes in brain metabolism.
In this article, we review research on the relation of the brain’s organization in microcircuits and networks to the spread of AD on a background of aging-related changes in energy metabolism. We consider the evidence for adaptive changes in microcircuit and network activation in response to pathologic processes, such as the balance of excitatory/inhibitory synaptic activity and neurotrophic factor production, and show how these adaptations relate to regional neuroenergetics. Finally, we relate these processes to whole-organism energetics and show how a positive energy balance caused by excessive caloric intake and a sedentary lifestyle favors cognitive aging and the AD cascade by impairing adaptive responses.
Selective vulnerability in AD: connectivity and energetics
A body of neuroimaging research has established that manifestations of early AD relate to specific network dysfunction resulting from atrophy27 and hypometabolism within critical nodes.28 MRI-documented atrophy in early AD is most prominent in medial temporal lobe (MTL), extending over time into inferior temporal, temporal pole, inferior parietal, superior frontal, posterior medial cortex (PMC, consisting of posterior cingulate cortex and precuneus), inferior frontal, and superior parietal regions.29 Hypometabolism on FDG-PET evolves temporally through a similar regional pattern.30 The early involvement of parietal cortex correlates with decline in processing visuospatial information,31 whereas, the early involvement of MTL and PMC nodes of the episodic memory and default mode networks are responsible for key AD deficits in episodic and semantic memory (Figure 1). It is still unclear what determines this selective regional vulnerability in AD. Nevertheless, mounting evidence suggests that pathologic changes spread into regions that are energetically challenged and receive neuronal projections from regions already exhibiting pathology.
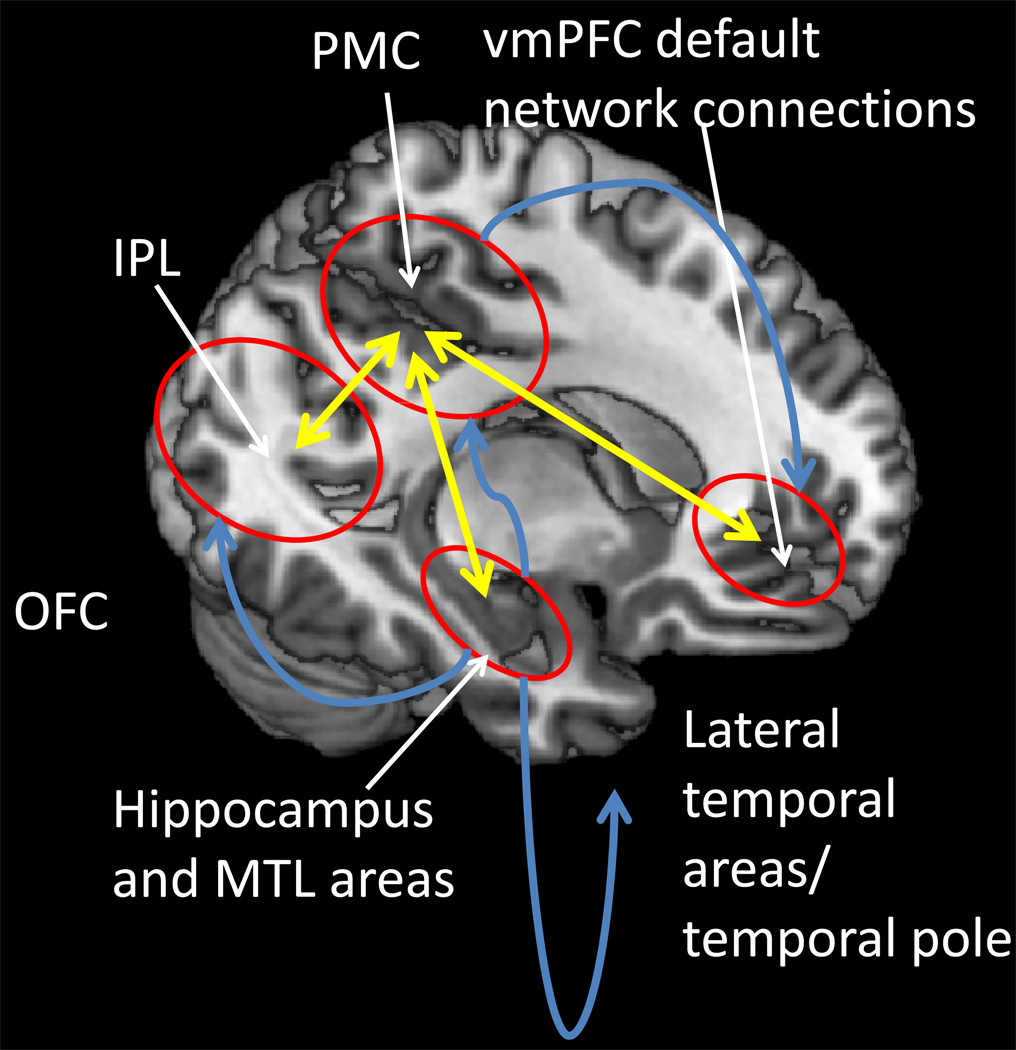
Figure 1.
Spread of the neuropathology in AD. Neurofibrillary tangles and neurodegeneration first appear in entorhinal cortex, and then in other medial temporal lobe (MTL) structures; fibrillary Aβ deposits and plaques first appear in transmodal areas [such as the posterior medial cortex (PMC), the inferior parietal lobule (IPL) and the lateral temporal lobe and temporal pole] that maintain reciprocal connections (illustrated by yellow arrows) with the entorhinal cortex. Spread of neurofibrillary tangles and neurodegeneration (illustrated by blue arrows) does not correlate with the spread of fibrillary Aβ deposition and plaque formation.
We share the view that a cohesive narrative for the temporal progression of AD cannot be constructed without reference to connectivity and energetics.26 Connectivity is necessary (although not sufficient) to explain the differential spatial and temporal spread of the two pathological landmarks of AD: extracellular deposits of amyloid β-peptide (Aβ), assuming various plaque formations, and intracellular neurofibrillary tangles, NFTs, consisting of hyperphosphorylated self-aggregating tau.24,32,33 There are conspicuous anatomical connections between sequentially affected areas in AD. Aβ deposition occurs at a constant slow rate at various neocortical locations in some older individuals34 (Figure 2). NFTs, on the other hand, appear first at the subiculum and entorhinal cortex (layer II/III and layer IV) accompanied by synaptic and cellular loss.24,32 Cells in layer II and adjacent parts of layer III affected by NFTs are precisely those that receive lateral connections from (transmodal) neocortex, and, in turn, project to the hippocampus proper or cornu ammonis (CA).24,35 Layer III neurons generate the glutamatergic perforant path pathway that terminates on distal dendrites of CA1 neurons, while layer II neurons project to CA3 pyramidal neurons that, in turn, give rise to the Schaffer collateral pathway that terminates on the apical dendrites of CA1 neurons. The loss of enthorhinal neurons deprives the hippocampus of neocortical input35 and directly impairs its function and plasticity.36 On the other hand, the affected cells in layer IV send lateral connections to transmodal neocortex;24 loss of these cells deprives the PMC and other transmodal cortices from hippocampal input.
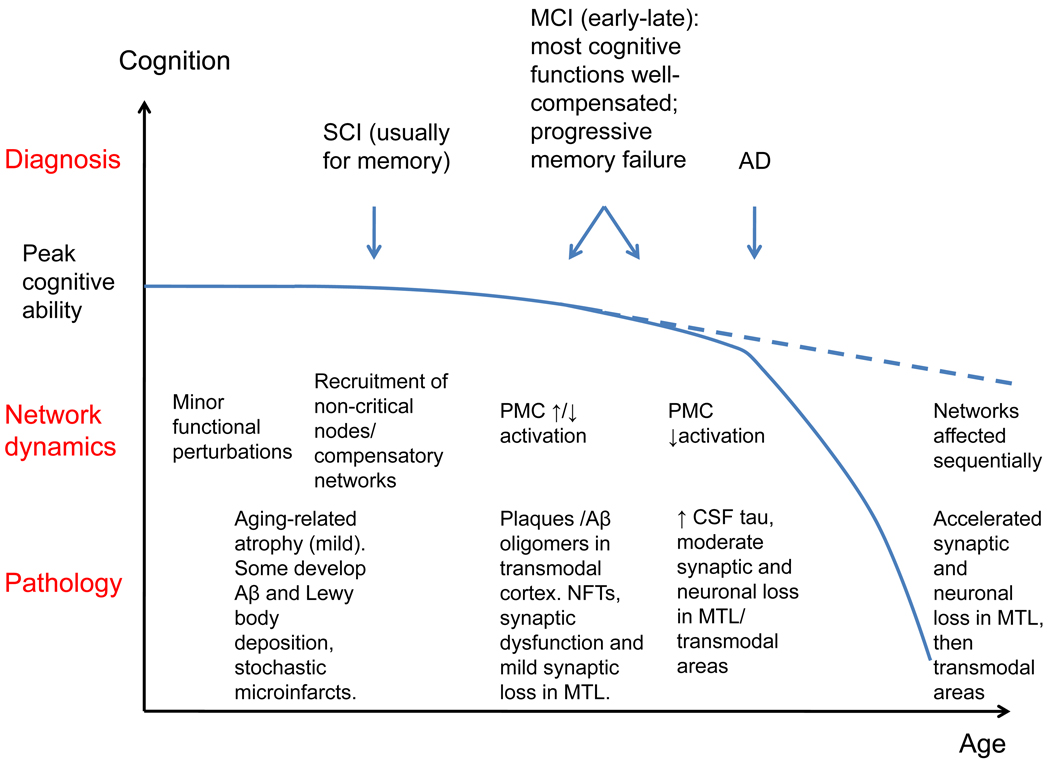
Figure 2.
Evolution of cognitive ability with age, in the presence or absence of AD pathology: schematic progression of pathology, brain network dynamics and clinical manifestations. SCI: subjective cognitive impairment; MCI: mild cognitive impairment; AD: clinical Alzheimer’s disease; NFTs: neurofibrillary tangles; PMC: posterior medial cortex; MTL: medial temporal lobe.
Neocortical involvement in AD can be largely attributed to connectivity to MTL allocortex; transmodal areas directly linked to it are the most vulnerable, whereas motor and primary sensory areas that are not directly connected to it are least affected24 (Figure 1). This selective vulnerability is not attributable to cytoarchitecture: transmodal areas not directly linked to MTL (such as the portion of anterior cingulate cortex, which is linked with motor areas) are spared.37 Moreover, lack of involvement of a brain region in AD does not imply resistance to neurodegenerative cascades in general, since frontoinsular and anterior cingulate areas are spared in AD but degenerate in behavioral variant Frontotemporal Dementia.38 Supporting the notion that connectivity determines regional vulnerability in AD, a pattern of involvement similar to the cortex is seen in the thalamus: Aβ deposits and NFTs are confined to nuclei with limbic connections.39
What could the mechanisms be for the spread of AD through anatomical connections? Cellular and animal studies have shown that soluble Aβ oligomers accumulate at the synapses40, where they impact a delicate balance of excitation/inhibition41,42, impair long term potentiation43 and facilitate long-term depression (two types of synaptic plasticity critical for learning and memory).44,45 Axons and synapses are selectively vulnerable to intracellular accumulation of pathologic substrates and may be the site where the nerve cell death process is triggered.46 Aβ oligomer accumulation in synapses may, therefore, result in tau hyperphosphorylation and aggregation in axons, which may be transferred to the neuronal soma in the form of a NFT, far from the site of Aβ deposition.
While connectivity partly explains the spread of AD, it does not account for the origin of the disease in specific neocortical and MTL areas. Instead, this localization may partly be accounted for by aging and age-related metabolic disease, which render MTL neurons particularly vulnerable to the energetic stress related with AD extracellular and intracellular deposits. Neuroimaging evidence suggests reduced efficiency of energy metabolism and disproportionate metabolic cost for cognitive processing in the hippocampus, parahippocampal gyrus and amygdala (as well as PMC, frontal and temporal transmodal nodes).23 Animal studies have shown that hippocampal pyramidal neurons have the highest energy requirements of any neurons in the brain47 and may therefore be at risk under conditions of unmet metabolic needs. Aging-related cognitive impairment in rats is associated with down-regulation of insulin signaling and glucose utilization pathways.48 In hippocampal pyramidal neurons, aging and chronic hyperinsulinemia synergistically up-regulate the gene for the glucocorticoid receptor (GR) and genes for inflammatory/immune pathways and down-regulate insulin signaling genes, thereby blocking glucose utilization and decreasing mitochondrial function.6 The end-result of chronic hyperinsulinemia is MTL atrophy.49
Turning our attention to affected neocortical areas, the PMC and medial prefrontal hubs of the default mode network show early functional impairment in AD,5,50 associated with Aβ deposition.51,52 We view this vulnerability as the result of their recruitment to compensate for failing hippocampal function with aging and in the early stages of AD. Successful memory encoding depends on the dynamic balance of hippocampal activation and PMC deactivation.53 PMC deactivation decreases with age-related cognitive impairment53 and successful memory encoding can only be maintained by hippocampus hyperactivation. Similarly, in aging and early AD, greater activation of the hippocampus, PMC and frontal areas is required for successful memory retrieval.54,55 This increased hippocampal recruitment presumably translates into a chronic increase of the energy requirements of its neurons. With AD progression (clinically at the stage of late MCI), PMC deactivation during encoding is attenuated further,56, especially among APOE ε4 carriers, suggesting that it represents an aspect of AD pathophysiology. Given that unrestrained episodic retrieval and semantic processing occupy brain activity whenever the brain is not engaged in specific cognitive tasks [representing the “default” functional state of the brain], this lack of deactivation translates into a chronic increase in the energy requirements of default network nodes. Eventually, atrophy due to neuronal death occurs in affected default mode network nodes resulting in severe hypometabolism.28
Excitatory and inhibitory signaling dysregulation in aging and AD
Given that much of the energy consumed by neurons is used for synaptic signaling,57 neuronal energetics are intricately linked to neurotransmission. The vast majority of the brain’s neurons and synapses deploy either the excitatory neurotransmitter glutamate or the inhibitory neurotransmitter GABA, while other neurotransmitters (serotonin, norepinephrine, dopamine and acetylcholine) and neuropeptides (somatostatin, corticotrophin-releasing hormone, neurokinins, etc.) fine tune the activity in neural networks.58 Nerve cell microcircuits within different brain regions are organized in a fundamentally similar fashion (Figure 3). Glutamate released from presynaptic terminals activates AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) receptors resulting in depolarization of the postsynaptic membrane and Ca2+ influx through NMDA (N-methyl-D-aspartate) receptor channels and voltage-dependent Ca2+ channels (Figure 4). Ca2+ serves as a second messenger that activates cascades of enzymes (protein kinases, nitric oxide synthase and proteases) and transcription factors (CREB, AP1 and others) that mediate rapid or delayed biochemical and structural changes, and may also increase the resistance of the neurons to disease. Perturbations in the balance between glutamatergic and GABAergic signaling occur early in the development of age-related cognitive impairment and AD, resulting from and contributing to disturbed cellular metabolism. Moreover, normal synaptic activity reduces Aβ production and protects synapses against Aβ-related alteration.59
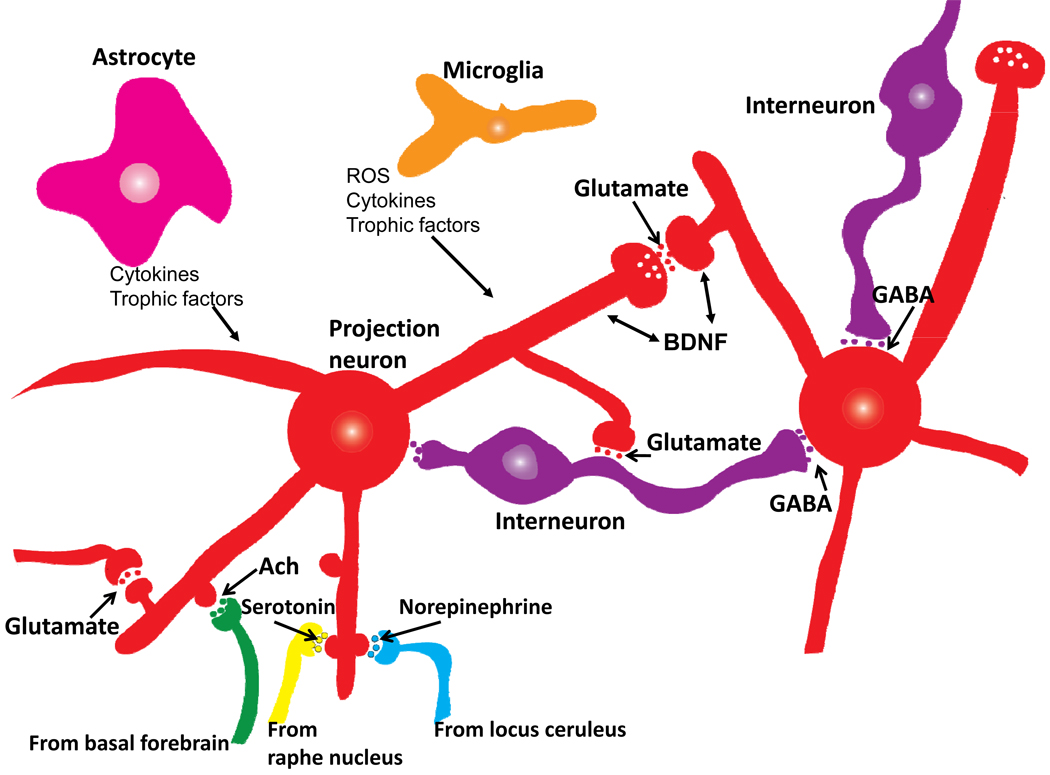
Figure 3.
Basic organization of neuronal microcircuits that control information flow through all brain regions involved in cognitive processing. The major excitatory projection neurons are glutamatergic with long axons that synapse on dendrites of other glutamatergic neurons that may, in turn, project their axons to a different brain region. GABAergic interneurons receive excitatory inputs from glutamatergic neurons and form synapses on the cell bodies of the same or other glutamatergic neurons. Glutamatergic neurons also receive synaptic inputs from noradrenergic, serotonergic and cholinergic neurons whose cell bodies are located in the locus ceruleus, raphe nucleus and basal forebrain, respectively. Neurons in all brain regions also interact with glial cells including astrocytes and microglia which produce trophic factors and cytokines which may normally play important roles in synaptic plasticity. However, excessive production of pro-inflammatory cytokines and reactive oxygen species (ROS) by glial cells has been implicated in the pathogenesis of cognitive impairment and AD.
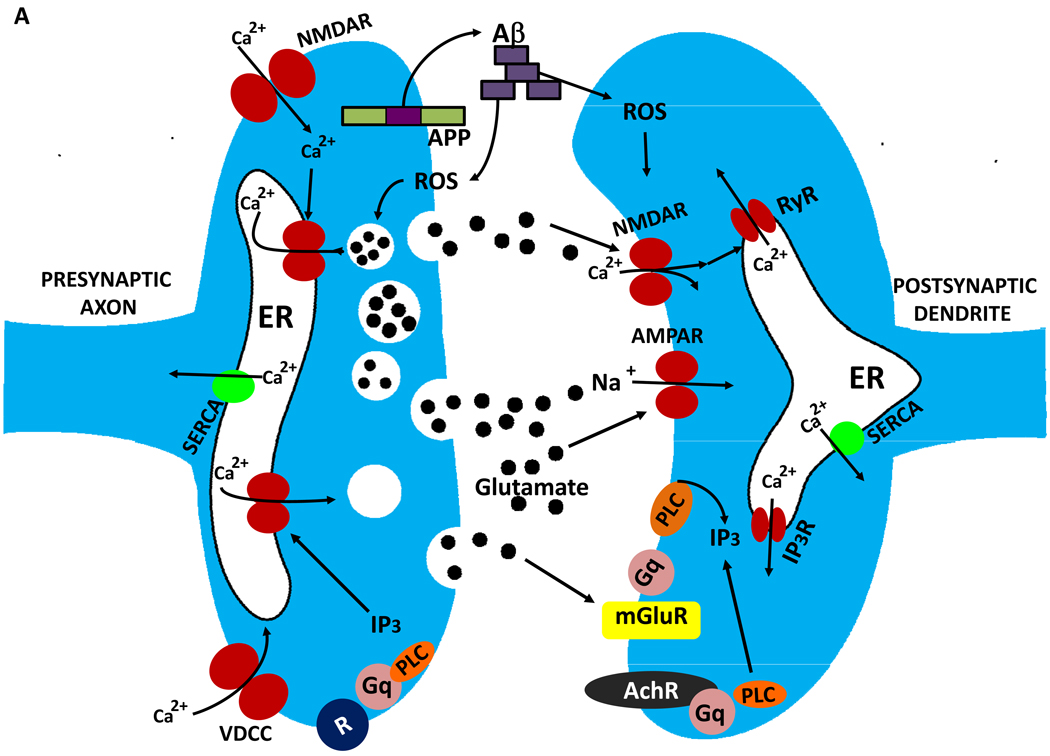
Figure 4.
Mechanisms of synaptic dysfunction in aging and Alzheimer’s disease. The β-amyloid precursor protein is axonally transported and so is present in high amounts in presynaptic terminals. In properly functioning synapses the APP is proteolytically cleaved in the middle of the Aβ sequence by the α-secretase, thereby preventing the production of Aβ. During normal aging, and more so in AD, APP is cleaved at the N- and C-termini of Aβ by β-secretase and γ-secretase, respectively, resulting in the production and self-aggregation of Aβ. Aggregation of Aβ on the membrane generates ROS resulting in membrane lipid peroxidation, which then impairs the function of membrane ion-motive ATPases thereby promoting membrane depolarization and Ca2+ influx through NMDA receptor channels and voltage-dependent Ca2+ channels. Sustained elevation of cytoplasmic Ca2+ levels promotes depletion of presynaptic glutamate stores resulting in impaired synaptic transmission and damage to axons and dendrites. In addition, perturbed mitochondrial function caused by aging, oxidative stress and Aβ results in energy depletion in neurons which exacerbates synaptic dysfunction and degeneration of neurons. Further contributing to the demise of neurons in AD is dysregulation of endoplasmic reticulum (ER) function that results in depletion of ER Ca2+ stores and accumulation of misfolded proteins.
An inhibitory imbalance (induced by GABA receptor agonists or glutamate receptor antagonists) impairs synaptic plasticity and associated learning and memory processes in animals and human subjects.60 Conversely, excitatory imbalance resulting from excessive glutamate receptor activation and/or reduced GABAergic signaling can result in seizures and degeneration of synapses and neurons.61 Complex microcircuit alterations affecting regional excitatory/inhibitory balance do occur in aging and AD in the hippocampus and cortex. Studies in animals have demonstrated that aging decreases GABAergic signaling in the hippocampus,62 resulting in excitatory imbalance, while at the same time aging impairs neuronal glucose uptake, causes mitochondrial dysfunction, and activates glucocorticoid pathways6 rendering neurons vulnerable to glutamate-induced damage.63 The excitatory imbalance of aging may be exacerbated by AD, since Aβ promotes membrane depolarization and renders human cortical neurons vulnerable to glutamate-mediated Ca2+ overload in vitro,64 which may lead to emergence of hyperactive neuronal clusters in the vicinity of plaques, as has been demonstrated in a rat model of AD.42 The emergence of such clusters may account for the increased metabolism in nodes of the default mode network associated with high regional PIB binding, which is seen transiently in the AD before atrophy prevails.28 The excitatory imbalance in AD may also result in increased occurrence of epilepsy in AD patients.65 Paradoxically, AD may also cause a regional inhibitory imbalance: hippocampal synaptic plasticity is impaired in AD mice due to reduced NMDA receptor activity,66 whereas Aβ can reduce seizure-like activity in cultured hippocampal neurons induced by GABA receptor antagonists.67 To reconcile these facts, we should note that, in mouse models of AD, there is initially increased hippocampal and cortical excitability followed by GABAergic sprouting, increased inhibitory transmission and impaired synaptic plasticity.41
The expression of genes encoding proteins involved in the regulation of neuronal excitability is altered in brain aging and AD. Age-related modifications of gene promoter regions are associated with reduced expression of genes encoding proteins involved in synaptic plasticity (e.g., glutamate and GABA receptor subunits, synaptic vesicle proteins) and cellular Ca2+ homeostasis (Ca2+ binding proteins, Ca2+ dependent kinases and Ca2+ transporters) in humans.68 Alterations in the expression of genes involved in synaptic plasticity and Ca2+ metabolism regulation have been documented in animal models of aging and AD and older humans.69,70 The down-regulation of inhibitory signaling and Ca2+ binding proteins may render the neurons vulnerable to Ca2+ overload. Our group has recently demonstrated that chronic experimental silencing of cortical neurons in vitro results in molecular changes similar to those seen in aging and AD, including reduced expression of genes involved in GABAergic transmission, inhibitory neuropeptides, calcium buffering, and calmodulin- and CREB-mediated signaling.71
The events downstream of perturbed network activity and dysregulated cellular energy metabolism that lead to neuronal death may also include accumulation of DNA damage and impaired removal of damaged proteins and organelles. For example, the expression of genes involved in DNA repair and removal of damaged proteins are suppressed in multiple regions in the mouse brain during aging.72 Physiological levels of glutamate receptor activation up-regulates DNA repair gene expression73 and induces the movement of proteasomes from dendritic shafts into synaptic spines74 in cultured rat neurons, protecting themagainst the accumulation of damaged DNA and proteins. Nerve cell microcircuit perturbations in AD may impair these important housekeeping processes.
The survival and growth of neurons is supported by neurotrophic factors produced by neurons or glial cells. Brain-derived neurotrophic factor (BDNF) is produced by neurons throughout the brain, where it is released in an activity-dependent manner (Figure 5A). BDNF plays pivotal roles in synaptic plasticity, learning and memory and neurogenesis, and can protect neurons against metabolic and oxidative insults.46 In addition, BDNF enhances intracellular energy availability to cultured mouse neurons by increasing expression of glucose transporters and stimulating amino acid transport.75 Studies of transgenic mouse models of AD indicate that large Aβ oligomers suppress BDNF production,76 disrupt its ability to activate the transcription factor CREB,77 and block the retrograde trafficking of BDNF from synaptic terminals to the nucleus impairing its ability to promote neuronal survival.78 Selective blockade of NMDA receptors mimics the abnormal molecular phenotypes of electrically silenced neurons, and treatment with BDNF reverses the perturbations caused by chronic suppression of neuronal activity. These findings suggest that activity-dependent neurotrophic signaling is impaired in brain aging and AD. Indeed, it was reported that cerebrospinal fluid BDNF declines in humans with aging and even more so in AD patients.79
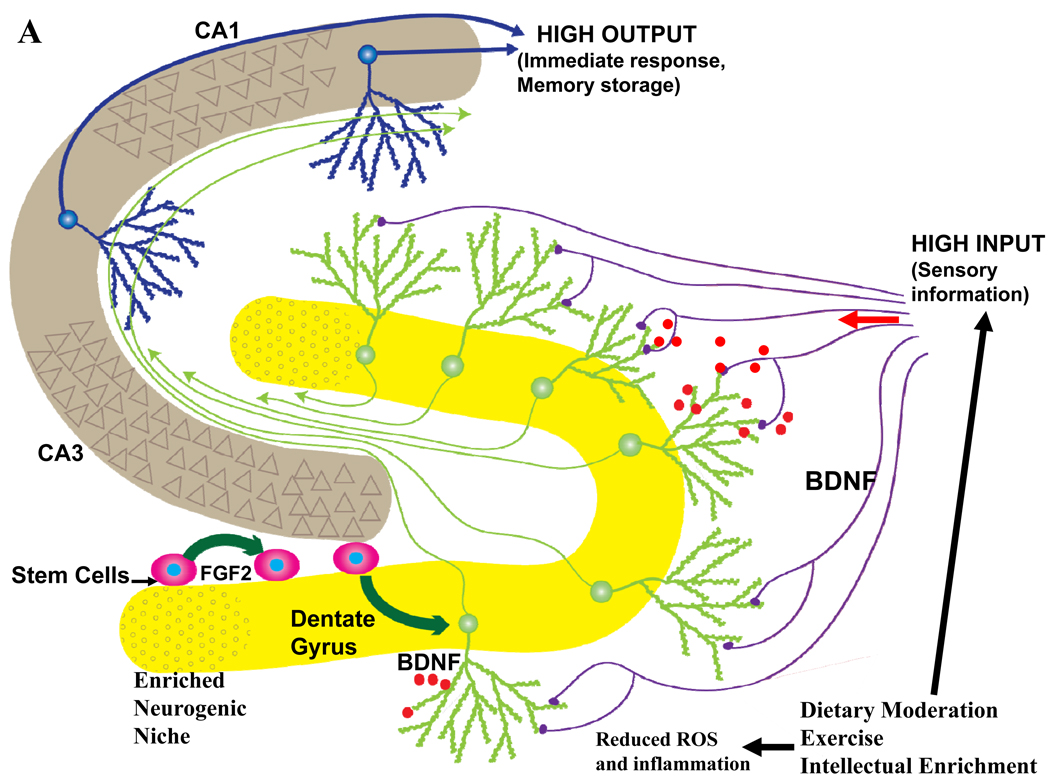
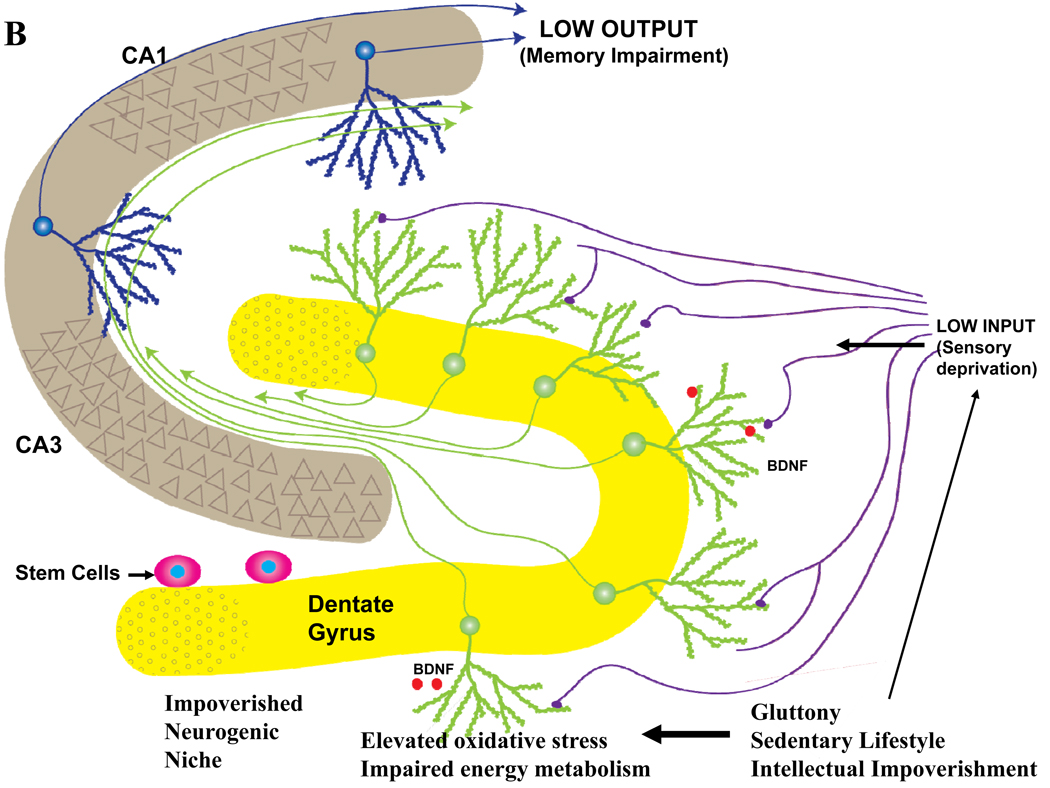
Figure 5.
The impact of lifelong ‘brain healthy’ and unhealthy lifestyles on late life hippocampal plasticity and cognitive function. Information from multimodal sensory association cortices enters the hippocampus from the entorhinal cortex via perforant path axons which synapse on dendrites of dentate granule neurons. The axons of granule neurons synapse on dendrites of pyramidal neurons which, in turn, may synapse on additional pyramidal projection neurons which then exit the hippocampus and innervate neurons in regions of the cerebral cortex involved in the long-term storage and processing of memories. A. Behaviors believed to promote healthy brain aging include moderation of dietary energy intake, regular exercise and engaging in intellectually challenging occupations and hobbies. Data suggest that these behaviors increase activity in hippocampal circuits and impose a mild cellular stress on neurons resulting in the activation of signaling pathways that induce the production of neurotrophic factors such as BDNF. As a consequence, synaptic plasticity and neurogenesis are enhanced and the resistance of neurons to aging and disease processes is increased. B. Behaviors that may contribute to cognitive impairment include excessive dietary energy intake, a sedentary lifestyle and a low level of cognitively challenging experiences. The latter lifestyle promotes diabetes and obesity, and can impair hippocampal synaptic plasticity and neurogenesis, thereby rendering neurons vulnerable to dysfunction and degeneration during aging.
Molecular alterations in AD are associated with perturbed neuronal energy metabolism
The process of Aβ oligomer formation in neuronal rat cultures generates hydrogen peroxide and hydroxyl radical, which then induce lipid peroxidation in the plasma membrane of neurons and glial cells and impair the function of ion-motive (Ca2+ and Na+/K+) ATPases and glucose transporters; as a result cellular Ca2+ and energy homeostasis are perturbed and synaptic function is impaired (Figure 4).80 In addition, AD pathogenesis may be linked with excessive accumulation of Ca2+ in the endoplasmic reticulum, which contributes to synaptic dysfunction and neuronal degeneration.81 In vivo imaging of intracellular Ca2+ levels in cortical neurons in a mouse model of AD revealed that some neurons are hypoactive, whereas neurons in the vicinity of Aβ plaques are hyperactive.42 The latter findings are consistent with the excitoxicity/energy depletion hypothesis of neuronal degeneration in AD.46
Impairment of mitochondrial function occurs in vulnerable neurons in MCI and AD and likely results from a combination of factors including Aβ oligomer accumulation, oxidative stress and a deficit in neurotrophin signaling.82 A reduction in the activity of several mitochondrial enzymes (e.g., α-ketoglutarate, pyruvate and isocitrate dehydrogenases) is evident in brain tissue samples from AD patients,83 and experimental findings in animal models of aging and AD suggest that mitochondrial dysfunction is both necessary and sufficient for impaired cognitive function.7,8 Mitochondria are located in presynaptic terminals and dendrites where they play important roles in local Ca2+ signaling and associated processes involved in synaptic plasticity.82,84 These synapse-associated mitochondria may be particularly vulnerable, and so may be compromised early in the AD process.
Perturbed cellular energy metabolism and associated oxidative stress are also involved in the hyperphosphorylation and self-aggregation of tau. In a mouse model of Down syndrome with a subset of triplicated human chromosome 21 ortholog genes (including amyloid precursor protein, APP), mitochondrial membrane potential and ATP production are reduced in brain cells and tau is hyperphosphorlyated due to an increase in GSK3β and JNK activities.85 Among the many kinases that can phosphorylate tau, data from AD mouse models strongly implicate GSK3β in the pathogenesis of AD.86 GSK3β may play important roles in Aβ processing87 and in linking perturbed cellular energy metabolism and cognitive decline in aging and AD. Agents that inhibit GSK3β reduce tau hyperphosphorylation, enhance cognitive function and reduce Aβ production in mouse models of AD.88 GSK3β suppression enhances glucose uptake by several cell types89 and increases brain insulin-like growth factor 1 (IGF-1), which is decreased in AD,90 in mouse model of AD. These data suggest an important link between AD pathogenesis and brain energy metabolism amenable to pharmacologic interventions.
Impact of energy intake and expenditure on cognitive aging
High-energy diets and diabetes may have adverse effects on cognitive function in aging and AD, whereas dietary energy restriction may have beneficial effects (Figure 5). Here we review experimental data in animals supporting these claims. In rhesus monkeys, aging is associated with decreased number (or activity) of functional mitochondria in the hippocampus and a negative correlation exists between metabolic syndrome severity and oxidative function of these mitochondria.6 Rodents fed with fats and/or simple sugars exhibit poor learning and memory compared to animals on lower energy diets,9 and even in young animals excessive weight impairs some cognitive domains.10 On the other hand, in a mouse model of accelerated aging, caloric restriction attenuated age-related deficits in learning and memory.91 Life-long caloric restriction in mice prevents age-related declines in learning,92 and preserves spatial and non-spatial and working memory in aged rats.93 Even when initiated in mid-life, dietary energy restriction preserves cognitive functions in aging mice.94 In mouse models of AD, high-energy diets exacerbate Aβ deposition and memory impairment,95 whereas dietary energy restriction prevents96 or attenuates97 the development of cognitive impairment and Aβ and tau pathologies.
Three general mechanisms by which excessive energy intake adversely affects cognitive function are increased oxidative stress, inflammatory processes and impaired adaptive cellular stress responses. Oxidative damage to proteins and DNA is elevated in brain cells of animals on high-energy diets98 and reduced in animals on low energy diets.99 High-energy diets promote inflammatory processes in the brain associated with cognitive impairment.100 On the other hand, dietary energy restriction protects neurons and synapses in animal models in which neurotoxicity is mediated by oxidative stress.101 Alternate day fasting reduced brain damage and improved functional outcome in an animal model of stroke by a mechanism involving suppression of brain inflammation. The effectiveness of dietary restriction was reduced in older animals, perhaps as a result of age-related impairment of adaptive cellular stress response pathways.102
Particularly interesting is emerging evidence that excessive dietary energy intake impairs,100 whereas dietary energy restriction increases BDNF signaling.102 Animal studies in which BDNF or its receptor trkB have been genetically manipulated, or BDNF has been administered to the brain, have demonstrated major roles for BDNF in synaptic plasticity, learning and memory and neuronal resistance to oxidative, metabolic and excitotoxic insults relevant to cognitive dysfunction and AD.46 BDNF also enhances neurogenesis in the hippocampus, which may contribute to maintenance of hippocampal neurons and preservation of cognitive function during aging.103 Leptin receptor mutant diabetic mice that have reduced BDNF levels exhibit cognitive impairment and impaired synaptic function and neurogenesis.11 BDNF signaling also plays major roles in energy metabolism and cognitive function in humans as demonstrated by the cases of human subjects with BDNF haploinsufficiency104 and with de novo trkB mutations105 who are obese, insulin resistant and cognitively impaired.
Impaired cellular energy metabolism accompanies increased oxidative stress, as indicated by reduced expression and/or activity of mitochondrial proteins and oxidative genomic damage.106 The most common metabolic disease, diabetes impairs learning and memory in animals by inducing multiple alterations in hippocampal microcircuits, including reduced dendritic spine density, impaired synaptic plasticity and reduced neurogenesis.11 Diabetes may impair cognitive function, in part, by hyperactivation of the hypothalamic-pituitary-adrenal axis and lowering glucocorticoid levels can restore cognitive function, synaptic plasticity and neurogenesis.11 The links between diabetes and AD are complex and likely also involve inflammatory mechanisms: in double-mutant AD transgenic and diabetic mice, the onset of diabetes exacerbates AD-like cognitive dysfunction without an increase in brain Aβ burden, but in association with cerebrovascular inflammation.107
Animal studies have demonstrated benefits of exercise on cognitive function during normal aging and in models of insulin resistance/diabetes and AD, and have elucidated the underlying mechanisms (Figure 5). Several studies have documented beneficial effects of exercise in mouse models of AD, such as improved cognitive performance.18 Exercise reduces glucocorticoid levels and enhances hippocampal neurogenesis.19 Mild metabolic challenges associated with exercise induce the expression of genes encoding proteins that enhance the ability to resist perturbations and cellular plasticity, therefore enhancing learning. Exercising rats exhibit increased levels of proteins involved in cellular energy metabolism and synaptic plasticity in the hippocampus.20 The hippocampal transcriptome of old mice that have been running lifelong exhibit greater learning-induced activation of synaptic plasticity and mitochondrial function genes, and down-regulation of oxidative stress and lipid metabolism genes; running also modulates genes involved in cell excitability, energy metabolism, and insulin signaling.108 We should take particular notice of the fact that exercise impacts cognition by enhancing learning. This may explain why certain studies have failed to show a beneficial effect of exercise on cognition independent of cognitive stimulation.109
In regards to the cellular and molecular mechanisms underlying the cognition-enhancing effects of exercise, BDNF plays a pivotal role. Even short-term exercise may improve memory in rats, associated with increased hippocampal BDNF.110 Exercise and caloric restriction each increase hippocampal dendritic spine density and BDNF levels in diabetic mice and exercise significantly enhances the effect of caloric restriction on spine density and BDNF levels.111 Exercise-induced BDNF may strengthen existing synapses, promote synaptogenesis and stimulate neurogenesis.19,46 The effects of exercise may not occur simultaneously across cognitive domains; instead, memory retention appears best immediately after a period of exercise, associated with BDNF elevation, whereas memory acquisition is improved after a post-exercise delay.112 A second crucial mediator of the brain effects of exercise is the peripherally produced IGF-1, which induces plastic and neuroprotective brain changes and stimulates hippocampal neurogenesis.113 Finally, two proteins that play important roles in cognitive processes, mitogen-activated protein kinase and the transcription factor CREB (cyclic AMP response element-binding protein), are also increased in the hippocampus of rats in response to exercise.114
Energy-based therapeutic interventions in cognitive aging and AD
Thus far, most of the funds for basic and translational research on AD have been invested in developing treatments to halt the production of Aβ or enhance its removal, which have, thus far, failed in clinical trials. Here we consider alternative approaches that show promise in preclinical and preliminary clinical studies and aim at prophylaxis and slowing of cognitive decline based on modulating adaptive cellular stress response pathways and energy metabolism.
The considerable evidence that diabetes is a risk factor for cognitive impairment and AD has led to preclinical studies aimed at establishing the efficacy of anti-diabetic treatments in animal models.93,96,115 At the cellular level, insulin was shown to decrease binding of Aβ oligomers at the synapses and the oxidative stress and synaptic spine deterioration they cause.116 Several small studies have suggested that insulin treatment improves cognitive function in patients with MCI or AD. In one study, subcutaneous insulin-treated patients with coincident AD exhibited significantly less cognitive decline compared to placebo-treated patients.117 In another study, intranasal insulin improved cognitive performance in AD patients.118 Despite the disappointment caused by the negative trial of the insulin-sensitizing agent rosiglitazone in AD,119 modulation of insulin signaling pathways continues to appear as a promising target for AD therapeutics. Particularly promising for the treatment of cognitive impairment and AD, particularly in insulin resistant subjects, are the GLP-1 (glucagon-like peptide 1) receptor agonists. GLP-1 receptors are widely expressed in neurons throughout the brain and data suggest that their activation enhances synaptic plasticity and cognitive performance and promotes neuronal survival.120 Recent preclinical studies have demonstrated beneficial effects of GLP-1 receptor agonists in animal models of AD, including protective and restorative effects on synaptic plasticity and cognitive function.115,121 Similarly, treatment of AD mice with sitagliptin (which inhibits the enzyme that inactivates GLP-1 in the blood, DPP4) resulted in increased brain levels of GLP-1, ameliorated memory deficits and reduced levels of oxidative stress.122 A protease-resistant analog of GLP-1 called Exendin-4 was developed and is now widely used for treatment of diabetes. Because of its dual actions on glucose metabolism and neurons affected in AD, clinical trials to test the efficacy of Exendin-4 in human subjects with MCI and early AD have recently been initiated.
Given the multiple neuroprotective actions of neurotrophic factors, such as nerve growth factor (NGF) and BDNF, they have great potential as therapeutic agents in AD, as well as against aging-related cognitive decline. Unfortunately, they exert pleiotropic effects and it is difficult to deliver them at the site of pathology. Therefore, small molecules selectively targeting specific neurotrophin receptors show greater promise for modulating neurotrophin signaling via systemic delivery.123 An alternative approach is gene therapy. Intraparenchymal NGF gene delivery to the basal forebrain of aged rhesus monkeys restored cholinergic neuronal markers to levels of young monkeys,124 whereas NGF gene transfer into the septum of aged rats increased the number of cholinergic neurons and acetylcholine release.125 NGF and recombinant hNGF-61 were successfully delivered via ocular and intranasal administration to transgenic AD mice, in which they suppressed AD pathology.126,127 In aged rats and non-human primates, local BDNF delivery reverses neuronal atrophy and ameliorates age-related cognitive impairment, whereas in transgenic AD mice, BDNF gene delivery reverses synapse loss, partially normalizes aberrant gene expression, improves cell signaling and restores learning and memory.128
Finally, there is a potential for sustaining and restoring functional circuits in the aging brain by providing neurons with chemicals that elevate levels of the energy substrates adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+). Best known for its ability to preserve ATP levels in muscle cells thereby enhancing endurance, creatine can also protect neurons against oxidative and metabolic insults, including Aβ toxicity,129 in vitro or against traumatic brain injury in vivo.130 Administration of nicotinamide, which increases cellular NAD+ levels in the brain, improved cognitive function in old rats131 and in a mouse model of AD.132 Preliminary human studies suggest that dietary niacin, which consists of nicotinamide and nicotinic acid, can reduce the risk of age-related cognitive decline and AD.133 Another approach to enhancing neuronal bioenergetics is to target mitochondrial potassium channels; our group has recently demonstrated improvements in cognitive function and reductions in Aβ and tau pathologies in AD mice treated with the mitochondrial potassium channel opener diazoxide 134. Because creatine, nicotinamide and diazoxide are all approved for use in humans, clinical trials in subjects with MCI and AD could be initiated without delay.
Conclusions
From this review, it is evident that multiple mechanisms that largely depend on the organism’s state of energy metabolism adaptively modify neuronal and brain networks. The value of a lifestyle that stimulates the brain’s adaptive responses via regular exercise, moderation of dietary energy intake and intellectual vigor cannot, in our view, be overstated. The available evidence suggests that these three brain-healthy habits protect cells against the adversities of aging and AD by engaging cellular stress response pathways that induce the expression of genes encoding proteins involved in cytoprotection and synaptic and neurogenic plasticity (Figure 5A). Approaches that enable such brain-healthy lifestyles should be developed and widely implemented. Novel patterns of food intake should be considered in light of the recent evidence that alternate day caloric restriction diets can be adhered to and improve health dramatically.135 Finally, from a drug discovery for AD perspective we propose an alternative focus to Aβ metabolism, to the levels of whole body and cellular energy metabolism and stimulation of adaptive cellular stress responses. Pharmacological approaches should also pursue reasonable targets, such as agents that suppress inflammation or enhance mitochondrial function.136 Whether these conceptual changes are going to be successful in preventing and treating cognitive aging and AD is an open question and a challenge.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH. We thank P. Rapp and L. Mucke for their valuable comments on the manuscript.
Footnotes
Search strategy: PubMed articles published between 1990 and the time of writing. Searches included various combinations of the following terms: Alzheimer, cognitive, energy metabolism, network, excitatory, GABA, glutamate, amyloid, mitochondria, neurotrophic factors, diabetes, exercise, oxidative stress.
Author Contributions: D. K. and M. P. M. made equal contributions to the organization and writing of this manuscript.
Conflicts of Interest: Neither author has any conflicts of interest to declare.
Contributor Information
Dimitrios Kapogiannis, Email: kapogiannisd@mail.nih.gov.
Mark P. Mattson, Email: mattsonm@grc.nia.nih.gov.
References
- 1.Gunstad J, Lhotsky A, Wendell CR, et al. Longitudinal Examination of Obesity and Cognitive Function: Results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 2010;34:222–2229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Archives of neurology. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 3.Toro P, Schonknecht P, Schroder J. Type II diabetes in mild cognitive impairment and Alzheimer's disease: results from a prospective population-based study in Germany. J Alzheimers Dis. 2009;16:687–691. doi: 10.3233/JAD-2009-0981. [DOI] [PubMed] [Google Scholar]
- 4.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouquet M, Desgranges B, Landeau B, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132:2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blalock EM, Grondin R, Chen KC, et al. Aging-related gene expression in hippocampus proper compared with dentate gyrus is selectively associated with metabolic syndrome variables in rhesus monkeys. J Neurosci. 2010;30:6058–6071. doi: 10.1523/JNEUROSCI.3956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont M, Ho DJ, Calingasan NY, et al. Mitochondrial dihydrolipoyl succinyltransferase deficiency accelerates amyloid pathology and memory deficit in a transgenic mouse model of amyloid deposition. Free Radic Biol Med. 2009;47:1019–1027. doi: 10.1016/j.freeradbiomed.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quick KL, Ali SS, Arch R, et al. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiology of aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behavioural brain research. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 10.Mielke JG, Nicolitch K, Avellaneda V, et al. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behavioural brain research. 2006;175:374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Stranahan AM, Arumugam TV, Cutler RG, et al. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature neuroscience. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Archives of neurology. 67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etgen T, Sander D, Huntgeburth U, et al. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 170:186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 14.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology. 67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 16.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 18.Nichol K, Deeny SP, Seif J, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannangara TS, Lucero MJ, Gil-Mohapel J, et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Q, Vaynman S, Souda P, et al. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. The European journal of neuroscience. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 21.Mesulam M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Annals of neurology. 2008;64:367–378. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Wang J, Wang L, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17–e13. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold SE, Hyman BT, Flory J, et al. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 25.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson DK, Storandt M, Morris JC, et al. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Wegiel J, Bobinski M, Tarnawski M, et al. Fibrillar amyloid-beta affects neurofibrillary changes but only in neurons already involved in neurofibrillary degeneration. Acta Neuropathol. 2001;101:585–590. doi: 10.1007/s004010000334. [DOI] [PubMed] [Google Scholar]
- 34.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 35.Hyman BT, Van Hoesen GW, Damasio AR, et al. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science (New York, N.Y. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 36.Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- 37.Scheff ScheffSW, Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 38.Seeley WW, Allman JM, Carlin DA, et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007;21:S50–S57. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- 39.Braak H, Braak E. Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol. 1991;81:261–268. doi: 10.1007/BF00305867. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Chang L, Viola KL, et al. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science (New York, N.Y. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 43.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Hong S, Shepardson NE, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behavioural brain research. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaManna JC, Harik SI. Regional comparisons of brain glucose influx. Brain research. 1985;326:299–305. doi: 10.1016/0006-8993(85)90039-3. [DOI] [PubMed] [Google Scholar]
- 48.Rowe WB, Blalock EM, Chen KC, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu W, Brickman AM, Luchsinger J, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Annals of neurology. 2008;64:698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sperling RA, LaViolette PS, O'Keefe K, et al. Amyloid Deposition Is Associated with Impaired Default Network Function in Older Persons without Dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller SL, Celone K, DePeau K, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodard JL, Seidenberg M, Nielson KA, et al. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielson KA, Douville KL, Seidenberg M, et al. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiology of aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 58.Reis HJ, Guatimosim C, Paquet M, et al. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr Med Chem. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- 59.Tampellini D, Rahman N, Gallo EF, et al. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009;29:9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maubach K. GABA(A) receptor subtype selective cognition enhancers. Curr Drug Targets CNS Neurol Disord. 2003;2:233–239. doi: 10.2174/1568007033482779. [DOI] [PubMed] [Google Scholar]
- 61.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 62.Vela J, Gutierrez A, Vitorica J, et al. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. Journal of neurochemistry. 2003;85:368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 63.Armanini MP, Hutchins C, Stein BA, et al. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain research. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- 64.Mattson MP, Cheng B, Davis D, et al. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larner AJ. Epileptic seizures in AD patients. Neuromolecular Med. 2010;12:71–77. doi: 10.1007/s12017-009-8076-z. [DOI] [PubMed] [Google Scholar]
- 66.Hu NW, Klyubin I, Anwy R, et al. GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20504–20509. doi: 10.1073/pnas.0908083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sepulveda FJ, Opazo C, Aguayo LG. Alzheimer beta-amyloid blocks epileptiform activity in hippocampal neurons. Mol Cell Neurosci. 2009;41:420–428. doi: 10.1016/j.mcn.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 69.Loerch PM, Lu T, Dakin KA, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy PH, McWeeney S, Park BS, et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 71.Gleichmann M, Zhang Y, Wood WH, et al. Molecular changes in brain aging and Alzheimer's disease are mirrored in experimentally silenced cortical neuron networks. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.08.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu X, Zhan M, Duan W, et al. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8:R234. doi: 10.1186/gb-2007-8-11-r234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang JL, Tadokoro T, Keijzers G, et al. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. J Biol Chem. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- 75.Burkhalter J, Fiumelli H, Allaman I, et al. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng S, Garzon DJ, Marchese M, et al. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J Neurosci. 2009;29:9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tong L, Balazs R, Thornton PL, et al. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24:6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poon WW, Blurton-Jones M, Tu CH, et al. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiology of aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li G, Peskind ER, Millard SP, et al. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS ONE. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mark RJ, Pang Z, Geddes JW, et al. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mattson MP. ER calcium and Alzheimer's disease: in a state of flux. Sci Signal. 2010;3:pe10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bubber P, Haroutunian V, Fisch G, et al. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Annals of neurology. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 84.Li Z, Okamoto K, Hayashi Y, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Shukkur EA, Shimohata A, Akagi T, et al. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for Down syndrome. Hum Mol Genet. 2006;15:2752–2762. doi: 10.1093/hmg/ddl211. [DOI] [PubMed] [Google Scholar]
- 86.Muyllaert D, Kremer A, Jaworski T, et al. Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav. 2008;7 Suppl 1:57–66. doi: 10.1111/j.1601-183X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 87.Martinez A, Perez DI. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer's disease? J Alzheimers Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- 88.Sereno L, Coma M, Rodriguez M, et al. A novel GSK-3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 89.Buller CL, Loberg RD, Fan MH, et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836–C843. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bolos M, Fernandez S, Torres-Aleman I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem. 2010;285:17693–17700. doi: 10.1074/jbc.M109.096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Komatsu T, Chiba T, Yamaza H, et al. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Experimental gerontology. 2008;43:339–346. doi: 10.1016/j.exger.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Ingram DK, Weindruch R, Spangler EL, et al. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 93.Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiology of aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- 94.Means LW, Higgins JL, Fernandez TJ. Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav. 1993;54:503–508. doi: 10.1016/0031-9384(93)90243-9. [DOI] [PubMed] [Google Scholar]
- 95.Cao D, Lu H, Lewis TL, et al. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 96.Halagappa VK, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 97.Wu P, Shen Q, Dong S, et al. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiology of aging. 2008;29:1502–1511. doi: 10.1016/j.neurobiolaging.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 98.Bruce-Keller AJ, White CL, Gupta S, et al. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49:22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hyun DH, Emerson SS, Jo DG, et al. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo Z, Ersoz A, Butterfield DA, et al. Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. Journal of neurochemistry. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- 102.Arumugam TV, Phillips TM, Cheng A, et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Annals of neurology. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gray J, Yeo GS, Cox JJ, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yeo GS, Connie Hung CC, Rochford J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature neuroscience. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 106.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stranahan AM, Lee K, Becker KG, et al. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiology of aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolf SA, Kronenberg G, Lehmann K, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biological psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 111.Stranahan AM, Lee K, Martin B, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- 114.Shen H, Tong L, Balazs R, et al. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer'slinked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plastino M, Fava A, Pirritano D, et al. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci. 2010;288:112–116. doi: 10.1016/j.jns.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 118.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 119.Alderton C, Zvartau-Hind M, Ritchie S. 12th Int Conf Alzheimer's Dis Relat Disord (ICAD) 20090 (ed Int Conf Alzheimer's Dis Relat Disord (ICAD)) Abst P1-263; (Int Conf Alzheimer's Dis Relat Disord (ICAD)). [Google Scholar]
- 120.Perry TA, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr Drug Targets. 2004;5:565–571. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- 121.Gengler S, McClean PL, McCurtin R, et al. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 122.D'Amico M, Di Filippo C, Marfella R, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Experimental gerontology. 2010;45:202–207. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 123.Longo FM, Yang T, Knowles JK, et al. Small molecule neurotrophin receptor ligands: novel strategies for targeting Alzheimer's disease mechanisms. Current Alzheimer research. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 124.Nagahara AH, Bernot T, Moseanko R, et al. Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery. Experimental neurology. 2009;215:153–159. doi: 10.1016/j.expneurol.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu K, Meyers CA, Guerra NK, et al. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol Ther. 2004;9:262–269. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 126.Capsoni S, Covaceuszach S, Ugolini G, et al. Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimers Dis. 2009;16:371–388. doi: 10.3233/JAD-2009-0953. [DOI] [PubMed] [Google Scholar]
- 127.Covaceuszach S, Capsoni S, Ugolini G, et al. Development of a non invasive NGF-based therapy for Alzheimer's disease. Current Alzheimer research. 2009;6:158–170. doi: 10.2174/156720509787602870. [DOI] [PubMed] [Google Scholar]
- 128.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature medicine. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. Journal of neurochemistry. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan PG, Geiger JD, Mattson MP, et al. Dietary supplement creatine protects against traumatic brain injury. Annals of neurology. 2000;48:723–729. [PubMed] [Google Scholar]
- 131.Rex A, Spychalla M, Fink H. Treatment with reduced nicotinamide adenine dinucleotide (NADH) improves water maze performance in old Wistar rats. Behavioural brain research. 2004;154:149–153. doi: 10.1016/j.bbr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 132.Green KN, Steffan JS, Martinez-Coria H, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline. Journal of neurology, neurosurgery, and psychiatry. 2004;75:1093–1099. doi: 10.1136/jnnp.2003.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu D, Pitta M, Lee JH, et al. The KATP channel activator diazoxide ameliorates Abeta and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer“s disease. J Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-101017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bolognesi ML, Matera R, Minarini A, et al. Alzheimer's disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009;13:303–308. doi: 10.1016/j.cbpa.2009.04.619. [DOI] [PubMed] [Google Scholar]