Abstract
Nitric oxide (NO) drives pro-survival responses in vascular cells and limits platelet adhesion, enhancing blood flow and minimizing thrombosis. The matricellular protein thrombospondin-1 (TSP1), through interaction with its receptor CD47, inhibits soluble guanylyl cyclase (sGC) activation by NO in vascular cells. In vascular smooth muscle cells (VSMCs) both intracellular cGMP and cAMP regulate adhesion, contractility, proliferation, and migration. cGMP can regulate cAMP through feedback control of hydrolysis. Inhibition of the cAMP phosphodiesterase-4 selectively interfered with the ability of exogenous TSP1 to block NO-driven VSMC adhesion but not cGMP accumulation, suggesting that cAMP also contributes to VSMC regulation by TSP1. Inhibition of phosphodiesterase-4 was sufficient to elevate cAMP levels, and inhibiting guanylyl cyclase or phosphodiesterase-3, or adding exogenous TSP1 reversed this increase in cAMP. Thus, TSP1 regulates VSMC cAMP levels in part via cGMP-dependent inhibition of phosphodiesterase-3. Additionally basal cAMP levels were consistently elevated in both VSMCs and skeletal muscle from TSP1 null mice, and treating null cells with exogenous TSP1 suppressed cAMP levels to those of wild type cells. TSP1 inhibited both forskolin and isoproterenol stimulated increases in cAMP in VSMCs. TSP1 also abrogated forskolin and isoproterenol stimulated vasodilation. Consistent with its ability to directly limit adenylyl cyclase-activated vasodilation, TSP1 also limited cAMP-induced dephosphorylation of myosin light chain-2. These findings demonstrate that TSP1 limits both cGMP and cAMP signaling pathways and functional responses in VSMCs and arteries, by both phosphodiesterase-dependent cross talk between these second messengers and by inhibition of adenylyl cyclase activation.
Keywords: cAMP, cGMP, thrombospondin-1, vascular smooth muscle cells
1. Introduction
Nitric oxide (NO) is a central modulator of vascular health. NO produced by endothelial nitric oxide synthase (eNOS) promotes blood flow and tissue perfusion by relaxing the vascular smooth muscle cells (VSMCs) of resistance arteries, reducing endothelial inflammation and platelet aggregation and promoting angiogenesis [1]. Recently we reported that the matricellular protein thrombospondin-1 (TSP1) limits the canonical NO pathway in VSMCs [2]. By suppressing NO signaling, TSP1 is a regulator of both acute and subacute physiologic responses. At the level of arterial tone, TSP1 directly modulates NO-stimulated VSMC relaxation [3] and thus acutely controls tissue blood flow [4,5] and limits both blood pressure and cardiac dynamics [6,7].
The major molecular target of NO in this context is soluble guanylyl cyclase (sGC). NO binding to the prosthetic heme in sGC activates the enzyme to synthesize cGMP [8]. TSP1 inhibits NO-stimulated sGC activation in both endothelial and VSMCs through binding to the cell surface receptor CD47 [9]. TSP1-CD47 interaction is necessary for this inhibitory signal since cells lacking CD47 are resistant to TSP1 inhibition. cGMP in turn activates cGMP-dependent-protein kinase (PKG) and, at least in platelets, TSP1 directly inhibits nucleotide activation of this downstream enzyme [10]. New work also describes a role for circulating blood-borne TSP1 to inhibit endothelial based NO production by inhibiting eNOS activation [7] and VEGF receptor-2 activation [11]. TSP1 then, as a regulator of the canonical NO pathway at multiple levels, can control acute vascular tone, blood flow [3,6] and angiogenic remodeling [12].
Recently we reported that both cGMP and cAMP levels are elevated in tissues from TSP1 and CD47 null mice compared to wild type controls [6]. A number of mechanisms could account for the elevated cAMP. In several cell types, including T cells [13] and porcine thyroid cells [14], CD47 has been found to limit adenylyl cyclase and thus cAMP levels through interaction with heterotrimeric G proteins (Gi). In VSMCs [15] and platelets [16] a TSP1 based peptide sequence, 4N1K (K-1016RFYVVMWK1024-K), that was derived from the C-terminal globular domain of TSP1 and contains a Val–Val–Met motif that is required for binding to CD47, decreased cAMP. In contrast, sickle cell red blood cells treated with 4N1K did not experience a drop in cAMP levels [17]. Alternatively, CD47 through modulation of cGMP levels could regulate phosphodiesterases that hydrolyze cAMP to 5′-AMP [18,19].
In this study, we examine the potential for TSP1-CD47 signaling to regulate cAMP levels in endothelial and VSMCs. In VSMCs TSP1 potently blocks NO-stimulated increases in cell adhesion independent of effects on cGMP hydrolysis. Likewise in VSMCs (but not endothelial cells) physiologically relevant amounts of TSP1 block cGMP-stimulated increases in cellular cAMP and also blocks isoproterenol and forskolin-stimulated increases in cAMP. At a functional level, soluble TSP1 potently inhibits both isoproterenol and forskolin-stimulated endothelial independent vasodilation. Thus in VSMCs TSP1 modulates cellular cAMP levels directly via inhibition of adenylyl cyclase activation and indirectly via cGMP-dependent cross talk.
2. Materials and Methods
2.1. Animals
C57BL/6 wild type and TSP1 null mice aged 14 to 18 weeks were housed under pathogen free conditions and had ad libitum access to filtered water and standard rat chow. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committees of the University of Pittsburgh School of Medicine.
2.2. Cells and Reagents
Primary aortic VSMCs were harvested from wild type and TSP1 null mice as previously reported [2], cultured in growth medium (SM-GM, Lonza, Switzerland) and used within passage 2. Human aortic VSMCs (Lonza, Switzerland) were maintained in smooth muscle cell growth medium supplemented with the manufacturer's additives (SM-GM, Lonza, Switzerland) and 5% fetal calf serum (FCS) in 5% CO2 at 37°C. Cells were utilized between passages 4 through 9. Rat aortic VSMCs were obtained from freshly isolated vessels via tissue digestion and selective growth medium conditions. Phenotype purity was confirmed via staining for α-smooth muscle actin. Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza and grown in E-GM + 2% bovine serum (Lonza, Switzerland). Purity of cultures was monitored by immunochemical staining with monoclonal human anti-CD31 antibody (Sigma, St. Louis, MO). Cells were used at passages 3 through 6. Di-Bu-cAMP, 8-Br-cAMP, 8-Br-cGMP and 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ), isoproterenol and forskolin were obtained from Sigma (St. Louis, MO). Diethylamine NONOate (DEA/NO) was kindly provided by Drs. Joseph Saavedra and Larry Keefer (NIH, Frederick, Maryland). The phosphodiesterase inhibitors rolipram (PDE4 inhibitor) and trequinsin (PDE3 inhibitor) were obtained from Calbiochem (San Diego, CA). TSP1 was prepared from human platelets obtained from the NIH blood bank as previously described [20] or purchased from Athens Research Technology (Athens, GA). Intracellular cGMP and cAMP were measured by immunoassay kits obtained from Amersham Biosciences (GE Healthcare, Franklin, NJ).
2.3. F-actin and Myosin Light Chain-2 (MLC-2) Staining of VSMCs
Adherent rat aortic VSMCs were grown on 5 μg/ml collagen I coated glass coverslips (Lab-Tek, Nunc). Since TSP1 has a serum response element in its promoter region, cells were serum starved 24 h in basal medium (SM-GM without serum and additives) + 0.1% BSA and treated as indicated. Cells were then fixed in 2% paraformaldehyde in PBS (pH 7.4), and permeabilized in 0.1% Triton X100 in PBS for 15 min. Cells were blocked 45 min in 2% BSA in PBS and incubated 18 h in polyclonal phospho-Myosin Light Chain 2 (ser19) antibody (Cell Signaling, Boston, MA) followed by a secondary antibody (Alexa 546) labeled anti-rabbit in combination with Cy3 labeled phalloidin and Hoechst staining (Molecular Probes, Invitrogen, Carlsbad, CA). Images were obtained using an inverted confocal Olympus microscope at 60x magnification.
2.4. Cell Adhesion Assay
Type I collagen (2.5 μg/ml) in PBS was plated in 8 μl spots on 1 cm volume sterile culture plates (Falcon, Becton-Dickinson, Franklin Lakes, NJ) and allowed to adhere overnight at 4°C. The following day the matrix substrate was aspirated and plates blocked with 1% BSA in PBS for 30 minutes at 37°C. Human or rat VSMCs were then plated in basal medium + 0.1 % BSA at a density of 1 × 105 cells per ml per plate and allowed to adhere for 1 h at 37°C. Plates were then washed gently with PBS and cells fixed with glutaraldehyde (1%) for 30 min and stained with DiffQuik (Dade Berhing, Newark, DE) for 10 min, washed with distilled water and air dried. Cell adhesion was then determined by counting cells per high powered field. Alternatively, cell adhesion was carried out in 96-well culture plates. After pre-coating wells with collagen, harvested cells were plated at a density of 1 × 104 cells/well in medium plus 0.1% BSA and treatment agents then incubated in 5% CO2 for 1 h. Wells were washed with PBS, and cells fixed with glutaraldehyde (1%) for 10 min, washed and stained with crystal violet (1%) for 20 min. Excess stain was rinsed away, adherent cells were treated with acetic acid (10%), and plates were read at 570 nm.
2.5. Intracellular Cyclic Nucleotide Measurement
Human aortic VSMCs (104 cells/well) grown overnight in 96-well culture plates were pretreated for 24 h with medium containing 2% FCS and weaned off serum over 24 h before treatment with a NO donor and other agents in serum/growth factor free medium (SM-GM + 0.1% BSA) for the indicated time periods. In some experiments aortic VSMCs were grown to 80% surface saturation in 6-well plates, weaned off serum over 24 h and treated in basal medium. Intracellular cGMP or cAMP levels were determined using an enzyme immunoassay kit (GE Healthcare, Franklin, NJ). HUVECs (104 cells/well) were plated in 96-well culture plates in E-GM + 2% FCS and likewise weaned off serum over 24 h prior to treatment. Cells were treated with a NO donor and agents in serum/growth factor free E-GM + 0.1% BSA and intracellular cAMP measured by immunoassay. In other experiments aortic-derived VSMCs from age and sex matched C57BL/6 wild type and TSP1 null mice (104 cells/well) were plated in full growth medium and grown to 80% surface saturation, deprived of serum and growth factors overnight and treated with TSP1 (2.2 nM) and DEA/NO (10 μM) in basal medium + 0.1% BSA. Intracellular cyclic nucleotide levels were then determined via immunoassay. Total protein was determined via a BCA reaction (Pierce, Madison, WI) and results normalized accordingly.
2.6. Tissue cAMP Determination
Thigh muscle biopsies were harvested from age matched male C57BL/6 wild type and TSP1 null mice, snap frozen with liquid nitrogen, pulverized and suspended in chilled lysis buffer. The mixtures were sonicated on wet ice to ensure complete lysis, centrifuged 10 min at 13,000 g at 4°C and the supernatant was used for cAMP determination via ELISA immunoassay (GE Healthcare, Franklin, NJ). Total protein was determined via a BCA reaction and cAMP results normalized accordingly.
2.7. Western Blot Analysis
Cells for Western blot analysis were homogenized and suspended in lysis buffer [50 mM Tris HCl (pH 7.4), 125 mM NaCl, 1 mM EDTA, 1% NP40, 1 mM sodium orthovanadate, 20 mM NaF, 10mg/ml PMSF/protease inhibitor mixture (Sigma P8340)]. Insoluble material was removed by centrifugation at 12,000 ×g for 10 min at 4°C. Forty micrograms of protein from cell lysates in SDS sample buffer were electrophoresed in gels and transferred to PVDF membranes before immunoblotting with antibodies against phospho-MLC-2 (Cell Signaling, Boston, MA) and β-actin.
2.8. In Vitro Arterial Vasorelaxation Assay
Adult male age matched wild type C57BL/6 mice were euthanized by cervical dislocation. After trans-section of the abdominal vena cava, the vasculature was gently flushed with Krebs buffer via puncture of the left ventricle to remove blood and the thoracic aorta cleaned of surrounding connective tissue in situ then dissected free. To remove the endothelial layer vessels were cannulated over their entire length with a specially designed blunt needle that had a roughed outer metal surface. Segments (3 mm in length) were then mounted in a dual wire myograph system (Multi Myograph Model 610M). Vessels were allowed to equilibrate in standard Krebs buffer (in mM: 130 NaCl, 14.9 NaHCO3, 5.5 dextrose, 4.7 KCl, 1.17 MgSO4, 1.18 KH2PO4, 1.6 CaCl2, pH 7.4) bubbled with 5% CO2 + 95% O2 at 37°C. Tension of the arteries was set to 9.98 mN. Vessels were then allowed to equilibrate until baseline tension remained constant (30-60 min). Vessel viability was confirmed by a contractile response to KCl buffer (in mM: 34.7 NaCl, 100 KCl, 1.18 KH2PO4, 1.17 MgSO4, 14.9 NaHCO3, 5.5 dextrose, 1.6 CaCl2, pH 7.4), repeated twice for 5 min each. Dose-response curves to phenylephrine were carried out and a dose that produced 80% maximum contraction (EC80) was chosen for establishing baseline vascular tone prior to additional treatments. Vessels achieving less than 15% relaxation to 10-6 M acetylcholine were considered to be denuded of endothelium.
2.9. Statistics
Studies were repeated in three independent experiments and results presented as the mean ± SD, with analysis of significance done using one-way ANOVA to compare multiple unmatched data points and a P<0.05 taken as significant. Treatment conditions for adhesion assays in some instances (where indicated) were performed in duplicates and each experiment independently repeated three times with results presented as the mean ± SD. Results of arterial relaxation studies were analyzed by 2-way ANOVA with the Bonferroni post hoc test to determine significance.
3. Results
3.1. Exogenous TSP1 inhibits cAMP-driven actin reorganization in VSMCs
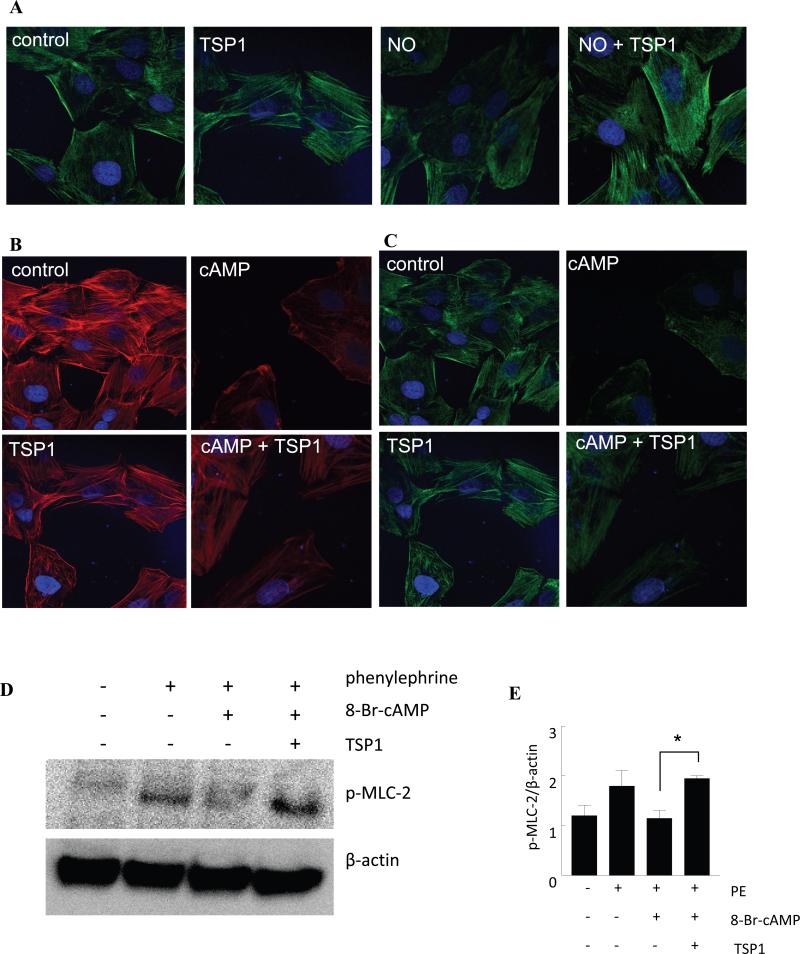
NO-stimulated VSMC adhesion on collagen is blocked by TSP1 [2]. NO also induces morphologic alterations in VSMCs [3]. Consistent with the previously reported inhibition by TSP1 of NO-induced cytoskeletal changes in endothelial cells [12], treatment with 10 μM DEA/NO decreased actin filament organization in rat aortic VSMCs, and this was reversed by exogenous TSP1 (2.2 nM) (Fig. 1A). Similar results were obtained in human aortic VSMCs (data not shown).
Fig. 1. cAMP-driven actin reorganization in VSMC is inhibited by exogenous TSP1.
(A) Rat aortic VSMCs were treated in serum free medium plus 0.1% BSA (control), TSP1 (2.2 nM), DEA/NO (10μM) or DEA/NO + TSP1 (2.2 nM) for 1 h, then fixed, permeabilized and stained for p-MLC-2. Representative images three separate experiments are presented. Magnification - 60x. Cells were treated in serum free medium plus 0.1% BSA (control), TSP1 (2.2 nM), di-Bu-cAMP (100 μM) or di-Bu-cAMP + TSP1 (2.2 nM) for 1 h, then fixed, permeabilized and stained for F-actin phalloidin (B) and p-MLC-2 (C). Images are representative of three separate experiments. Magnification – 60x. (D) Aortic VSMCs at 90% confluence on 3.5 cm culture dishes were serum/additive starved over 24 h and in basal medium plus 0.1% BSA treated with TSP1 (2.2 nM) ± 8-Br-cAMP (100 μM) ± phenylephrine (10 μM), lysates prepared and Western blot analysis of p-MLC-2 determined. A representative blot of 3 separate experiments is presented. (E) Western blot expression was quantified by densitometry calculated by measuring intensity of bands using Image J and normalized to β-actin. *P<0.05, PE + 8-Br-cAMP + TSP1 versus PE + 8-Br-cAMP.
Arterial vasoconstriction requires coordinated interactions between VSMC actin and myosin heavy chains. NO/cGMP signaling promotes vasorelaxation by preventing phosphorylation of the myosin regulatory subunit MLC-2 [21]. We previously showed that TSP1 inhibits NO-stimulated dephosphorylation of MLC-2 in human VSMCs [3]. TSP1 treated rat aortic VSMCs show increased association of phospho-MLC-2 (p-MLC-2) with stress fibers (Fig. 1A) compared to control cells. As expected, exogenous NO decreased p-MLC-2 association with stress fibers, and this was significantly abrogated by TSP1 (Fig. 1A).
In several cell types, changes in cellular cAMP can regulate cell morphology and actin organization [22]. In myocardial cells and VSMCs these cytoskeletal dynamics are mediated by cAMP in part through altering the phosphorylation and dephosphorylation of MLC-2 [23][24]. Treatment of rat aortic VSMCs with the cAMP analogue di-Bu-cAMP (and forskolin, which increases cellular cAMP by directly activating adenylyl cyclase, data not shown) resulted in a marked decrease in F-actin organization which exogenous TSP1 significantly abrogated (Fig. 1B). Likewise, rat aortic VSMCs treated with di-Bu-cAMP showed decreased p-MLC-2, which TSP1 significantly abrogated (Fig.1C). In VSMCs, NO inhibits sphingosine-1-phosphate-stimulated phosphorylation of MLC-2 [3]. Myosin light chain kinase (MLCK) phosphorylates MLC-2 in a Ca2+-dependent manner and controls VSMC contraction [25]. Western blotting confirmed that TSP1 inhibits cAMP-stimulated decreases of MLC protein phosphorylation in phenylephrine-stimulated rat aortic VSMCs (Fig. 1D, E).
3.2. cAMP-stimulated arterial relaxation is inhibited by TSP1
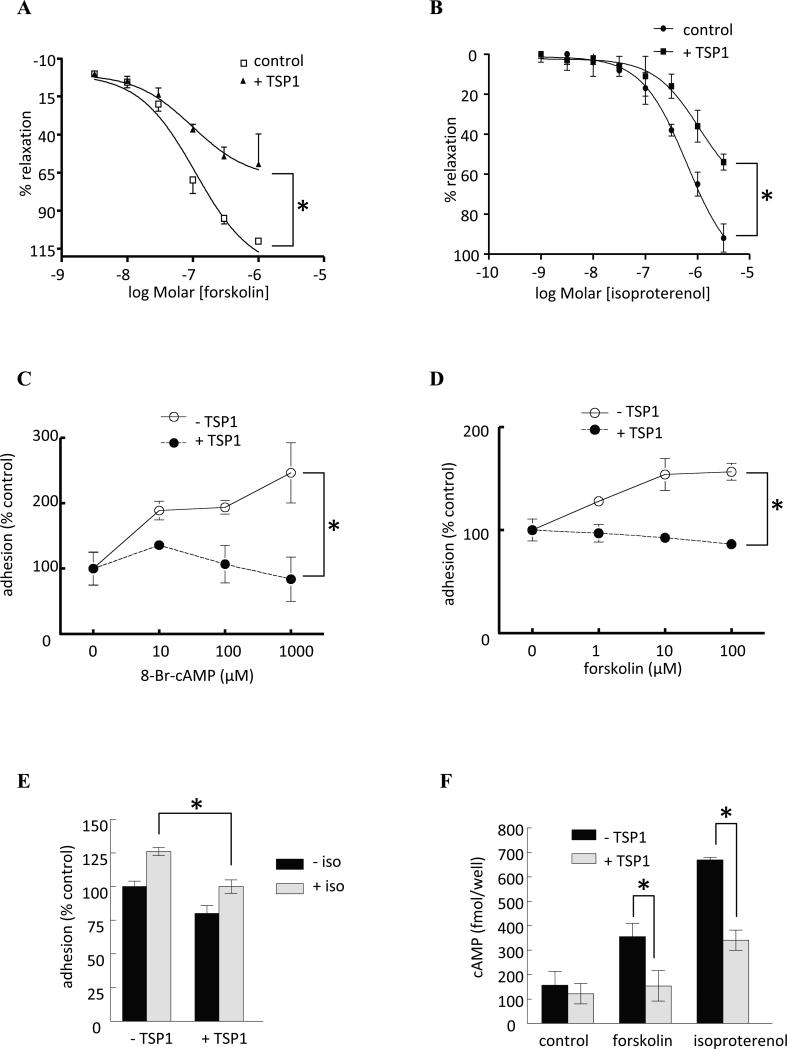
cAMP regulation of MLCK activity controls actin-myosin cross bridge cycling [23,24,26], which stimulates arterial vasodilation [27]. Both forskolin and isoproterenol can increase VSMC cAMP levels through effects on adenylyl cyclase. To assess whether TSP1 can acutely regulate cAMP-mediated vasodilation, fresh murine aortic arterial segments were denuded of endothelium (removing the effects of endogenous NO via eNOS) and treated with exogenous TSP1. Treating denuded arterial segments with either agent led to an expected dose-dependent stimulated arterial vasodilation, and this was inhibited by physiologically relevant amounts (2.2 nM) of TSP1 (Fig. 2A, B). Interestingly, pre-treatment with pertussis toxin (a specific blocker of Gi signaling) rendered endothelial deficient arterial segments insensitive to TSP1 inhibition (data not shown).
Fig. 2. TSP1 inhibits cAMP-stimulated vasodilation and VSMC adhesion, and agonist-induced cAMP accumulation.
Freshly harvested aortas from 12 week old male wild type C57BL/6 mice underwent disruption of the endothelium which was confirmed by lack of vasorelaxation in response to acetylcholine. Vessels were then pre-contracted and arterial vasorelaxation to a dose response curve of (A) forskolin or (B) isoproterenol ± TSP1 (2.2 nM) determined. Data represent the mean ± SD of 4 vessels in each treatment group. *P<0.005, TSP1 treated versus control. Fitted curves were analyzed by two-way ANOVA followed by the Bonferroni post test. (C) Human aortic VSMC (2 × 105 cells/ml) attachment was determined on dishes coated with type I collagen (2.5 μg/ml) in the presence of the cell permeable cAMP analog 8-Br-cAMP ± TSP1 (2.2 nM). Results presented are the mean ± SD of three experiments. *P<0.05, TSP1 treated versus control. VSMC (2 x 105 cells/ml) attachment was determined on dishes coated with type I collagen (2.5 μg/ml) in the presence of the indicated doses of (D) forskolin ± TSP1 (2.2 nM) or (E) isoproterenol (10 μM) ± TSP1 (2.2 nM). Results presented are the mean ± SD of three experiments. * P<0.05, TSP1 treated versus control (D); *P<0.05 TSP1 + isoproterenol versus isoproterenol (E). (F) Human aortic VSMCs were plated at 80% confluence and serum deprived over 24 h. In basal medium + 0.1% BSA cells were treated with isoproterenol (10 μM) or forskolin (1 μM) ± TSP1 (2.2 nM) and cAMP levels determined via ELISA. Results are the mean ± SD of three experiments. *P<0.05, TSP1 + isoproterenol (or forskolin) versus isoproterenol (or forskolin).
3.3. TSP1 blocks cAMP-stimulated VSMC adhesion
cAMP regulates integrin-mediated cell adhesion in a substrate-specific manner [28] Human aortic VSMC adhesion to collagen substrate was enhanced in a dose-dependent manner by 8-Br-cAMP, and 2.2 nM TSP1 blocked this response (Fig. 2C). TSP1 was also very effective at blocking both foskolin (Fig. 2D) and isoproterenol (Fig. 2E) stimulated increases in VSMC adhesion to type I collagen. Here too, pertussis toxin treatment rendered VSMC less sensitive to TSP1 inhibition of adenylyl cyclase-stimulated adhesion (data not shown).
3.4. TSP1 inhibits drug- or receptor-mediated cAMP synthesis
Several mechanisms could account for the above activities of TSP1 to antagonize cAMP-stimulated VSMC adhesion and adenylyl cyclase-dependent vasodilation. TSP1 binding to CD47 induces dissociation of Giα from CD47 [16], which could inactivate adenylyl cyclase and decrease cAMP levels. Consistent with these findings, isoproterenol (10 μM) and forskolin (1 μM) stimulated increases in cAMP in human aortic VSMCs were sensitive to inhibition by exogenous TSP1 (2.2 nM) (Fig. 2F). Thus cAMP signaling in VSMCs is sensitive to inhibition by TSP1, and Gi-mediated inhibition of adenylyl cyclase may account for the functional antagonism of cAMP signaling by TSP1 in vascular smooth muscle.
3.5. TSP1 regulates cAMP levels in part via PDE cross talk
TSP1-CD47 signaling in VSMCs prevents activation of sGC by NO, thereby lowering cGMP levels [2]. PDE3 is known to hydrolyze both cAMP and cGMP, however, with variable selectivity [29,30], and cGMP is a competitive inhibitor of PDE3 hydrolysis of cAMP. The result is that cGMP and cAMP levels track together in VSMCs with increased cGMP leading to increased cAMP and vice versa. Given the identified role TSP1 has in limiting vascular cell cGMP levels, TSP1 inhibition of cAMP-stimulated VSMC responses might also occur through cGMP-mediated control of cAMP specific PDEs.
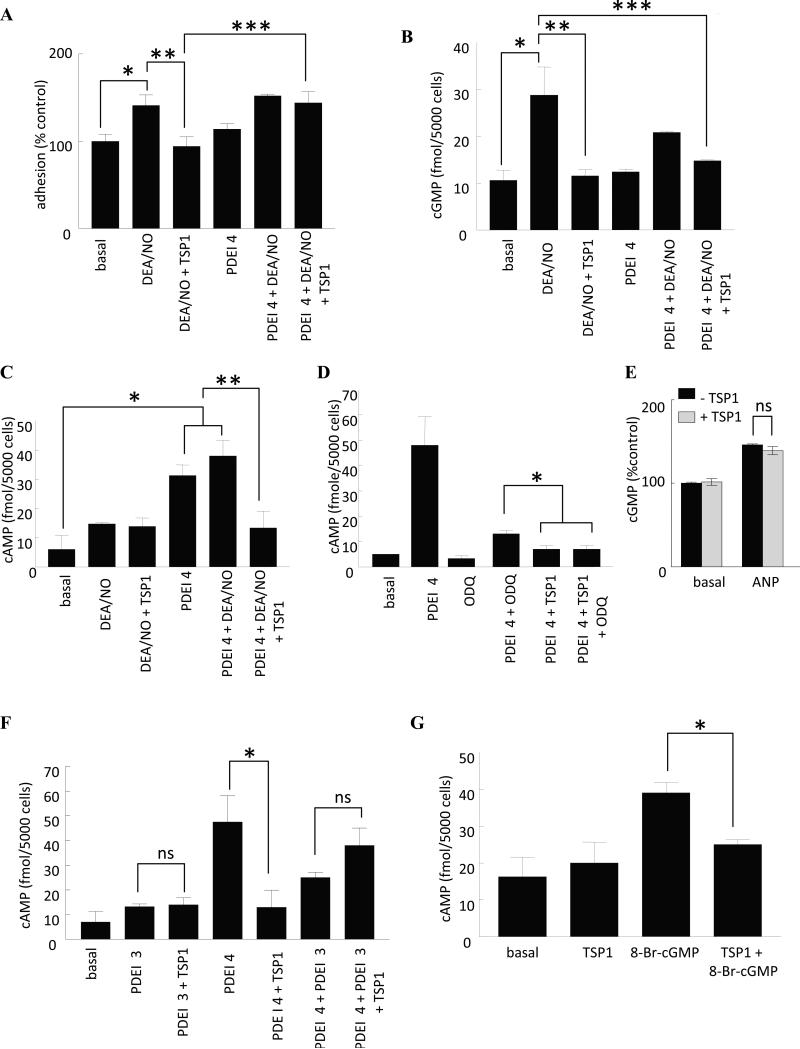
We first assessed PDE-mediated cross talk between cGMP and cAMP in human aortic VSMCs. Hydrolysis of cGMP is mediated by the cGMP phosphodiesterases PDE1, PDE3, and PDE5 [31]. Of these, PDE5 is the major cGMP-hydrolyzing PDE in VSMCs [32]. Previously we found that inhibition of these PDEs moderately increased basal adhesion of VSMCs, but did not prevent TSP1 from inhibiting NO-stimulated adhesion [2]. Thus, TSP1 does not inhibit NO-induced cGMP levels by enhancing the activity of any of the cGMP-specific PDEs. Inhibition of the cAMP PDE4 by rolipram did not, in and of itself, change basal or NO-stimulated increases in VSMC adhesion. However, rolipram reversed the TSP1 inhibition of NO-stimulated VSMC adhesion (Fig. 3A).
Fig. 3. TSP1 regulates VSMC cAMP levels via PDE cross talk.
(A) Human aortic VSMC (2 × 105 cells/ml) attachment was determined on dishes coated with type I collagen (2.5 μg/ml) in the presence of the indicated treatment agents at the following concentration - DEA/NO (10 μM), PDE4 inhibitor (1 μM), TSP1 (2.2 nM). Results presented are the mean ± SD of three experiments. *P<0.05, basal versus DEA/NO; **P<0.05, DEA/NO versus DEA/NO + TSP1; ***P<0.05, DEA/NO + TSP1 versus DEA/NO + TSP1 + PDEI 4. (B) VSMCs were incubated for 5 min in basal medium (SM-GM without serum and additives) + 0.1% BSA in the presence of the indicated treatment agents at the following concentrations - DEA/NO (10 μM), PDE4 inhibitor (1 μM), TSP1 (2.2 nM) and intracellular cGMP measured by immunoassay. Results presented are representative of three experiments. *P<0.05, basal versus DEA/NO; **P<0.05, DEA/NO versus DEA/NO + TSP1; ***P<0.05, DEA/NO versus DEA/NO + TSP1 + PDEI 4. (C) VSMCs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium + 0.1% BSA in the presence of the indicated treatment agents at the following concentrations - DEA/NO (10 μM), PDE4 inhibitor (1 μM), TSP1 (2.2 nM) and intracellular cAMP measured via immunoassay . *P<0.05, basal versus PDEI 4 or DEA/NO + PDEI 4; **P<0.05, PDEI 4 or DEA/NO + PDEI 4 versus DEA/NO + TSP1 + PDEI 4. VSMCs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium + 0.1% BSA in the presence of the indicated treatment agents at the following concentrations - DEA/NO (10 μM), PDE4 inhibitor (1 μM), TSP1 (2.2 nM), ODQ (10 μM) (D) and intracellular cAMP measured via immunoassay. *P<0.05, PDEI 4 + ODQ versus PDEI 4 + TSP1 or PDEI 4 + TSP1 + ODQ. VSMCs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium + 0.1% BSA and ANP (10 μM) ± TSP1 (2.2 nM) and cGMP measured by immunoassay (E). ns = not significant, ANP versus ANP + TSP1. (F) VSMCs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium + 0.1% BSA in the presence of the indicated treatment agents at the following concentrations - DEA/NO (10 μM), PDE4 inhibitor (1 μM), PDE3 inhibitor (2 μM), TSP1 (2.2 nM) and intracellular cAMP measured via immunoassay *P<0.05, PDEI 4 versus PDEI 4 + TSP1 or PDEI 4 + PDEI 3. ns = not significant, PDEI 3 versus PDEI 3 + TSP1 and PDEI 4 + PDEI 3 versus PDEI 4 + PDEI 3 + TSP1. (G) VSMCs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium + 0.1% BSA in the presence of 8-Br-cGMP (10 μM) ± TSP1 (2.2 nM) and cAMP levels determined via immunoassay. * P<0.05, TSP1 + 8-Br-cAMP versus 8-Br-cAMP.
Because PDE4 is a cAMP specific phosphodiesterase, we did not expect rolipram to directly alter cGMP levels. This was confirmed in cells treated with NO donor and/or exogenous TSP1 with or without the PDE4 inhibitor (Fig. 3B). Rolipram treatment did not alter cGMP levels, excluding feedback regulation through cAMP inhibition of PDE5. NO-induced cGMP was slightly, though not significantly, decreased in the presence of rolipram, but exogenous TSP1 still blocked the NO-stimulated increase in cGMP in the presence of this inhibitor (Fig. 3B). Therefore, rolipram does not block TSP1 inhibition of NO signaling by elevating cGMP or by preventing TSP1 modulation of sGC.
3.6. TSP1 inhibition of NO-driven VSMC responses involves cGMP-cAMP cross talk
Because cGMP-mediated inhibition of PDE3 in VSMCs can elevate cAMP [29,30] the PDE4-sensitive adhesive response to TSP1 could involve this second messenger. NO alone did not significantly increase human aortic VSMC cAMP levels, and adding TSP1 had no effect (Fig. 3C). As expected, the PDE4 inhibitor significantly elevated cAMP levels in untreated cells or cells treated with an NO donor (Fig. 3C). Interestingly, TSP1 in the presence of NO and rolipram reproducibly inhibited cAMP levels (Fig. 3C).
If inhibition of this cAMP response by TSP1 was mediated by the known inhibition of the cAMP-hydrolyzing PDE3 by cGMP, it should require guanylyl cyclase activity. This was confirmed using ODQ, which reversed rolipram-stimulated cAMP accumulation in the presence of NO (Fig. 3D). To further demonstrate that TSP1 modulation of cAMP is sGC-specific and rule out effects via other enzymatic sources of cGMP, VSMCs were treated with atrial natriuretic peptide (ANP), a specific activator of particulate guanylyl cyclase (pGC). In this case TSP1 did not limit ANP-stimulated changes in cellular cGMP further confirming the specificity of TSP1 inhibition of sGC (Fig 3E). Interestingly, TSP1 still had a residual inhibitory effect on cAMP even when cGMP cross talk was blocked (Fig. 3D) supporting the findings that TSP1 also inhibits forskolin and isoproterenol-stimulated increases in VSMC cAMP and arterial vasodilation putatively through Gi-dependent inhibition of adenylyl cyclase. This result suggested that PDE3 becomes the limiting cAMP PDE when PDE4 is blocked. Consistent with this mechanism, adding the PDE3 inhibitor alone in the presence of NO did not alter cAMP, but inhibiting PDE3 abrogated the rolipram-dependent increase in cAMP and diminished the ability of TSP1 to inhibit cAMP levels under this condition (Fig. 3F). Furthermore, addition of the cell permeable analogue 8-Br-cGMP increased intracellular cAMP levels in VSMCs presumably through competition with cAMP for PDE3 hydrolysis, and exogenous TSP1 abrogated this increase (Fig. 3G). Taken together, these results indicate that TSP1 directly modulates adenylyl cyclase-activated formation of cAMP, but under conditions of NO-stimulation and increased cGMP, TSP1 can also lower cAMP by preventing cGMP-mediated inhibition of PDE3.
3.7. Sensitivity of NO-dependent cAMP signaling to TSP1 is specific for VSMCs
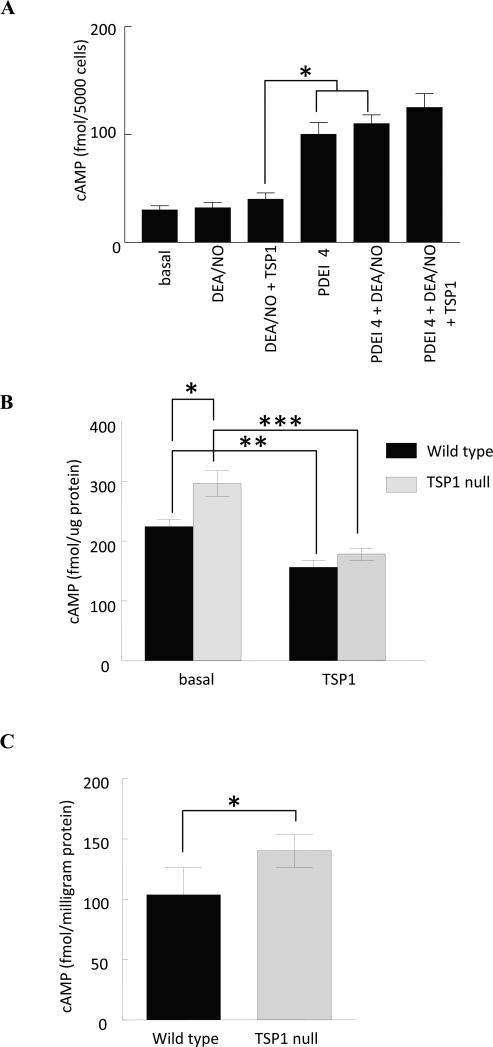
Endothelial and VSMCs express different PDE variants and family members [33], suggesting that cell type-specific cross talk between cyclic nucleotide pathways mediated by these PDEs could account for differential responses to TSP1 and NO. Although cGMP responses of VSMCs appear identical to those of endothelial cells, cross talk with cAMP differed (Fig. 4A). Consistent with a previous report [34], HUVEC cAMP levels did not increase in response to NO. Inhibiting PDE4 did increase cAMP in the absence or presence of NO as in VSMCs (compare with Fig. 3C). Most importantly, in contrast to VSMCs, elevated HUVEC cAMP levels in the presence of rolipram and NO was not inhibited by TSP1.
Fig. 4. Inhibition of NO-dependent cAMP signaling by TSP1 is specific for VSMC.
(A) HUVECs (5000 cells/well) were incubated in 96-well plates for 5 min in basal medium (E-GM without serum and additives) + 0.1% BSA in the presence of the indicated treatment agents at the following concentrations - DEA/NO (10 μM), PDE4 inhibitor (1 μM), TSP1 (2.2 nM) and intracellular cAMP measured via immunoassay *P<0.05, DEA/NO + TSP1 versus PDEI 4 or DEA/NO + PDEI 4. Results are representative of three separate experiments. (B) Murine derived aortic VSMCs from wild type and TSP1 null age matched C57BL/6 mice were grown to 80% surface saturation on 6-well culture plates (Nunc) and serum and growth factor deprived overnight in SM-GM (Lonza, Switzerland) and 0.1% BSA. The next morning cells were treated with TSP1 (2.2 nM) for 15 min, lysed and cAMP determined via ELISA immunoassay (GE Healthcare, Franklin, NJ). Results were normalized to total protein and are presented as the mean ± SD of three separate experiments. *P<0.05, TSP1 null versus wild type; **P<0.05, wild type + TSP1 versus wild type; ***P<0.05 TSP1 null + TSP1 versus TSP1 null. (C) Vastus lateralis skeletal muscle biopsies were obtained from age matched male C57BL/6 wild type and TSP1 null mice, snap frozen in liquid nitrogen, pulverized and suspended in lysis buffer. Following sonication cAMP levels were determined from the supernatant via ELISA immunoassay with results normalized to total protein. Results represent the mean ± SD of 6 wild type and 6 TSP1 null muscle biopsies. * P<0.05, TSP1 null versus wild type.
3.8. VSMC and skeletal muscle cAMP levels are limited by endogenous TSP1
Basal cGMP levels are continuously modulated by endogenous TSP1-CD47 signaling [7,9]. In murine TSP1 and CD47 null endothelial and VSMCs basal cGMP levels are greater than in wild type cells [9,12]. Likewise temporary gene silencing of CD47 in porcine VSMCs elevates basal cGMP above control [5]. Interestingly, cAMP levels are increased significantly in TSP1 null aortic VSMCs compared to wild type (Fig. 4B). Adding back exogenous TSP1 suppresses cAMP levels both in wild type and TSP1 null cells. Together these results are consistent with both cGMP driven regulation of PDE3 leading to decreased hydrolysis of cAMP and direct inhibition of adenylyl cyclase by TSP1. Similarly, cAMP levels were significantly elevated in skeletal muscle samples from TSP1 null mice compared to wild type (Fig. 4C).
4. Discussion
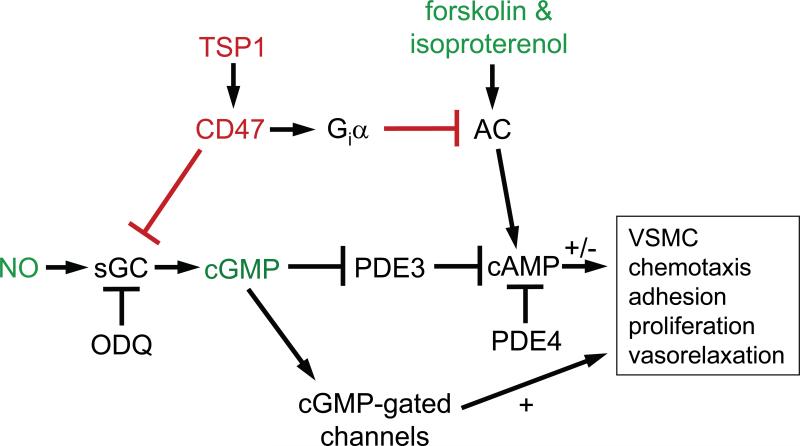
The sGC/cGMP pathway is an important molecular target for antagonism between TSP1 and NO to control angiogenic responses of endothelial cells [12], VSMC contractility [2], and platelet activation [10]. TSP1, as an inhibitor of the canonical NO pathway, acutely regulates tissue blood flow and hemostasis, and limits the ability of higher animals to survive fixed ischemic and ischemia-reperfusion injuries [3,4,35]. However, vascular smooth muscle tone also depends on cAMP signaling [22,36], and previous reports of elevated cAMP levels in tissues from TSP1 and CD47 null mice [6] suggested that TSP1-CD47 signaling could also control vascular responses via this second messenger. The present results identify dual mechanisms by which TSP1 alters cAMP responses in VSMC – (i) via Gi-mediated inhibition of adenylyl cyclase, and (ii) via cGMP-dependent regulation of cAMP-specific PDE activity (Fig. 5).
Fig. 5. TSP1 controls VSMC response through modulating both cAMP and cGMP.
TSP1 acts at the level of guanylyl cyclase (GC) to inhibit NO-induced VSMC adhesion, migration, proliferation and contraction [2,3]. NO signaling also elevates cAMP through inhibiting PDE3 activity in a cGMP-dependent manner. TSP1 can block this elevation under conditions where PDE3 is limiting for cAMP degradation. Acting through cGMP-independent pathways, TSP1 also inhibits VSMC cAMP accumulation and limits cAMP-dependent vasodilation. TSP1 control of VSMC cAMP, through cGMP cross talk and in a cGMP-independent fashion by inhibiting adenylyl cyclase activation represents additional and novel mechanisms by which TSP1 regulates VSMC contractility, arterial diameter and blood flow emphasizing the redundant nature of TSP1 regulation of vascular tone. (AC, adenylyl cyclase; Giα, G protein-coupled receptor)
CD47 ligation was previously shown to induce dissociation of Giα, which was associated with increased integrin activation. In the context of NO, however, TSP1 ligation of CD47 decreases VSMC adhesion. Therefore, Gi-mediated integrin activation can not explain the present data. CD47 ligation is also known to modulate cAMP levels in several cell types including platelets [15]. In thyroid cells, the TSP1-derived peptide 4N1K (RFYVVMWK) by binding to CD47 prevented a ceramide-induced decrease in cAMP [14]. Conversely, several CD47 ligands including a derivative of the above peptide decreased cAMP levels in platelets, malignant breast epithelial cells, and T cells and, in the latter two cell types, induced cell death in a protein kinase A-dependent manner [13]. The latter studies are consistent with our results in VSMC and suggest that CD47 ligation directly lowers cAMP synthesis via G-protein regulation of adenylyl cyclase (review in [37]). Our data in cultured VSMCs (that lack eNOS) and endothelial free arterial segments further suggests direct coupling of CD47 to adenylyl cyclase activity.
Cellular cGMP and cAMP levels are linked through feedback via PDEs [38]. The importance of cross talk between cGMP and cAMP to TSP1 modulation of NO-driven cell signals was demonstrated by the finding that inhibition of PDE4 prevented TSP1 downregulation of NO-driven responses. PDE4 is responsible for hydrolysis of cAMP and does not directly alter cGMP levels. Under these conditions, cGMP inhibits PDE3 and thereby elevates intracellular cAMP (Fig. 5). Interestingly TSP1 regulation of cAMP via cGMP-dependent cross talk may be specific for VSMCs in that we did not see a similar coupling between cAMP and cGMP levels in vascular endothelial cells. Regulation of PDE4 activity and subcellular localization (review in [39-41]), therefore, may be a key to determine whether NO and TSP1 signals can regulate cAMP in a specific cell type. Furthermore, the ability of a Src kinase, which is another target of TSP1 signaling in vascular cells [42,43], to regulate a PDE4 variant [44] suggests that PDE4 may directly participate in TSP1 signaling. The above results also suggest that VSMC responses to TSP1 may be sensitive to drugs that selectively modify PDE activity. Importantly, studies using VSMCs from knockout mice confirmed a role for endogenous TSP1 in modulating cellular cAMP levels. Consistent with earlier reports of elevated cGMP levels in the absence of endogenous TSP1 [12] and CD47 [9], VSMCs from TSP1 null animals demonstrated significantly greater basal cAMP levels, which were suppressed by exogenous TSP1. Skeletal and cardiac muscle responses are known to be regulated by cAMP-cGMP cross talk [45]. The finding that TSP1 null skeletal muscle biopsies display increased cAMP levels and the identified role cAMP has in regulating mitochondrial metabolism [46] suggests a possible further role for TSP1 in regulating muscle energetics.
TSP1 can both stimulate and inhibit VSMC responses. In part, these opposing responses may be explained by competing signals from different TSP1 receptors. Positive proliferative and chemotactic responses of VSMCs to TSP1 have been ascribed to the TSP1 receptor αβ1 integrin [47,48], which recognizes the N-terminal domain of TSP1. The C-terminal domain of TSP1 stimulates VSMC chemotaxis through αvβ3 integrin and CD47 [47,49,50]. Responses of VSMCs to PDGF are also dependent on endogenous levels of TSP1 [48]. Generally, these positive activities of TSP1 require nanomolar or greater concentrations of the protein. Conversely, inhibition of NO signaling in vascular cells via CD47 occurs at 10-100 pM concentrations of TSP1. Therefore, the latter may be the dominant activity of TSP1 for VSMCs when physiological concentrations of NO are present.
Both cGMP and cAMP are known to promote arterial vasodilation [1,51]. TSP1 blocks eNOS activation and endogenous NO production [7], NO-dependent activation of sGC and cGMP-stimulated de-phosphorylation of MLC-2 in VSMCs and limits NO-stimulated vasodilation in mice [3]. TSP1, as we show, can also limit cellular cAMP in both a cGMP-dependent and cGMP-independent manner (Fig. 5). These findings suggest that in vivo circulating TSP1, by suppressing endothelial derived NO production (through inhibiting eNOS activation), also limits cAMP-mediated signaling in vessels. Additionally, we find that TSP1 regulates VSMC contraction by directly resisting cAMP-dependent modulation of contractile proteins. Consistent with this novel role in regulating VSMC contractility, we show that exogenous TSP1 in endothelium free arteries inhibits forskolin and isoproterenol stimulated vasodilation. These findings indicate that TSP1 can modulate VSMC contractile proteins in cultured cells and arterial tone in vessels through regulation of cAMP in a cGMP-independent manner [51]. This is particularly relevant given the enhanced expression of TSP1 in the extracellular matrix of aged and atherosclerotic arteries [52-55] and suggests that TSP1 limits both cGMP- and cAMP-stimulated vasodilation. This may partly explain the reported decrease in cAMP and cGMP stimulated activation of protein kinase G-I (PKG-I) in aged VSMCs and suggests increased age-driven arterial expression of the TSP1-CD47 nexus could account for loss of arterial vasodilation in older individuals[56]. Then blocking the TSP1-CD47 signaling nexus, by selectively enhancing regional cyclic nucleotide levels of both cGMP and cAMP, may represent a novel means of increasing arterial vasodilation and blood flow and of altering blood pressure.
5. Conclusions
The matrix-cellular protein thrombospondin-1 has an identified role in limiting cGMP levels in vascular cells. We now demonstrate that TSP1 can limit vascular smooth muscle cell cAMP levels by an NO-dependent pathway through cGMP mediated alterations of cAMP hydrolysis. Additionally, TSP1 potently suppresses activation of VSMC adenylyl cyclase and cAMP-stimulated vasodilation and thus has NO-independent effects on vascular cAMP. TSP1 thus redundantly modulates cyclic nucleotide levels in the vasculature.
Acknowledgements
This research was supported by the following funding: NIH grant K22 CA128616 (JSI) and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (DDR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
JSI is a consultant to and Chair of the Scientific Advisory Board of Vasculox, Inc. (St. Louis, MO) and founder of Radiation Control Technologies, Inc. (Pittsburgh, PA).
References
- 1.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 2.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD. Blocking thrombospondin-1/cd47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD. Gene silencing of cd47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: Implications for human disease. Ann Surg. 2008;247:860–868. doi: 10.1097/SLA.0b013e31816c4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and cd47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting enos activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq218. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 9.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. Cd47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cgmp signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with cd47. J Biol Chem. 2010 doi: 10.1074/jbc.M110.172304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cgmp-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manna PP, Frazier WA. The mechanism of cd47-dependent killing of t cells: Heterotrimeric gi-dependent inhibition of protein kinase a. J Immunol. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- 14.Rath GM, Schneider C, Dedieu S, Sartelet H, Morjani H, Martiny L, El Btaouri H. Thrombospondin-1 c-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int J Biochem Cell Biol. 2006;38:2219–2228. doi: 10.1016/j.biocel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J Cell Biol. 1999;147:389–400. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier WA, Gao AG, Dimitry J, Chung J, Brown EJ, Lindberg FP, Linder ME. The thrombospondin receptor integrin-associated protein (cd47) functionally couples to heterotrimeric gi. J Biol Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- 17.Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of cd47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279:42393–42402. doi: 10.1074/jbc.M407631200. [DOI] [PubMed] [Google Scholar]
- 18.Rao YJ, Xi L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol Sin. 2009;30:1–24. doi: 10.1038/aps.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto T, Kobayashi T, Kamata K. Phosphodiesterases in the vascular system. J Smooth Muscle Res. 2003;39:67–86. doi: 10.1540/jsmr.39.67. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DD, Cashel J, Guo N. Purification of thrombospondin from human platelets. J Tissue Cult Methods. 1994;16:217–222. [Google Scholar]
- 21.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. Cgmp-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res. 2007;101:712–722. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier S, Julien C, Popoff MR, Lamarche-Vane N, Meloche S. Cyclic amp induces morphological changes of vascular smooth muscle cells by inhibiting a rac-dependent signaling pathway. J Cell Physiol. 2005;204:412–422. doi: 10.1002/jcp.20308. [DOI] [PubMed] [Google Scholar]
- 23.Wu LL, Tang C, Liu MS. Altered phosphorylation and calcium sensitivity of cardiac myofibrillar proteins during sepsis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R408–416. doi: 10.1152/ajpregu.2001.281.2.R408. [DOI] [PubMed] [Google Scholar]
- 24.Pfitzer G. Signal transduction in smooth muscle: Invited review: Regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 25.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–1780. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- 26.Raina H, Zacharia J, Li M, Wier WG. Activation by ca2+/calmodulin of an exogenous myosin light chain kinase (mlck) in mouse arteries. J Physiol. 2009 doi: 10.1113/jphysiol.2008.165258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabbani G, Vijay V, Sarabu MR, Gupte SA. Regulation of human internal mammary and radial artery contraction by extracellular and intracellular calcium channels and cyclic adenosine 3', 5' monophosphate. Ann Thorac Surg. 2007;83:510–515. doi: 10.1016/j.athoracsur.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Tasken K. The camp-epac-rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem. 2004;279:44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- 29.Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. Role of phosphodiesterase 3 in no/cgmp-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res. 2003;93:406–413. doi: 10.1161/01.RES.0000091074.33584.F0. [DOI] [PubMed] [Google Scholar]
- 30.Delpy E, Coste H, Gouville AC. Effects of cyclic gmp elevation on isoprenaline-induced increase in cyclic amp and relaxation in rat aortic smooth muscle: Role of phosphodiesterase 3. Br J Pharmacol. 1996;119:471–478. doi: 10.1111/j.1476-5381.1996.tb15696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic gmp phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 32.Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families pde iii and pde iv to attenuate proliferation of rat vascular smooth muscle cells. Biochem Pharmacol. 1994;48:827–835. doi: 10.1016/0006-2952(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 33.Maurice DH. Cyclic nucleotide phosphodiesterase-mediated integration of cgmp and camp signaling in cells of the cardiovascular system. Front Biosci. 2005;10:1221–1228. doi: 10.2741/1614. [DOI] [PubMed] [Google Scholar]
- 34.Boulanger C, Schini VB, Hendrickson H, Vanhoutte PM. Chronic exposure of cultured endothelial cells to eicosapentaenoic acid potentiates the release of endothelium-derived relaxing factor(s). Br J Pharmacol. 1990;99:176–180. doi: 10.1111/j.1476-5381.1990.tb14673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/cd47 signaling. Surgery. 2008;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lillestll IK, Helle KB, Aardal S. Relaxing effects of cyclic gmp and cyclic amp-enhancing agents on the long-lasting contraction to endothelin-1 in the porcine coronary artery. Scand J Clin Lab Invest. 1998;58:625–634. doi: 10.1080/00365519850186058. [DOI] [PubMed] [Google Scholar]
- 37.Watts VJ, Neve KA. Sensitization of adenylate cyclase by galpha i/o-coupled receptors. Pharmacol Ther. 2005;106:405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Zaccolo M, Movsesian MA. Camp and cgmp signaling cross-talk: Role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- 39.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 40.Maurice DH. Dynamic regulation of camp signaling by cgmp in the cardiovascular system: Roles of phosphodiesterase 2 and phosphodiesterase 3 enzymes. Proc West Pharmacol Soc. 2003;46:32–36. [PubMed] [Google Scholar]
- 41.Baillie GS, Houslay MD. Arrestin times for compartmentalised camp signalling and phosphodiesterase-4 enzymes. Curr Opin Cell Biol. 2005;17:129–134. doi: 10.1016/j.ceb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 43.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via cd36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPhee I, Yarwood SJ, Scotland G, Huston E, Beard MB, Ross AH, Houslay ES, Houslay MD. Association with the src family tyrosyl kinase lyn triggers a conformational change in the catalytic region of human camp-specific phosphodiesterase hspde4a4b. Consequences for rolipram inhibition. J Biol Chem. 1999;274:11796–11810. doi: 10.1074/jbc.274.17.11796. [DOI] [PubMed] [Google Scholar]
- 45.Movsesian MA. Camp-mediated signal transduction and sarcoplasmic reticulum function in heart failure. Ann N Y Acad Sci. 1998;853:231–239. doi: 10.1111/j.1749-6632.1998.tb08271.x. [DOI] [PubMed] [Google Scholar]
- 46.Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by camp, akaps and the proteasome. Trends Cell Biol. 2008;18:604–613. doi: 10.1016/j.tcb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Lymn JS, Patel MK, Clunn GF, Rao SJ, Gallagher KL, Hughes AD. Thrombospondin-1 differentially induces chemotaxis and DNA synthesis of human venous smooth muscle cells at the receptor-binding level. J Cell Sci. 2002;115:4353–4360. doi: 10.1242/jcs.00119. [DOI] [PubMed] [Google Scholar]
- 48.Isenberg JS, Calzada MJ, Zhou L, Guo N, Lawler J, Wang XQ, Frazier WA, Roberts DD. Endogenous thrombospondin-1 is not necessary for proliferation but is permissive for vascular smooth muscle cell responses to platelet-derived growth factor. Matrix Biol. 2005;24:110–123. doi: 10.1016/j.matbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Nesselroth SM, Willis AI, Fuse S, Olson ET, Lawler J, Sumpio BE, Gahtan V. The c-terminal domain of thrombospondin-1 induces vascular smooth muscle cell chemotaxis. J Vasc Surg. 2001;33:595–600. doi: 10.1067/mva.2001.112318. [DOI] [PubMed] [Google Scholar]
- 50.Lee T, Nesselroth SM, Olson ET, Esemuede N, Lawler J, Sumpio BE, Gahtan V. Thrombospondin-1-induced vascular smooth muscle cell chemotaxis: The role of the type 3 repeat and carboxyl terminal domains. J Cell Biochem. 2003;89:500–506. doi: 10.1002/jcb.10524. [DOI] [PubMed] [Google Scholar]
- 51.Gerthoffer WT, Trevethick MA, Murphy RA. Myosin phosphorylation and cyclic adenosine 3',5'-monophosphate in relaxation of arterial smooth muscle by vasodilators. Circ Res. 1984;54:83–89. doi: 10.1161/01.res.54.1.83. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio M, De Rosa G, Pelicci PG, Napoli C. P66shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein e knockout mice. Endothelium. 2008;15:276–287. doi: 10.1080/10623320802487791. [DOI] [PubMed] [Google Scholar]
- 53.Riessen R, Kearney M, Lawler J, Isner JM. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/s0002-8703(98)70105-x. [DOI] [PubMed] [Google Scholar]
- 54.Roth JJ, Gahtan V, Brown JL, Gerhard C, Swami VK, Rothman VL, Tulenko TN, Tuszynski GP. Thrombospondin-1 is elevated with both intimal hyperplasia and hypercholesterolemia. J Surg Res. 1998;74:11–16. doi: 10.1006/jsre.1997.5209. [DOI] [PubMed] [Google Scholar]
- 55.Canfield AE, Farrington C, Dziobon MD, Boot-Handford RP, Heagerty AM, Kumar SN, Roberts IS. The involvement of matrix glycoproteins in vascular calcification and fibrosis: An immunohistochemical study. J Pathol. 2002;196:228–234. doi: 10.1002/path.1020. [DOI] [PubMed] [Google Scholar]
- 56.Lin CS, Liu X, Tu R, Chow S, Lue TF. Age-related decrease of protein kinase g activation in vascular smooth muscle cells. Biochem Biophys Res Commun. 2001;287:244–248. doi: 10.1006/bbrc.2001.5567. [DOI] [PubMed] [Google Scholar]