Abstract
The etiology of major depression remains unknown, but dysfunction of serotonergic signaling has long been implicated in the pathophysiology of this disorder. p11 is an S100 family member recently identified as a serotonin 1B (5-HT1B) and serotonin 4 (5-HT4) receptor binding protein. Mutant mice in which p11 is deleted show depression-like behaviors, suggesting that p11 may be a mediator of affective disorder pathophysiology. Using somatic gene transfer, we have now identified the nucleus accumbens (NAcc) as a key site of p11 action. Reduction of p11 with adeno-associated virus (AAV)-mediated RNA interference (RNAi) in the NAcc, but not in the anterior cingulate, of normal adult mice resulted in depression-like behaviors nearly identical to those seen in p11 knockout mice. Restoration of p11 expression specifically in the NAcc of p11 knockout mice normalized depression-like behaviors. Human NAcc tissue shows a significant reduction of p11 protein in depressed patients when compared to matched healthy controls. These results suggest that p11 loss in rodent and human NAcc may contribute to the pathophysiology of depression. Normalization of p11 expression within this brain region with AAV-mediated gene therapy may be of therapeutic value.
Introduction
The neuroanatomical and molecular substrates underlying major depressive disorder remain poorly understood. Serotonin is considered to be a key neurotransmitter in depression-like states, and the most commonly prescribed antidepressant therapies increase extracellular levels of serotonin. Abnormalities of the serotonergic system underlie an array of psychiatric pathologies, including depression, anxiety, alcoholism, and impulsive behavior(1). Recently, the serotonin receptors 5-HT1B and 5-HT4 were reported to interact with p11, a member of the S100 family of cytoplasmic, small acidic EF-hand type helix-loop-helix proteins(2, 3). p11 facilitates cell membrane localization and ligand-mediated activation of these receptors, a function analogous to those served by p11 for several other proteins and ion channels, such as the NaV1.8 sodium and TASK-1 potassium channels (4). p11 knockout (KO) mice exhibit depression-like phenotypes, such as increased immobility times on tail suspension test (TST) and forced swim test (FST), while transgenic overexpression of p11 recapitulates the actions of pharmacological antidepressant treatment (2).
To better understand the involvement of p11 action in depressive-like states and suggest p11-based antidepressant therapies, we have used viral vector-mediated gene transfer to focally alter p11 expression in normal adult mouse brain. Several brain regions have been implicated in the pathogenesis of depression from animal, human imaging and therapeutic studies such as stereotactic surgery. These include the ventral striatum/nucleus accumbens (NAcc), subgenual cingulate (area 25), hippocampus, amygdala, habenula and anterior cingulate cortex(5-7). Two of these regions, the NAcc and anterior cingulate cortex, were chosen for further study. The NAcc was chosen on the basis of both human functional imaging and results from therapeutic deep brain stimulation (DBS), which support animal studies suggesting that reduced activity within this region may promote human depression(5, 7, 8). In addition, the ability of serotonin to inhibit glutamatergic synaptic transmission in the NAcc is blunted in p11 KO mice. p11 KO mice are also relatively resistant to the effects of 5-HT1B agonists on abnormal movements induced by L-dopa following dopaminergic denervation of the dorsal striatum (9). Given the anatomic and physiological similarities between the dorsal and ventral striatum, these results further support a potential role for p11 in the function of the ventral striatum(2, 9). The anterior cingulate was chosen as the second focal region to be studied, on the basis of findings in our original report of reduced p11 expression in the anterior cingulate cortex of depressed human patients compared with controls and up-regulated p11 expression in this region after various antidepressant therapies in rodents(2).
Results
To alter p11 levels focally within the adult brain, we utilized adeno-associated virus (AAV)-mediated gene transfer as described (10, 11). A small interfering RNA (siRNA) was created which efficiently blocked expression of a p11-GFP fusion protein in HEK 293 cells, while a control siRNA against the luciferase gene had no effect (Fig. 1A). A GFP expression cassette contained within both siRNA plasmid constructs yielded equivalent expression of unmodified GFP in the two samples. The second band in the siLuc lane corresponds to an artifactual splice variant of the fusion protein. These siRNA sequences were then cloned into AAV2 vectors as small hairpin RNAs (shRNA), which also expressed YFP cassettes (AAV.sip11.YFP or AAV.siluc.YFP) to monitor the location of AAV transduction within the brain. AAV.sip11.YFP efficiently blocked endogenous p11 expression in primary NAcc neurons in culture (Fig. 1B) while control AAV.siluc.YFP had no effect. Infusion into the NAcc in vivo following optimization of infusion parameters confirmed efficient focal transduction of the AAV vectors (Fig. 1C) and consequent reduction in neuronal p11 expression (Fig. 1D). A vector containing a p11 cDNA was used for overexpression, which also harbored a control luciferase siRNA expression cassette (AAV.siLuc.p11) so that it would be matched with the AAV.sip11.YFP vector. This permitted use of a third vector encoding both luciferase siRNA and YFP (AAV.siLuc.YFP) as a single negative control for both AAV.sip11.YFP and AAV.siLuc.p11 vectors.
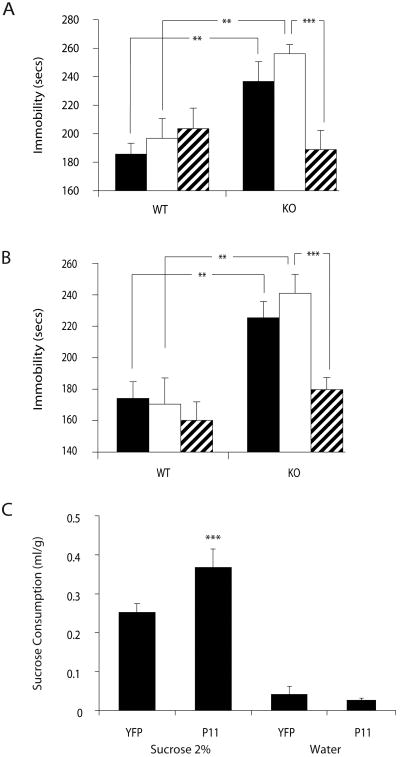
Fig 1. Focal inhibition of p11 expression in adult mouse NAcc induces depression-like behaviors.
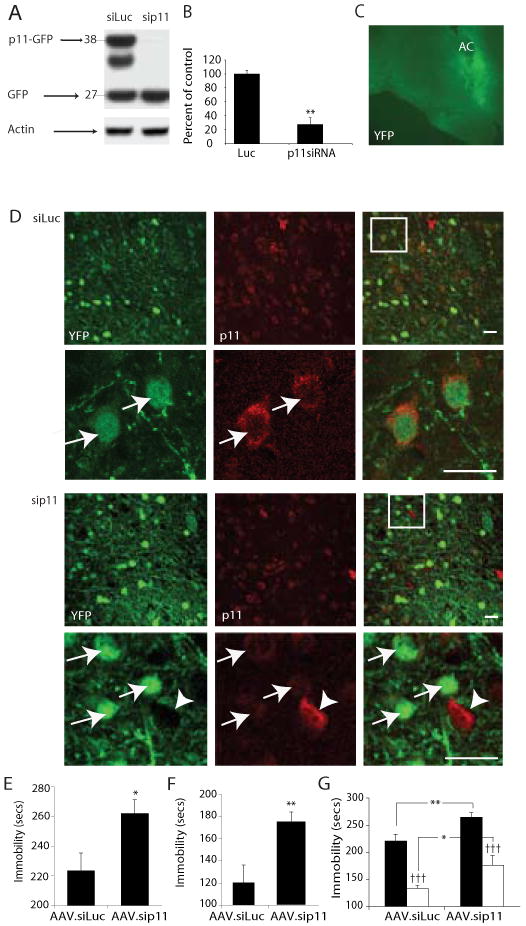
(A)Western blot analysis confirming effective knockdown of a p11-GFP fusion protein in HEK 293 cells co-transfected with shRNA to p11 but not with luciferase shRNA, analyzed using an antibody to GFP. Band sizes are in kDa. (B) Quantitative PCR analysis of endogenous p11 mRNA levels in primary NAcc neurons following AAV-mediated gene transfer of p11 shRNA compared with luciferase control shRNA (C) Coronal brain section of 3 month old male mouse with injection of p11 shRNA virus co-expressing YFP confirms local transduction within the NAcc following YFP immunostaining. AC=Anterior commissure. (D) Confocal micrographs of YFP (green) and p11 (red) immunofluorescense in the NAcc after injection of control virus (AAV.siLuc.YFP, top 2 panels) and knockdown virus (AAV.sip11.YFP, bottom 2 panels) in vivo. Arrows, YFP-positive cells; arrowhead, YFP-negative cell still expressing p11 at normal levels (scale bars, 20 μm). (E-G) Induction of depression-like behaviors in adult mice following AAV shRNA-mediated focal knockdown of p11 in NAcc. (E) Increased immobility of p11 knockdown compared with controls on tail suspension test. (F) Increased immobility of p11 knockdown compared with controls on forced swim test * p<0.05, ** p<0.01, two-tailed t-test. (G) Effect of focal p11 knockdown in the NAcc on behavioral response to anti-depressant treatment in the tail suspension test. The loss of NAcc p11 expression does not abolish the anti-depressant effect of imipramine (open bars) compared with saline controls (closed bars) ††† p<0.001 post-imipramine compared with matched saline control, two-tailed t-test. However, there remains a significant increase in immobility following drug treatment in p11 siRNA mice compared with siluc controls *p<0.05, **p<0.01, two-tailed t-test.
Behavioral effects of localized p11 knockdown and over-expression in C57Bl/6 mice after AAV-mediated gene transfer were examined with an automated, unbiased video monitoring system to measure immobility times on the tail suspension test (TST) and forced swim test (FST). These are commonly used as depression-related behavioral paradigms, as time of immobility in each assay is reduced by treatment with several anti-depressants used in current clinical practice, while factors associated with human depression, such as chronic or early life stress exposure, post-partum state or genetic predisposition, all increase TST and/or FST immobility times (12, 13). Focal reduction of p11 in the NAcc of otherwise normal adult mice with AAV-mediated RNA interference resulted in a significant increase in immobility in the TST (Fig. 1E). The time spent immobile and percent change from control were comparable to previous observations in p11 KO mice(2). A similar increase in immobility was observed in the FST (Fig. 1F). The increased immobility seen in the TST and FST was not due to a defect in locomotor activity, since horizontal movement in an open field did not differ between control and p11 knockdown groups (Fig. S1). Infusion of the p11 knockdown vector into the anterior cingulate (Fig. S2A) did not yield any significant change in these behaviors (Fig. S2B).
To evaluate the possible involvement of NAcc p11 in the behavioral effects of antidepressant medications, a separate group of mice received p11 knockdown or control viruses or no surgery, followed by acute treatment with the tricyclic anti-depressant imipramine or saline prior to behavioral testing. Imipramine blocks reuptake of both serotonin and norepinephrine, but was chosen for this study in order to directly compare our results with prior data obtained with imipramine in p11 KO mice(2). When mice were treated with saline, the previously observed effect of focal NAcc p11 knockdown on increasing TST immobility times was replicated. As expected, imipramine treatment of mice in both the no-surgery and control vector groups resulted in significant reductions in TST immobility times (Fig 1G). The p11 knockdown mice also responded to imipramine with a reduction in TST immobility (Fig 1G). However, there was still a significant increase in immobility time compared with control mice treated with the antidepressant (Fig 1G). These results are similar to data obtained with the p11 knockout mice, which remained responsive to imipramine, but retained higher immobility compared to wild-type littermates(2).
To determine whether the behavioral consequences of congenital loss of p11 expression can be reversed, a virus containing p11 cDNA was injected into the NAcc of p11 KO mice, which restored p11 expression selectively in this brain region (Fig. 2A). To confirm that this p11 was functional, we quantified 5-HT1B binding using receptor autoradiography (Fig. 2B,C; Fig. S5). Increased 5-HT1B receptor binding was found in the terminal regions of NAcc neurons in the hemisphere injected with p11 overexpressing vector compared with the hemisphere injected with the control AAV vector expressing the YFP marker gene. The increased ligand binding in the axon terminal fields is consistent with the known distribution of 5-HT1B receptors(14, 15). The magnitude of this effect was similar to that seen when comparing p11 KO mice with wild-type littermates(2).
Fig 2. Overexpression of p11 in NAcc neurons potentiates 5-HT1B agonist binding and signaling.
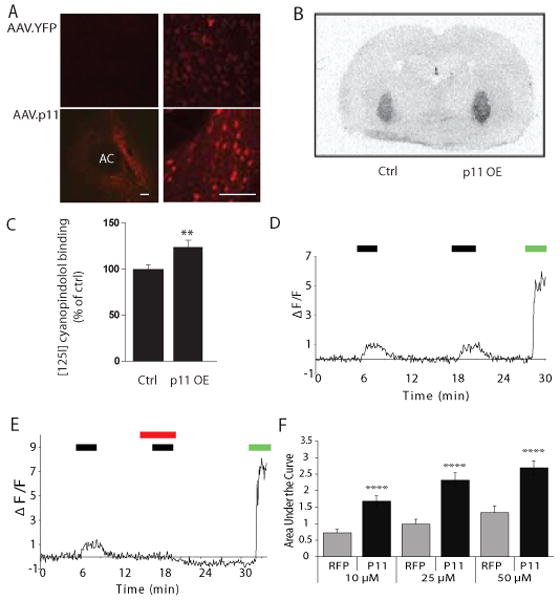
(A) Immunohistochemistry (left, low power magnification; right, high-power magnification) demonstrates focal restoration of p11 expression in the NAcc of p11 KO mice following AAV mediated gene therapy with a p11 cDNA but not with control YFP, which demonstrates only light background staining. AC=Anterior commissure (scale bars= 80 μm). (B,C) 5-HT1B receptor binding levels in p11 KO mice after intraccumbal injection of p11 overexpression (OE) vector (AAV.siLuc.p11) or the control vector (AAV.siLuc.YFP) using 10pM [125I]-cyanopindolol. (B) Autoradiography revealed increased binding in the pallidum terminal regions of accumbens neurons in the hemisphere overexpressing p11 following AAV-mediated gene transfer (p11 OE) compared with the control hemisphere expressing the YFP marker gene (ctrl). (C) Quantification confirmed the significant increase in 5-HT1B binding in the p11 overexpressing hemisphere (** p<0.01, two-tailed t-test). (D) Treatment of primary striatal neurons with the 5HT1B-agonist anpirtoline (10μM) resulted in a repeated increase in fluorescence as measured by the area under the curve using the calcium-sensitive dye Fluor 4 (black bar=exposure to anpirtoline; green bar=exposure to glutamate as a positive control for calcium fluorescence). (E) Effect of anpirtoline (25μM; black bar) was completely blocked by exposure to the 5-HT1B antagonist GR127935 (10μM, red bar); green bar=exposure to glutamate as a positive control for calcium fluorescence. (F) Calcium fluorescence was significantly increased by AAV-mediated overexpression of p11 (black bars) in ventral striatal neurons compared with control RFP (gray bars) expression after exposure to 10μM, 25μM and 50μM anpirtoline (****p<0.0001).
We further examined the effect of p11 overexpression on 5-HT1B receptor function using calcium imaging of primary ventral striatal neuronal cultures (Figs. S3A,B). Although AAV can transduce neurons in primary culture, this is very inefficient, and since gene expression requires several days after transduction, glial overgrowth can often complicate studies. Therefore, we developed a method of direct intrastriatal injection of AAV vectors in post-natal day 2 living rat pups (Fig. S3C) followed by harvest one week later. This resulted in efficient gene transfer into cultured ventral striatal neurons with no overgrowth of glial cells since the neurons could be studied almost immediately (Fig. S3D). As an assay for 5-HT1B function, we used calcium imaging since prior studies have indicated that activation of this receptor can activate pathways which increase intracellular calcium(16, 17). Treatment of cultured ventral striatal neurons with the 5-HT1B agonist anpirtoline resulted in a reproducible increase in calcium signal in a dose dependent fashion expressed as the area under the curve (Figs. 2D, S3A,B). This was a specific 5-HT1B agonist response: subsequent responses could be completely blocked by addition of the 5-HT1B antagonist GR127935 (Fig. 2E). Overexpression of p11 in ventral striatal neurons from normal mice resulted in a significant increase in anpirtoline-induced calcium responses in a dose-dependent fashion compared with control neurons transduced with red fluorescent protein (RFP) (Fig. 2F). This confirmed not only that AAV.p11 increases 5HT1B binding in vivo, but also that it increases the functional response of ventral striatal neurons to a 5HT1B agonist as well.
Having confirmed the functional efficacy of our AAV.p11 vector, we then examined the behavioral effects of restoring p11 expression exclusively in the ventral striatum of p11 KO mice. Focal replacement of p11 expression in the NAcc bilaterally with AAV-mediated gene therapy normalized TST immobility times of p11 KO animals (Fig. 3A); the performance of these mice on TST was indistinguishable from all wild-type littermates, including those with no surgery as well as those receiving control or p11 over-expressing virus. As expected, p11 KO mice receiving no surgery or control AAV had significantly increased TST immobility times compared with wild-type littermates. Results similar to those observed with the TST were also obtained on the FST, confirming the normalization of depressed phenotypes in these mice (Fig. 3B). Anhedonia is also a key component of human depression that can be tested in mice with a sucrose preference test. p11 KO mice consume less sucrose, consistent with an anhedonic phenotype(2). Reintroduction of p11 into the NAcc of p11 KO mice resulted in a significant increase in sucrose preference compared with mice receiving the control vector (Fig. 3C). Overall activity and feeding behavior were all unaffected by transgenic knockdown of p11 and by overexpression of p11 in the NAcc of p11 KO and wild-type mice (Fig. S4). Thus, several depression-like behaviors resulting from the absence of p11 during development can be fully overcome through reintroduction of p11 into the adult NAcc without influencing non-specific activities.
Fig 3. Focal restoration of NAcc p11 via AAV gene therapy reverses depression-like behaviors in transgenic p11 KO mice.
(A) Immobility times on tail suspension test following AAV gene therapy to restore NAcc p11 expression (hatched bars). Depression-like behaviors were reversed in these animals compared with both untreated mice (filled bars) and control AAV-treated mice (open bars). (B) Immobility times on the forced swim test in the same animals as in fig. 3A. (C) Increased sucrose preference in p11 KO mice following restoration of NAcc p11 expression. Volume of sucrose intake over 24 hrs. per gram of body weight was significantly increased in p11 KO mice compared with controls while there was no significant difference between groups in water intake. The percent of sucrose as a measure of overall fluid intake was also significantly increased (not shown). **p<0.01, ***p<0.005, two-tailed t-test.
In view of these results implicating the NAcc in the behavioral effects of p11 in mice, we also examined p11 expression in post-mortem NAcc of depressed human patients compared with controls matched for age, sex and post-mortem interval using methodology described previously(18). Blinded Western blot analysis of extracts from NAcc tissue (N=17 per group) revealed a significant reduction in p11 protein in depressed patients compared with controls (Fig. 4). The original report linking p11 to depression noted a similar reduction in p11 expression in the anterior cingulate of depressed patients(2), while another study noted reductions in p11 mRNA in hippocampus, frontal cortex and amygdala of depressed patients (2, 19). The present data support these earlier observations implicating reduced p11 expression as a feature of clinical depression.
Fig 4. p11 expression in post-mortem human NAcc brain tissue.
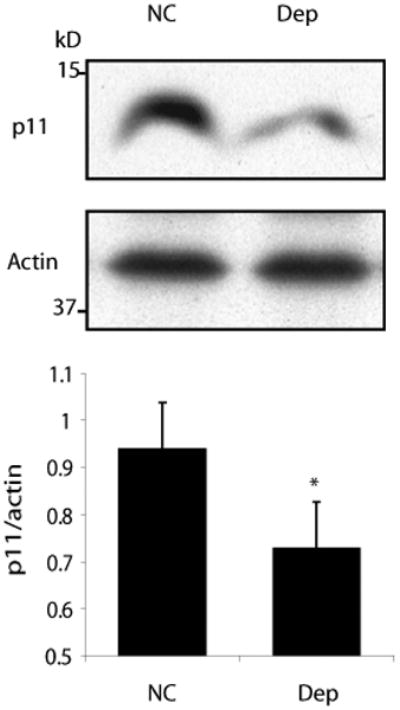
(A) Representative western blot of protein lysates from post-mortem NAcc brain tissue from normal controls (NC) and depressed patients (Dep) (N=17 per group). (B) Quantitative densitometry of western blots demonstrates reduced expression of p11 in post-mortem NAcc tissue from depressed patients (DEP) compared with normal controls (NC). *p<0.05, two-tailed t-test.
Discussion
Our data identify the NAcc as a key brain region mediating the ability of p11 to prevent the development of depression-like phenotypes in mice. Some regional specificity is suggested by the lack of behavioral effect of p11 knockdown in the anterior cingulate. It should be noted that the mouse anterior cingulate is not equivalent to human area 25, which has also been implicated in human depression and is thought to correspond to the mouse infralimbic cortex(20). Furthermore, since AAV2 is highly selective for neurons, these results indicate that p11 loss within neurons of the NAcc induces depression-like behaviors (10, 21). These results also demonstrate that the effects of p11 deletion in p11 KO mice (2) are not likely to be attributable to developmental consequences of embryonic p11 loss, since we observed similar behavioral effects from somatic blockade of p11 in normally developed adult mice.
The effect of p11 loss specifically in NAcc neurons in the current study provides support for the importance of this region as a mediator influencing the development of depression-like behaviors. Other brain structures, such as the hippocampus and frontal cortex, have received the majority of attention in experimental studies of the neurobiology of depression(22). The NAcc is a key component of the mesolimbic dopamine reward circuitry, receiving dopamine inputs from the ventral tegmental area (VTA), and as such has been most frequently studied in drug addiction and reward research(7). While data regarding the relationship between this area and depression remain limited, several studies have identified protein alterations within the NAcc which correlate with depression-like behaviors in rodents(7). Viral vectors have also been used to focally overexpress genes, such as the transcription factors CREB and ATF3, to induce depression-like behaviors, while expression of antagonizing genes such as dominant negative CREB or ATF2 resulted in anti-depressant responses(23). To our knowledge, the results reported here represent the first demonstration of depression-like behaviors which mimic whole-animal, embryonic deletion of a gene by somatic blockade of gene expression exclusively within a specific brain region. This is also the first report that depression-like behaviors observed in a genetic mouse model can be reversed with gene therapy to restore gene expression within such a focal region. Since p11 is expressed in other areas of the brain which have been linked to depression, including the hippocampus and infralimbic cortex, it is likely that there is a role for p11 in the functioning of these other key regions as well(24). Furthermore, since the NAcc is anatomically linked to these and other brain regions relevant to depression, altered NAcc p11 could indirectly influence the activity of other areas(25, 26). Our results not only advance understanding of the mechanism of p11 action within the brain, but provide strong support for the NAcc as a target for development of novel antidepressant therapies.
Modeling complex behaviors such as depression can be challenging in rodents, and results of our current and previous studies suggest that both the p11 KO mouse and AAV-mediated inhibition of p11 expression may be useful as new genetic models for depression research. The tests used here to quantify depression-like behaviors (TST, FST) use immobility as a measure of lack of motivation and they are the most widely used assays for depression-like behaviors in rodents. A number of interventions known to be involved in the susceptibility to or induction of major depression in humans increase immobility times on TST and FST, which further supports the relevance of these tests to human depression(12). A variety of other depression models have been described in rodents, including chronic mild stress and social defeat. However, these models use environmental stresses to induce behaviors that have been suggested to reflect human depression. In the current study, we have used the TST and FST as assays to quantify depression-like behaviors that occurred spontaneously following genetic manipulation of p11 levels (either transgenic or somatic) and reversed following restoration of p11 expression. Similarly, sucrose intake is generally used as a measure of anhedonia in mice, since intake of sweets is generally pleasurable and preferred by rodents(12). While other substances (ex. sweet milk) or novel environments have also been used as alternatives, sucrose preference remains the most widely used measure of anhedonic responses in rodents. Both environmental factors and genetic predisposition are likely to influence the risk of developing depression in humans, and therefore it will be of great interest in the future to determine if NAcc p11 levels alter sensitivity to developing depression-like behaviors in various models of chronic stress.
Serotonin signaling has long been of interest in depression studies, in part due to the mechanism of action of effective human antidepressant medications(1). This was in fact the basis for originally studying factors which could influence 5-HT1B receptor function, which resulted in the identification of p11(2). Our studies demonstrate that p11 does improve the response of NAcc neurons to 5-HT1B agonists, since overexpression of p11 increased the response of NAcc neurons to 5-HT1B agonists in primary culture and increased 5-HT1B ligand binding in NAcc terminal fields in live mouse brain. These observations support previous suggestions of a role for 5-HT1B in depression, and also confirm that the p11 ovexpressed by AAV vectors in these studies was functional in NAcc neurons. Nonetheless, these data do not conclusively demonstrate that the mechanism of p11 action in the NAcc is through modulation of 5-HT1B receptor activity. p11 can also influence 5-HT4 receptor binding, although the density of 5-HT4 receptors in the NAcc is far lower than that of 5-HT1B receptors(3, 24). There are also a variety of other ion channels and proteins which are modulated by p11 binding, although the relevance of most of these to NAcc function remains unclear(4). It is also possible that p11 interacts with one or more as yet unidentified factors which are critical to NAcc regulation of mood. Therefore, while our data demonstrate that the NAcc is a key site of p11 action to prevent the development of depression-like behaviors in rodents, further studies will be necessary to determine if p11 functions within these neurons through interactions with 5-HT1B receptors and/or other gene products.
The reduction in p11 expression in NAcc from depressed humans suggests that this may be a pathophysiological feature of major depression in at least some patients. The behavioral consequences of reduced p11 expression throughout the brain of p11 KO mice were reversed by reintroducing p11 expression focally in the NAcc. These combined data suggest that gene therapy to normalize p11 expression in the human NAcc may be useful as a treatment for select patients with major depression who prove refractory to other types of antidepressant treatment. Together with recent reports using the same AAV2 vector for human gene therapy in the brain for Parkinson's disease and pediatric genetic disorders, the present data highlight the therapeutic potential of normalizing p11 expression in the NAcc(10, 27).
Materials and Methods
Western Blotting
HEK293 cells were cultured in DMEM with 10% FBS for 24-48 h, followed by 2-h incubation with serum-free DMEM. Cells were washed with ice-cold phosphate-buffered saline (PBS), and then lysed in lysis buffer (20mM Tris pH 8.0, 1% Triton X-100, 150 mM NaCl, protease and phosphatase inhibitors) (Sigma). Samples were cleared by centrifugation, and equal amounts of protein (15–30 μg) were denatured in a sample buffer and resolved on a NuPAGE 4-12% gradient gel (Invitrogen). Blots were incubated with various amounts of anti-mouse S100A10 from R&D Systems followed by secondary antibodies and enhanced chemiluminescent detection (Pierce Thermo Scientific).
AAV vectors
AAV vectors were constructed as described(10, 11). The following oligonucleotides were annealed and cloned immediately downstream from the H1 promoter of AAV.H1.YFP into BamH1 and HindIII sites to generate AAV.H1.siLuc.YFP, AAV.H1.sip11.YFP and AAV.H1.siLuc.p11, respectively: siLuc (5′- GATCCCCCCGCTGGAGAGCAACTGCAT CTTCCTGTCA ATGCAGTTGCTCTCCAGCGGTTTTTGGAA-3′ and 5′-CTAGTTCCAAAAACCGCTGGAGAGCAACTGCAT TGACAGGAAG ATGCAGTTGCTCTCCAGCGGGGG-3′), sip11 (5′- TGGATCCTCTGGCTGTGGACA TTCAAGAGATGTCCACAGCCAGAGGATCC TTTTTTC-3′), p11 (5′- ATGCCATCCCAAATGGAGCACGCCATGGAAACCATGATGCTTACGTTTCACAGGTTTGCAGGCGACAAAGACCACTTGACAAAGGAGGACCTGAGAGTGCTCATGGAACGGGAGTTCCCTGGGTTTTTGGAAAATCAAAAGGATCCTCTGGCTGTGGACAAAATAATGAAGGACCTGGACCAGTGCCGAGATGGCAAAGTGGGCTTCCAGAGCTTTCTATCACTAGTGGCGGGGCTCACCATTGCATGCAATGACTATTTTGTAGTAAACATGAAGCAGAAGGGGAAGAAA -3′). All siRNA expression cassettes were verified by sequencing. Virus stocks were prepared by packaging the vector plasmids into AAV serotype 2 particles using a helper-free plasmid transfection system. The vectors were purified by using heparin affinity chromatography and dialyzed against PBS. AAV titers were determined by quantitative PCR using primers to a fragment of the AAV backbone and adjusted to 1012 genomic particles per ml.
Animals and Histology
C57BL/6 male mice (11 weeks old) were used in this study. All animal studies were approved by the Insitutional Animal Care and Use Committee at Weill Cornell Medical College and followed all institutional guidelines. Stereotaxic surgical procedures were performed under Ketamine/Xylazine anesthesia. After each mouse was placed in a stereotaxic frame, 1 μl of each virus (1×1012 genomic particles) in PBS was injected into the NAcc (anteroposterior +1.3, mediolateral ±0.9, dorsoventral −4.7 from bregma) over 10 min using a 10-ml Hamilton syringe and an infusion pump (World Precision Instruments). These coordinates, and this volume and rate of infusion, were determined empirically to adequately cover the nucleus accumbens without significant spread to other regions in pilot studies using a variety of volumes, flow rates and minor coordinate adjustments. The needle was left for an additional 5 min and then slowly withdrawn. Animals received bilateral injections of either AAV.siLuc.YFP (control) or AAV.sip11.YFP (knockdown) or AAV.siLuc.p11 (over-expression). Mice were sacrificed by perfusion with 4% paraformaldehyde and PBS. The brains were analyzed by immunohistochemistry by using a free floating sections method. Coronal slices were blocked with 2% donkey serum (Vector Laboratories) before being incubated overnight with primary antibody 1:100 (goat anti-S100A10, R&D Systems). Sections were incubated in secondary antibody 1:200 (Alexa-fluor 546 donkey anti-goat, Molecular Probes/Invitrogen) for 1 hour before being mounted with anti-fade media (Molecular Probes/Invitrogen).
5-HT1B Receptor Autoradiography
Cryostat sections (12μm thick) were made from p11 KO mice injected with p11 overexpressing vector (AAV.siLuc.p11) or control vector (AAV.siLuc.YFP) in the right and left hemispheres respectively as described above. 5-HT1B receptors were detected by incubating the sections in 170mM Tris/150mM NaCl pH7.4 (25°C) containing the antagonist [125I]cyanopindolol (10pM; 2200 Ci/mmol), 100nM 8-OH-DPAT as a 5-HT1A blocker, and 30μM isoproterenol as a β-adrenergic receptor blocker, for 2 hours. Non-specific binding was determined by measurements in the presence of 100μM serotonin. Sections were then rinsed 2×5 min in cold binding buffer, dipped in distilled water at 4°C and dried under cold air. The sections were apposed to Biomax MR film for 3-5 days. Optical density measurements were obtained using the NIH Image 1.63 image analysis system. Specific binding was calculated by digital subtraction of non-specific labeling from total binding.
Rat pup intracranial injection
At post natal day 2, male rat pups were anesthetized by isofluorane inhalation and kept on ice for the time of the procedure. A hole was made with a 26G needle through skin and bone at the injection point. The nucleus accumbens injection site was assessed relative to bregma using a 0.05% w/v bromophenol blue solution in PBS and a stopper was placed in the 33G injection needle to asses reproducible injection depth. A total of 5.109 genomic units(1 μl in PBS) of adeno-associated virus serotype 2 expressing p11 or DsRed were manually injected into the NAc over 4 minutes by using a microinfusion pump attached to an injection holder (World Precision Instruments). After procedure the animals were warmed and returned to the home cage.
Primary NAcc cultures
NAcc neurons were isolated from postnatal male rats (7-10 days old). Briefly, the brains were quickly removed and dissected at room temperature in Hibernate A (BrainBits). NAcc was dissected, minced and transferred to a solution containing papain (2 mg/ml; Worthington) and DNAse1 (1 U/ml), and incubated for 30 min at 37°C with gentle rocking. The tissue was then washed twice with warm Hibernate A containing 1% BSA, and dissociated by trituration in Hibernate A media containing DNAse1 (1 U/ml). The suspension was placed on top of a solution of 10% BSA in Hibernate A and centrifuged for 5 min at 200 × g. After centrifugation the cells were resuspended in 250 μl of Hibernate A and the debris was allowed to settle for 10 min. The top 200 μl of cell suspension were cleared again through 10% BSA in Hibernate. The cell pellet was then washed once with 1 ml of Hibernate A. The pellet was resuspended in 20 μl of Hibernate A, and 2 μl were plated onto poly-D-lysine-coated gridded coverslips, which were then placed in a 6-well plate with 2 ml astrocyte conditioned Neurobasal A/10% FBS medium (Invitrogen) at 37°C. The cells were allowed to attach for 3-4 h and then the medium was replaced with astrocyte conditioned Neurobasal A/2% B-27 medium (Invitrogen).
Calcium imaging
Measurements of intracellular Ca2+ fluctuations were performed by digital-imaging fluorescence microscopy 24 to 48 hours after plating. The coverslips were rinsed with HBSS buffer (Invitrogen), 1.25 mM probenecid, pH 7.4 and incubated with 1 μM Fluor4 (Molecular Probes) for 25 min at RT. The cells were then washed twice with the loading buffer and incubated for an additional 25 min before mounting in a recording chamber. The cells were continuously perfused with HBSS at a rate of 1 ml/min and were allowed to equilibrate in the chamber for another 15 min prior to recording. All assays were performed at room temperature (20-25 °C). Fluorescence images were acquired every 6 sec with an Olympus BX61 inverted microscope using a 20×/0.75 objective. At the end of the experiment the cells were fixed with 4% PFA for 2 min directly in the recording chamber and then immunolabelled for neuronal b-tubulin (Tuj1, 1:2000, Covance), P11 (AF2377, 1:200, R&D systems), or DSRed (C-20, 1:500, Santa Cruz). Image stacks were created and analyzed with imageJ. Results are presented as ΔF/F=[(Fx- B)-(Fbaseline-B)]/(Fbaseline-B) where B is background intensity, Fx is intensity at any given time and Fbaseline is the average baseline intensity (before any treatment).
Tail Suspension Test (TST)/Forced Swim Test (FST)
All mice were tested 6 weeks post-AAV injection. Behavioral tests took place between 0.5-2 hours into the dark (awake) half of their 24 hour light cycle. The TST and FST were carried out at least 7 days apart and the TST always preceded the FST. For the TST, mice were individually suspended by the tail to a horizontal bar using adhesive tape (distance from floor of testing box was 5 cm). Mice were positioned such that the base of the tail was aligned with the bottom of the bar. For the FST, mice were placed in a round tank of water, 29 cm in diameter. Water was 23-25°F, 15 cm deep. Water was replaced between animals. (Mice did not experience a “priming” swim the day prior to testing consistent with prior reports(3)) In experiments with imipramine (10 mg/kg), animals were tested 15 minutes post-injection. A 6 minute test session was videotaped and scored by using automated behavioral analysis software from Clever Systems. This software extracts a negative image from the rodent's silouhette and tracks body orientation as well as head, tail, and limb movement. The parameter recorded was the number of seconds spent immobile. The investigator doing these studies was blinded to the animal identity code until data were obtained and analysis was completed.
Sucrose Preference Test
The sucrose preference test was performed with a two-bottles procedure, during which mice had free access to both water and a sucrose solution. Group-housed animals were first habituated for 48 h both to consume fluids from a small bottle and to adjust to the 2% sucrose solution. After habituation, the sucrose preference was measured during three days. Each day, group-housed mice were individually caged and two bottles were presented to them for 24 h; one with tap water and one with a 2% sucrose solution. Consumption of water or sucrose solution was measured by weighing the bottles before and after the sessions. Bottles were counterbalanced across the left and the right sides of the cage, and their position was alternated from test to test. Animals were weighed after the last day of test. Sucrose preference was calculated in two ways, Preference=[average sucrose solution intake (ml) / animal weight (g)] and, Preference (percent) =[average sucrose solution intake (ml) / average total fluid intake (ml)] × 100.
Open Field Activity
Mice were tested for 30 min in an open field apparatus (27.9 × 27.9 cm; Med Associates, Inc.) under dim red light during the dark phase of the cycle. Distance traveled (horizontal activity) based upon light beam breaks within the open field device was recorded for each mouse.
24-Hour Eating/Activity
24 hours prior to the initiation of recording, each mouse was single-caged and allowed to acclimate to the cage, novel food hopper and large cabinet in which testing was performed. Lighting conditions were maintained according to the mouse's established circadian rhythm. Each cage contained a water spout, bedding, nesting pads, and a food hopper that prevented scattering of crumbs. Additionally, food intake was measured during the 24 hour acclimation period and was comparable to intake during the subsequent 24 hour, recorded period. Rodent activity was analyzed using automated software from Clever Systems, Inc., as described above.
Characteristics of Human Subjects
Human brain specimens were obtained from the Dallas Brain Collection(18). Briefly, after obtaining next of kin permission, brain tissue was collected from cases at the Dallas County Medical Examiners Office, Transplant Service Center and Willed Body Program at the University of Texas Southwestern Medical Center. Blood toxicology screens were conducted in each case. Subjects with known history of neurological disorders or head injury were excluded. Clinical records and collateral information from direct telephone interviews with a primary caregiver were obtained for each case. Two board certified psychiatrists carried out an extensive review of the clinical information and made independent diagnoses followed by a consensus diagnosis using DSM IV criteria. Demographic characteristics associated with the tissue are presented in tabular form in Table S1. The collection of human brain specimens was approved by the Institutional Review Board of University of Texas Southwestern Medical Center. 17 normal controls and 17 cases of major depression were included in this study. The two groups were matched as closely as possible for race, gender, age, pH, postmortem interval (PMI) and RIN (RNA integrity number) (detailed in Table S1).
Human Tissue Preparation
In each case, cerebral hemispheres were cut coronally into 1-2cm blocks. The nucleus accumbens was dissected from the appropriate coronal section and immediately placed in a mixture of dry ice and isopentane (1:1, v:v). The frozen tissue was then pulverized on dry ice and stored at -80°C. To determine pH and RIN, tissue weighing approximately 150 mg was punched from the cerebellum, homogenized in 5 ml of ddH2O (pH adjusted to 7.00) and centrifuged for 3 min at 8000g at 4 °C. pH of the supernatant was measured in duplicate (Thermo-Electron Corporation). RIN determination was performed by isolating total RNA using Trizol (Invitrogen) followed by analysis with an Agilent 2100 Bioanalyzer. The samples were sonicated in 1% SDS and boiled for 10 min. Small aliquots of the homogenate were retained for protein determination by the bicinchoninic acid protein assay method. Equal amounts of protein were processed by using 16% acrylamide gels. Immunoblotting was carried out with monoclonal antisera against p11 (1/1000) and polyclonal antisera against actin (1/1000). Antibody binding was detected by enhanced chemiluminescence and quantified by densitometry, using NIH IMAGE 1.63 software. The concentration of p11 was normalized to the concentration of actin. All data are presented as normalized levels. The investigator completing the Western blots was blinded as to sample group assignment until the data were obtained and analysis was complete.
Statistical Analyses
Two-tailed t-test was used for statistical comparison of all animal groups, quantitative histological measures and human patient samples, with statistical significance set as less than a p value of 0.05.
Supplementary Material
Acknowledgments
We thank M. Heiman and A. Schaefer for providing helpful comments on the final manuscript. Funding: The study was supported by Department of Defense grants W81XWH-09-0381 (M.G.K.), W81XWH-08-1-0111 (P.G.) and W81XWH-09-1-0401 (J.W-S), National Institutes of Health grants MH074866 (P.G.) and MH062236 (C.T.), The Skirball Foundation grant and NARSAD Distinguished Investigator award (P.G.), NARSAD Young Investigator awards (M.F., P.S.), Vetenskapsrådet (P.S.) and Swedish Royal Academy of Sciences (P.S.). Author contributions: M.G.K. and P.G. were responsible for the overall design of the study, data interpretation and primary writing of the manuscript, with assistance of B.A. and J.W-S. The animal surgeries and most behavioral studies were completed by B.A. with assistance of M.V. M.S. provided advice and technical assistance regarding behavioral studies. S.M. provided reagents and technical advice regarding viral vector preparation. J.W-S completed most of the histological studies. M.A-L. performed the sucrose preference test and developed and performed the primary cell culture assays for gene transfer. M.F. provided p11 reagents and technical advice as well as assistance with data interpretation. P.S. and T.E. completed the 5-HT1B binding and autoradiography as well as Western blot and blinded analysis of the human tissue extracts, while C.T. and T.E. obtained and processed the human tissue and maintained the blinded code. Competing Interests: M.G.K. is a founder and paid consultant for Neurologix, Inc., which has licensed intellectual property rights to p11 gene therapy for behavioral disorders, and P.G. is a founder and equity holder in Intracellular Therapies Inc., which has licensed intellectual property rights to p11. M.G.K. and B.A. are inventors on a patent application assigned to Cornell University related to p11 gene therapy for behavioral disorders and P.G. and P.S. are inventors on a patent application assigned to The Rockefeller University related to the p11 gene and behavioral disorders.
Footnotes
No other author reports competing financial interest.
References and Notes
- 1.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 2.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science (New York, NY. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 3.Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Current opinion in pharmacology. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkotter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Andren PE, Greengard P, Svenningsson P. Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2163–2168. doi: 10.1073/pnas.0711839105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 12.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 13.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 15.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Wurch T, Pauwels PJ. Coupling of canine serotonin 5-HT(1B) and 5-HT(1D) receptor subtypes to the formation of inositol phosphates by dual interactions with endogenous G(i/o) and recombinant G(alpha15) proteins. J Neurochem. 2000;75:1180–1189. doi: 10.1046/j.1471-4159.2000.0751180.x. [DOI] [PubMed] [Google Scholar]
- 17.Zgombick JM, Borden LA, Cochran TL, Kucharewicz SA, Weinshank RL, Branchek TA. Dual coupling of cloned human 5-hydroxytryptamine1D alpha and 5-hydroxytryptamine1D beta receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertussis toxin-sensitive G protein(s) Mol Pharmacol. 1993;44:575–582. [PubMed] [Google Scholar]
- 18.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett JS, Samulski RJ, McCown TJ. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, Nestler EJ. Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 25.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 27.Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.