Abstract
Achilles allografts have become popular for anterior cruciate ligament (ACL) reconstructions in older patients. Primary ACL reconstructions using Achilles tendon allografts in patients age 30 years and older are successful in restoring the knee to “normal” or “near normal.” During a three-year period, the two senior authors performed 65 primary ACL reconstructions using Achilles tendon allografts in patients aged 30 years and older. Our exclusion criteria were periarticular fracture, ipsilateral/contralateral knee ligament injury, and previous or concomitant osteotomy or cartilage restoration procedure. Each patient was evaluated via physical examination, functional and arthrometric testing, and radiographic and subjective outcome. Knees were considered normal, near normal, or abnormal based on the International Knee Documentation Committee (IKDC) system. Forty-three patients were examined at an average of 33 months (minimum, 24 months) postoperatively. At the time of ACL reconstruction, 35% had normal articular cartilage in all three compartments and 70% had meniscal tears. No re-ruptures occurred. While 24% had mean maximal translation differences less than or equal to 3 mm, none had side-to-side differences greater than 5 mm. Postoperative IKDC, Activities of Daily Living, and Activity Rating Scale scores averaged 88, 94, and 7.7, respectively. Despite the overall favorable outcomes, 29% had worsened radiographic grades at follow-up. Using an Achilles allograft for ACL reconstruction in patients older than 30 years, we restored over 90% of knees to normal or near normal while limiting postoperative complications. Poor subjective results may be related less to instability and more to pain, which may result from progressive arthritis.
Keywords: anterior cruciate ligament reconstruction, allograft
Introduction
Anterior cruciate ligament reconstruction is successful in restoring knee stability in 80–95% of patients [3, 7, 11, 20, 25, 26, 30, 35, 36, 38, 39, 42, 47, 48]. While there are several graft options, including both autografts and allografts, no single graft provides superior results in all patients [34, 52].
Because of their better mechanical properties, autograft tendons are generally preferred over allografts for the treatment of anterior cruciate ligament ruptures in young patients, who are more prone to re-rupture. Despite this advantage, the potential for donor-site morbidity is a major concern. Complications attributed to the harvest of either patellar tendon and/or hamstring autografts include anterior knee pain [49], loss of anterior knee sensitivity [32], flexion weakness [49, 51], disruptions to the normal mechanics of the patellofemoral joint [9, 28], and the potential of patellar fracture [33] and patellar tendon rupture [8]. These comorbidities may explain the slower return to sporting activities among older athletes who had autograft (versus allograft) anterior cruciate ligament reconstructions [6].
Reconstruction using allograft tissue eliminates the possibility of donor-site morbidity. However, in addition to the obvious risk of disease transmission (reported at 1 in 1,667,000 for HIV in properly screened patients [10]), slower incorporation times [27, 43], higher re-rupture rates, and increased residual laxity have been reported [5, 30, 34, 36, 47, 48, 52].
Over the past decade at our institution, Achilles tendon allografts have been used commonly for the reconstruction of the anterior cruciate ligament. While high rates of successful anterior cruciate ligament reconstruction have been reported using patellar tendon allografts, the decision to use Achilles tendon allografts was based on its ease of use and relative strength. In the senior authors’ combined experience, the Achilles tendon allograft is generally technically easier to use because (a) the bone plug is more predictable than for patellar tendon allografts, (b) there is no potential for graft-tunnel length mismatch, (c) the length of the graft allows for easier salvage if graft sutures are cut during insertion of tibial interference screw, and (d) the graft diameter can be more easily matched to the patient. An Achilles tendon allograft is usually more cylindrical than a patellar tendon allograft. Therefore, for a given diameter, the Achilles allograft has more cross-sectional area [47], which correlates with greater strength. Limited clinical data comparing Achilles and patellar tendon allografts confirm this relationship: patellar tendon allografts have 4.4% and 10.4% rates of laxity and re-rupture failure, respectively, compared to the respective values of 2.5% and 4.8% for Achilles allografts [48].
We are unaware of any series detailing the use of Achilles tendon allografts for anterior cruciate ligaments reconstruction in a cohort of older athletes. The purpose of this study is to evaluate the midterm subjective, objective, functional, and radiographic results of using Achilles tendon allografts to primarily reconstruct the anterior cruciate ligament in patients age 30 years and older.
Materials and Methods
This study was approved by our hospital’s Institutional Review Board. We present a consecutive cohort of patients who underwent anterior cruciate ligament reconstruction using Achilles tendon allograft. All subjects were operated upon by one of the two senior authors (DW Altchek, RF Warren) during a 3-year period. We recommended anterior cruciate ligament reconstruction using allograft for (a) those aged 35 years or older and (b) those older than 30 years old who wished to minimize postoperative pain and hasten immediate postoperative recovery due to work considerations.
Patient Population
All patients aged 30 years and older and who underwent a primary anterior cruciate ligament reconstruction using an Achilles tendon allograft were considered for the study. We excluded all those with a tibial plateau or distal femoral fracture, recent or previous injury to any of the other ipsilateral or contralateral knee ligaments, and/or previous or concominant osteotomy or procedure (e.g., microfracture, mosaicplasty, autologous chondrocyte implantation) to restore an articular cartilage defect of the knee. An ipsilateral meniscal procedure, such as a partial meniscectomy or meniscal repair, was not an exclusionary criterion.
Seventy-seven patients met our inclusion criteria. After the review of medical records, nine patients were excluded due to multiligamentous knee injury (three), concurrent microfracture procedures (three), tibial plateau fracture (one), contralateral below knee amputation from prior injury (one), and contralateral anterior cruciate ligament (ACL) rupture (one). Three other patients were removed from consideration due to pregnancy (two) and death (one) during the follow-up interval. Therefore, a total of 65 patients fulfilled our study criteria.
Surgical Technique
At our institution, patients were anesthetized with an epidural–spinal blockade. Prior to any incisions, all patients received prophylactic intravenous antibiotics and underwent an examination under anesthesia. All reconstructions were arthroscopically assisted and all, but one, were performed using a single-incision technique.
All grafts were inserted retrograde with the calcaneal bone block lodged within the femoral tunnel. All bone blocks were fashioned to a diameter of 10 or 11 mm and length between 20 and 30 mm. The femoral and tibial tunnels were drilled to diameters of either 10 or 11 mm and 10 to 12 mm, respectively, using commercially available aiming devices. Femoral tunnels were drilled using a transtibial technique. A metallic cannulated interference screw, usually 7 mm wide by 20 mm long, was used for femoral tunnel fixation; in most patients, a combination of interference (Bio-RCI Screw, Smith & Nephew, Andover, MA, USA) and cortical fixation (DW Altchek: Richards Ligament button; RF Warren: Richards Staple [Smith and Nephew Richards, Memphis, TN, USA]) was used to secure the soft tissue portion of the graft within the tibial tunnel. Ligament buttons and staples were used to augment fixation in 28 and 13 patients, respectively. Two patients had interference fixation only.
Allografts
The four tissue banks that supplied the Achilles tendon allografts used in this study were all were licensed in New York State and approved by the American Academy of Tissue Banks. All allografts were (a) aseptically procured, (b) fresh-frozen, (c) washed with a company-specific proprietary wash, and (d) all, but five, were irradiated with a dose between 1.5 and 2.5 Mrad (Table 1). Community Tissue Service (Dayton, OH, USA) routinely sterilized grafts with gamma irradiation from a Cobalt-60 source, while the Musculoskeletal Tissue Foundation (Eatontown, NJ, USA) and American Red Cross (Washington, DC, USA) only terminally irradiated grafts only if the graft was culture-positive following processing or was flagged by screening questionnaire.
Table 1.
Allograft sources
| Company | Total | Number irradiated |
|---|---|---|
| American Red Cross (Washington, DC) | 17 | 14 |
| Community Tissue Service/Blood Center (Dayton, OH) | 16 | 16 |
| Musculoskeletal Transplant Foundation (Eatontown, NJ) | 9 | 7 |
| Ohio Valley Tissue and Skin | 1 | 1 |
Rehabilitation
All patients were enrolled in a supervised physical therapy program. Weight-bearing was protected for the initial 2 weeks and then gradually increased. Range of motion and quadriceps exercises, such as straight-leg raises, were initiated immediately after surgery. A hinged knee brace was removed when acceptable quadriceps control was achieved. Closed chain activities were used for the first 8 weeks. Light exercise and jogging were allowed by 16 weeks with a gradual return to full activities by 20 weeks.
Outcome Evaluation
All outcome evaluations were performed by one of the authors other than the attending surgeons and at a minimum of 2 years following the reconstructive procedure. Each patient was evaluated within five domains: (a) physical examination, (b) functional testing, (c) arthrometric testing, (d) radiographic outcome, and (e) subjective outcome. All five outcome domains have been commonly used to assess outcome following anterior cruciate ligament reconstruction.
-
Physical Examination
To eliminate surgeon bias, all follow-up physical examinations were performed by an author other than the primary surgeon. Each evaluation consisted of assessments of (a) range of motion measured with a goniometer, (b) thigh circumference measured 10 cm proximal to the superior pole of the patella, (c) ligamentous stability (Lachman and pivot shift tests), (d) presence of an effusion, (e) patellar mobility as compared to the contralateral side, and (f) peripatellar tenderness. While the pivot shift was determined to be 0 (no glide), 1+ (glide), 2+ (jump), and 3+ (locked subluxation), the Lachman test was graded as 1 (0–5 mm), 2 (6–10 mm), or 3 (greater than 10 mm) and A (firm end point) or B (no end point).
-
Functional Testing
Under the supervision of a physical therapist (M. Levinson), all patients performed bilateral knee functional testing using a single one-leg hop test for distance. Each subject was required to perform three single one-leg hops for each leg. Each hop was measured for distance with the three trials averaged for each leg.
-
Arthrometric Testing
A physical therapist (M. Levinson) experienced with the use of a KT-1000 arthrometer (MEDmetric Corp) performed all testing. Arthrometric testing of each knee was conducted using a KT-1000 arthrometer and previously published protocols at 15, 20, and 30 ft-lb [14, 15]. Side-to-side comparisons were made based on mean maximal translation. The compliance index defined as the ratio of translations with applied forces of 15 and 20 ft-lb was calculated for each knee. A ratio of less than 1.0 was considered normal compliance.
-
Radiographic Outcome
Using standing posteroanterior, lateral, and merchant views, radiographs were obtained of the affected knee at the follow-up visit. Each series of radiographs was assessed for evidence of (a) arthritic progression and (b) tunnel widening. All radiographs were interpreted by a sports medicine fellow and a senior staff radiologist.
A modified International Knee Documentation Committee (IKDC) scale was used to grade all available preoperative and follow-up radiographs. “Mild” arthritis indicated minimal changes, such as small osteophytes, slight sclerosis, or mild flattening of the femoral condyles. “Moderate” arthritis has those “mild” changes combined with radiographically detectable joint-space narrowing. “Severe” changes included a joint space narrowed to 2 mm or a level 50% less than its normal value.
Tunnel widening was assessed using the system previously described by Fahey and Indelicato [17]. The reamer size used to create the tunnel tibial tunnel was recorded as the tunnel size at the time of the operation, while the diameter of the sclerotic rim outlining the tibial tunnel was measured as the follow-up tunnel width. A radiographic magnification ratio was determined by comparing the measured screw diameter on the posteroanterior and lateral views with the actual screw size. All values were adjusted using this magnification ratio. “Tunnel widening” was considered to be postoperative tunnel diameter greater than 15% on the lateral alone or averaged for the posteroanterior and lateral radiographs.
-
Subjective Outcome
At the most recent follow-up, a validated set of questionnaires, including the 2000 IKDC Subjective Knee Evaluation Form [2], the Knee Outcome Survey Activities of Daily Living (ADL) Scale, and the Activity Rating Scale [31], were completed by each subject.
Overall Outcome
The overall outcome was based on the scheme presented in the 2000 IKDC Knee Examination Form. The reconstructed knee was considered “normal” knee if all of the following criteria were satisfied: (a) lack of an effusion, (b) passive motion deficits less than 3° of extension and less than or equal to 5° of flexion, (c) difference in mean maximal translation less than 3 mm on KT-1000 testing, and (d) single-leg hop ratio of reconstructed to normal knee greater than 0.9. A knee was considered “abnormal” if any of the following criteria were met: (a) presence of a moderate or severe effusion, (b) passive motion deficits greater than 5° of extension and 15° of flexion, (c) difference in mean maximal translation greater than 5 mm on KT-1000 testing, or (d) single-leg hop ratio of reconstructed to normal knee less than 0.76.
Results
Patient Population
Of the 65 eligible, 43 patients (66%), consisting 21 males and 22 females, agreed to participate in the follow-up study. The mean follow-up interval was 33 months (range, 24–47).
The average patient age at the time of anterior cruciate ligament reconstruction was 47 years (range, 30–68). The mean time from most recent injury to surgery was 30 weeks (range, 2–206) with 22 reconstructions occurring within the initial 12 weeks following the injury. The mechanism of injury was identified in all but three cases. Recreational athletics accounted for at least 81% (35/43) of these injuries; trip and fall accounted for five injuries. Of the recreational athletic injuries, skiing was the most common (20) followed by tennis (4), flag football (3), soccer (2), softball (2), basketball (1), volleyball (1), karate (1), and biking (1). Subsequent procedures were performed on two patients: one patient underwent irrigation and debridement with graft retention to treat a superficial wound infection, while another patient had meniscal debridement following meniscal repair. Both patients were retained in the study.
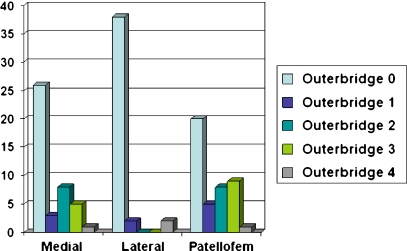
During arthroscopy at the time of the reconstruction, 35% (15/43) were noted to have normal articular cartilage in all three knee compartments. Of the remaining 28 patients with chondromalacia, 13 had severe chondromalacia (Outerbridge classification grade 3 or 4) in at least one compartment. The degree of chondromalacia in the medial, lateral, and patellofemoral compartment was recorded for each subject (Fig. 1).
Fig. 1.
Nearly two thirds (65%, 28/43) of patients had degenerative changes involving at least one compartment at the time of the ACL reconstruction. The medial and patellofemoral compartments were more commonly involved than the lateral compartment
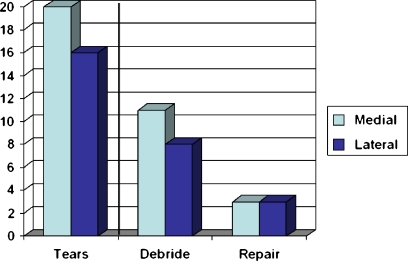
A meniscal tear was identified during the reconstruction in 70% (30/43). Fourteen patients had an isolated medial meniscal tear, ten had isolated lateral meniscal tears, and six had tears involving both menisci. The treatment of these tears was tabulated (Fig. 2).
Fig. 2.
Seventy percent (30/43) of patients had a meniscal tear identified at the time of ACL reconstruction. The vast majority (80%) were debrided
Outcome Evaluation
A summary of outcomes is presented in Table 2.
-
Physical Examination
Only two patients (5%) had passive motion deficits defined as motion loss greater than 2° of extension and 4° of flexion compared to the contralateral limb. Both subjects had isolated deficits in terminal extension, measuring 3° and 10°, respectively.
The affected extremity had a thigh circumference average of 0.23 cm smaller (range, 2.0 cm to −2.0 cm) than the contralateral limb. Four patients (9%) had a thigh circumference deficit greater than 1 cm on the operative extremity.
At the follow-up examination, 81% (35/43) of patients had a Lachman exam grade restored to 1A; the other 19% (8/43) had grade 2A stability. Only one patient, who reported a follow-up Activity Rating Scale score of 12, had a pivot shift graded as 2 or greater, though 11 others had a grade 1 pivot shift that was not found in the contralateral knee.
While none had an effusion or peripatellar tenderness, two patients (5%) had a decrease in patellar mobility in the reconstructed knee. Two patients had peripatellar tenderness in the unaffected knee.
-
Functional Exam
The hop ratio averaged 0.97 (range, 0.57–1.10). Eight (19%) had a hop ratio less than 0.90 than the contralateral limb; four of those patients had a hop ratio less than 0.76. Over half (23/43) actually hopped farther using the affected knee.
-
Arthrometric Testing
One patient did not undergo KT-1000 testing. Thirty-three (76%) patients had mean maximal translations restored to less than 3 mm of the contralateral knee. While ten patients (24%) had mean maximal translation differences 3 mm or more, none had a side-to-side difference greater than 5 mm with maximal translation. The age at surgery for those with a mean maximal translation greater than 3 mm averaged 52.0 years. The compliance index was normal in 93%.
-
Radiographic Outcome
Preoperative radiographs were available for review in only 72% (31/43 patients). Among these 31 preoperative radiographs, 7 subjects showed no evidence of arthritis, 22 “mild” arthritis, and two “moderate” arthritis within at least one compartment. At follow-up, 29% (9/31) had a worsened radiographic grade postoperatively. Among these nine patients, three advanced from no to “mild” arthritis, five from “mild” to “moderate,” and one from no to “moderate”. Seven of those who had progression of arthritis underwent meniscal surgery at the time of ACL reconstruction; however, only three had meniscal surgery within the same compartment that deteriorated radiographically. Three of the patients who had radiographic progression from “mild” to “moderate” had a meniscal surgery prior to ACL reconstruction. Additionally, among the six patients with “moderate” arthritis at follow-up, three (50%) had Outerbridge grade 3 or 4 articular cartilage injuries within the same compartment documented at the time of anterior cruciate ligament reconstruction. Conversely, 4 (31%) of the 13 patients who had Outerbridge grade 3 or 4 articular cartilage injuries noted intraoperatively had “moderate” arthritis radiographically.
At the most recent follow-up, “tunnel widening” existed in 45% (19/42). The mean percent widening was 29% (range, 15–84%).
-
Subjective Outcome
The mean postoperative IKDC, ADL, and Activity Rating Scale scores were 88 (range, 58–100), 94 (range, 71–100), and 7.7 (range, 0–16), respectively. Eighty-six percent (37/43) had a postoperative IKDC score 80 points or higher, while 77% (33/43) had an ADL score 90 points or higher. All six patients with an IKDC score less than 80 points had an ADL score less than 90 points. Fifty-one percent (22/43) had an Activity Rating Scale Score greater than eight points.
Table 2.
Summary of results
| “Successful” criterion | Percentage |
|---|---|
| Restoration of normal motion | 95 |
| Restoration of thigh circumference | 91 |
| Grade 1A Lachman exam | 81 |
| Grade 0 or 1 pivot shift | 98 |
| Hop ratio ≥ 0.90 | 81 |
| KT-1000 mean maximal translation <3 mm | 76 |
| No radiographic progression of arthritis | 71 |
| IKDC score ≥ 80 | 86 |
| ADL score ≥ 90 | 77 |
| Activity Rating Scale score > 8 | 51 |
Overall Outcome
During the follow-up period, no re-ruptures occurred within this cohort group. Twenty-five patients (41.7%) with a mean age at time of surgery of 47.2 years met all the criteria required for a “normal” knee. Only four patients (7.0%), who had an average age of 57.2 years at surgery, had a knee rated as “abnormal.” All of the patients with an “abnormal” knee were greater than age 50 years at the time of ACL reconstruction.
Discussion
The goal of anterior cruciate ligament reconstruction is to prevent secondary instability episodes, which may result in articular and/or meniscal damage in an active population [13, 16, 29, 37]. A number of graft types, including various autograft and allograft tissues, have been successfully used to restore stability in 80% to 95% of patients [3, 7, 11, 20, 25, 26, 36, 39]. Because of lower rates of re-rupture and residual instability, autografts, particularly patellar tendons, are favored in young athletes, who are generally more active and therefore at higher risk of re-rupture.
Patients over age 30 years may have a different set of concerns that influence the treatment of ACL. Older patients are prone to engage in activities that require less secure stability than their younger counterparts who often return to contact sports. Therefore, older individuals may be more willing to sacrifice a degree of stability for a lower risk of donor-site morbidity and a faster recovery. When faced with the prospect of an autograft and the associated donor-site morbidity, older patients with financial and familial obligations may elect for nonoperative treatment and activity modification.
For those that wish to return to athletic activity, allograft tissue can eliminate the recurrent instability often seen in nonoperative treatment and the donor-site morbidity associated with autograft reconstruction. As the availability of allograft tissue and the accuracy of donor-screening tests have improved, the popularity of allografts for anterior cruciate ligament reconstruction has increased. During the 3-year period of this study, the use of Achilles tendon allograft for primary and revision anterior cruciate ligament reconstruction grew from 12% to 41% at the senior authors’ institution. While the proportion of revision surgeries remained 5% throughout the time period, the total number of reconstructions per year increased from 517 to 581, and the average patient age rose from 31 to 33 years. These latter two statistics suggest that the increased use of allografts has extended the indications for reconstructive surgery in the older, athletic population.
The rise in the use of allografts at our institution was due to the overall impression of the senior authors that most older athletes have a “good” outcome following allograft reconstruction. Our finding that over 90% of knees returned to a “normal” or “near normal” level following anterior cruciate ligament reconstruction with Achilles tendon allograft supports our assertion. The outcomes from this study compare favorably with the senior author’s series on anterior cruciate ligament reconstruction using patellar tendon autograft (Table 3) [11].
Table 3.
Comparison of anterior cruciate ligament reconstruction studies from our institution
| This study | Buss et al. [11] | |
|---|---|---|
| Graft | Achilles tendon allograft | Patellar tendon autograft (+/− iliotibial band fascial sling) |
| Subjects | 43 | 69 |
| Average age (range) | 47 years (30 to 68) | 24 years (16 to 40) |
| Follow-up (range) | 33 months (24 to 47) | 32 months (24 to 42) |
| Passive extension, normal | 95% | 93% |
| Lachman, grade 1A | 81% | 88% |
| Pivot shift, grade 1 or 2 | 97% | 93% |
| Maximal translation difference < 3 mm | 83% | 84% |
While the objective data from our study can be compared to other studies, it is difficult to directly compare the subjective results from this series to others. First, 16 assessment instruments exist that allow patients to rate their overall knee function [21]. Though a recent study has suggested that the Mohtadi questionnaire is the most appropriate to gauge outcome following anterior cruciate ligament reconstruction [50], this instrument has not been widely used in previously published papers. Second, no clearly established, validated thresholds exist to define a “good” or “poor” result and/or to accurately determine the return to pre-injury sporting level [21]. Despite a stable knee, many patients may self-restrict from pursuing certain athletic activities due to fear of recurrent injury. The contribution of these factors would presumably be much larger in a series with a larger number of older patients.
The mismatch between objective failure and subjective scores in our series attests to the imperfect metrics applied for evaluation of anterior cruciate ligament reconstruction. Despite 65% and 70% rates of chondral and meniscal damage, respectively, more than 75% of patients in this series had an IKDC score greater than 80 points and an ADL score greater than 90 points. However, among the six patients with scores below both of these levels, all had KT-1000 mean maximal translation differences of less than 3 mm. Furthermore, persistent pain, not instability, was the most consistent negative finding among this group. In terms of return of function, over half of patients had an Activity Rating Scale score greater than 8, the score which may be the minimum required to indicate return to sports [23].
The relationship between restoration of stability and radiographic progression of osteoarthritis also appears multifactoral. In this series, nearly one third (9/31) demonstrated progression in the degree of osteoarthritis. Among those, six (19%) advanced to “moderate” arthrosis by an average of 33 months postoperatively. The subset of patients with radiographic deterioration was similar to the cohort overall in terms of age, follow-up, injury mechanism, rate of meniscal injury, and degree of chondromalacia encountered at the time of arthroscopy. Furthermore, the compartment deemed most at-risk by intraoperative assessment of articular or meniscal damage was not necessarily the compartment in which progression of arthritis occurred radiographically. It is unclear whether the injury, surgery, patient age, or a combination or some other factor contributed to the high rate of arthritis seen in our series.
At our institution, early degenerative changes are not considered a contraindication to anterior cruciate ligament reconstruction. The cartilage may continue to degenerate despite a functionally stable knee [1, 12, 18, 44]. While no clear relationship between reconstructed ligament and the progression of arthritis exists, this study confirms previous reports that an Achilles allograft anterior cruciate reconstruction in an older patient can restore functional stability and allow return to athletic activity in this population [4, 18, 45, 46].
Though the overall results among our cohort were generally favorable, another recent study of patients undergoing ACL reconstructions using Achilles tendon allografts reported a 21% (5/24) failure rate [24]. This study grouped together (a) primary reconstructions among those patients at least 30 years of age and, unlike our study, (b) revision surgeries performed in any aged patient. Among their five failures, three occurred in revision reconstructions; the other two reconstructions, both primary reconstructions, had satisfactory subjective outcome scores and were deemed failures based on KT-1000 values alone [24]. Furthermore, our study used a combination of interference and cortical fixation, while the tibial fixation in the comparison study was interference fixation alone [24]. Recent works suggest that a combination of interference and cortical fixation may have increased strength compared to aperture fixation alone for fixation of soft tissue within the tibial tunnel [40, 41].
There are several weaknesses to this study. First, the major limitation is its retrospective design, which limited our ability to obtain information on preoperative and/or immediate postoperative subjection function and the radiographic and/or the magnetic resonance imaging appearance of the knee. The conclusions that can be drawn are limited by the fact that the study is retrospective and uncontrolled with loss of one third of the patients to follow-up. Second, the inclusion of patients with a previous meniscectomy may have negatively impacted radiographic progression and subjective scores; however, the rate of radiographic progression (30.0% among those with a previous meniscectomy versus 28.6% of those without a previous meniscectomy) and subjective scores were not significantly different. Third, while the number of tissue banks used and the associated heterogeneity of graft sterilization techniques may be viewed as weaknesses, it does allow for a more general application of the data. None of the grafts were irradiated above 2.5 Mrad, which was within the current guidelines for allograft tissue and below the 3 Mrad threshold at which all tissue properties are negatively affected [19, 22].
Despite these shortcomings, this study confirms that the Achilles tendon allograft is a viable option for anterior cruciate ligament reconstruction in patients age 30 years and older. In this series, we were able to restore greater than 90% of knees to a “normal” or “near normal” state while limiting postoperative complications, such as graft re-rupture, extension loss, and peripatellar pain. Poor subjective results appeared to be related less to instability and more to pain, which may be the result of a higher rate of progressive arthritis seen in this patient population.
Footnotes
This study was supported by outside funding or grant(s) from the Institute for Sports Medicine Research, New York, NY.
None of the researchers or an affiliated institute has received (or agreed to receive) from a commercial entity something of value (exceeding the equivalent of US $500) related in any way to this manuscript or research.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participating in the study was obtained.
Research was performed at the Hospital for Special Surgery, New York, NY.
References
- 1.Aït Si, Selmi T, Fithian D, Neyret P. The evolution of osteoarthritis in 103 patients with ACL reconstruction at 17 years follow-up. Knee. 2006;13(5):353–358. doi: 10.1016/j.knee.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ. The International Knee Documentation Committee Subjective Knee Evaluation Form. Normative data. Am J Sports Med. 2006;34:128–135. doi: 10.1177/0363546505280214. [DOI] [PubMed] [Google Scholar]
- 3.Bach BR, Jr, Aadalen KJ, Dennis MG, Carreira DS, Bojchuk J, Hayden JK, Bush-Joseph CA. Primary anterior cruciate ligament reconstruction using fresh-frozen, nonirradiated patellar tendon allograft: minimum 2-year follow-up. Am J Sports Med. 2005;33(2):284–292. doi: 10.1177/0363546504267347. [DOI] [PubMed] [Google Scholar]
- 4.Barber FA, Elrod BF, McGuire DA, Paulos LE. Is an anterior cruciate ligament reconstruction outcome age dependent? Arthroscopy. 1996;12(6):720–725. doi: 10.1016/S0749-8063(96)90177-2. [DOI] [PubMed] [Google Scholar]
- 5.Barbour SA, King W. The safe and effective use of allograft tissue—an update. Am J Sports Med. 2003;31(5):791–797. doi: 10.1177/03635465030310052801. [DOI] [PubMed] [Google Scholar]
- 6.Barrett G, Stokes D, White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505–1512. doi: 10.1177/0363546504274202. [DOI] [PubMed] [Google Scholar]
- 7.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renström P. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am. 2002;84-A(9):1503–1513. doi: 10.2106/00004623-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bonamo JJ, Krinick RM, Sporn AA. Rupture of the patellar ligament after use of its central third for anterior cruciate reconstruction. J Bone Joint Surg Am. 1984;66:1294–1297. [PubMed] [Google Scholar]
- 9.Breitfuss H, Frohlich R, Povacz P, Resch H, Wicker A. The tendon defect after anterior cruciate ligament reconstruction using the midthird patellar tendon—a problem for the patellofemoral joint? Knee Surg Sports Traumatol Arthrosc. 1996;4:194–198. doi: 10.1007/BF01466615. [DOI] [PubMed] [Google Scholar]
- 10.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res 1989;(240):129–36. [PubMed]
- 11.Buss DD, Warren RF, Wickiewicz TL, Galinat BJ, Panariello R. Arthroscopically assisted reconstruction of the anterior cruciate ligament with use of autogenous patellar-ligament grafts. Results after twenty-four to forty-two months. J Bone Joint Surg Am. 1993;75(9):1346–1355. doi: 10.2106/00004623-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Cohen M, Amaro JT, Ejnisman B, Carvalho RT, Nakano KK, Peccin MS, Teixeira R, Laurino CF, Abdalla RJ. Anterior cruciate ligament reconstruction after 10 to 15 years: association between meniscectomy and osteoarthrosis. Arthroscopy. 2007;23(6):629–634. doi: 10.1016/j.arthro.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 13.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 14.Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720–726. [PubMed] [Google Scholar]
- 15.Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401–407. doi: 10.1177/036354658501300607. [DOI] [PubMed] [Google Scholar]
- 16.Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG. The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med. 2004;32(8):1906–1914. doi: 10.1177/0363546504265006. [DOI] [PubMed] [Google Scholar]
- 17.Fahey M, Indelicato PA. Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med. 1994;22(3):410–414. doi: 10.1177/036354659402200318. [DOI] [PubMed] [Google Scholar]
- 18.Ferretti A, Conteduca F, Carli A, Fontana M, Mariani PP. Osteoarthritis of the knee after ACL reconstruction. Int Orthop. 1991;15(4):367–371. doi: 10.1007/BF00186881. [DOI] [PubMed] [Google Scholar]
- 19.Fideler BM, Vangsness CT, Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23(5):643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- 20.Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR., Jr Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2–11. doi: 10.1177/03635465030310011501. [DOI] [PubMed] [Google Scholar]
- 21.Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAMASK Trial Team Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414–1423. doi: 10.1093/rheumatology/keh362. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res. 1991;9(2):209–218. doi: 10.1002/jor.1100090209. [DOI] [PubMed] [Google Scholar]
- 23.Gobbi A, Francisco R. Factors affecting return to sports after anterior cruciate ligament reconstruction with patellar tendon and hamstring graft: a prospective clinical investigation. Knee Surg Sports Traumatol Arthrosc. 2006;14(10):1021–1028. doi: 10.1007/s00167-006-0050-9. [DOI] [PubMed] [Google Scholar]
- 24.Grafe MW, Kurzweil PR. Anterior cruciate ligament reconstruction with Achilles tendon allografts in revisions and in patients older than 30. Am J Orthop. 2008;37(6):302–308. [PubMed] [Google Scholar]
- 25.Harner CD, Olson E, Irrgang JJ, Silverstein S, Fu FH, Silbey M. Allograft versus autograft anterior cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop Relat Res 1996 Mar;(324):134–44. [DOI] [PubMed]
- 26.Indelli PF, Dillingham MF, Fanton GS, Schurman DJ. Anterior cruciate ligament reconstruction using cryopreserved allografts. Clin Orthop Relat Res 2004 Mar;(420):268–75. [DOI] [PubMed]
- 27.Jackson DW, Grood ES, Goldstein JD, Rosen MA, Kurzweil PR, Cummings JF, Simon TM. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 28.Järvelä T, Paakkala T, Kannus P, Järvinen M. The incidence of patellofemoral osteoarthritis and associated findings 7 years after anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29(1):18–24. doi: 10.1177/03635465010290010701. [DOI] [PubMed] [Google Scholar]
- 29.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior–posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883–888. [PubMed] [Google Scholar]
- 30.Linn RM, Fischer DA, Smith JP, Burstein DB, Quick DC. Achilles tendon allograft reconstruction of the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21(6):825–831. doi: 10.1177/036354659302100611. [DOI] [PubMed] [Google Scholar]
- 31.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 32.Mastrokalos DS, Springer J, Siebold R, Paessler HH. Donor site morbidity and return to the preinjury activity level after anterior cruciate ligament reconstruction using ipsilateral and contralateral patellar tendon autograft: a retrospective, nonrandomized study. Am J Sports Med. 2005;33(1):85–93. doi: 10.1177/0363546504265926. [DOI] [PubMed] [Google Scholar]
- 33.McCarroll JR. Fracture of the patella during a golf swing following reconstruction of the anterior cruciate ligament. Am J Sports Med. 1983;11:26–27. doi: 10.1177/036354658301100107. [DOI] [PubMed] [Google Scholar]
- 34.Miller SL, Gladstone JN. Graft selection in anterior cruciate ligament reconstruction. Orthop Clin North Am. 2002;33(4):675–683. doi: 10.1016/S0030-5898(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 35.Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon-patellar bone autograft. Am J Sports Med. 2006;34(4):553–564. doi: 10.1177/0363546505281812. [DOI] [PubMed] [Google Scholar]
- 36.Noyes FR, Barber-Westin SD. Reconstruction of the anterior cruciate ligament with human allograft. Comparison of early and later results. J Bone Joint Surg Am. 1996;78(4):524–537. doi: 10.2106/00004623-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154–162. doi: 10.2106/00004623-198365020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Plancher KD, Steadman JR, Briggs KK, Hutton KS. Reconstruction of the anterior cruciate ligament in patients who are at least forty years old. A long-term follow-up and outcome study. J Bone Joint Surg Am. 1998;80(2):184–197. doi: 10.2106/00004623-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Poehling GG, Curl WW, Lee CA, Ginn TA, Rushing JT, Naughton MJ, Holden MB, Martin DF, Smith BP. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774–785. doi: 10.1016/j.arthro.2005.04.112. [DOI] [PubMed] [Google Scholar]
- 40.Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376–384. doi: 10.5435/00124635-200807000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320–1325.e6. doi: 10.1016/j.arthro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Roe J, Pinczewski LA, Russell VJ, Salmon LJ, Kawamata T, Chew M. A 7-year follow-up of patellar tendon and hamstring tendon grafts for arthroscopic anterior cruciate ligament reconstruction: differences and similarities. Am J Sports Med. 2005;33(9):1337–1345. doi: 10.1177/0363546504274145. [DOI] [PubMed] [Google Scholar]
- 43.Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448–458. doi: 10.1016/j.arthro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Seon JK, Song EK, Park SJ. Osteoarthritis after anterior cruciate ligament reconstruction using a patellar tendon autograft. Int Orthop. 2006;30(2):94–98. doi: 10.1007/s00264-005-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelbourne KD, Benner RW. Isolated anterior cruciate ligament reconstruction in the chronic ACL-deficient knee with degenerative medial arthrosis. J Knee Surg. 2007;20(3):216–222. doi: 10.1055/s-0030-1248046. [DOI] [PubMed] [Google Scholar]
- 46.Shelbourne KD, Stube KC. Anterior cruciate ligament (ACL)-deficient knee with degenerative arthrosis: treatment with an isolated autogenous patellar tendon ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 1997;5(3):150–156. doi: 10.1007/s001670050043. [DOI] [PubMed] [Google Scholar]
- 47.Shino K, Nakata K, Horibe S, Inoue M, Nakagawa S. Quantitative evaluation after arthroscopic anterior cruciate ligament reconstruction. Allograft versus autograft. Am J Sports Med. 1993;21(4):609–616. doi: 10.1177/036354659302100421. [DOI] [PubMed] [Google Scholar]
- 48.Siebold R, Buelow JU, Bos L, Ellermann A. Primary ACL reconstruction with fresh-frozen patellar versus Achilles tendon allografts. Arch Orthop Trauma Surg. 2003;123(4):180–185. doi: 10.1007/s00402-003-0476-1. [DOI] [PubMed] [Google Scholar]
- 49.Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE., Jr Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986–1995. doi: 10.1177/0363546504271211. [DOI] [PubMed] [Google Scholar]
- 50.Tanner SM, Dainty KN, Marx RG, Kirkley A. Knee-specific quality-of-life instruments: which ones measure symptoms and disabilities most important to patients? Am J Sports Med. 2007;35(9):1450–1458. doi: 10.1177/0363546507301883. [DOI] [PubMed] [Google Scholar]
- 51.Tashiro T, Kurosawa H, Kawakami A, Hikita A, Fukui N. Influence of medial hamstring tendon harvest on knee flexor strength after anterior cruciate ligament reconstruction. A detailed evaluation with comparison of single- and double-tendon harvest. Am J Sports Med. 2003;31(4):522–529. doi: 10.1177/31.4.522. [DOI] [PubMed] [Google Scholar]
- 52.West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]