Abstract
Nuclear factor of activated T-cells (NFAT) proteins are, calcium-regulated transcription factors, key regulator of stimulation-dependent gene activation. In our microarray analysis for the genes expressed in human black and white hairs, NFAT2 was significantly upregulated in the white hair, compared to the black hair. The aim of this study was to investigate functional role of NFAT2 in melanogenesis. Western blot analysis was performed to investigate the expression of NFAT2 protein in B16 melanoma cells. Our data showed that NFAT2 expression was increased in the hypopigmented B16 cells, while tyrosinase and MITF expression was decreased. To investigate the potential role of NFAT2, the recombinant adenovirus expressing microRNA specific for NFAT2 was transduced into the cultured B16 melanoma cells. Consistently, inhibition of NFAT2 enhanced tyrosinase activity and melanin content. Moreover, cyclosporine A, which is known as a calcineurin inhibitor blocking NFAT activation, enhanced tyrosinase activity and melanin content. These data suggest that NFAT2 may play an important role in regulation of melanogenesis in melanocyte.
Keywords: Melanogenesis, Melanocyte, B16 melanoma cells, NFAT2, Cyclosprorin A
Introduction
In mammals, color of skin and hair is caused by melanin synthesis and distribution. Although melanin plays a crucial protective role in human skin by absorbing free radicals and shielding from UV light, abnormal changes of melanin synthesis such as hypermelanotic or hypomelanotic resulted in a vast number of skin disease and disorders. Current therapies for skin pigmentation diseases are unsatisfactory. Thus the search for key molecules to modulate the mechanism of pigmentation is of great interest. We previously performed cDNA microarray to screen differentially expressed genes in the human black and white hair, and found that Nuclear Factor of Activated T cells (NFAT) 2 significantly upregulated in the white hair, compared to the black hair.
NFAT proteins are a group of calcium-regulated transcription factors that play a pivotal role in development and function of immune system as well as during the timing and activation of many vertebrate tissues (Kuo & Leiden, 1999; Oh-hora 2009; Na et al., 2010). These gene family consists of five members designated NFATc1 (NFAT2) through NFATc4 and NFAT5. These proteins contain an N-terminal regulatory domain as well as a DNA-binding motif with homology to the Rel domain of NF-kB (Wolfe et al., 1997; Gauthier & Degnan, 2008). In unstimulated cells, NFAT proteins are localized to the cytoplasm by hyperphosphorylation of the N-terminal regulatory domain. Signaling pathways that promote a sustained influx of calcium activate the phosphatase calcineurin, which dephosphorylates the regulatory domain and exposes a nuclear localization sequence (Pores-Fernando & Zweifach, 2009; Rinne et al., 2009). Thus, calcineurin inhibitors, like cyclosporine A (CsA) and FK506, have been used for the last 2 decades to probe this pathway. In the nucleus, NFAT proteins cooperate with other transcription factors, like activator protein-1 (AP-1) family members, to regulate gene transcription (Macián et al., 2001; Wu et al., 2010). NFAT proteins affect many of cellular activities including cell growth and proliferation, cell differentiation, apoptosis, angiogenesis, and tissue invasion and migration.
The expression and putative role of NFATs in skin was first described by Fung-Leung et al. in 1995 (Fung-Leung et al., 1995), and subsequently regulation of keratinocyte differentiation by calcineurin and NFATs was recently reported (Santini et al., 2001; Mammucari et al., 2005). Al-Daraji et al. have very rescently described expression of calcineurin and NFAT1 by a variety of cell types in normal and psoriatic skin (Al-Daraji et al., 2009). In addition, NFAT1 can contribute to regulation of hair growth and associated with regulation of hair cycle (Gafter-Gvili et al., 2003). NFAT2 and 4 are expressed and can be an important upstream regulator of COX-2 in metastatic melanoma (Flockhart et al., 2009). It has been known that NFAT3 is responsible for the up-regulation of the pro-inflammatory cytokine tumor necrosis factor (TNF) in the immune system (Ouyang et al., 2007).
Although many functional roles of NFATs proteins are implicated in various biological systems, the expression and putative role of NFAT2 in pigmentation has not been elucidated yet. In this study, we investigate the functional role of NFAT2 in melanoma cells using adenovirus mediated gene knockdown system.
Materials and Methods
Cell culture and CsA treatment
B16 melanoma cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, Rockville, MD) at 37℃ under a humidified atmosphere containing 5% CO2. After incubation for 24 h, the cells were further incubated with 20 µM of CsA or vehicle (dimethyl sulfoxide, DMSO). After 24 h, melanin content assay was performed and tyrosinase activity was measured.
WTS assay
The viability of cells was assessed by a WTS assay using EZ-Cytox Cell Viability Kit (Daeil Labservice, Korea) based on the cleavage of the tetrazolium salt to water soluble formazan by succinate-tetrazolium reductase. In brief, cells were subcultured in 24-well plates at roughly 1×105 cells per well and allowed to adhere for 24 h at 37℃ before being treated with adenovirus. After adenovirus treatment, the cells were incubated with 50 µl of the EZ-Cytox solution for 1 h at 37℃. During the incubation period, the viable cells converted the EZ-Cytox solution to a water-soluble formazan dye. Thereafter, the resulting formazan was quantified using an ELISA reader. The optical density (O.D.) was determined at 450 nm, after automatic subtraction of all background signals.
Measurement of melanin content
B16 melanoma cells were individually treated with the test preparations or adenovirus for each indicated time. Cells were incubated at 4℃ for 24 h in 1 ml of 1 N NaOH and then vortexed to solubilize the melanin, after which the samples were centrifuged for 10 min at 14,000 rpm. Spectrophotometric analysis of the melanin content was then conducted at 405 nm. Next, the melanin content was determined based on the absorbance/µg of protein. The protein concentration of the cells was determined using a BCA protein assay kit.
Assay of cellular tyrosinase activity
B16 melanoma cells were treated with the individual test drugs or adenovirus for indicated time, after which the cells were washed with 0.1 M of potassium phosphate-buffered saline (PBS) and collected with lysis buffer. Next, the cells were then ruptured by freezing and thawing and the lysate was clarified by centrifugation at 14,000×rpm for 20 min. The protein content was then determined using a BCA Protein Assay Kit (Thermo, Rockford, IL). After quantifying the protein levels, the concentrations were adjusted to contain the same amount of protein (20 µg). Each well of the 96-well plate contained the lysate and 1 mM of L-DOPA in phosphate solution. Following incubation at 37℃ for 1 h, the absorbance was measured at 475 nm using a spectrophotometer.
Construction of recombinant adenovirus expressing microRNA specific for NFAT2 and knock-down of NFAT2 in the B16 cells
Three pre-microRNA sequences were designed that targeted the 3' untranslated region (UTR) of the human NFAT2 mRNA using an online tool, Invitrogen's RNAi Designer. The double-stranded DNA oligonucleotides corresponding to the three different NFAT2-specific pre-microRNAs and a control sequence were individually cloned into the parental vector pcDNA6.2-GW/miR (Invitrogen, Carlsbad, CA) to generate pcDNA6.2-miR-NFAT2, and pcDNA6.2-miR-control, respectively. An expression vector for a GFP epitope fusion protein of miR-NFAT2 was generated from pENTR/GFP. Recombinant adenovirus was produced using the ViraPower Adenoviral Expression System (Invitrogen, Carlsbad, CA), according to the manufacturer's instruction. Briefly, the recombination region of each pcDNA6.2-GW/miR-based expression vector was transferred to the Gateway Vector pAd/CMV/V5-DEST using the transfer vector pDONR221 in an in vitro recombination reaction. The recombined adenoviral plasmids generated from pAd/CMV/V5-DEST in this manner were transformed into competent DH5α (Toyobo, Osaka, Japan). After selection, a single clone of DH5α was isolated and expanded. The recombinant adenoviral plasmid was purified, and then transfected into 293A cells. After a sufficient cytopathic effect was observed in 293A cells, adenovirus was purified using the Adeno-X Virus Purification Kit (Clontech, Mountain View, CA). The recombinant adenoviruses Ad-miR-NFAT2, Ad-miR-control were generated from pcDNA6.2-miR-NFAT2, and pcDNA6.2-miR-control, respectively. All insertion sequences were confirmed by nucleotide sequencing. The B16 cells were transduced with the adenovirus expressing scrambled microRNA (Ad/miScr) and microRNA specific for NFAT2 (Ad/miNFAT2) at the 10 multiplicity of infection for 6 h.
Western blot analysis
Cells were lysed in Proprep buffer (Intron, Daejeon, Korea). After vigorous pipetting, extracts were centrifuged for 15 min at 13,000 rpm. Total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA). Samples were run on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes and incubated with appropriate antibodies for overnight at 4℃ with gentle agitation. Blots were then incubated with peroxidase-conjugated secondary antibodies for 30 minutes at room temperature, and visualized by enhanced chemiluminescence (Intron). Anti-NFAT2, anti-tyrosinase, and anti-microphthalmia-associated transcription factor (MITF) antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA), and anti-actin antibody was purchased from Sigma (St. Louis, MO). Proteins were separated by 8% reducing SDS-PAGE and then immune blotted onto nitrocellulose membranes in 20% methanol, 25 mM Tris, and 192 mM glycine. Membranes were then blocked with 5% non-fat dry milk and incubated with primary antibody overnight. Subsequently, membranes were washed in Tween-Tris buffer saline (TTBS), incubated with horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit (1 : 4,000) antibodies for 4 h, rewashed, and finally developed using an enhanced ECL system (KPL Inc., Gaithersburg, MD). The membranes were then reprobed with β-actin antibody as a control for protein loading.
Statistical analysis
The data for viability assay, the melanin synthesis, and tyrosinase activity assay were statistically evaluated using an ANOVA-test. The data are given as the means±SEM.
Results
Expression of NFAT2, tyrosinase, and MITF in B16 melanoma cells
In our previous study, NFAT2 mRNA expression was markedly increased in white hair bulb compared to black hair bulb in cDNA microarray, which gave us the idea that NFAT2 might effect on synthesis of melanin in any way. To confirm this postulation, we tried to investigate whether NFAT2 differentially expressed in the hypopigmented B16 cells, compared to the melanogenic B16 cells. From our previous experiment, we already knew that color of B16 cells was faded by increase of cell passage number. We first confirmed that melanin content of B16 cells was decreased by cell passage (Fig. 1A). Consistent with this data, Western blot analysis showed that tyrosinase and MITF, which major markers for melanogenesis, were markedly decreased in over-passaged B16 cells, compared with under passaged mother cells (Fig. 1B). Furthermore, expression of NFAT2 protein was also increased in the hypopigmented B16 cells. These results indicate that NFAT2 might effect on melanogenesis in B16 melanoma cells.
Fig. 1.
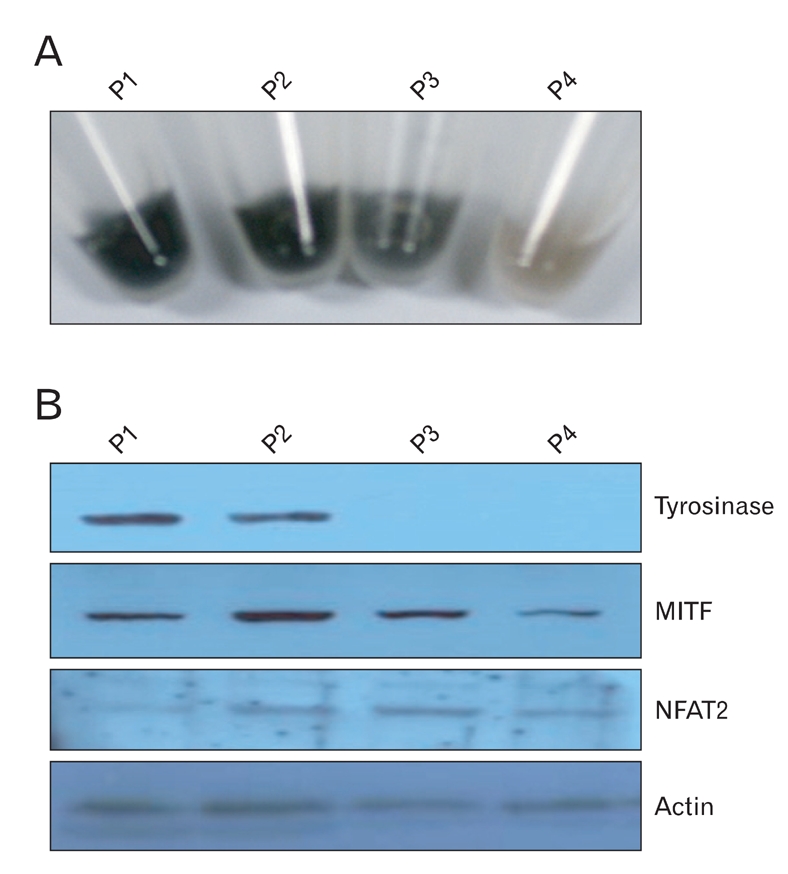
Expression of NFAT2, tyrosinase, and MITF proteins in the hypopigmented B16 melanoma cells. (A) Photographs of the pellets from the B16 melanoma cells. Cells (2×106) were seeded onto 100mm dishes and cultured for 2 to 3 days [passage (P)1]. When the cells reached approximately 90% confluence, they were trypsinized, counted, and passaged into new dishes (P2). These procedures were repeated 2 times over a period of 2 weeks (P3, P5). Each culture was prepared for following experiment. (B) Expression of NFAT2, tyrosinase, and MITF in the hypopigmented B16 cells. Cell lysates from each culture were separated on polyacrylamide gels, transferred onto nitrocellulose membranes, and then reacted with the anti-NFAT2, anti-tyrosinase and anti-MITF antibodies. Anti-actin antibody was used as a loading control.
NFAT2 is knockdowned through the microRNA
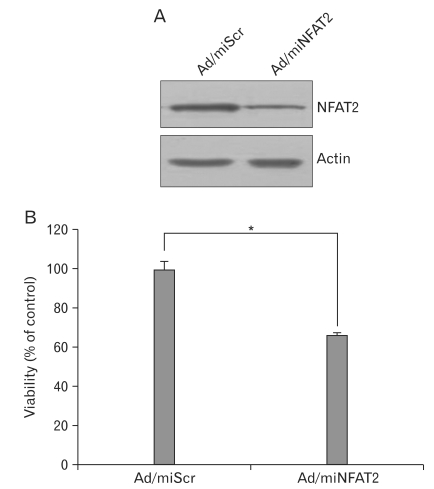
To investigate the role of NFAT2 in pigmentation, we made a recombinant adenovirus expressing microRNA specific for NFAT2 (Ad/miNFAT2) to knockdown expression of this gene. The B16 cells were harvested two days after transduction with Ad/miNFAT2 or Ad/miScr. NFAT2 protein level was measured by Western blotting using NFAT2 antibody. MicroRNA specific for NFAT2 significantly reduced its protein level, whereas scrambled microRNA did not (Fig. 2A). To determine whether NFAT2 affects cell viability of B16 cells, we carried out cell proliferation assay. After transduction with Ad/miNFAT2 and Ad/miScr, cell viability assay was performed by WTS assay. As shown in Fig. 2B, downregulation of NFAT2 affected cell proliferation, consistant with a previous report. These results indicate that NFAT2 microRNA used in this study could specifically down-regulate NFAT2 expression level, and NFAT2 plays in partly in cellular proliferation in B16 cells.
Fig. 2.
Knock-down of NFAT2 by infection of adenovirus expressing microRNA specific for NFAT2. (A) The B16 cells were transduced with adenovirus expressing microRNA specific for NFAT2 (Ad/miNFAT2) at the 10 multiplicity of infection (MOI) for 6 h. After washing twice with PBS, cells were refed with growth medium and incubated for 24 h. The expression of NFAT2 was verified by Western blot using the anti-NFAT2 antibody and anti-actin antibody. Adenovirus expressing scrambled microRNA (Ad/miScr) was used as a negative control. (B) Cell survival was measured by WTS assay. Data are expressed as percentage of control (Ad/miScr at 1 day). The mean values±SEM are averages of triplicate measurements. *Statistically significant difference (P<0.05).
Regulation of pigmentation by NFAT2
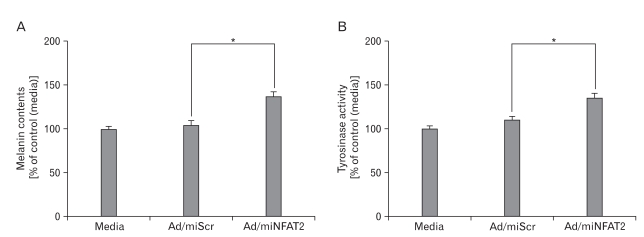
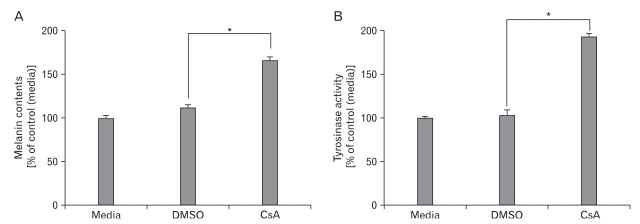
Previous reports showed that NFAT2 expressed in epidermal keratinocyte and regulated induction of COX2 during apoptosis by UV radiation (Flockhart et al., 2008). However, the roles of NFAT2 in epidermal melanocyte are poorly characterized, especially in pigmentation. As shown in our present data, expression of NFAT2 was increased in the hypopigmented B16 cells. We speculated that melanogenesis/pigmentation may be related with endogenous NFAT2 level. To test this idea, NFAT2 microRNA or scrambled microRNA was transduced into B16 cells, and alteration of melanogenesis potential (pigmentation) was examined using melanin contents and tyrosinase activity assays. Following 2 days of microRNA transduction, colors of culture medium dramatically altered from bright red into blackish red color (data not shown) and furthermore, melanin contents and tyrosinase activity were significantly increased by NFAT2 microRNA (Figs. 3A and B). These results indicate that NFAT2 microRNA could specifically upregulated melanogenesis related proteins and NFAT2 may play an important role for regulation of melanogenesis in B16 melanoma cells. To more validate this information, we pretreated B16 cells with CsA, a selective inhibitor for Calcineurin/NFATsignaling. As expectedly, treatment with CsA increased melanin content and tyrosinase activity (Figs. 4A and B), consistent with the previous data.
Fig. 3.
Effect of NFAT2 knockdown on melanogenesis in B16 melanoma cells. The B16 cells were transduced with adenovirus expressing scrambled microRNA (Ad/miScr) and microRNA specific for NFAT2 (Ad/miNFAT2) at the 10 multiplicity of infection (MOI) for 6 h. After incubation for 48 h, alteration of pigmentation by NFAT2 knockdown verified by melanin content assay (A) and tyrosinase activity (B). Data are expressed as percentage of control (Ad/miScr). The mean values±SEM are averages of triplicate measurements. *Statistically significant difference (P<0.05).
Fig. 4.
Effect of CsA on melanogenesis in B16 melanoma cells. The B16 cells were transduced with adenovirus expressing scrambled microRNA (Ad/miScr) and microRNA specific for NFAT2 (Ad/miNFAT2) at the 10 multiplicity of infection (MOI) for 6 h. After incubation for 24 h, Cells were further incubated with 20 µM of CsA. After 24h, melanin content assay (A) and tyrosinase activity (B) were performed as described in the materials and methods. The control was assessed by measurement of protein concentration. Data are expressed as percentage of control. The mean values±SEM are averages of triplicate measurements. *Statistically significant difference (P<0.05).
Discussion
NFAT2 expression was increased in the hypopigmented B16 cells, while tyrosinase and MITF expression was decreased. Inhibition of NFAT2 expression with microRNA specific for NFAT2 enhanced tyrosinase activity and melanin content. Then, CsA blocked NFAT2 activation and enhanced tyrosinase activity and melanin content in our experiment.
Many investigations have concentrated on the molecular mechanisms related to melanogenesis for developing new therapeutic agents for hair graying and skin pigmentation abnormalities. Melanin synthesis in mammals is a complex process that takes place in the melanosome (Murakami et al., 2009), an organelle that contains pigment-producing enzymes; and the process is known to be regulated by a number of factors, including hormones differentiation factors, growth factors, cytokines, as well as compounds from natural sources (Costin & Hearing, 2007). In addition, melanin synthesis is stimulated by a large number of effectors, including 1-oleyl-2-acetyl-glycerol (Friédmann et al., 1990), UVB radiation (Roméro-Graillet et al., 1996), cAMP-elevating agents such as forskolin, IBMX, a-MSH, glycyrrhizin (GR) (Hunt et al., 1994). In common, melanin content correlates directly with the activity of tyrosinase and the protein levels of tyrosinase (Maeda et al., 1997; Shibahara et al., 2000). Although there is a lot of information about mechanism of melanogenesis, the overall picture underlying the regulation of melanin biosynthesis is not yet clear. Since dysregulation of melanogenesis may cause cancer (Kadekaro et al., 2006), clarification of the causal factors is thus of great importance. In this report we investigate the putative role of NFAT2 during melanogenesis by melanin contents and tyrosinase activity.
Recently, it has been known that NFAT/calcineurin could affected in hair follicle activation (Horsley et al., 2008). Initial interest in calcium/calcineurin signaling in the epidermis came from the observation that increased calcium triggers epidermal differentiation but appears to inhibit hair follicle cycling. This speculation of calcium regulation supported by additional observations that CsA caused hair cycling apart from its effects on the immune system in mice and humans (Sawada et al., 1987). Further studies using conditional mutants implicated NFAT signaling in hair cycling by the demonstration that calcineurin B mutants showed cycling alopecia (Mammucari et al., 2005). The epidermis lacking NFAT2 develops normally, but after initiating telogen, it prematurely enters the next anagen. The observation that stem cell and differentiation markers are not affected in the mutant mice suggests that NFAT2 acts specifically on hair cycling.
Based on these results, we speculate that NFAT2 plays an authentic role as melanogenesis regulator in melanotic melanoma cells. Interestingly, we demonstrated that expression of NFAT2 was markedly increased in the hypopigmented B16 cells in this study. Furthermore, we demonstrated that CsA increases melanogenesis level in melanoma cells, and that knockdown of NFAT2 expression by siRNA also increased melanin content and tyrosinase activity. It is well known that CsA inhibit the phosphatase activity of calcineurin, which regulates nuclear translocation and subsequent activation of NFAT transcription factors. In addition to the calcineurin/NFAT pathway, recent studies indicated that CsA also block the activation of BRAF/MEK/ERK signaling, so inhibited Cox-2 promoter activation and protein induction in metastatic melanoma cells (Flockhart et al., 2008). It would be interesting to know whether CsA can increased pigmentation. Interestingly, we found that melanin content and tyrosinase activity was increased by CsA, suggesting that calcineurin/NFAT cross-talk with melanogenesis signaling.
In summary, we demonstrated that NFAT2 expression was increased by regression of pigmentation, and that knockdown of NFAT2 reduced the B16 proliferation and increased melanin content and tyrosinase activity and tyrosinase expression. Our results suggest that NFAT2 has a role for regulation of melanogenesis in melanocyte.
Acknowledgements
This study was financially supported by research fund of Chungnam National University in 2008.
References
- 1.Al-Daraji WI, Afolayan J, Zelger BG, Abdellaoui A, Zelger B. Modulation of NFAT-5, an outlying member of the NFAT family, in human keratinocytes and skin. Am J Transl Res. 2009;1:184–202. [PMC free article] [PubMed] [Google Scholar]
- 2.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 3.Flockhart RJ, Armstrong JL, Reynolds NJ, Lovat PE. NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma. Br J Cancer. 2009;101:1448–1455. doi: 10.1038/sj.bjc.6605277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flockhart RJ, Diffey BL, Farr PM, Lloyd J, Reynolds NJ. NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure. FASEB J. 2008;22:4218–4227. doi: 10.1096/fj.08-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friédmann PS, Wren FE, Matthews JN. Ultraviolet stimulated melanogenesis by human melanocytes is augmented by di-acyl glycerol but not TPA. J Cell Physiol. 1990;142:334–341. doi: 10.1002/jcp.1041420216. [DOI] [PubMed] [Google Scholar]
- 6.Fung-Leung WP, Pope BL, Chourmouzis E, Panakos JA, Lau CY. Tepoxalin, a novel immunomodulatory compound, synergizes with CsA in suppression of graft-versus-host reaction and allogeneic skin graft rejection. Transplantation. 1995;60:362–368. doi: 10.1097/00007890-199508270-00011. [DOI] [PubMed] [Google Scholar]
- 7.Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003;284:C1593–C1603. doi: 10.1152/ajpcell.00537.2002. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier M, Degnan BM. The transcription factor NF-kappaB in the demosponge Amphimedon queenslandica: insights on the evolutionary origin of the Rel homology domain. Dev Genes Evol. 2008;218:23–32. doi: 10.1007/s00427-007-0197-5. [DOI] [PubMed] [Google Scholar]
- 9.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt G, Todd C, Cresswell JE, Thody AJ. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 11.Kadekaro AL, Wakamatsu K, Ito S, Abdel-Malek ZA. Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006;11:2157–2173. doi: 10.2741/1958. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999;17:149–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 13.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, Yokokawa Y, Hatao M, Naganuma M, Tomita Y. Comparison of the melanogenesis in human black and light brown melanocytes. J Dermatol Sci. 1997;14:199–206. doi: 10.1016/s0923-1811(96)00575-0. [DOI] [PubMed] [Google Scholar]
- 15.Mammucari C, Tommasi di Vignano A, Sharov AA, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–676. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Murakami M, Matsuzaki F, Funaba M. Regulation of melanin synthesis by the TGF-beta family in B16 melanoma cells. Mol Biol Rep. 2009;36:1247–1250. doi: 10.1007/s11033-008-9304-6. [DOI] [PubMed] [Google Scholar]
- 17.Na IK, Markley JC, Tsai JJ, et al. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood. 2010;116:e18–e25. doi: 10.1182/blood-2009-12-259432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231:210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang W, Hu Y, Li J, et al. Direct evidence for the critical role of NFAT3 in benzo[a]pyrene diol-epoxide-induced cell transformation through mediation of inflammatory cytokine TNF induction in mouse epidermal Cl41 cells. Carcinogenesis. 2007;28:2218–2226. doi: 10.1093/carcin/bgm115. [DOI] [PubMed] [Google Scholar]
- 20.Pores-Fernando AT, Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev. 2009;231:160–173. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 21.Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol. 2009;47:400–410. doi: 10.1016/j.yjmcc.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roméro-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem. 1996;271:28052–28056. doi: 10.1074/jbc.271.45.28052. [DOI] [PubMed] [Google Scholar]
- 23.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987;56:684–686. [PubMed] [Google Scholar]
- 25.Shibahara S, Yasumoto K, Amae S, et al. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000;13(Suppl 8):98–102. doi: 10.1034/j.1600-0749.13.s8.18.x. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe SA, Zhou P, Dötsch V, et al. Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature. 1997;385:172–176. doi: 10.1038/385172a0. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Nguyen BC, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]