Abstract
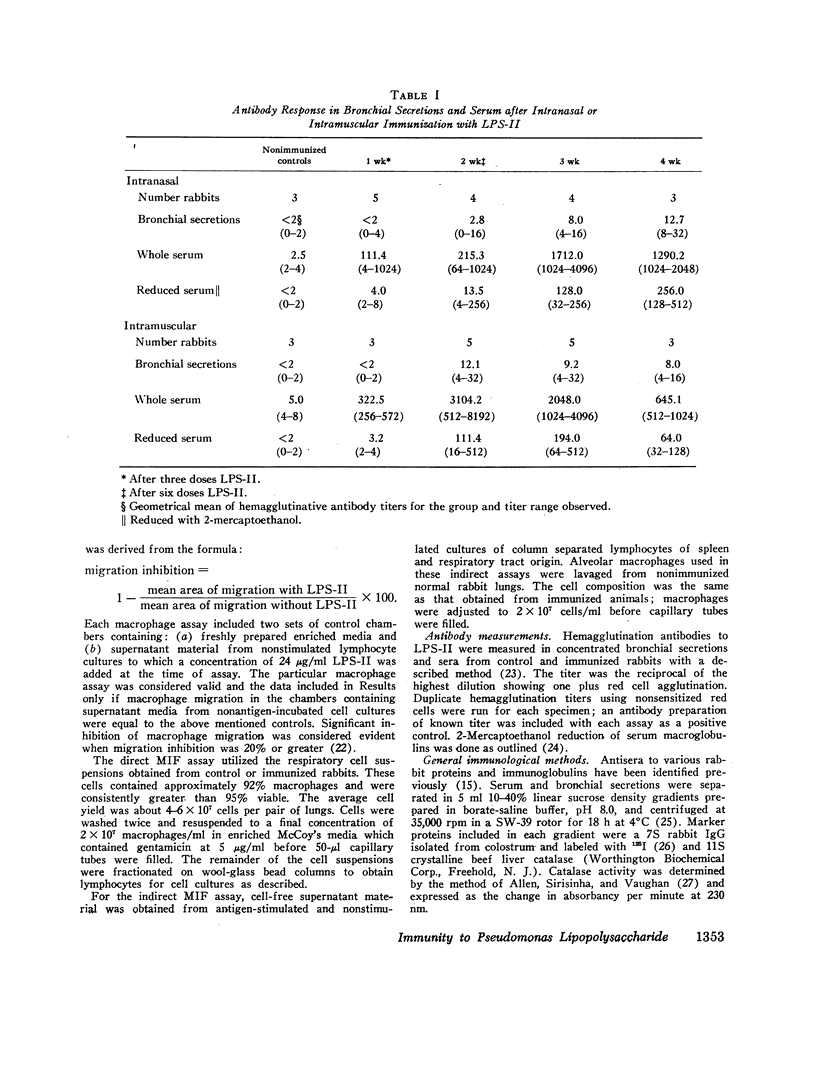
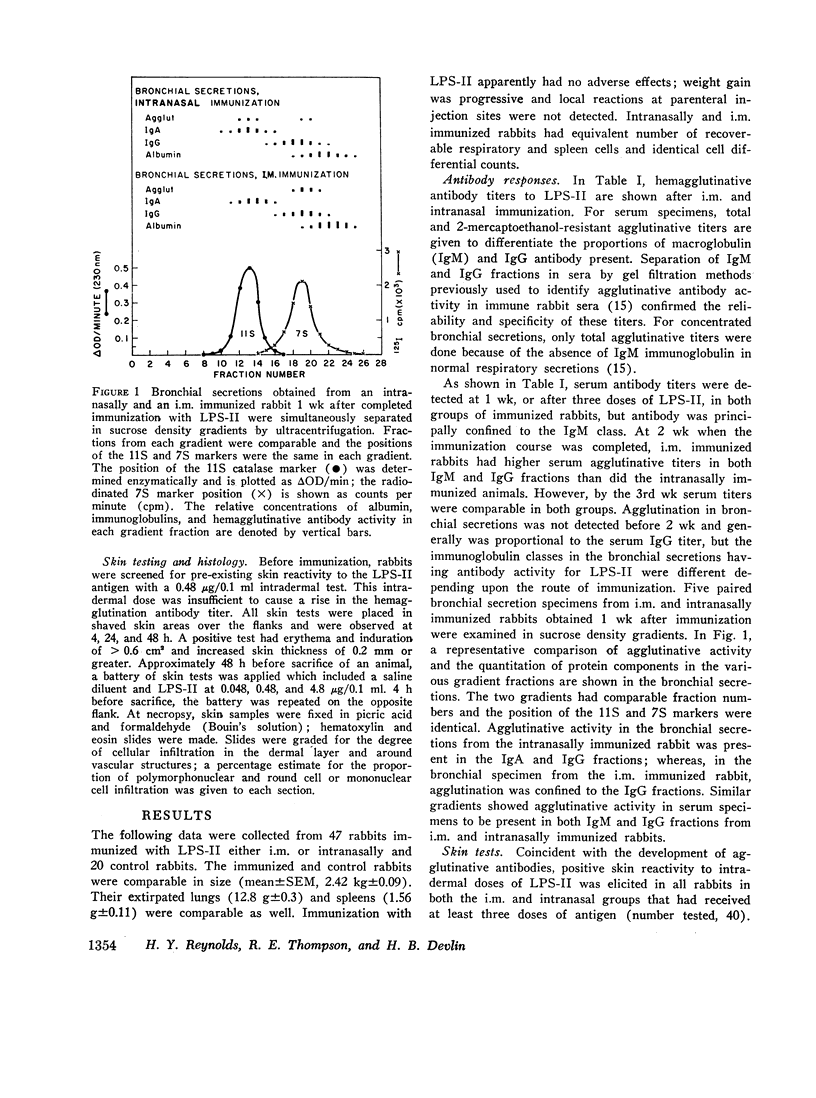
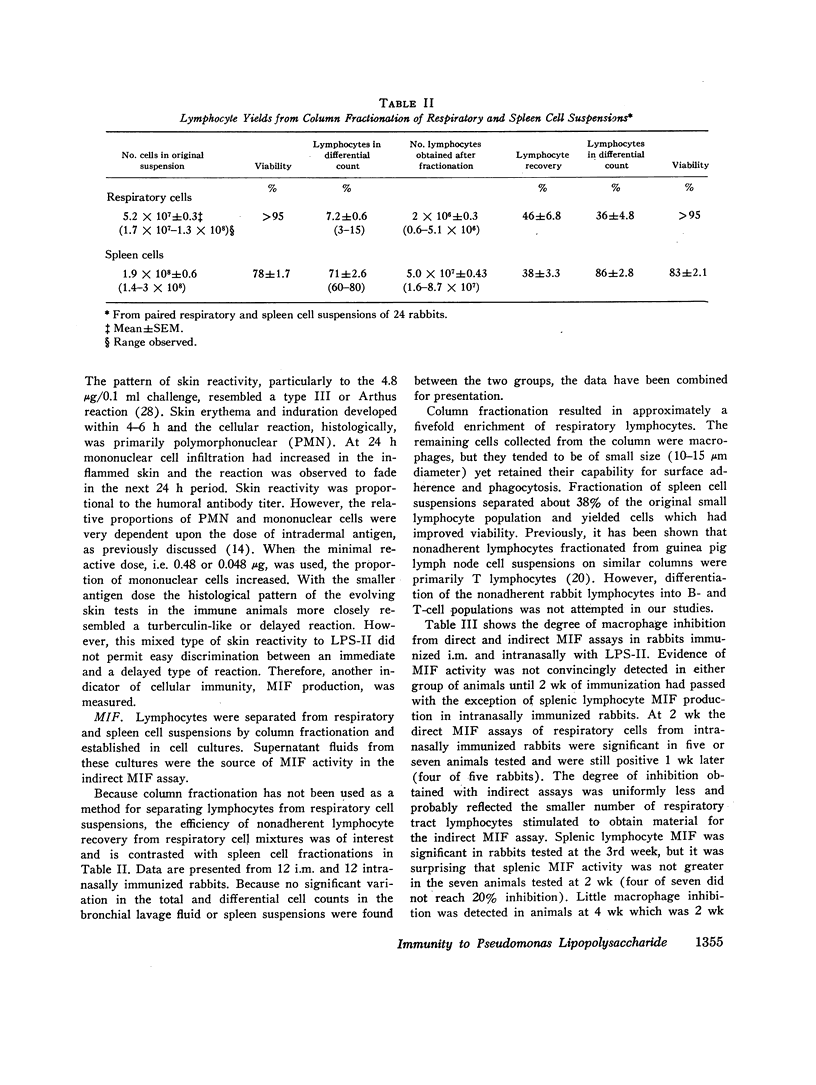
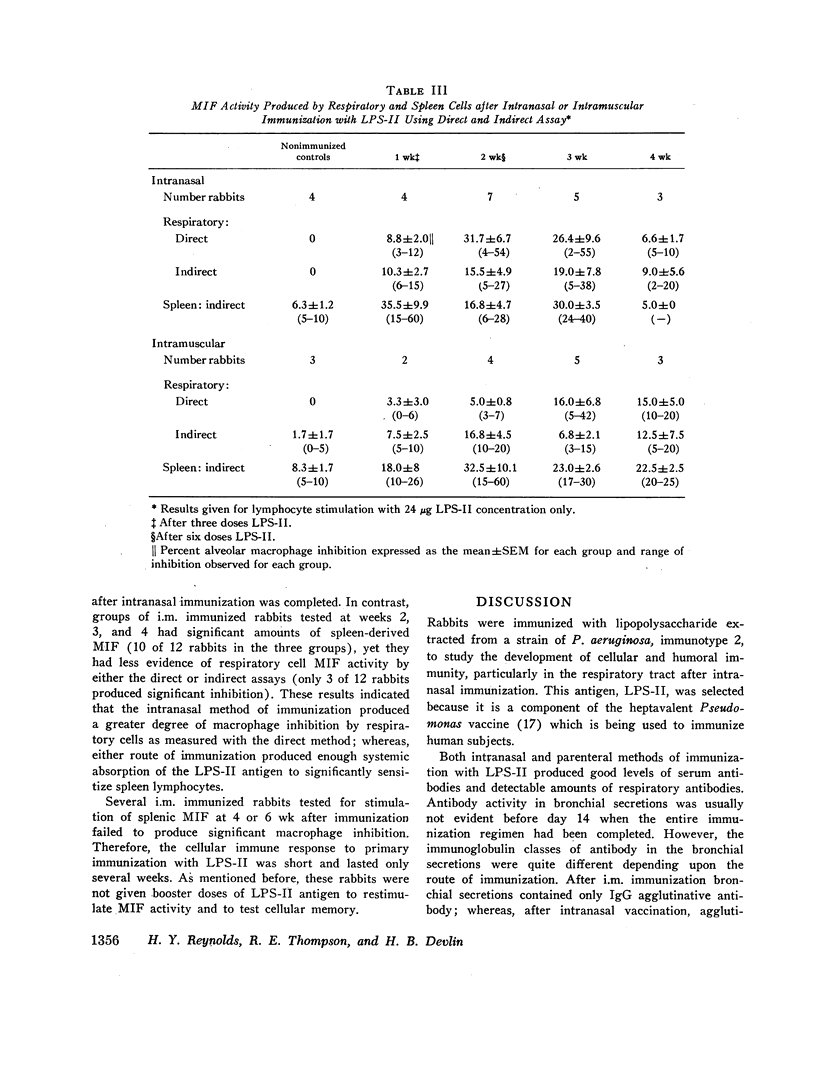
Immunization with Pseudomonas lipopolysaccharide induced both cellular and humoral immunity in rabbits, particularly in the respiratory tract after intranasal immunization. Either parenteral (i.m.) or intranasal immunization elicited an IgG antibody response in respiratory secretions, but only intranasal immunization produced secretory IgA antibody. Immunization by both routes stimulated serum IgM and IgG agglutinative antibodies. Because both methods of immunization produced skin test reactivity which had components of both Arthus and tuberculin-like reactions, cellular immunity was more readily assessed by the measurement of migration inhibitory factor (MIF) released from immune lymphocytes in respiratory and spleen cell suspensions after challenge with the lipopolysaccharide antigen. After intranasal vaccination, MIF activity was detected in the respiratory tract by direct assay; in contrast, i.m. immunized rabbits did not produce respiratory MIF. Both modes of immunization resulted in splenic MIF activity. However, lymphocytes were only capable of producing MIF for short periods after primary immunization had ended, apparently losing this function in about 2-3 wk. Therefore, it was concluded that cellular immunity by in vitro assay was transient after primary immunization with this Pseudomonas antigen in contrast to the more persistent humoral immunity. The biological significance of immune lymphocytes as part of the coordinated host defense of the lung needs further evaluation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Sirisinha S., Vaughan J. H. Immunochemical studies on equine antibodies to human gamma-2-globulin. J Immunol. 1965 Nov;95(5):918–928. [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder J. G., Fisher M. W., White A. Type-specific immunity in pseudomonas diseases. J Lab Clin Med. 1972 Jan;79(1):47–54. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- Greisman S. E., Hornick R. B. Cellular inflammatory responses of man to bacterial endotoxin: a comparison with PPD and other bacterial antigens. J Immunol. 1972 Dec;109(6):1210–1222. [PubMed] [Google Scholar]
- HUEBNER K. F., GENGOZIAN N. DEPRESSION OF THE PRIMARY IMMUNE RESPONSE BY DL-PENICILLAMINE. Proc Soc Exp Biol Med. 1965 Feb;118:561–565. doi: 10.3181/00379727-118-29905. [DOI] [PubMed] [Google Scholar]
- Hanessian S., Regan W., Watson D., Haskell T. H. Isolation and characterization of antigenic components of a new heptavalent Pseudomonas vaccine. Nat New Biol. 1971 Feb 17;229(7):209–210. doi: 10.1038/newbio229209a0. [DOI] [PubMed] [Google Scholar]
- Harris J. C., Dupont H. L., Hornick R. B. Fecal leukocytes in diarrheal illness. Ann Intern Med. 1972 May;76(5):697–703. doi: 10.7326/0003-4819-76-5-697. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med. 1970 Oct 1;283(14):739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Johnson J. S. Structural units of canine serum and secretory immunoglobulin A. Biochemistry. 1971 Jul 20;10(15):2821–2827. doi: 10.1021/bi00791a003. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. II. Interaction of respiratory antibodies with Pseudomonas aeruginosa and alveolar macrophages. J Immunol. 1973 Aug;111(2):369–380. [PubMed] [Google Scholar]
- Reynolds H., Carta-Sorcini M., Mage R. The immunoglobulins derived from a VH-CH recombinant rabbit and its normal relatives. I. Measurements of heavy chain molecular weights and allotypes of colostral IgA. Immunochemistry. 1973 Jul;10(7):443–447. doi: 10.1016/0019-2791(73)90014-1. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Rosenthal A. S., Davie J. M., Rosenstreich D. L., Blake J. T. Depletion of antibody-forming cells and their precursors from complex lymphoid cell populations. J Immunol. 1972 Jan;108(1):279–281. [PubMed] [Google Scholar]
- Salomon P. F., Tamlyn T. T., Grieco M. H. Escherichia coli pneumonia. Case report. Am Rev Respir Dis. 1970 Aug;102(2):248–257. doi: 10.1164/arrd.1970.102.2.248. [DOI] [PubMed] [Google Scholar]
- Seravalli E., Taranta A. Release of macrophage migration inhibitory factor(s) from lymphocytes stimulated by streptococcal preparations. Cell Immunol. 1973 Jul;8(1):40–54. doi: 10.1016/0008-8749(73)90091-9. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson J. R., Lerner A. M. Characteristics of nonbacteremic Pseudomonas pneumonia. Ann Intern Med. 1968 Feb;68(2):295–307. doi: 10.7326/0003-4819-68-2-295. [DOI] [PubMed] [Google Scholar]
- Tillotson J. R., Lerner A. M. Characteristics of pneumonias caused by Escherichia coli. N Engl J Med. 1967 Jul 20;277(3):115–122. doi: 10.1056/NEJM196707202770302. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]


