Abstract
Cancer patients often report impaired sleep quality. Impaired sleep quality may be due to increased levels of sleep-mediating cytokines resulting from cancer treatment. Exercise may have a positive influence on sleep-mediating cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and soluble tumor necrosis factor-alpha receptor (sTNF-R), which may improve sleep quality. This two-arm pilot study compared the influence of a home-based exercise intervention with standard care/control on sleep quality and mediators of sleep. Breast and prostate cancer patients (n = 38) beginning radiation therapy were randomized to a 4-week exercise program or no exercise arm. Global sleep quality, subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction were assessed with the Pittsburgh Sleep Quality Index. IL-6, TNF-α, and sTNF-R were measured before and after intervention. There was a greater improvement in sleep quality in the exercise group from pre- to postintervention, although the difference was not significant. Additionally, there were associations between IL-6 and sleep efficiency and duration, suggesting that regulation of sleep-mediating cytokines by exercise may mediate improvements in sleep-quality components.
Subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction are components of global sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI). Poor sleep quality has been associated with multiple negative consequences such as worse health, fatigue, mood disturbances, and immunosuppression.1,2 Poor sleep quality is common among cancer patients and several times more prevalent than in the general population.3,4
Radiation therapy in particular leads to a multitude of side effects including sleep problems.5 Radiation targets the tumor and surrounding tissue. 6 Symptomatic injury can result from irradiating normal tissues surrounding the tumor.6 For example, radiation activates cellular signaling pathways that lead to the expression of proinflammatory cytokines.6,7 In particular, radiation causes an increase in interleukin-6 (IL-6)8 and tumor necrosis factor-α (TNF-α)9,10 production. IL-6 and TNF-α help regulate the inflammatory process locally and trigger central nervous system-mediated responses to inflammation.11 TNF-α activity promotes inflammation, and levels are high when systemic inflammation and catabolic conditions are observed.12 IL-6 functions as either a pro- or anti-inflammatory cytokine, depending on the stimulus. In response to tissue injury, IL-6 is proinflammatory. Local tissue injury involves cytokine production at the site of inflammation. The systemic low-grade inflammatory response to local inflammation is known as the acute-phase response.13 IL-6 and TNF-α, as proinflammatory cytokines, are associated with fever, pain, cognitive impairment, fatigue, insomnia, and decreased eating14–16 and may be, at least in part, responsible for cancer-related symptoms. 15–17
Cytokines also play a role in sleep regulation by interacting with the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis initiates the secretion of cortisol, a sleep-regulating glucocorticoid. IL-6 and TNF-α activate the HPA axis, which can lead to adrenal secretion of cortisol. 11 As a result, high levels of circulating cytokines can lead to abnormal cortisol fluctuations. Abnormal cortisol secretion is associated with short sleep duration and high sleep disturbance. 18 Ultimately, cancer and its treatments disturb cytokine secretion and, as a consequence, affect the sleep/wake cycle2 and immune function.19
Physical exercise has been shown to improve sleep quality in cancer survivors. A clinical trial in breast cancer patients receiving hormonal treatment who participated in a four-times-per-week, 20-minutes-per-day home-based walking exercise intervention, described as moderate intensity, had improved self-reported sleep quality after 4 weeks of participation. 20 In another study, patients within 2 years of diagnosis of a variety of cancers who had completed or were currently undergoing various cancer treatments participated in a supervised exercise intervention 2 days per week for 12 weeks. The exercise intervention was modeled after phase II cardiac rehabilitation and consisted of treadmill walking, stair stepping, and upper body exercises, which were all made progressively more challenging as patients adapted. Self-reported sleeping difficulty significantly decreased following the exercise intervention.21
Exercise may improve sleep quality through the regulation of proinflammatory cytokines.22 Regular exercise training reduces low-grade inflammation23,24 by triggering the immediate but transient release of IL-6 from skeletal muscle.25 IL-6, released in proportion to exercise intensity, duration, muscle mass used during exercise, and training status, functions as an anti-inflammatory when released during and immediately after an acute bout of exercise. When acting as an anti-inflammatory, IL-6 triggers the release of soluble TNF-α receptor (sTNF-R), which has anti-inflammatory effects, and inhibits the production of TNF-α, an inflammatory cytokine.13,25,26
Physical exercise of moderate intensity may have a positive influence on sleep quality through altered plasma concentrations of mediators of sleep. This subject has not been fully explored in cases of impaired sleep quality stemming from cancer and its treatments. In this secondary analysis, we investigated the relationship between mediators of sleep and subjective sleep quality in breast and prostate cancer patients undergoing radiation treatments.
Methods
The primary outcomes and methods from this randomized, controlled phase II pilot clinical trial were previously reported by Mustian et al27 in 2009 and are briefly described below.
Participants
Breast and prostate cancer patients starting radiation treatments were recruited for participation in this study at the University of Rochester James P. Wilmot Cancer Center, Rochester, NY. Eligibility criteria were (1) a primary diagnosis of breast or prostate cancer; (2) no distant metastases; (3) no recurrent disease; (4) no conditions prohibiting participation in a low-to-moderate intensity walking or resistance exercise program or physical fitness testing, as assessed by the patient’s radiation oncologist (or physician designee); (5) completion of enrollment and baseline assessments before the end of the first calendar week of radiation treatments; (6) at least 30 scheduled radiation treatments (6 weeks); (7) sedentary lifestyle (no regular exercise or fewer than two exercise sessions per week); and (8) signed informed consent. The Research Subjects Review Board approved the study protocol and consent procedures prior to enrollment of any participants.
Procedures
Baseline assessments of patients in this clinical trial were obtained during the first week of radiation therapy. Demographic information was drawn from the clinical record. Patients completed the PSQI, a symptom inventory, and a daily diary. A pedometer assessed steps walked daily, and blood was drawn for measurement of cytokine levels.
Patients were randomized after stratification based on breast or prostate cancer diagnosis to either (1) the control condition (radiation treatment alone), ie, standard care (SC), or (2) the intervention condition: radiation treatment and a 4-week home-based exercise (HBEX) intervention that included progressive walking and resistance band exercises. The exercise intervention was performed off-site from the University of Rochester Medical Center in a patient-selected home-based environment.
Patients in the HBEX arm of the study were given a 45-minute instructional session led by an exercise physiologist. During this session, participants were provided with an exercise kit that contained materials needed to complete the home-based walking and progressive resistance training intervention (ie, written instructions, a pedometer, and therapeutic resistance bands).
SC patients were encouraged to remain only as active as they were prior to study inclusion and were not given a pedometer during the study intervention period to reduce the risk of exercise contamination. Participants in both groups were monitored by weekly contact with study personnel and by review of daily diary entries. Baseline assessments were repeated following 4 weeks of SC or moderate-intensity HBEX and again 3 months later.
Exercise prescription
The American College of Sports Medicine (ACSM) guidelines for exercise testing and prescription were followed in the design of the HBEX program.28 The progressive walking component of the exercise intervention was designed to be performed at a moderate intensity. Participants wore a pedometer during the initial 1-week assessment period and recorded their daily number of steps. Using the average number of steps taken during the baseline assessment as a guide, participants in the exercise arm were instructed to increase the number of steps attained each day by 5%–20% while performing activity at a moderate intensity. Moderate-intensity exercise was described as a score of 3–5 on the ACSM revised rating of perceived exertion (RPE) scale, with 0 representing no exertion and 10 representing maximal exertion. An individualized table, developed to increase compliance and provide motivation, was given to exercise arm participants at the beginning of the intervention. The table included the average number of steps taken at baseline and the corresponding 5%, 10%, 15%, and 20% increases.
The resistance training portion of the exercise intervention was designed to be performed at a low to moderate intensity, 7 days per week, for 4 weeks, with the aim of maintaining upper body muscular strength. A 7-day-per-week intervention was chosen because the low- to moderate-intensity regimen—designed to maintain, not improve, strength—was performed with a minimal number of sets and repetitions. Upper body resistance exercises were the focus due to the reliance on the lower body for the progressive walking portion of the intervention.
Three therapeutic bands offering low to moderate resistance were provided to allow for individualized resistance and progression. During the 45-minute instructional session, patients were shown proper resistance band use and body mechanics for the 11 resistance exercises, which included bicep curls, tricep extensions, overhead press, rows, chest press, internal and external rotation of the shoulder, front and lateral raises, and horizontal abduction and adduction of the shoulder. Progression was determined by the participants, as they were instructed to increase the intensity by shortening the band length or changing the band color, with the aim of increasing from the initial sets and repetitions to a maximum of 4 sets of 15 repetitions per exercise.
Measures
Demographic and medical information
Demographic information collected from the on-study and clinical record forms were age, gender, race, partnered status, job status, and educational level, height, weight, and cancer treatment history.
Sleep
Subjective sleep quality was assessed using the PSQI,29 a 19-item questionnaire used to assess sleep quality during the past month. Global scores range from 0 to 21 with lower scores indicating better sleep quality. A global score of greater than 5 is generally accepted as an indicator of impaired global sleep quality.29 Seven components, individually scored from 0 to 3, make up the global score: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction. Psychometric evaluation of the PSQI in cancer patients has established internal consistency, reliability, and construct validity. Researchers have found internal consistency coefficients (Cronbach alpha) of 0.81 (n = 170) and 0.77 (n = 249) in diverse samples of cancer patients.30 Internal consistency, reliability, and construct validity of the PSQI were also demonstrated in a study of 472 cancer patients, with an internal consistency coefficient of 0.80.31 PSQI scores were moderately to highly correlated with sleep quality and sleep problems, exceeding r = 0.69 with various other measures, and poorly correlated, r ≤ 0.37, with unrelated constructs such as nausea and vomiting in cancer patients.31
Biologic mediators of sleep
Blood samples were collected during a fasting state at baseline and post intervention and were centrifuged to allow serum collection. Serum samples were aliquotted and stored at −80 °C. Enzyme-linked immunosorbent assays (ELISAs) were used to determine the concentrations of IL-6 in serum (IL-6s), IL-6 in plasma (IL-6p), TNF-α, and sTNF-R using commercially available kits from Becton, Dickinson and Company, Franklin Lakes, NJ.
Adherence and compliance
Adherence and compliance were assessed in both groups. Daily steps walked and daily minutes of resistance exercise, from the self-report daily diaries, were used to determine the amount of exercise each patient performed during the study assessment periods. The level of exercise was determined specifically by frequency (number of times per week), duration/volume (length of time/volume of the exercise bout), mode (type of exercise), and intensity (difficulty level of the effort put forth to perform the exercise; ACSM RPE scale).28
The frequency of exercise was determined by adding the total numbers of days per week that a patient reported doing any exercise. The total daily duration was identified by using a pedometer to determine daily steps walked and, from that information, estimating duration, then adding the minutes of resistance exercise performed. The mode was determined from the patient’s record of aerobic and resistance exercise modalities. The intensity was assessed by having patients record an RPE for the exercise sessions.28
Adherence to and compliance with the walking program were also assessed by pedometers, set to zero each morning and worn throughout the day. Patients recorded the daily steps walked in a diary each night before going to bed.
Data analysis
Data analyses were conducted using SPSS PASW version 18 and R Version 2.9.1 software. All statistical tests were performed at a two-tailed 5% level of significance. Data were coded and cleaned by independent data managers using Teleforms scanned into a Microsoft Access database; data managers visually audited the data. Patient data were analyzed on an intent-to-treat basis. Assumptions underlying all analyses were checked, and analyses included all evaluable patients. Missing data were minimal, and no imputations were necessary for analyses. Immediately after consent, two participants withdrew with no data provided; therefore, data from these two participants were not used in the analyses.
Descriptive statistics, frequency distributions, means, mean change scores, and standard deviations (SDs) for measures were assessed in the two study arms. Independent-sample t-tests were used to compare baseline characteristics of the two groups. Analysis of covariance (ANCOVA) was used to examine differences between HBEX and SC groups in the outcomes measured. All ANCOVA analyses controlled for mean-centered baseline and age, because they may influence sleep and inflammatory markers. Outcomes assessed were global sleep quality, duration of sleep, sleep disturbances, sleep latency, daytime dysfunction due to sleepiness, habitual sleep efficiency, overall sleep quality, use of sleep medication, IL-6, TNF-α, sTNF-R, and patient-reported cancer-related and/or cancer treatment-related side effects. These ANCOVA models included an arm*baseline interaction term. Significant interaction terms were left in the model, and nonsignificant ones were removed. Values are reported as mean ± SD. The exploratory analyses used to assess associations between sleep quality and mediators of sleep were performed using Spearman correlations with P values and 95% confidence intervals (CIs) based on Fisher’s Z transformation procedure. All hypothesis tests were two-tailed at the 0.05 level of significance.
Results
Participants
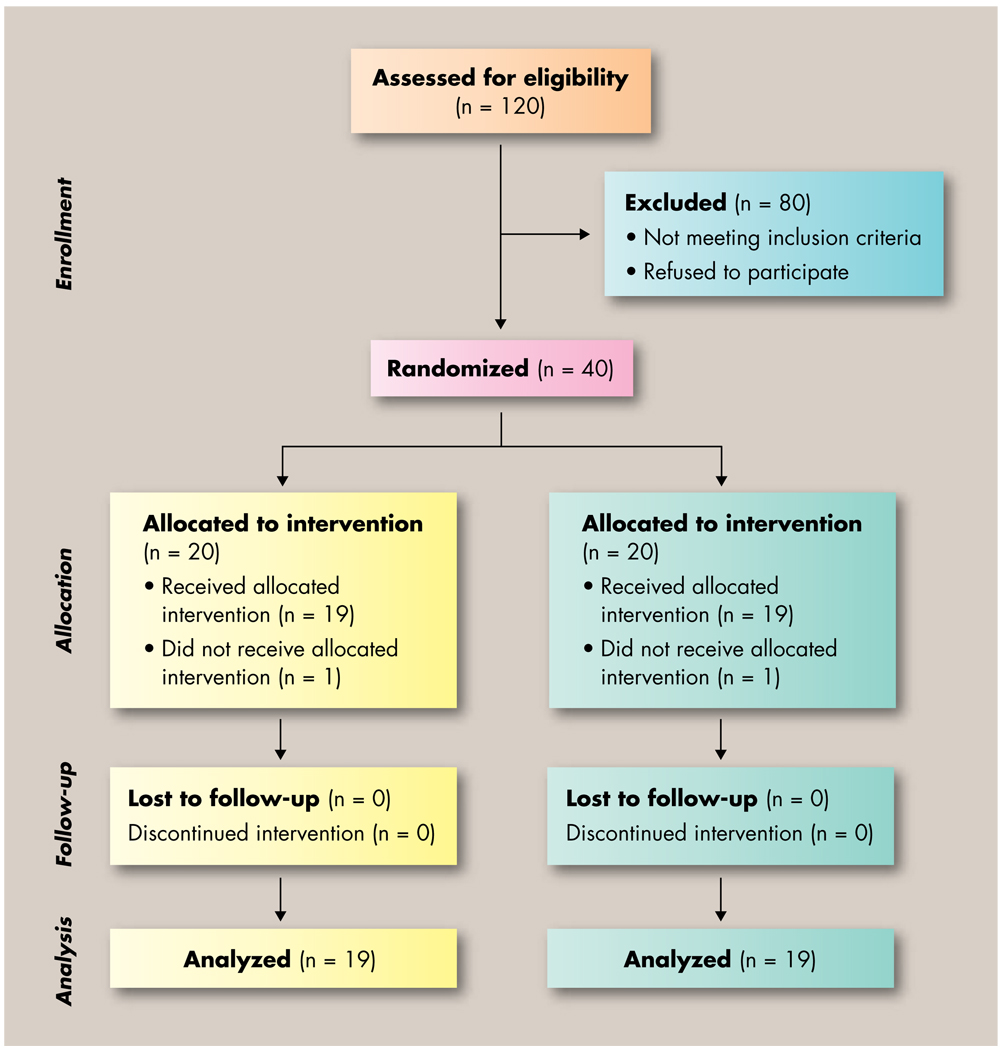
From a total of 120 screened participants, 82 were potentially eligible for participation in the study. Study coordinators approached 61 patients following physician or nurse referral, and of them, 40 were eligible and agreed to participate. Reasons for not enrolling the 21 remaining patients included ineligibility due to maintaining an exercise program prior to enrollment or patient declining to participate. Two participants, one from each study arm, did not complete any study-related testing and therefore were not included in the analyses, leaving 38 fully evaluable patients. Figure 1 illustrates the recruitment efforts, enrollment, and completion of the study by participants.
Figure 1.
Flow of participants through the study
There were no significant differences between groups at baseline in demographic variables, including age, height, weight, body mass index, gender, race, marital status, education, employment status, weekly hours worked, or Karnofsky performance status. There were no differences in clinical variables, including previous surgery, chemotherapy or hormone therapy, or total radiation dose. The demographic variables have previously been presented in Mustian et al.27 There were no significant differences between groups at baseline in study outcome variables, including global sleep quality and the seven components that contribute to the global score: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction. Additionally, no group differences were found in baseline levels of the potential sleep mediators IL-6, TNF-α, and sTNF-R. Patients all had early-stage cancer with no distant metastases or recurrent disease. See Table 1 for demographic and treatment-related characteristics of participants.
TABLE 1.
Demographic and treatment-related characteristics of the study population
| Characteristic | Exercise group (n = 19) |
Control group (n = 19) |
All (n = 38) |
|---|---|---|---|
| Cancer type, n (%) | |||
| Prostate | 6 (32%) | 5 (26%) | 11 (29%) |
| Breast | 13 (68%) | 14 (74%) | 27 (71%) |
| Race, n (%) | |||
| White | 16 (84%) | 18 (95%) | 34 (90%) |
| Asian | 2 (11%) | 0 (0%) | 2 (5%) |
| Black | 1 (5%) | 1 (5%) | 2 (5%) |
| Currently employed, n (%) | 17 (90%) | 12 (63%) | 29 (76%) |
| Marital status, n (%) | |||
| Married | 14 (74%) | 9 (47%) | 23 (61%) |
| Divorced | 2 (11%) | 5 (26%) | 7 (19%) |
| Single | 2 (11%) | 2 (11%) | 4 (10%) |
| Widowed | 1 (5%) | 3 (16%) | 4 (10%) |
| Some college education or higher, n (%) | 16 (84%) | 12 (63%) | 28 (74%) |
| Previous surgery, n (%) | 16 (84%) | 16 (84%) | 16 (84%) |
| Previous chemotherapy, n (%) | 9 (47%) | 10 (53%) | 19 (50%) |
| Current hormone therapy, n (%) | 1 (5%) | 2 (10%) | 3 (8%) |
| Karnofsky performance status, mean ± SD | 96.3 ± 6.8 | 93.7 ± 10.1 | 95.0 ± 8.6 |
| Age, years, mean ± SD | 56.6 ± 13.7 | 63.3 ± 9.4 | 60.0 ± 12.1 |
| Height, inches, mean ± SD | 64.7 ± 3.6 | 65.0 ± 3.5 | 64.8 ± 3.5 |
| Weight, pounds, mean ± SD | 173.7 ± 46.8 | 188.3 ± 43.9 | 181.0 ± 45.4 |
| Body mass index, kg/m2, mean ± SD | 28.7 ± 5.4 | 31.3 ± 6.8 | 30.0 ± 6.2 |
| Total radiation dose, Gy, mean ± SD | 66.6 ± 13.1 | 61.7 ± 7.9 | 64.1 ± 11.0 |
SD = standard deviation
Exercise adherence and compliance
Aerobic exercise
Fifteen of the 19 patients assigned to HBEX reported increased daily steps walked. The mean number of daily steps walked in this group was 7,222 at baseline, 11,200 at postintervention, and 12,878 at 3-month follow-up. In addition, the mean increase in daily steps walked from baseline to postintervention was 5,959 steps and from baseline to 3-month follow-up, 7,095 steps. Patients in the SC group had mean daily steps of 5,544 at baseline, 4,796 at postintervention, and 5,180 at 3-month follow-up. Their mean change in daily steps walked from baseline to postintervention was −572 steps and from baseline to 3-month follow-up, −64 steps. ANCOVAs with mean daily steps as the response and baseline daily steps as the covariate showed significantly more daily steps walked post intervention and at the 3-month follow-up in the HBEX group, compared with the SC group (all P < 0.05).
Resistance exercise
Of the 19 patients in the HBEX group, 12 reported doing resistance training during the intervention, for an average of 17 minutes per day, 3 days per week, at an RPE of 4 (moderate intensity). Eight of the HBEX participants reported doing resistance exercise at the 3-month follow-up for an average of 18 minutes per day, 1.5 days per week. In the HBEX group, time spent in resistance training increased by 9.4 minutes from baseline to postintervention and by 6.81 minutes from postintervention to 3-month followup. None of the SC patients reported performing resistance training at postintervention, and only one patient reported doing resistance training at the 3-month follow-up. In the SC group, time spent in resistance training decreased by 1.6 minutes from baseline to postintervention and by 1.0 minutes from postintervention to 3-month follow-up. ANCOVAs comparing mean daily minutes of resistance training and mean days of resistance training per week with baseline daily minutes and training days per week as covariates showed significantly more daily minutes of resistance training and mean days of resistance training in the HBEX group, compared with the SC group at postintervention and 3-month follow-up (all P < 0.05). See Table 2 for exercise at baseline and postintervention.
Table 2.
Exercise and sleep
| Measurement | Baseline ± SD | 95% CI | Post ± SD | 95% CI | Change | P value |
|---|---|---|---|---|---|---|
| Exercise group (n = 19) | ||||||
| Exercise level | ||||||
| Daily steps | 7,222.2 ± 2,691.3 | 5,925–8,520 | 11,200.1 ± 5,851.8 | 8,380–14,021 | +3,977.6 | 0.009 |
| Daily resistance, min | 1.16 ± 2.95 | 0.00–2.58 | 10.59 ± 11.37 | 5.11–16.06 | +9.43 | 0.002 |
| Days/wk of resistance | 0.21 ± 0.54 | 0.00–0.47 | 3.26 ± 2.92 | 1.85–4.67 | +3.05 | < 0.001 |
| PSQI scores | ||||||
| Global sleep quality | 7.06 ± 4.26 | 4.87–9.25 | 6.00 ± 3.87 | 4.08–7.92 | −1.06 | 0.37 |
| Subjective sleep quality | 1.00 ± 0. 69 | 0.66–1.34 | 1.11 ± 0.74 | 0.75–1.46 | +0.11 | 0.50 |
| Sleep latency | 1.39 ± 1.24 | 0.77–2.01 | 1.00 ± 1.33 | 0.34–1.66 | −0.39 | 0.24 |
| Sleep duration | 1.00 ± 0.77 | 0.62–1.38 | 0.89 ± 0.57 | 0.62–1.17 | −0.11 | 0.54 |
| Sleep efficiency | 0.94 ± 1.11 | 0.39–1.50 | 0.63 ± 0.90 | 0.20–1.06 | −0.31 | 0.11 |
| Sleep disturbance | 1.61 ± 0.78 | 1.22–2.00 | 1.32 ± 0.58 | 1.04–1.60 | −0.39 | 0.06 |
| Use of sleep medication | 0.39 ± 0.98 | 0.00–0.88 | 0.39 ± 0.98 | 0.00–0.88 | 0.00 | 1.00 |
| Daytime dysfunction | 0.65 ± 0.70 | 0.29–1.01 | 0.78 ± 0.81 | 0.38–1.18 | +0.13 | 0.50 |
| Control group (n = 19) | ||||||
| Exercise level | ||||||
| Daily steps | 5,544.9 ± 2,746.7 | 4,221–6,869 | 4,796.9 ± 2,613.9 | 3,404–6,190 | −572.3 | 0.30 |
| Daily resistance, min | 1.57 ± 4.73 | 0.00–3.85 | 1.57 ± 4.73 | 0.00 ± 0.00 | −1.57 | 0.16 |
| Days/wk of resistance | 0.21 ± 0.63 | 0.00–0.51 | 0.00 ± 0.00 | 0.00 ± 0.00 | −0.21 | 0.16 |
| PSQI scores | ||||||
| Global sleep quality | 7.79 ± 4.00 | 5.86–9.71 | 7.44 ± 4.72 | 5.10–9.79 | −0.35 | 0.60 |
| Subjective sleep quality | 1.26 ± 0.73 | 0.78–1.56 | 1.17 ± 0.79 | 0.78–1.56 | −0.09 | 0.50 |
| Sleep latency | 1.11 ± 1.05 | 0.62–1.71 | 1.17 ± 1.10 | 0.62–1.71 | +0.06 | 1.00 |
| Sleep duration | 1.21 ± 0.86 | 0.47–1.30 | 0.89 ± 0.83 | 0.47–1.30 | −0.32 | 0.03 |
| Sleep efficiency | 0.95 ± 1.08 | 0.41–1.59 | 1.00 ± 1.19 | 0.41–1.59 | +0.05 | 1.00 |
| Sleep disturbance | 1.47 ± 0.51 | 1.15–1.85 | 1.50 ± 0.71 | 1.15–1.85 | +0.03 | 0.67 |
| Use of sleep medication | 0.58 ± 1.17 | 0.03–1.30 | 0.67 ± 1.28 | 0.03–1.30 | +0.09 | 0.41 |
| Daytime dysfunction | 1.21 ± 0.98 | 0.66–1.45 | 1.06 ± 0.80 | 0.66–1.45 | +0.15 | 0.10 |
SD = standard deviation; CI = confidence interval; PSQI = Pittsburgh Sleep Quality Index (lower PSQI scores indicate better sleep)
Sleep quality
Global sleep quality is scored from 0 to 21, with lower scores indicating better sleep quality. The seven components of sleep are scored from 0 to 3, with 0 indicating no difficulty and 3 indicating severe difficulty.
Baseline
At baseline, approximately 60% (n = 10) of participants in the HBEX group and 74% (n = 14) of participants in the SC group reported scores of greater than 5 for global sleep quality as measured by the PSQI, indicating impaired global sleep quality. Self-reported global sleep quality was 7.06 in the HBEX group, and 7.79 in the SC group, with no significant difference between groups at baseline. Subjective sleep quality was reported as fairly bad or very bad by 22% (n = 4) of HBEX participants and 32% (n = 6) of SC participants. The majority of participants in each group slept for a duration of 6 or more hours per night, with 17% (n = 3) of HBEX participants and 26% (n = 5) of SC participants reporting 6 or fewer hours of sleep per night. Sleep efficiency, the percentage of time spent in bed actually sleeping, was reported as less than 75% in 33% (n = 6) of HBEX participants and 21% (n = 4) of SC participants. The use of sleep medication three or more times per week was reported by 11% (n = 2) of participants in the HBEX group and 16% (n = 3) in the SC group.
Postintervention
The percentage of participants who reported impaired global sleep quality in the HBEX group postintervention dropped to 50% (n = 9), compared with 60% at baseline. There was a slight reduction in the number of SC participants who reported impaired global sleep quality postintervention (baseline = 74%; postintervention = 67%). Using ANCOVA, with baseline values and age as covariates, the difference between groups was not statistically significant (HBEX = 6.00 ± 3.87; SC = 7.44 ± 4.72; P = 0.50). The HBEX group reported better subjective sleep quality postintervention than did the SC group, but there was a 0.09 improvement in subjective sleep quality in the SC group at postintervenion, compared with a 0.11 reduction in the HBEX group. The HBEX group reported less sleep latency than the SC group postintervention. Sleep latency fell by 0.39 points throughout the intervention in the HBEX group, whereas the SC group reported a 0.06 point increase in sleep latency. The number of patients reporting 6 or fewer hours of sleep per night decreased from 17% at baseline to 10% (n = 2) postintervention in the HBEX group and from 26% at baseline to 11% (n = 2) postintervention in the SC group. At postintervention, in the HBEX group, only 16% (n = 3) of participants reported having less than 75% sleep efficiency, compared with 33% at baseline, whereas 22% (n = 4) of SC participants reported less than 75% sleep efficiency. Sleep medication usage did not change in the HBEX or SC group from baseline to postintervention. See Table 2 for means, SDs, and 95% CIs for global sleep quality and the seven components of sleep.
Biological mediators of sleep
Baseline
Baseline levels of IL-6p (HBEX = 1.08 pg/mL; SC = 3.60 pg/mL; P = 0.31); IL-6s (HBEX = 5.74 pg/mL; SC = 6.28 pg/mL; P = 0.31); TNF-α (HBEX = 0.57 pg/mL; SC = 9.43 pg/mL; P = 0.31); and sTNF-R (HBEX = 760.62 pg/mL; SC = 766.30 pg/mL; P = 0.96) did not differ between groups.
Postintervention
Postintervention levels of IL-6p increased slightly in both groups. ANCOVA adjusted for baseline values and age revealed that between-group differences of postintervention IL-6s were significant, with the HBEX group having lower IL-6s levels (HBEX = 6.33 pg/mL; SC = 9.26 pg/mL; P = 0.04). TNF-α increased in both groups from baseline to postintervention. Postintervention TNF-α was lower in the HBEX group compared with the SC group, although the difference was not significant when evaluated by adjusted ANCOVA (HBEX = 2.82 pg/mL; SC = 9.58 pg/mL; P = 0.61). sTNF-R decreased by 80 pg/mL in the HBEX group and increased by nearly 20 pg/mL in the SC group from baseline to postintervention. See Table 2 for means, SDs, and 95% CIs for the HBEX and SC groups in exercise and sleep. See Table 3 for sleep mediator means and ranges.
TABLE 3.
Plasma/serum levels of sleep mediators
| Mediator | Baseline | Range | Post | Range | Change | P value |
|---|---|---|---|---|---|---|
| Exercise group (n = 19) | ||||||
| IL-6p level, pg/mL | 1.08 | 0.06–2.97 | 1.38 | 0.29–6.41 | +0.30 | 0.47 |
| IL-6s level, pg/mL | 5.74 | 0.83–48.10 | 6.33 | 0.61–24.05 | +0.59 | 0.94 |
| TNF-α level, pg/mL | 0.57 | 0.00–4.18 | 2.82 | 0.00–35.99 | +2.25 | 0.31 |
| sTNF-R level, pg/mL | 760.62 | 448.64–1,476.21 | 680.52 | 361.68–1,319.53 | −80.10 | 0.59 |
| Control group (n = 19) | ||||||
| IL-6p level, pg/mL | 3.60 | 0.00–8.81 | 3.75 | 0.00–7.76 | +0.15 | 0.59 |
| IL-6s level, pg/mL | 6.28 | 0.08–12.47 | 9.26 | 1.79–16.74 | +2.98 | 0.24 |
| TNF-α level, pg/mL | 9.43 | 0.00–28.84 | 9.58 | 0.00–29.18 | +0.15 | 0.67 |
| sTNF-R level, pg/mL | 766.30 | 598.72–933.87 | 783.98 | 600.99–966.97 | +17.68 | 0.66 |
IL-6p = plasma interleukin-6 (IL-6); IL-6s = serum IL-6; TNF-α = tumor necrosis factor-alpha; sTNF-R = soluble TNF-α receptor
Relationship between sleep and mediators of sleep
Baseline
In participants as a whole, sTNF-R was negatively associated with subjective sleep quality (r = −0.36; P = 0.026, CI = −0.610, −0.046); sleep disturbances (r = −0.42; P = 0.009; CI = −0.651, −0.115); and the use of sleep medications (r = −0.33; P = 0.045; CI = −0.586, −0.008). IL-6p was positively associated with sleep duration (r = 0.35; P = 0.031; CI = 0.035, 0.603) and sleep efficiency (r = 0.39; P = 0.015; CI = 0.081, 0.613).
In the HBEX group, there was a negative association between subjective sleep quality and sTNF-R (r = −0.53; P = 0.020; CI = −0.792, −0.097); sleep disturbances and IL-6p (r = −0.63; P = 0.004; CI = −0.884, −0.249); and use of sleep medication and IL-6p (r = −0.54; P = 0.017; CI = −0.799, −0.114). There were two data points, one at baseline and one at postintervention, that were clearly IL-6s outliers. These values were removed. However, the results with and without those values were similar. Higher levels of IL-6p and lower levels of sTNF-R were associated with poorer sleep quality for all variables.
Postintervention
In the study sample as a whole, there was a positive association between TNF-α and subjective sleep quality (r = 0.33; P = 0.041; CI = 0.015, 0.590) and TNF-α and sleep latency (r = 0.36; P = 0.028; CI = 0.042, 0.607).
In the HBEX group, there was a negative association between subjective sleep quality and sTNF-R (r = −0.50; P = 0.030; CI = −0.058, −0.777) and sleep efficiency and IL-6p (r = −0.49; P = 0.034; CI = −0.772, −0.045) postintervention, indicating that better subjective sleep quality and sleep efficiency were associated with higher levels of sTNF-R and IL-6p, respectively.
In the SC group postintervention, there was a positive association between sleep duration and IL-6p (r = −0.49; P = 0.036; CI = 0.039, −0.769), indicating that higher levels of IL-6p were associated with impaired sleep duration.
There was a positive association between changes from baseline in the SC group in TNF-α and changes in sleep latency (r = 0.50; P = 0.031; CI = 0.054, 0.775) and use of sleep medications (r = 0.58; P = 0.009; CI = 0.176, 0.820). No significant associations were observed between changes in sleep measures and changes in inflammatory markers in the HBEX group.
Discussion
The results from this secondary analysis of a randomized clinical trial provide support for the use of a home-based walking and resistance training exercise program during radiation therapy for breast and prostate cancers to positively influence sleep quality, possibly by regulating mediators of inflammation. The mean global sleep quality of 6.00 in the HBEX group at postintervention was less than the self-reported global sleep quality in a healthy group of participants of similar age, reported in a different study.1 These findings are in accordance with previous research suggesting that exercise may improve sleep in cancer patients20,21,32 and contribute to the growing body of literature supporting the safety and benefits of exercise during radiation treatment.33–36
The exploratory analyses of the relationships between mediators of sleep and sleep quality revealed an association between IL-6 and sleep efficiency, sleep duration, and the use of sleep medication. In participants from both groups, increases in fasting IL-6, an inflammatory marker and mediator of sleep, were correlated with reduced sleep duration and reduced sleep efficiency. In the SC group, IL-6p increased from baseline and was associated with impaired sleep duration postintervention. Postintervention IL-6p levels in the HBEX group (1.38 pg/mL) were similar to IL-6p levels in a healthy sample of women who reported exercising one or more times per week (1.25 pg/mL),37 whereas postintervention IL-6p levels in the SC group were more than two times greater (3.75 pg/mL).
TNF-α, another inflammatory marker and mediator of sleep, also increased in the SC group and was associated with longer sleep latency and greater use of sleep medication. Conversely, in the HBEX group, an increase in sTNF-R correlated with better subjective sleep quality and global sleep quality, suggesting that sTNF-R may be a mediator of sleep.
The outcomes of this study lead us to hypothesize that the relationship between the mediators of sleep and sleep quality may be mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Radiation treatment can activate signaling pathways that lead to the expression of IL-68 and TNF-α.6,7,9,10 IL-6 and TNF-α can activate the HPA axis, which can lead to the adrenal secretion of cortisol.11 Therefore, high levels of circulating cytokines can result in abnormal cortisol fluctuations, and abnormal cortisol secretion is associated with short sleep duration and sleep disturbance. 18 However, exercise results in reduced low-grade inflammation23,24 by triggering the immediate but transient release of IL-6 from skeletal muscle.25 Functioning as an anti-inflammatory when released during and immediately after an acute bout of exercise, IL-6 triggers the release of IL-1 receptor antagonist (IL-1ra), sTNF-R, and IL-10, which have anti- inflammatory effects, and IL-6 inhibits the production of TNF-α.13,25,26 Exercise may improve sleep quality through the regulation of pro-inflammatory cytokines that would otherwise stimulate the HPA axis and promote cortisol production.22
The results presented should be considered preliminary, as these analyses were not the primary aim of the study but were exploratory. By the same token, the small size of this pilot study limited our power to detect differences and correlations, which means we may have missed important relationships among exercise, inflammatory markers, and sleep measures. These results should not be generalized to a broader population of cancer survivors or to survivors of nonmetastatic breast and prostate cancers receiving treatment other than radiation. Additionally, we were not able to control for comorbid conditions or medications that may have influenced sleep. The use of an objective measure of rest such as actigraphy, in addition to subjective self-reported sleep quality as assessed by the PSQI, would add strength to future studies of this problem.
Although participants were randomized to the HBEX and SC groups, those who agreed to participate may have been more highly motivated to begin an exercise intervention that included walking and resistance training. Therefore, these beneficial results may not necessarily translate into improvements in all breast and prostate cancer survivors beginning radiation treatment, as there may be a fair amount of resistance to partaking in a regular exercise program in the general public, although it has many positive healthy effects in general. Finally, the study was not placebo controlled or fully blinded. Participant expectancy and experimenter bias may have played a role in improvements.
Despite the limitations, the positive outcomes from this pilot study provide evidence supporting home-based aerobic and resistance exercise training during radiation treatment for nonmetastatic breast and prostate cancer patients. Future phase III randomized controlled trials are needed with larger samples to fully investigate these relationships and confirm these findings related to sleep quality and markers of inflammation. Additionally, research comparing the effects of various modes of exercise on sleep and inflammation in cancer patients undergoing radiation is needed.
Acknowledgments
Funded by grants 1R25CA102618 and K07CA120025 from the National Cancer Institute.
Footnotes
Conflicts of interest: The authors have nothing to disclose.
References
- 1.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell JF. Insomnia in cancer patients. Clin Cornerstone. 2004;6 suppl 1D:S6–S14. doi: 10.1016/s1098-3597(05)80002-x. [DOI] [PubMed] [Google Scholar]
- 3.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 4.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 7.Herskind C, Bamberg M, Rodemann HP. The role of cytokines in the development of normal-tissue reactions after radiotherapy. Strahlentherapie Onkologie. 1998;174 suppl 3:12–15. [PubMed] [Google Scholar]
- 8.Brach MA, Gruss HJ, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. J Biol Chem. 1993;268:8466–8472. [PubMed] [Google Scholar]
- 9.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- 12.Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med (Auckland, NZ) 2001;31:115–144. doi: 10.2165/00007256-200131020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 15.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? a cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 17.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- 18.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassilopoulou-Sellin R. Endocrine effects of cytokines. Oncology (Williston Park) 1994;810:43–46. 49–50. [PubMed] [Google Scholar]
- 20.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- 21.Young-McCaughan S, Mays MZ, Arzola SM, et al. Research and commentary: change in exercise tolerance, activity and sleep patterns, and quality of life in patients with cancer participating in a structured exercise program. Oncol Nurs Forum. 2003;30:441–454. doi: 10.1188/03.ONF.441-454. [DOI] [PubMed] [Google Scholar]
- 22.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 23.Fallon KE, Fallon SK, Boston T. The acute phase response and exercise: court and field sports. Br J Sports Med. 2001;35:170–173. doi: 10.1136/bjsm.35.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 25.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57 suppl 10:43–51. [PubMed] [Google Scholar]
- 26.Santos RVT, Tufik S, De Mello MT. Exercise, sleep and cytokines: is there a relation? Sleep Med Rev. 2007;11:231–239. doi: 10.1016/j.smrv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol. 2009;7:158–167. [PMC free article] [PubMed] [Google Scholar]
- 28.Dwyer GB, Davis SE. ACSM’s Health-Related Physical Fitness Assessment Manual. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. American College of Sports Medicine. [Google Scholar]
- 29.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 32.Tang MF, Liou TH, Lin CC. Improving sleep quality for cancer patients: benefits of a home-based exercise intervention. Support Care Cancer. 2010;18:1329–1339. doi: 10.1007/s00520-009-0757-5. [DOI] [PubMed] [Google Scholar]
- 33.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- 34.Mock V, Pickett M, Ropka ME, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9:119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 35.Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14:464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 36.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101:550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]