Abstract
Cardiac mitochondria, the main source of energy as well as free radicals, are vital organelles for normal functioning of the heart. Mitochondrial number, structure, turnover and function are regulated by processes such as mitochondrial protein quality control, mitochondrial fusion and fission and mitophagy. Recent studies suggest that abnormal changes in these mitochondrial regulatory processes may contribute to the pathology of heart failure (HF). Here we discuss these processes and their potential as therapeutic targets.
Introduction
Heart failure (HF) is characterized by a reduced ability to fill the left ventricle and/or eject blood to match the body’s demands [1]. Myocardial infarction, hypertension, diabetes, idiopathic cardiomyopathy, bacterial, viral or parasitic myocarditis, and congenital disease represent various etiologies of HF in humans [2]. Identifying potential mechanisms that contribute to the pathogenesis of HF has led to a number of therapeutic approaches [3]. We examine here a novel hypothesis that mitochondrial dysfunction may be a common participant in the pathogenesis and progression of HF.
How mitochondrial metabolic defects, ROS generation and apoptosis may contribute to the pathogenesis of HF will be briefly discussed, followed by a description of emerging research regarding mitochondrial protein quality control, control of mitochondrial number through fusion and fission and turnover through a process called mitophagy, and their potential role in HF.
Heart failure, a defect in mitochondrial energy generation
Cardiac mitochondria are the primary source of energy and maintain intracellular ATP levels through β-oxidation of fatty acids or by glycolysis to produce acetyl-CoA molecules, which enter the tricarboxylic acid (TCA) cycle and produce NADH and FADH2. NADH and FADH2 cofactors, in turn, transfer electrons in the mitochondrial electron transport chain, ultimately generating ATP. Decrease in energy production and the associated cardiac dysfunction is also found in animal models and in patients with HF and cardiomyopathies [4–7]. Further, knockout mice lacking particular mitochondrial proteins exhibit cardiac dysfunction and cardiomyopathy [8]. In addition to reduction in energetics, mitochondrial dysfunction in the presence of oxygen results in uncoupling of the electron transport chain and oxidative phosphorylation, thus generating cell-damaging reactive oxygen species (ROS) [6,9–11].
Mitochondrial ROS-mediated damage
Dysfunctional mitochondria are a major source of ROS, such as superoxide and hydrogen peroxide [10,11], that are generated mainly by mitochondrial complex I and complex III [12]. These ROS cause cardiac damage. Reduction of ROS levels by over-expression of metallothionein, manganese superoxide dismutase (MnSOD) or catalase protects against mitochondrial dysfunction and cardiomyopathy [13–15].
Elevated levels of humoral factors, like catecholamines and renin-angiotensin-aldosterone, are detrimental in HF irrespective of etiology and many drugs that inhibit these factors or their effect are commonly used as HF treatments. These humoral factors act, at least in part, by exacerbating mitochondrial dysfunction. For example, angiotensin II increases mitochondrial dysfunction, ROS production, mitochondrial depolarization and decreases respiration in cultured cells [10]. Further, mitochondrial release of H2O2 activates NADPH oxidase, resulting in increased intracellular superoxide production and reduced nitric oxide bioavailability [10]. Mitochondrial ROS production and its role in the pathogenesis of HF are reviewed elsewhere [16], and relevant to this discussion, these studies demonstrate that current therapies may indirectly benefit the heart by reducing mitochondrial dysfunction.
Mitochondria-mediated apoptosis
The role of mitochondria in cardiac apoptosis is well documented [17,18]. The release of mitochondrial enzymes and proteins such as cytochrome c, endonuclease G, apoptosis inducing factor and Smac triggers caspase activation, nuclear DNA fragmentation, and cell death [18]. Leakage of cytochrome c and Smac from mitochondria occurs after mitochondrial outer membrane integrity has been breached and mitochondrial pores are formed in response to stress stimuli. Targeting apoptotic factors, enzymes and kinases that contribute to mitochondrial pore formation reverses cardiomyopathy and the associated mitochondrial dysfunction. For example, an increase in Bcl-2 attenuates ischemia-reperfusion-induced apoptosis and protects against myocardial injury [19]. Bcl-2 also confers anti-apoptotic effects by increasing the Ca2+ threshold for mitochondrial membrane pore opening and decreasing mitochondrial Ca2+ efflux due to Na+-dependent Ca2+ exchanger in mouse heart mitochondria [19]. Bcl-2 over-expression in desmin-deficient mice dramatically ameliorates the cardiomyopathic phenotype by restoring electron transport chain function and decresing mitochondrial sensitivity to Ca2+ exposure [20].
In summary, the contribution of mitochondrial dysfunction to HF through reduction in ATP generation, increased ROS production and mitochondria-dependent apoptosis are well documented. However, recent work has identified changes in new mitochondrial processes such as mitochondrial protein quality control, mitochondrial fusion and fission and mitophagy as synergistic contributors to the pathology of HF.
New developments in the mitochondrial field and their implication in the study of heart failure
1. Mitochondrial protein quality control
ROS can directly inactivate mitochondrial proteins or generate lipid peroxidation and glycoxidation products that create inactivating adducts on proteins [21,22]. The modified and often misfolded proteins accumulate in the mitochondria and further impair mitochondrial functions [23]. Therefore identification and removal of these damaged mitochondrial proteins is important to maintain normal mitochondrial functions. The mitochondria have a set of chaperones and proteases that form a protein quality control (PQC) system, which degrades the damaged proteins or reverses the damage [24,25].
Molecular chaperones or heat shock proteins (HSP) confer resistance to cardiac stress. Members of the HSP70, HSP60 and HSP100 families reside in the mitochondrial matrix. They stabilize unfolded proteins in an ATP-dependent manner, enabling protein refolding to functional proteins or preventing aggregation of these misfolded proteins [26]. Multiple types of mitochondrial ATP-dependent proteases that participate in mitochondrial PQC mainly belong to the AAA (ATPases associated with a number of cellular activities) protein family. AAA proteases are arranged in two major complexes: the i-AAA protease faces the inter-membrane space, whereas the m-AAA protease complex faces the matrix. Both complexes bind unfolded proteins and catalyze their degradation. This proteolytic response is critical for the turnover of dysfunctional mitochondrial proteins and maintenance of PQC [21].
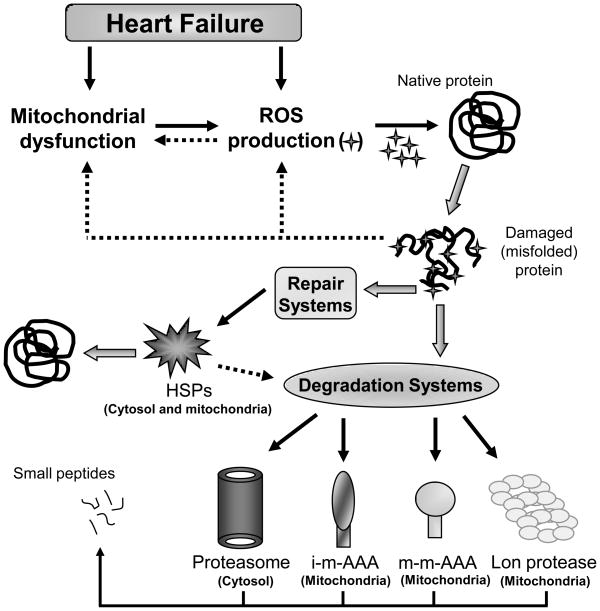
The contribution of AAA protease dysfunction to accumulation of unfolded proteins as well as its effect on mitochondrial function has been investigated in ageing and neurodegenerative diseases [27]. Continual cardiovascular stress contributes to cardiac wear and tear and results in accumulation of damaged cardiac proteins, which further increases mitochondrial dysfunction and the progression of HF [28]. The changes that occur during HF, which result in improper protein quality control, and the protein repair and recycle machinery system are summarized schematically in Figure 1. Future studies focusing on turnover of misfolded mitochondrial proteins and the contribution of AAA proteases and PCQ dysfunctions to HF are required. Another process that contributes to quality control of the mitochondria is mediated by fission of damaged mitochondria from the mitochondrial network. The regulation of the fission process and the destiny of these damaged mitochondria are discussed in the next two sections.
Figure 1. Schematic representation of the protease and chaperone systems involved in mitochondrial protein quality control.
Oxidation by reactive oxygen species damages mitochondrial proteins in either reversible or irreversible manners, and accumulation of oxidized/damaged proteins can lead to mitochondrial dysfunction in heart failure. Intracellular elimination of oxidatively modified proteins is achieved in the mitochondria and cytosol by either degradation (Lon, proteasome and m-AAA proteases) or specific repair systems (HSPs-heat shock proteins).
2. Mitochondrial fusion and fission
Because the mitochondria are dynamic organelles that constantly divide and fuse to maintain their numbers [29], disruption of this process in the heart can also contribute to HF. Further, mitochondrial fusion mixes mitochondrial contents, thus enabling protein complementation, mitochondrial DNA (mtDNA) repair and equal distribution of metabolites [30,31]. In contrast, fission enables the segregation of mitochondria into daughter organelles to increase the number of mitochondria and enhance their distribution along cytoskeletal elements [30,31].
Key components of the mitochondrial fusion and fission machinery in mammals have been identified. Mitochondrial fusion is regulated by three large GTPase proteins: Mitofusin 1 (MFN1), mitofusin 2 (MFN2) and optic atrophy protein 1 (OPA1) [29,31]. During mitochondrial fusion, through interactions of their coiled-coil domains, MFN1 and MFN2 form homo-oligometric and hetero-oligomeric complexes, and thus tether outer membranes of neighboring mitochondria together [32]. Fusion of the inner mitochondrial membrane is mediated by OPA1, a protein that faces the inter-membrane space [33]. To complete mitochondrial fusion both OPA1, MFN1, but not MFN2, are required [34].
For mitochondrial fission, the opposing process to fusion, at least two proteins are required. These are dynamin-related protein 1, Drp1, and the mitochondrial outer membrane protein, Fis1 [35]. Drp1 is primarily a cytosolic protein and is recruited to punctate spots on the mitochondrial surface [36]. Upon translocation to mitochondria, where it binds to Fis1, the Drp1 molecule assembles future fission sites and then mediates severing of the mitochondrial membranes through a GTP hydrolysis-dependent mechanism [35,37,38]. Currently, how the mitochondrial inner membrane divides during fission is not known, although several proteins, such as MTP 18 [39] and MTGM [40] have been proposed to mediate that process. Recent studies demonstrate that mitochondrial dynamics is regulated by a number of signaling enzymes and post-translational modifications (see review [41])
Mitochondrial fusion and fission in the heart
A high incidence of abnormal mitochondrial morphologies has been reported in many cardiac diseases, suggesting that mitochondrial fusion and fission are required for normal cardiac function. In cultured neonatal ventricular myocytes, inhibition of mitochondrial fission by over-expressing a dominant negative mutant form of Drp1, Drp1-K38A, prevents over-production of reactive oxygen species (ROS), mitochondrial permeability transition pore formation and subsequent cell death under sustained high glucose conditions [42]. An increase in the level of cytosolic Ca2+ induced by thapsigargin (Tg) or potassium chloride (KCL) causes a rapid and transient mitochondrial fragmentation in neonatal and adult cardiomyocytes [43]. This induced cardiac mitochondrial fission is dependent on the Drp1-mediated pathway and is associated with increased ROS generation [43]. Because calcium overload is a common feature in HF, this may increase mitochondrial fission and dysfunction, thus further contributing to the decrease in the metabolic demand of the heart and increasing its injury.
Despite variations in mitochondrial size and shape in individual myocytes, very little is known about mitochondrial dynamics in the adult heart and in heart disease. Cardiac mitochondria are highly organized and compacted between contractile filaments and next to T-tubules, or right under the sarcolemma [43]. Disorders in mitochondrial organization and the presence of abnormally small and fragmented mitochondria have been noted in end-stage dilated cardiomyopathy, myocardial hibernation and ventricular-associated congenital heart disease [44–48]. Similarly, the mitochondria in failing rat hearts are small and fragmented as compared with normal hearts [49]. These pathological changes in mitochondrial morphology are associated with a decrease in OPA1 levels in cardiac myocytes and an increase in apoptosis in post-myocardial infarction-induced HF [49]. Further, intra-mitochondrial structural changes including disorganized cristae and/or reduced cristae density were also observed in the mitochondria of rats with failing hearts as compared with sham-operated rats [12]. Together, these studies demonstrate disruption of mitochondrial dynamics and structure in HF, suggesting a role for this process in the pathogenesis of HF.
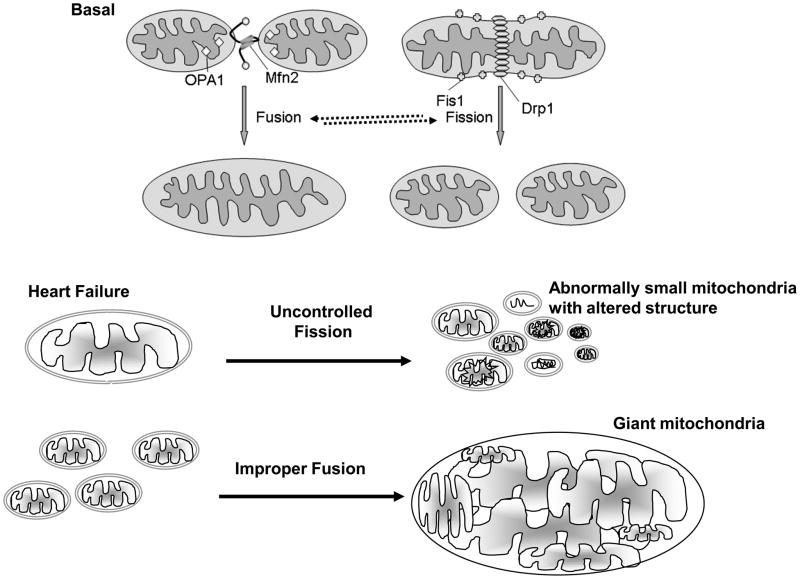
The molecular events that regulate mitochondrial dynamics and the mechanism by which disruption of the equilibrium between fusion and fission occurs under pathological conditions remain to be elucidated. Nevertheless, the mitochondrial fission and fusion machinery are emerging as potentially important contributors in HF (Figure 2). Mitochondrial fission may result in the generation of two non-identical daughter mitochondria, one with a high membrane potential and the other with a low membrane potential. The latter can be targeted for degradation in the lysosme (autophagy) by a process termed ‘mitophagy’ [50].
Figure 2. Pathological consequences of fusion and fission.
Basal mitochondrial fusion and fission maintains adequate numbers of mitochondria in the heart. Uncontrolled fission leads to many small and rounded mitochondria with altered intra-mitochondrial content. Improper fusion leads to giant mitochondria with an abnormal structure and shape. Deregulated fusion and fission have been observed in HF.
3. Autophagy
Macroautophagy (hereafter referred to as autophagy) is a critical response to stress conditions such as nutrient deprivation, oxidative stress, hypoxia and pathogen infection [51]. Autophagy is initiated in the cytoplasm by the formation of a double membrane, which expands and sequesters cytoplasmic proteins and organelles and eventually matures into an autophagosome. Fusion of autophagosomes with the lysosome results in breakdown of the sequestered cytosolic components in a catabolic process, for subsequent reuse of amino acids, fatty acids and other cellular building blocks [52]. Two distinct pathways have been shown to regulate autophagosome formation. Class III phosphoinositide 3-kinase (PI3K) complexes with Beclin 1 stimulate autophagy whereas class I PI3K and the mammalian target of rapamycin (mTor) kinase inhibit autophagy. Beclin 1 and the Abl kinase family of non-receptor tyrosine kinases participate in the later stages of autophagy by regulating the fusion of autophagosomes with lysosomes [53] and the subcellular trafficking of lysosomal enzymes [54]. Mitophagy refers to the selective elimination of mitochondria by lysosomes. A number of proteins including a ubiquitin ligase (Parkin), a serine threonine kinase (Ulk1) and a BH3 family member (NIX), have recently been identified as mediators of selective mitophagy in mammalian cells. However, the mechanisms by which these proteins mediate mitophagy in mammalian cells are poorly understood. Interestingly, studies in yeast have identified a number of target proteins, which directly regulate the lysosomal-mediated degradation of mitochondria independently of macroautophagy [55]. The development of tools to distinguish between macroautophagy and other types of lysosomal-mediated mitochondrial degradation in mammalian cells may help to further our understanding of this emerging field of research. Autophagy studies described thus far in the heart do not distinguish between the bulk removal of proteins and organelles by autophagy and the selective elimination of mitochondria by mitophagy. The discussion below suggests that autophagy, and presumably mitophagy, can mediate both cardioprotection and cardiotoxicity in failing hearts.
Autophagy and cardioprotection
Several studies indicate that autophagy can act as a mediator of cardioprotection during HF, especially if mitophagy selectively removes damaged mitochondria, as suggested (for detailed review see [56]. Inhibition of autophagy in the mouse heart during pressure overload-induced HF has been shown to advance the progression of cardiac disease and coincide with mitochondrial aggregation and increased apoptosis in cardiomyocytes [57]. Glucose deprivation of cardiomyocytes induces autophagy and mediates cardioprotection in an AMP-activated protein kinase (AMPK)-dependent manner [58]. Inhibition of autophagy by siRNA-mediated knockdown of Beclin 1 results in reduced autophagy in the HL1 myocyte cell line following ischemia and reoxygenation injury and coincides with an increase in the activation of the proapoptotic protein, Bax [59]. Upregulation of autophagy in HL1 myocytes reduces bacterial lipopolysaccharide (LPS)-mediated ROS production and also protects myocytes against LPS toxicity [60]. Collectively, these studies suggest that autophagy may serve as an adaptive response to protect cardiac cells from injury during HF. Autophagy may serve to increase the turnover of misfolded proteins as well as damaged mitochondria produced in the failing heart which, if not rapidly removed, may induce apoptotic cell death. In addition, autophagy may provide the damaged myocardium with a source of energy in the absence of a supply of nutrients and thereby promote cell survival.
Cardiotoxic autophagy
Paradoxically, stress-induced autophagy has also been implicated in the pathogenesis of HF. Heterozygous disruption of the gene encoding Beclin 1 in mice (low Beclin 1) and over-expression of Beclin 1 in transgenic mice (high Beclin 1) demonstrate that Beclin 1-driven autophagy is up-regulated in a pressure overload-induced model of HF and contributes to pathological remodeling in the myocardium in a dose-dependent manner [61]. Autophagy and presumably, mitophagy, can also be induced in the mouse heart by ischemia and is further enhanced during reperfusion, at which time it has been shown to be associated with increased myocardial injury in a Beclin 1-dependent manner [58]. Furthermore, an inhibition of autophagy by Beclin 1-knockdown increases cell viability in H2O2-treated cardiomyocytes in culture [58]. Collectively, these studies suggest that autophagy can also serve as a maladaptive response to cardiac injury and contribute to disease pathogenesis in the heart. Autophagy may be utilized to promote cell death independently or in combination with other programmed cell death mechanisms. However, as discussed in the section above, an up-regulation of autophagy in cardiomyocytes has been shown to be cardioprotective in multiple studies (reviewed in [51]).
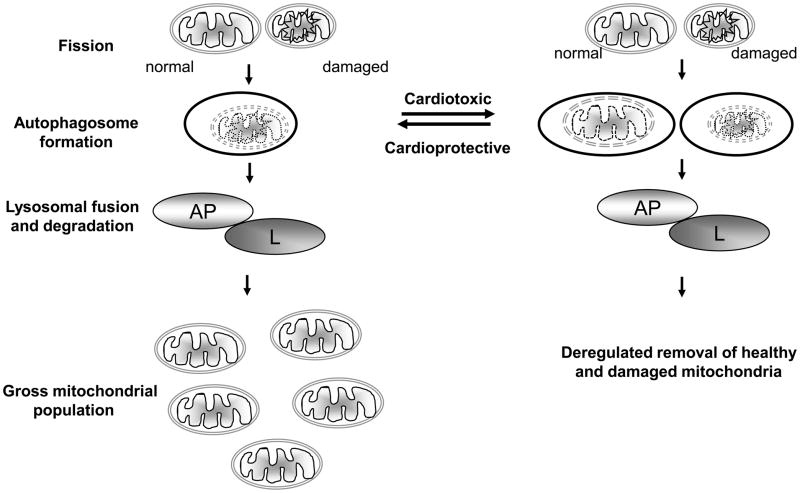
How then can autophagy and mitophagy mediate the opposing roles of cardioprotection and cardiotoxicity during HF? Perhaps low levels of autophagy are cytoprotective, and cytotoxic when over-activated (Figure 3). The pathways that regulate distinct stages of autophagy and selective mitophagy may determine if these processes are modestly increased and cardioprotective or strongly upregulated and cardiotoxic. Therefore, shifting the balance towards cardioprotective autophagy may provide a novel mechanism to maintain mitochondrial quality control and rescue failing hearts (Figure 3).
Figure 3. Mitophagy as a mediator of cardioprotection or cardiotoxicity.
Fission segregates damaged mitochondria from healthy (normal) mitochondria. Damaged mitochondria are then selectively sequestered into autophagosomes (AP) and targeted to the lysosomal compartment (L) for degradation by lysosomal hydrolases. Thus, mitophagy may be cardioprotective and serve to remove damaged mitochondria produced in the failing heart and thereby maintain a healthy mitochondrial population (left side). Alternatively, mitophagy may be over-active, non-selective and cardiotoxic, when removal of both damaged and healthy mitochondria occurs (right side).
Summary and Conclusion
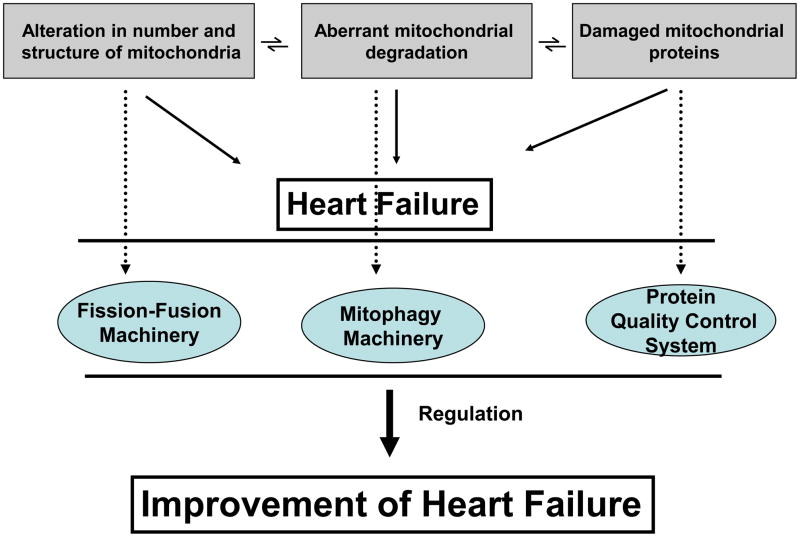
Mitochondria are crucial to myocardial function; they have an essential role in energy generation, cell redox potential, calcium homeostasis, fatty acid and glucose metabolism, ROS generation and mitochondria-dependent apoptosis. Various stress factors including humoral factors, mechanical pressure and toxins lead to abnormal mitochondrial metabolism and increased ROS generation and apoptosis and necrosis of cardiac cells. All these are detrimental to failing hearts regardless of their etiology. As important regulators of apoptosis and oxidative stress, mitochondria play a decisive role in cell death, thereby determining the extent of loss of cardiomyocytes, coronary endothelial cells and vascular smooth muscle cells. Loss of these cells leads to cardiac remodeling events such as cardiomyocyte hypertrophy, replacement fibrosis and inflammation and ultimately overt HF. So far, regulation of HF by targeting the mitochondria has mostly focused on preventing mitochondria-dependent apoptosis. Here we suggest that there are a number of other potential mitochondrial targets that may affect the progression of HF. To maintain normal mitochondrial structure, function and number, both under basal and under stress conditions, regulation of the PQC system, fusion-fission machinery and autophagy mechanisms are all required (Figure 4). In HF, aggregation and improper folding of proteins and damaged cell elements by protein adduct formation with free radicals are implicated in the disease pathogenesis. Therefore PQC holds significant importance in determining the mitochondrial fate (Table 1). Abnormal fusion and fission processes (either an increase or decrease) lead to a disproportionate number of mitochondria, altered intramitochondrial contents and disorganized shape during stress. Therefore drugs that shift the balance towards normal mitochondrial numbers and proper intra-mitochondrial contents and shape by regulating the fusion and fission machinery may provide novel therapeutic approaches for HF (Table 1). Removal of damaged mitochondria by mitophagy is crucial for maintaining a healthy population of mitochondria and could potentially be targeted for the treatment of HF (Table 1). Further studies are required to confirm whether maintaining or restoring normal function and structure and shape of this critical organelle by regulating the mitochondrial PQC system, the fusion-fission machinery and the autophagy machinery can serve as novel mitochondrial therapeutic targets for a variety of diseases associated with mitochondrial damage, including HF.
Figure 4. Mitochondrial regulatory mechanisms in heart failure.
Deregulation of the machinery responsible for protein quality control, fusion-fission and mitophagy leads to abnormal mitochondrial number, shape and structure, degradation and proteins in heart failure. Restoring these regulatory processes may ameliorate heart failure. (Dotted lines link with machinery responsible for particular processes.)
Table I.
Mitochondrial regulation-based therapeutic targets
| Strategic approach to target | Expected outcome | Some active research in the field | |
|---|---|---|---|
| Protein Quality Control System | To maintain the integrity of mitochondrial proteins | Proper mitochondrial function | [25,26], [21] |
| Fusion-Fission Machinery | Regulation of mitochondrial shape, structure and number | Quantity and quality of mitochondria retained. | [30–32], [42], [50] |
| Mitophagy Machinery | Removal of damaged mitochondria | Healthy mitochondrial population | [51,56], [57], [61] |
Acknowledgments
We thank Dr. Adrienne Gordon for critical review and editing of the manuscript.
Footnotes
DM-R is the founder and a share holder of KAI Pharmaceuticals, Inc, a company that plans to bring PKC regulators to the clinic. However, none of the work described in this study is based on or supported by the company. Other authors have no disclosure.
Conflict of Interest
DM-R is the founder of KAI Pharmaceuticals, Inc. However, none of the work in her academic laboratory is supported by the company. Other authors have no disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104 (24):2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97 (3):282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Kaye DM, Krum H. Drug discovery for heart failure: a new era or the end of the pipeline? Nat Rev Drug Discov. 2007;6 (2):127–139. doi: 10.1038/nrd2219. [DOI] [PubMed] [Google Scholar]
- 4.Doenst T, et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 86(3):461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 5.Marin-Garcia J, et al. Regional distribution of mitochondrial dysfunction and apoptotic remodeling in pacing-induced heart failure. J Card Fail. 2009;15 (8):700–708. doi: 10.1016/j.cardfail.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Murray AJ, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. 2008;44 (4):694–700. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Rosca MG, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80 (1):30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291 (4):H1489–1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 9.Jung C, et al. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77 (4):766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 10.Doughan AK, et al. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102 (4):488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 11.Ide T, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88 (5):529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 12.Bugger H, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 85(2):376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 13.Shen X, et al. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55 (3):798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 14.Ye G, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53 (5):1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 15.Ye G, et al. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52 (3):777–783. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmi SV, et al. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009;46 (6):421–440. [PubMed] [Google Scholar]
- 17.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77 (2):334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, et al. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280 (5):H2313–2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 20.Weisleder N, et al. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A. 2004;101 (3):769–774. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friguet B, et al. Mitochondrial protein quality control: implications in ageing. Biotechnol J. 2008;3 (6):757–764. doi: 10.1002/biot.200800041. [DOI] [PubMed] [Google Scholar]
- 22.Szweda PA, et al. Proteolysis, free radicals, and aging. Free Radic Biol Med. 2002;33 (1):29–36. doi: 10.1016/s0891-5849(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 23.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27 (2):306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugarte N, et al. Oxidized Mitochondrial Protein Degradation and Repair in Aging and Oxidative Stress. Antioxid Redox Signal. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 25.Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol. 2009;160 (9):718–725. doi: 10.1016/j.resmic.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Voos W, Rottgers K. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim Biophys Acta. 2002;1592 (1):51–62. doi: 10.1016/s0167-4889(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 27.Germain D. Ubiquitin-dependent and -independent mitochondrial protein quality controls: implications in ageing and neurodegenerative diseases. Mol Microbiol. 2008;70 (6):1334–1341. doi: 10.1111/j.1365-2958.2008.06502.x. [DOI] [PubMed] [Google Scholar]
- 28.Kohlhaas M, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 121(14):1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–144. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(Spec No 2):R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 31.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8 (11):870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 32.Eura Y, et al. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134 (3):333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 33.Meeusen S, et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127 (2):383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Cipolat S, et al. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101 (45):15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon Y, et al. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23 (15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrader M. Shared components of mitochondrial and peroxisomal division. Biochim Biophys Acta. 2006;1763 (5–6):531–541. doi: 10.1016/j.bbamcr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Smirnova E, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12 (8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James DI, et al. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278 (38):36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 39.Tondera D, et al. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci. 2005;118 (Pt 14):3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, et al. The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation. J Cell Sci. 2009;122 (Pt 13):2252–2262. doi: 10.1242/jcs.038513. [DOI] [PubMed] [Google Scholar]
- 41.Santel A, Frank S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60 (7):448–455. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 42.Parra V, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77 (2):387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 43.Hom J, et al. Regulation of mitochondrial fission by intracellular Ca(2+) in rat ventricular myocytes. Biochim Biophys Acta. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ausma J, et al. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem. 1998;186 (1–2):159–168. [PubMed] [Google Scholar]
- 45.Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46 (6):811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones M, et al. Ultrastructure of crista supraventricularis muscle in patients with congenital heart diseases associated with right ventricular outflow tract obstruction. Circulation. 1975;51 (1):39–67. doi: 10.1161/01.cir.51.1.39. [DOI] [PubMed] [Google Scholar]
- 47.Schaper J, et al. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83 (2):504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 48.Scholz D, et al. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5 (2):127–138. [PubMed] [Google Scholar]
- 49.Chen L, et al. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84 (1):91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27 (2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim I, et al. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462 (2):245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsunaga K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11 (4):385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 54.Yogalingam G, Pendergast AM. Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J Biol Chem. 2008;283 (51):35941–35953. doi: 10.1074/jbc.M804543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793 (9):1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Gottlieb RA, Carreira RS. AUTOPHAGY IN HEALTH AND DISEASE: V. Mitophagy as a Way of Life. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakai A, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13 (5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 58.Matsui Y, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100 (6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 59.Hamacher-Brady A, et al. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281 (40):29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 60.Yuan H, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296 (2):H470–479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu H, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117 (7):1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]