Summary
Multi-subunit protein complexes pose a challenge to the coordinated regulation of individual components. We show how the yeast transactivating factor Met4 functions as a component of the SCFMet30 ubiquitin ligase to synchronize its own activity with co-factor assembly. Cells maintain Met4 in a dormant state by a regulatory ubiquitin chain assembled by SCFMet30. Nutritional and heavy metal stress block Met4 ubiquitylation resulting in Met4 activation, which induces a stress response program including cell cycle arrest. Met4 relies on assembly with various co-factors for promoter binding. We report here that the stability of these DNA-binding co-factors is regulated by SCFMet30. Remarkably, the transcriptional activator Met4 functions as a substrate specificity factor in the context of SCFMet30/Met4 to coordinate co-factor degradation with its own activity status. Our results establish an additional layer for substrate recruitment by SCF ubiquitin ligases, and provide conceptual insight into coordinated regulation of protein complexes.
Introduction
Most if not all processes in the cell are governed by multi-subunit protein complexes (Alberts, 1998). They are assembled from individual proteins with distinct activities that together form the functional entity, and regulation of the individual components thus needs to be coordinated. Ubiquitylation regulates many aspects of protein complex dynamics, and relies itself on various multi-protein components. The covalent modification of substrate proteins with the small protein ubiquitin requires the E1-E2-E3 reaction cascade (Hershko and Ciechanover, 1998). The E3 ubiquitin ligases confer substrate specificity to this process. One large class of ubiquitin ligases are the SCF ligases (Petroski and Deshaies, 2005a). They are multi-protein complexes consisting of the RING-finger protein Rbx1/Hrt1/Roc1, the scaffold protein Cul1 (Cdc53 in yeast), and Skp1, which links the complex to the forth component, the substrate recruiting F-box protein (Bai et al., 1996). Diversity is achieved because cells express a variety of F-box proteins with different substrate binding characteristics. Each F-box protein is thought to form a distinct SCF ubiquitin ligase that regulates ubiquitylation of a defined set of substrates (Petroski and Deshaies, 2005a; Willems et al., 2004). Substrates of individual SCF ligases are often functionally unrelated and their ubiquitylation usually targets them for degradation by the 26S proteasome. A notable exception is SCFMet30, which ubiquitinates the transcriptional activator Met4 (Kaiser et al., 2000; Patton et al., 2000). Ubiquitylation of Met4 often directly blocks its ability to induce expression of target genes, but does not induce Met4 degradation (Chandrasekaran et al., 2006; Flick et al., 2004; Flick et al., 2006; Kuras et al., 2002). Proteolysis of polyubiquitylated Met4 is prevented by a cis-acting ubiquitin interacting motif (UIM) in Met4 that shields the canonical degradation signal, a K48-linked polyubiquitin chain, from recognition by the 26S proteasome (Flick et al., 2006). The SCFMet30 ubiquitin ligase and its substrate Met4 form the center of a regulatory network that coordinates the metabolic pathways of sulfur containing compounds with cell cycle progression (Kaiser et al., 2006). In addition, the cellular response to cadmium and arsenic stress is coordinated by the SCFMet30/Met4 system (Barbey et al., 2005; Wheeler et al., 2003; Yen et al., 2005), and aspects of phospholipid transport and tolerance to zinc deficiency were linked to this pathway (Schumacher et al., 2002; Wu et al., 2009).
During normal growth conditions Met4 is continuously polyubiquitylated and is maintained in the inactive, polyubiquitylated form in the nucleus. Under conditions where sulfur compounds are limiting or during cadmium and arsenic exposure, ubiquitylation of Met4 is inhibited, and deubiquitylation converts Met4 into an active transcription factor (Barbey et al., 2005). Active Met4 induces expression of a group of genes to increase biosynthesis of sulfur containing amino acids (Lee et al., 2009). In addition, fully active Met4 induces a complex cell cycle arrest to maintain cellular and genetic integrity under nutritional and heavy metal stress (Patton et al., 2000; Su et al., 2005; Yen et al., 2005). Being devoid of intrinsic DNA binding ability, Met4 requires several other proteins to coordinate this response. Therefore the functional Met4 transcription complex is formed with at least four other proteins, Met31, Met32, Met28, and Cbf1. Interestingly, all these proteins are required to regulate the biosynthesis pathways of methionine, cysteine, and SAM, but only Met32 is a potent cell cycle inhibitor during the stress response. Here we demonstrate that degradation of Met32 is important for cell cycle progression and that the bZIP transcription factor Met4 has a dual function as both transactivator and substrate specificity factor in the context of the ubiquitin ligase SCFMet30/Met4. The latter role of Met4 regulates degradation of its own DNA-binding co-factors Met32, Met31, and Cbf1, thereby controlling the abundance of the transcription factor complex. The dual role of Met4 achieves coordinated regulation of cell cycle and metabolic responses. Furthermore, our results add an additional functional layer to our understanding of ubiquitin- ligase function and demonstrate how substrate choice of SCF-type ubiquitin ligases is expanded and regulated.
Results
Regulation of the Met4-transcription complex by non-proteolytic roles of ubiquitylation and co-factor degradation
To analyze dynamics of the components of the Met4 transcription factor complex during nutritional stress, we grew cells in the presence of methionine, activated Met4 by shifting cells to growth medium lacking methionine and then re-repressed the response by addition of methionine (Figure 1A). Met4 activation and repression was obvious by changes in Met4 modifications, which has been shown to correlate well with the activity state such that polyubiquitylated Met4 specifies the repressed state, and de-ubiquitylated, phosphorylated Met4 characterizes the active state (Flick et al., 2004; Kaiser et al., 2000; Kuras et al., 2002). Steady-state Cbf1 levels were unchanged, Met31 protein abundance was slightly higher in methionine depleted growth media, and Met32 and Met28 protein levels changed dramatically being most abundant when Met4 was active (Figure 1A). Because MET28 expression is controlled by Met4 (Kuras et al., 1997; Lee et al., 2009), its changing protein levels are likely a reflection of transcriptional regulation. In contrast, Met31 and Met32 appears to be primarily regulated at the posttranscriptional level (Lee et al., 2009), although a transient increase in MET32 expression during methionine depletion can be observed (Figure S1). Regardless, the rapid disappearance of both Met28 and Met32 after addition of methionine suggested an active degradation pathway for these factors. Rapid degradation of Met32 was particularly interesting as it might be critical for efficient recovery from nutritional and heavy metal stress, because Met32 is a potent inducer of a cell cycle arrest under these conditions.
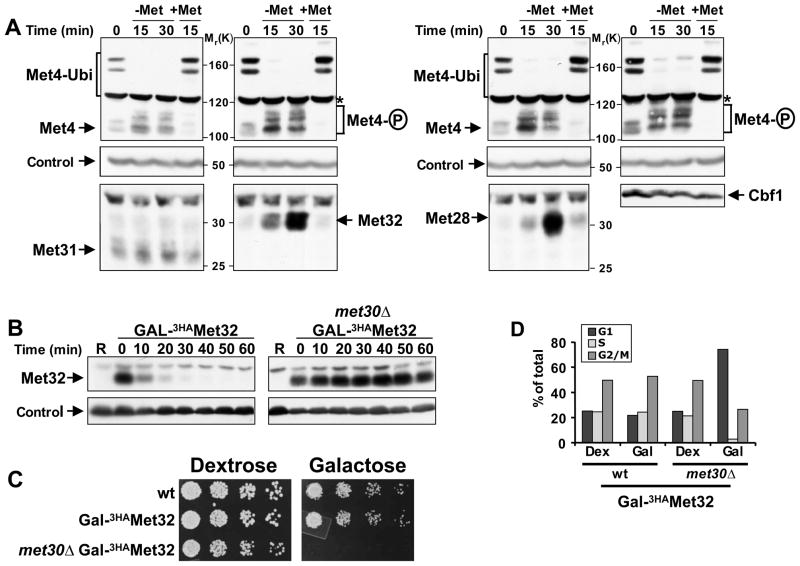
Figure 1. Met32 degradation by SCFMet30 is required for cell proliferation.
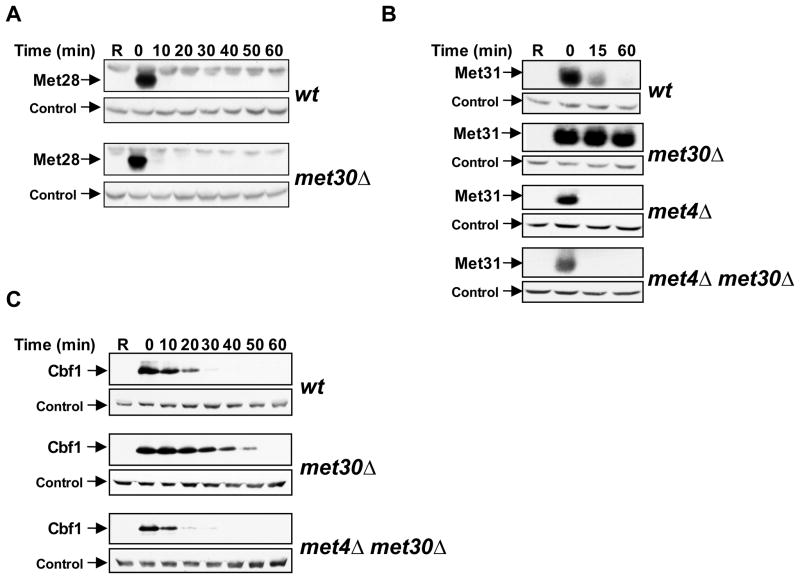
(A) Regulation of Met4 transcription complex components. Cells expressing Met313HA, Met323HA, Met283HA, or Cbf13HA from their endogenous loci were grown in minimal medium supplemented with 1mM methionine (+Met), shifted to medium without methionine (−Met) for 30′ to activate Met4, before methionine was added back to the cultures for an additional 15′ (+Met) to inactivate Met4. Cell lysates were analyzed by immunoblotting using anti-HA antibodies (Met28, Met32, Met31, Cbf1), and anti-Met4 antibodies to detect Met4 activation as indicated by loss of the ubiquitylated forms and appearance of phosphorylated species. The proteasome subunit Rpt1 was detected as a loading control.
(B) Met32 degradation depends on the F-box protein Met30. Met32 stability was analyzed by promoter shut-off experiments (“gal-shut-off“) and immunoblotting using anti-HA antibodies. R: uninduced sample grown in raffinose medium.
(C) Degradation of Met32 is essential for cell proliferation. Five fold serial dilutions of yeast strains as indicated were spotted onto YEP-dextrose (no Met32 expression) and YEP-galactose plates (Met32 expression). Plates were incubated at 30° C.
(D) Stabilization of Met32 induces a G1 cell cycle arrest. Yeast cells as indicated were grown in YEP-dextrose or YEP-galactose for 4hrs. Cell cycle distribution was analyzed by flow cytometry.
SCFMet30-dependent degradation of Met32 is required for cell proliferation
We sought to further characterize Met32 degradation, because this pathway could be a key component in induction of cell cycle arrest. Cells expressing Met32 under control of the tightly regulated GAL1 promoter were used to transiently induce expression of Met32 and protein stability was analyzed (Figure 1B). Met32 was very unstable but completely stabilized by deletion of the F-box protein Met30 (Figure 1B). Degradation of Met32 was essential for cell proliferation because its expression in cells deficient in Met32 degradation (met30Δ mutants) blocked cell division, whereas Met32 overexpression had no effect on wild-type cells, which have a potent Met32 degradation pathway (Figure 1B and 1C). Analysis of cell cycle profiles revealed that stabilization of Met32 induced primarily arrest in the G1 phase of the cell cycle with a smaller population arrested in G2/M (Figure 1D).
Met4 recruits Met32 to the SCFMet30 ubiquitin ligase
The F-box protein Met30 is a component of the SCFMet30 ubiquitin ligase. To address whether SCFMet30 is directly involved in Met32 ubiquitylation we conducted co-immunopurification experiments to probe for a physical interaction between the F-box protein Met30 and the putative substrate Met32 (Figure 2A). Met30 and Met32 interacted in vivo. It has previously been demonstrated that Met4 is a substrate for SCFMet30 and a Met30/Met4 complex can be detected in vivo (Kaiser et al., 2000; Rouillon et al., 2000). In addition, Met4 interacts with its co-factor Met32 in vivo and in vitro (Blaiseau and Thomas, 1998; Su et al., 2008), and it was therefore possible that the Met30/Met32 interaction we detected was indirect through the mutual binding partner Met4. Indeed, no interaction between Met30 and Met32 could be detected in met4Δ mutants, suggesting that Met4 mediates the Met30/Met32 interaction (Figure 2A).
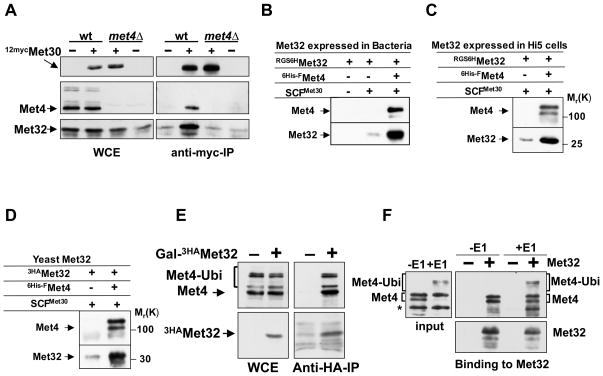
Figure 2. Met4 recruits Met32 to the SCFMet30 ubiquitin ligase in vivo and in vitro.
(A) In vivo interaction between Met30 and Met32 depends on Met4.
Wild-type cells and met4Δ mutants expressing endogenous 12mycMet30 and GAL1-3HAMet32 were grown in galactose medium containing 1mM. 12mycMet30 was immunopurified from total cell lysates and immunocomplexes were analyzed with anti-myc, anti-Met4, and anti-HA antibodies. Wild-type cells and met4Δ mutants expressing untagged Met30 were processed in parallel as controls.
(B and C) In vitro binding of recombinant Met32 expressed in E. coli and insect cells. Immobilized SCFMet30 and SCFMet30/Met4 complexes produced in insect cells were incubated with RGS6HMet32 expressed in E. coli (panel B), or insect cells (panel C). Bound proteins were eluted and analyzed by immunoblotting using anti-Met4 and anti-RGS4His antibodies.
(D) In vitro binding of Met32 expressed in yeast. Experiment as in panel B, except that 3HAMet32 expressed in yeast cells (met4Δ met30Δ) was used.
(E) Met32 binds to ubiquitylated and de-ubiquitylated Met4 in vivo. 3HAMet32 was immunopurified from yeast cells and immunocomplexes were analyzed by Western blotting using anti-HA and anti-Met4 antibodies. Cells expressing untagged Met32 were used as control.
(F) Met32 binds to ubiquitylated and de-ubiquitylated Met4 in vitro. Recombinant Met4 was bound to recombinant SCFMet30 and ubiquitylated in vitro to generate SCFMet30/Met4 containing ubiquitylated Met4. The same complex but containing non-ubiquitylated Met4 was obtained by omitting E1 from the reaction. Both complexes were incubated with immobilized RGS6His-3HAMet32 purified from E. coli. Bound fractions were analyzed by immunoblotting with anti-HA and anti-Met4 antibodies. Anti-HA beads without RGS6His-3HA Met32 were used as control.
Interactions between ubiquitin ligases and substrates are often transient because substrate binding initiates degradation. The lack of a detectable Met30/Met32 interaction in vivo in met4Δ mutants was therefore no conclusive proof for Met4-dependendent binding, although this result supported such a hypothesis. To directly address this question we used recombinant proteins to analyze binding in vitro. Components of SCFMet30 were co-expressed in insect cells and the recombinant SCFMet30 complex was purified. Consistent with previous reports (Chandrasekaran et al., 2006) Met4 expressed in insect cells efficiently associated with SCFMet30. Met32 was produced in E. coli, and in accordance with our in vivo findings, Met32 could only be efficiently recruited to SCFMet30 in the presence of Met4 (Figure 2B). Similar results were obtained using Met32 expressed in insect cells (Figure 2C), or yeast (Figure 2D). In both cases Met4 mediated efficient binding of Met32 to the ubiquitin ligase SCFMet30, excluding the possibility that posttranslational modifications on Met32 were required to create a recognition site for Met30. These results make the intriguing suggestion that the transcriptional activator Met4, which itself is a substrate of SCFMet30, also functions as a substrate specificity factor in the context of SCFMet30, and that Met32 ubiquitylation depends on the extended ubiquitin ligase SCFMet30/Met4.
The activity of Met4 as a transcription factor is strictly controlled by its regulatory polyubiquitin chain. We therefore asked whether the potential role of Met4 as an extended SCFMet30 substrate adaptor for Met32 is influenced by Met4 polyubiquitylation. We immunopurified Met32 from yeast cells and analyzed which modified species of Met4 were bound to Met32 (Figure 2E). No enrichment of any species of Met4 as compared to the total cell lysates was observed, suggesting that the substrate recruitment function of Met4 is not regulated by polyubiquitylation. To further address this question in vitro, we made use of a reconstituted, recombinant Met4 in vitro ubiquitylation assay (Aghajan et al., 2010; Chandrasekaran et al., 2006). Met4 was bound to recombinant SCFMet30 and ubiquitylated in vitro. The SCFMet30/Met4 complex was then incubated with immobilized Met32. Both ubiquitylated and non-ubiquitylated Met4 efficiently recruited Met32 confirming that the possible role of Met4 as a substrate adapter is independent of its ubiquitylation status (Figure 2F).
Two distinct ubiquitylation pathways regulate Met32 stability
Our results imply that Met4 is essential for ubiquitylation and degradation of Met32. Unexpectedly, Met32 was rapidly degraded in cells lacking MET4 (Figure 3A). Thus, a second, independent degradation pathway for Met32 must be active in met4Δ mutants because deletion of MET30 in met4Δ mutants had no effect on Met32 degradation, while it completely blocked Met32 degradation in cells containing Met4 (Figure 1B). Similarly, Met32 was significantly stabilized after inactivation of temperature sensitive Cdc34-3 or Cdc53-1, however degradation of Met32 was fully restored when MET4 was deleted in these mutants (Figure 3B). These experiments suggest two pathways for Met32 degradation. One pathway strictly depends on SCFMet30/Met4, and the second pathway requires neither Cdc53 nor Cdc34 and is thus most likely independent of SCF-type ubiquitin ligases. Importantly, binding of Met32 to Met4 must protect from the second pathway because Met32 was completely stabilized in met30Δ mutants (first pathway inactive) as long as Met4 was present.
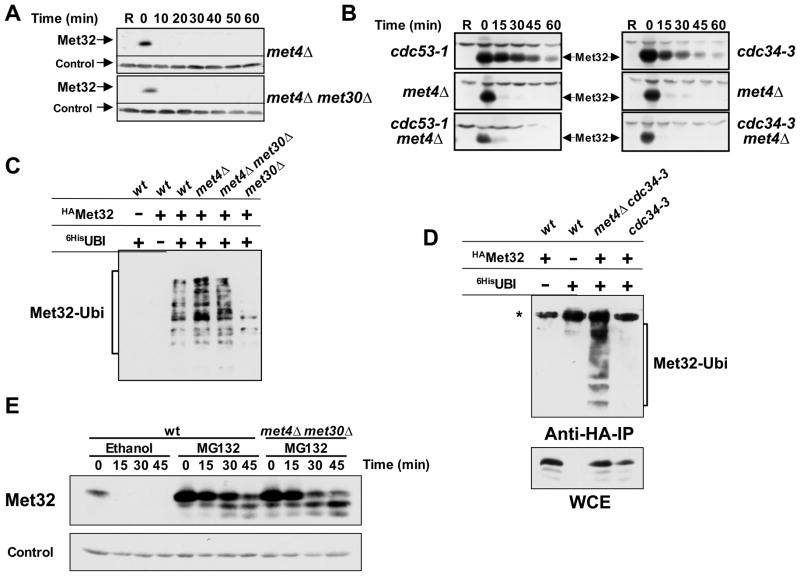
Figure 3. Met32 is degraded by two independent degradation pathways.
(A) Met30-independent degradation of Met32 in the absence of Met4. Met32 stability was assayed as described for figure 1B in met4Δ mutants and met4Δ met30Δ double mutants.
(B) The second degradation pathway of Met32 is independent of Cdc53 and Cdc34. Met32 degradation was analyzed in the mutants indicated as described for figure 1B, except that cells were shifted to non-permissive temperature (35°C) to inactivate Cdc53-1 or Cdc34-3 for 30min prior to the addition of galactose.
(C) Met32 ubiquitylation in vivo. Cells as indicated expressing GAL1-3HAMet32 and CUP1-6xHisubiquitinG76A were cultured in raffinose medium containing 1mM methionine. Expression of 6xHisubiG76A was induced for 2 hrs by addition of 100μM CuSO4, galactose was added for 1 h to induce 3HAMet32 expression, and cells were treated for 45 min with 50μM MG-132 to block proteasome activity. Ubiquitylated proteins were purified under denaturing conditions on Ni2+-sepharose beads and analyzed by immunoblotting using anti-HA antibodies. All strains were pdr5Δ mutants to increase permeability for MG-132 (Fleming et al., 2002).
(D) Cdc34 is required for Met32 ubiquitylation in vivo by the SCFMet30 pathway but not by the second degradation pathway. Experiment as in panel C, but cells were shifted to 35°C 30 minutes before addition of galactose to inactivate Cdc34-3. The asterisk indicates a cross-reacting band that was only visible when cells were grown at high temperature.
(E) Both Met32 degradation pathways are proteasome-dependent. Met32 stability was assayed as described for figure 1B except that cells were treated with 50μM MG-132 or solvent control (ethanol) 20 minutes before adding dextrose.
We next asked whether the second degradation pathway involved ubiquitylation of Met32. To this end we expressed 6xHis-tagged ubiquitin in various mutants, purified all ubiquitylated proteins under fully denaturing conditions on Ni2+-sepharose and detected ubiquitylated Met32 by immunoblotting (Figure 3C). Met32 was ubiquitylated in wild-type cells, met4Δ mutants, and met4Δ met30Δ double mutants to a similar extend. The latter two mutant backgrounds use only the second pathway for Met32 degradation, which is thus likely dependent on ubiquitylation. Almost no ubiquitylated Met32 was detected in met30Δ single mutants, consistent with Met32 stabilization in this mutant (Figure 1B). Similarly, Met32 ubiquitylation was blocked in cdc34-3 mutants in the presence of the SCFMet30/Met4 ligase, but restored when MET4 was deleted (Figure 3D). Furthermore, both Met32 degradation pathways were sensitive to the proteasome inhibitor MG-132 (Figure 3E). Together these results demonstrate dependence on the ubiquitin-proteasome system for both pathways. In addition, the in vivo ubiquitylation experiments (Fig. 3C and 3D) support the idea that Met4 binding prevents ubiquitylation of Met32 by the second, SCFMet30/Met4-independent pathway, which most likely only acts on unbound Met32. This pathway, hereafter referred to as the second Met32 degradation pathway, is probably important to eliminate unproductive co-factors, that is co-factors that are not in a complex with their transactivating partner Met4. Thus, Met4 promotes both degradation and stabilization of its co-factor Met32.
SCFMet30 is regulated by nutrient and heavy metal stress (Kaiser et al., 2006) and these signals are therefore likely to control degradation of Met32 via SCFMet30/Met4. In contrast, the second, SCFMet30/Met4-independent pathway, for Met32 degradation is constitutively active because degradation was unaffected by methionine or cadmium stress (Figure S2A).
We next attempted to identify components in the pathway that ubiquitylates unbound Met32. One attractive scenario was that Met4 binding induces a stable fold in Met32 and that unbound Met32 is degraded by a pathway that destroys misfolded proteins (Goldberg, 2003). However, Met32 was rapidly degraded in mutants deficient for either the cytosolic or the nuclear quality control pathway (Figure S2 B and C) (Gardner et al., 2005; Seufert and Jentsch, 1990). Furthermore, none of the other known E2s seemed to play a significant role in Met32 degradation by the SCFMet30/Met4-independent pathway (Figure S2), suggesting that degradation of unbound Met32 is mediated by several redundantly functioning E2s. Although these genetic experiments suggest that Met32 is properly folded even in the absence of Met4 we cannot exclude that structural disturbance in Met32 is detected and serves as degradation signal.
Even though we were unable to identify components of the pathway that targets unbound Met32, it is evident that Met4 is a central regulator in both Met32 degradation pathways such that Met4 stimulates degradation by SCFMet30/Met4 and prevents degradation by the second pathway. Together these results suggest a mechanism by which Met4 restricts its co-factor Met32 to the active transcription complex.
To more directly address the importance of the Met4/Met32 interaction for protection from the second degradation pathway, we deleted residues 374 to 403 in Met4, which have been shown to form the Met32 binding domain (Blaiseau and Thomas, 1998). Met32 degradation in met30Δ met4Δ double mutants is solely dependent on the second degradation pathway and, consistent with our model, expression of the wild type Met4 was protective and completely blocked Met32 degradation (Figure 4A). In contrast, expression of Met4Δ374–403 could not protect Met32 (Figure 4A), confirming that binding to Met4 shields Met32 from the second degradation pathway. Furthermore, the Met32 binding domain in Met4 was essential to induce cell cycle arrest because cells expressing Met4Δ374–403 failed to block cell proliferation even when Met32 was overproduced (Patton et al., 2000) (Figure 4B). These results further support the notion of two Met32 degradation pathways, which are important for cell proliferation and are inversely controlled by Met4.
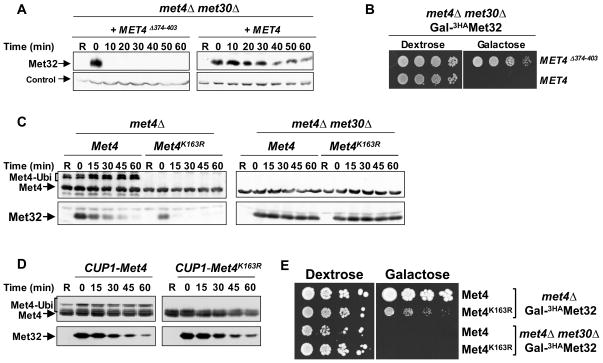
Figure 4. Met32 degradation by SCFMet30/Met4 is independent of Met4 ubiquitylation.
(A) Met4 binding blocks degradation of Met32 by the second degradation pathway. met4Δ met30Δ double mutants expressing GAL1-3HAMet32 and harboring centromeric plasmids expressing either wild-type Met4 or Met4Δ374-403 under control of the MET4 promoter, were analyzed for Met32 stability as described for figure 1B.
(B) The Met4/Met32 interaction is required for induction of cell cycle arrest. Serial dilutions of the yeast strains indicated were spotted onto either dextrose (Met32 expression off) or galactose (Met32 expressed) plates. Plates were incubated at 30°C.
(C) Met32 degradation by SCFMet30 is independent of Met4 ubiquitylation. Experiments as described for panel A except that the centromeric plasmids expressed Met4 and Met4K163R.
(D) Overexpression of Met4 or Met4163R stabilizes Met32. Experiment as in panel C except that Met4 and Met4163R were overexpressed from the CUP1 promoter, which was induced by addition of 25μM CuSO4 to the growth media.
(E) Stabilization of Met32 is important for cell cycle arrest. Serial dilutions of cells as indicated were spotted on dextrose containing (Met32 expression off) and galactose containing (Met32 expressed) plates and incubated at 30°C.
Met4 ubiquitylation regulates Met4 transcription factor activity but not its function in Met32 degradation
The activity of Met4 as a transcriptional activator is tightly regulated by its ubiquitylation status, such that polyubiquitylated Met4 is inactive and de-ubiquitylated Met4 is active (Flick et al., 2004; Kaiser et al., 2000; Kuras et al., 2002). Ubiquitylation of Met4 is catalyzed by SCFMet30 (Kaiser et al., 2000; Rouillon et al., 2000). We next asked whether the function of Met4 in promoting Met32 degradation is also regulated by the Met4 ubiquitylation status. To this end we used the Met4K163R mutant, which cannot be ubiquitylated due to the conversion of the single ubiquitin acceptor lysine into arginine (Flick et al., 2004). Expression of both the ubiquitylated wild-type form of Met4 and Met4K163R promoted efficient Met32 degradation (Figure 4C). Thus, unlike the transactivator function, the role of Met4 in substrate recruitment and Met32 degradation was not influenced by the ubiquitin chain attached to K163. The somewhat slower Met32 degradation rate in cells expressing wild-type Met4 is likely due to the higher Met4 level as compared to Met4K163R, which probably exceeds SCFMet30 capacity, and therefore protects a fraction of Met32 from the second degradation pathway. Accordingly, overexpression of wild-type Met4 or Met4K163R significantly delayed Met32 degradation (Figure 4D). Finally, deletion of MET30 in cells expressing Met4K163R or wild-type Met4 completely stabilized Met32 confirming that wild-type Met4 and Met4K163R promote SCFMet30/Met4-dependent Met32 degradation (Figure 4C).
SCFMet30 activity is essential for cell proliferation and its inactivation in response to nutritional and heavy metal stress induces a cell cycle arrest (Barbey et al., 2005; Kaiser et al., 2000; Patton et al., 2000; Su et al., 2005; Yen et al., 2005). An important aspect of the cell cycle block observed in response to SCFMet30 inactivation is the concomitant inhibition of Met4 ubiquitylation at lysine 163, because overexpression of Met4K163R is sufficient to induced cell cycle arrest even when SCFMet30 is active (Flick et al., 2004). However, overexpression of Met4 also leads to stabilization of Met32 (Figure 4D), and the individual contributions of Met4 activation and Met32 stabilization to the cell cycle arrest is therefore unclear. To differentiate between these two effects of SCFMet30 inactivation we analyzed cells expressing endogenous levels of Met4K163R, which have fully active Met4 and efficiently degrade Met32 (Figure 4C). Met4K163R expressing cells proliferated, albeit at a reduced rate, and arrested only when Met32 degradation was blocked by deletion of MET30 (Figure 4E, “Galactose”). Thus, both Met32 degradation and Met4 inactivation are critical cell cycle functions of SCFMet30.
Degradation of other Met4 co-factors
Met4 associates with at least four different co-factors to form an active transcription complex (Blaiseau and Thomas, 1998). We asked whether in addition to its role in Met32 degradation, Met4 also regulates stability of its other co-factors. Steady-state levels of Cbf1 during a methionine-depletion time course were unchanged, slightly regulated for Met31, and dramatically regulated for Met32 and Met28 (Fig. 1A). Determination of steady-state protein levels can only provide limited information about protein stability, and direct analysis of protein degradation showed that Cbf1, Met31, and Met28 were all unstable proteins (Figure 5A, B, and C). Met28 degradation was independent of SCFMet30, demonstrating that binding to Met4 is not sufficient for targeting to SCFMet30. Interestingly, Met31 and Cbf1, the two DNA-binding components besides Met32, were degraded in an SCFMet30-dependent manner (Figure 5B and C). Furthermore, in analogy to Met32, a second degradation pathway specifically targets the unbound fraction of Cbf1 and Met31, and this pathway is blocked by co-factor binding to Met4 (Figure 5B and 5C). Only the fraction of Met31 and Cbf1 that is bound to Met4 is degraded by SCFMet30, which allows selective control of the transcriptionally relevant population of these co-factors. Thus, inactivation of SCFMet30 might not dramatically alter total Met31 or Cbf1 levels because only a small fraction of these proteins might be targeted by SCFMet30/Met4. These experiments suggest a concerted regulation of co-factor stability by a dual mechanism mediated through Met4. In the context of the Met4 transcription complex the DNA binding components Cbf1, Met31, and Met32 are degraded by SCFMet30 (Figure 1B, 5B, and 5C). Active SCFMet30 thus prevents the formation of a stable transcription complex by constitutive degradation of the DNA-binding co-factors, which provides a molecular mechanism for the observation that Met4 does not stably associate with its target promoters during normal growth conditions (Kuras et al., 2002). Activation of the pathway during nutritional or heavy metal stress occurs through SCFMet30 inactivation, which results in selective stabilization of DNA-binding cofactors bound to Met4 and consequently promotes Met4-transcription complex recruitment to target promoters.
Figure 5. Met31 and Cbf1, but not Met28 are degraded by SCFMet30.
(A) Met28 degradation is independent of SCFMet30. Stability of Met28 was analyzed as described for figure 1B. MET32 was deleted in these cells to suppress lethality of met30 mutants (Patton et al., 2000). Control: anti-Rpt1.
(B, C) Met31 and Cbf1 are degraded by SCFMet30. Experiments as in panel A, but strains expressing 3HAMet31 (panel B), or 3HACbf1 (panel C) were analyzed.
In vitro reconstitution of the Met4-dependent ubiquitin ligase function of SCFMet30
Protein degradation and binding studies strongly support the idea that the transcriptional activator Met4 functions as both a non-proteolytic substrate and a substrate recruiting factor for SCFMet30. To unambiguously demonstrate a direct role of Met4 in co-factor degradation we reconstituted Met32 ubiquitylation in vitro. Active recombinant SCFMet30 was purified from insect cells (Figure 6A, left panel), and recombinant Met4 expressed in insect cells was bound to immobilized SCFMet30 as previously reported (Aghajan et al., 2010; Chandrasekaran et al., 2006). The ligase-substrate complex was eluted and the activity of the recombinant SCFMet30 complex confirmed by in vitro ubiquitylation of Met4 (Aghajan et al., 2010; Chandrasekaran et al., 2006) (Figure 6A, right panel). In vivo and in vitro binding results demonstrated that Met4 was required for Met32 recruitment to the SCFMet30 ligase (Figure 2). However, spatial proximity of a protein to the ligase is not sufficient for ubiquitylation because many SCF substrates are recruited as part of protein complexes, yet ubiquitylation is selective for the substrate component within the complex (Verma et al., 2001; Maniatis, 1999). Furthermore, the co-factor Met28 binds Met4, but unlike Met32 is not degraded in an SCFMet30-dependent manner (Figure 5A) (Kuras et al., 1997; Kuras et al., 1996). This is not due to an intrinsic resistance to degradation because Met28 is a highly unstable protein (Figure 5A). A specific topological presentation of proteins is probably required for efficient ubiquitylation. We therefore asked whether the SCFMet30/Met4 complex binds Met32 in a configuration that allows ubiquitin transfer. The immobilized SCFMet30/Met4 complex was incubated with recombinant Met32 purified from E. coli, the ligase substrate complex SCFMet30/Met4-Met32 was then eluted and the ubiquitylation reaction was initiated by addition of the reaction mix (Figure 6B). Both Met4 and Met32 were ubiquitylated in this reaction. We next asked whether ubiquitylation of Met4 is important for its function as a component of SCFMet30/Met4. To this end we used recombinant Met4K163R produced in insect cells to form the SCFMet30/Met4K163R complex. Replacing lysine at position 163 with arginine has been shown to block Met4 ubiquitylation in vivo and in vitro (Chandrasekaran et al., 2006; Flick et al., 2004). The SCFMet30/Met4K163R complex efficiently promoted Met32 ubiquitylation (Figure 6C). Together with in vivo experiments using cells expressing Met4K163R (Figure 4C), these results support the notion that in contrast to the transactivating activity, the function of Met4 in the context of the SCFMet30/Met4 ubiquitin ligase is not regulated by its ubiquitylation.
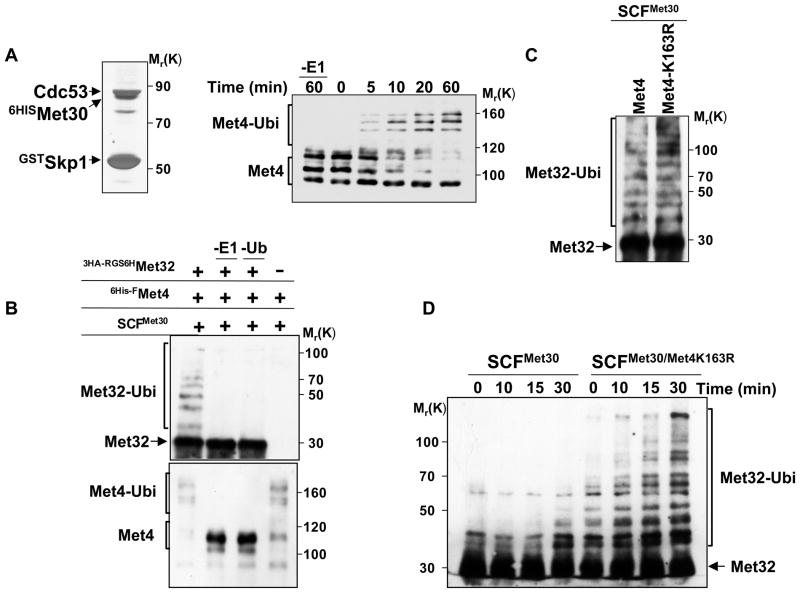
Figure 6. In vitro reconstitution of Met32 ubiquitylation by the SCFMet30/Met4 ubiquitin ligase.
(A) Recombinant, active SCFMet30. Recombinant SCFMet30 was purified on glutathione beads from insect cells co-expression GstSkp1, Cdc53, 6HisMet30, and Rbx1. Complexes were eluted, separated by SDS-PAGE and visualized by silver staining (left panel). The activity of the recombinant SCFMet30 was assayed by in vitro ubiquitylation of Met4 as described (Aghajan et al., 2010; Chandrasekaran et al., 2006). Reaction products were separated by SDS-PAGE and analyzed by immunoblotting using anti-Met4 antibodies. The asterisk indicates a cross-reacting band.
(B) In vitro ubiquitylation of Met32 by recombinant SCFMet30/Met4. Immobilized SCFMet30/Met4 complex were incubated with RGS6H-3HAMet32 purified from E. coli. The ligase-substrate complex was eluted with glutathione, and then incubated for 3 hrs at 30°C with ubiquitylation reaction mixture. Reactions were analyzed by immunoblotting using anti-HA and anti-Met4 antibodies.
(C) Met4K163R recruits Met32 to SCFMet30 and mediates Met32 ubiquitylation. Experiments as in panel B except that SCFMet30/Met4 and SCFMet30/Met4K163R were used as ubiquitin ligases.
(D) Met4 stimulates ubiquitylation of Met32.
Recombinant SCFMet30/Met4K163R complexes were purified as described above, eluted with glutathione and combined with purified RGS6H-3HAMet32 (25nM final concentration). Ubiquitylation reaction mixture was added and the reaction incubated at 30°C for the indicated time intervals. Reaction products were analyzed by immunoblotting using anti-HA antibodies.
Finally, to directly test the role of Met4 as a component of an ubiquitin ligase we compared Met32 ubiquitylation by SCFMet30 and SCFMet30/Met4K163R. The K163R mutant of Met4 was chosen in this experiment to specifically analyze the function of Met4 as a substrate-recruiting factor without being a substrate itself in this context. The SCF complexes with and without Met4K163R were purified from insect cells and eluted from glutathione beads. Purified Met32 and the ubiquitylation reaction mix were added to SCFMet30 or SCFMet30/Met4K163R, and Met32 ubiquitylation kinetics was analyzed (Figure 6D). Met4K163R greatly stimulated Met32 ubiquitylation by SCFMet30, likely due to increased Met32 recruitment. Together these results demonstrate that the transcriptional activator Met4 is a key component of the SCFMet30/Met4 ligase for ubiquitylation of its cofactor Met32, and provide evidence for expansion of substrate selection of SCF ligases by accessory factors. Importantly, our experiments show that these accessory factors can be themselves substrates of the same ligase, and can have distinct, additional biological activities, such as transactivation in the case of Met4.
Discussion
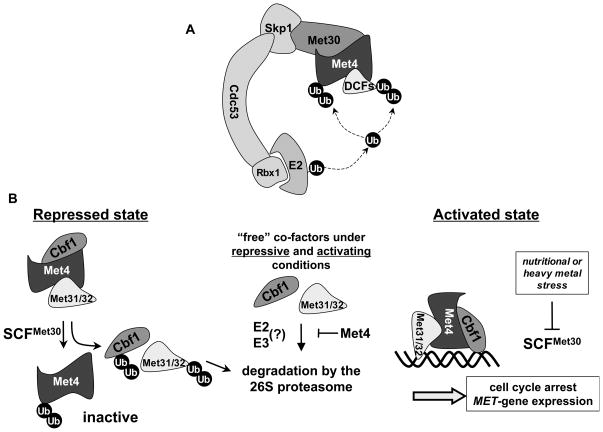
We report here that the transactivating factor Met4 controls polyubiquitylation and degradation of its DNA-binding co-factors by functioning as a substrate specificity factor for the ubiquitin-ligase SCFMet30/Met4. The concepts we describe in this study might ensure coordinated regulation of other multi-subunit protein complexes. In particular our results provide insight into how cells synchronize activation of a specific transcriptional program with cell cycle arrest in response to nutritional or heavy metal stress. Coordination is achieved by the triple role of Met4 as (i) a transactivating factor, (ii) a component of the ubiquitin ligase SCFMet30/Met4 that triggers degradation of the cell cycle inhibitor Met32, and (ii) as a binding partner that prevents co-factor degradation by other pathways (Figure 7). The abundance of Met32 and other DNA-binding cofactors in the Met4-transcription complex is synchronized with the activity state of Met4 as a transcription factor, which is inhibited by SCFMet30-dependent ubiquitylation (Figure 7). This avoids recruitment of non-productive transcription complexes to promoters. What is more, our model suggests a tight link between Met4 activity and Met32 stabilization at the single molecule level such that each deubiquitylated, active Met4 protein will protect its bound Met32 partner. We suggest that these mechanisms for concerted regulation at the level of individual protein complexes allow for a faster response to signals and are particularly receptive to fine-tuning.
Figure 7. Model for integrated transcription complex regulation.
(A) The transcriptional activator Met4 functions as a substrate recruitment component in the SCFMet30/Met4 ubiquitin ligase to regulate ubiquitylation and degradation of its own DNA-binding co-factors (DCFs).
(B) During repression Met4 recruits its co-factors to SCFMet30 for ubiquitylation and degradation. Met4 itself is inactivated as a transcription factor by a regulatory ubiquitin chain. Co-factors not bound to Met4 are degraded by a second degradation pathway. Stress conditions lead to inactivation of SCFMet30 and result in selective formation of productive Met4 transcription factor complexes. This is achieved because inhibition of SCFMet30 prevents both Met4 polyubiquitylation and degradation of Met4-bound cofactors. Note that degradation of co-factors not bound to Met4 is constitutive.
In addition to advancing conceptual understanding of protein complex regulation, our results provide new insight into substrate recruitment by SCF ubiquitin ligases. Eukaryotes have multiple F-box proteins (yeast cells about 20, mammals about 100), and are thus potentially able to form as many distinct SCF ligases. The temporally and spatially controlled ubiquitylation of likely hundreds of substrate proteins might require additional factors that modulate and/or dictate substrate recruitment to SCF-ligases (Yen and Elledge, 2008). Accordingly, an accessory factor for substrate recruitment to the human SCFSkp2 ubiquitin ligase has been described (Harper, 2001). The human cyclin dependent kinase (Cdk) inhibitor p27 is ubiquitylated by SCFSkp2/Cks1 (Ganoth et al., 2001; Spruck et al., 2001), whereby the small Cdk-binding protein Cks1 associates with Skp2 and significantly contributes to the formation of the substrate interaction surface for p27 (Hao et al., 2005). A similar role for cyclin T1 in recruitment of Cdk9 to SCFSkp2 has also been proposed but is controversial (Garriga et al., 2003; Kiernan et al., 2001). Our study describes that not only small, specialized protein binding factors such as Cks1 can be engaged to stabilize ligase substrate complexes, but that proteins with distinct and complex activities, such as transactivating factors like Met4, can be used as substrate recruitment factors in SCF ubiquitin ligases. Interestingly, Tian and colleagues (Tian et al., 2007) reported evidence that the transcription factor TAZ functions as a substrate recruitment factor for the transmembrane protein polycistin 2 in the context of SCFβ-Trcp. Even though the underlying biological rationale for engaging TAZ in polycystin 2 degradation is not obvious, these results are consistent with our study demonstrating that a transcription factor functions as a substrate specificity component in an SCF ubiquitin ligase.
Importantly, our results demonstrate that SCF ligases can use their own substrates to recruit additional targets. Thus, substrate selection by the cullin-RING ubiqutin ligases is more complicated than the current model of direct target recognition by substrate adapters proposes (Cardozo and Pagano, 2004; Petroski and Deshaies, 2005a; Willems et al., 2004). Mechanistically it is clear that proximity to the SCF complex is insufficient for ubiquitylation and degradation. This is exemplified by the stable CDKs that are bound to the highly unstable cyclins and CDK-inhibitors. More importantly, our results showed that in contrast to Cbf1, Met31, and Met32, the other Met4 co-factor, Met28, is not degraded in an SCFMet30-dependent manner. Nonetheless, Met28 is a highly unstable protein (Figure 5). Substrate recruitment factors like Met4 must thus have evolved to present ubiquitylation substrates in a particular configuration that facilitates ubiquitin transfer by the E2/SCF complex.
Together our results indicate that proteins with distinct cellular functions can act as substrate specificity factors of ubiquitin ligases and suggest a concept for how such dual activities coordinate regulation of individual components of protein complexes.
Experimental Procedures
Yeast strains and growth conditions
Yeast strains used in this study are listed in table S1 and are isogenic to 15DaubΔ (Reed et al., 1985). Standard culture conditions and yeast genetic methods were used (Guthrie and Fink, 1991). A general “gal-shut-off“ strategy was followed for determination of protein stability. Cells were grown in YEP medium supplemented with 2% raffinose to an A600= 0.3, galactose was added to a final concentration of 2% to induce expression controlled by the GAL1 promoter. After 60 minutes of gal-induction, cells were collected by filtration and resuspended in YEP with 2% dextrose to repress expression. Overexpression of Met4 and Met4163R under control of the CUP1 promoter was achieved by addition of 25μM CuSO4 final concentration. For cell spotting assays, cells were grown to mid-log phase, briefly sonicated, and 5-fold serial dilutions starting with 5000 cells were spotted onto agar plates.
Protein analyses and antibodies
Yeast protein extracts were prepared as previously described (Flick et al., 2006). We used polyclonal anti-Met4, 1:10000 (generous gift from M. Tyers), anti-myc and anti-HA (1:2000, Covance, Princeton, NJ) and anti-RGS4H (1:2000, Qiagen, Germantown, MD) for Western blotting and anti-Myc antibodies (SC-789-G, Santa Cruz Biotechnology, CA) for immunopurification.
Purification and analysis of ubiquitylated Met32
Cells expressing 6xHis-tagged ubiquitin-G76A (6xHisUbiG76A) under the control of the CUP1 promoter and carrying a GAL1-3HAMET32 allele were cultured in raffinose medium containing 1mM methionine to an A600= 0.3. Expression of 6xHisUbiG76A was induced for 2 hrs by addition of 100μM CuSO4 final concentration. For experiments using temperature sensitive cdc34-3 mutants, cells were shifted to 35°C for 30 min before addition of galactose. In either case galactose was added for 1 h to induce 3HAMet32 expression before addition of 50μM final concentration of the proteasome inhibitor MG-132 (American Peptide, Sunnyvale, CA) for 45 min. Total cellular ubiquitylated proteins were purified on Ni2+-sepharose beads under denaturing conditions (Kaiser and Tagwerker, 2005).
Recombinant proteins and protein complexes
Met32 was expressed in E. coli (BL21 DE3) or insect cells using the pQE-TriSystem His.Strep1 vector (Qiagen, Germantown, MD). The recombinant SCFMet30 complex was produced and purified from Hi5 insect cells as described (Aghajan et al., 2010; Chandrasekaran et al., 2006). The SCFMet30 concentration was approximately 0.15–0.2μM.
To make the recombinant SCFMet30/Met4 complex, SCFMet30 immobilized on glutathione sepharose beads was incubated for 2 hrs at 4 °C with total lysates of Hi5 insect cells expressing 6His-FlagMet4. The bead-bound SCFMet30/Met4 complex was either used for in vitro binding assays with recombinant Met32, or eluted for in vitro ubiquitylation assays as described (Aghajan et al., 2010; Chandrasekaran et al., 2006). Complexes containing Met4K163R were prepared similarly using insect cells expressing Met4K163R. Recombinant RGS6His-3HAMet32 and 6HisCdc34 were expressed and purified from E.coli (Petroski and Deshaies, 2005b).
In vitro binding of recombinant Met32 to SCFMet30/Met4
SCFMet30 and SCFMet30/Met4 were purified on glutathione sepharose beads, the bead-bound SCF complexes were incubated for 1 h at 4°C in 400μl lysis buffer with 50nM recombinant RGS6His-3HAMet32 purified from E. coli, or 500 μl of total lysates of Hi5 insect cells expressing RGS6HisMet32, or 1000μl of total yeast lysates (3mg/ml) prepared from a met4Δ met30Δ double mutant expressing 3HAMet32. Protein complexes were eluted from the beads and analyzed by immunoblotting. For binding of SCFMet30/Met4 to immobilized Met32, purified recombinant SCFMet30/Met4 was eluted from glutathione beads and incubated with ubiquitylation mix to generate ubiquitylated Met4. Non-ubiquitylated Met4 was treated identical except that E1 was lacking in the ubiquitylation reaction. RGS6His-3HAMet32 expressed in E. coli was immobilized on anti-HA beads and incubated with SCFMet30/Met4 containing ubiquitylated or de-ubiquitylated Met4.
In vitro ubiquitylation reaction
SCFMet30 and SCFMet30/Met4 were purified on glutathione sepharose beads and the bead-bound complexes were incubated for 1 h at 4°C in 400μl lysis buffer with 50nM recombinant RGS6His-3HAMet32 purified from E. coli. Complexes were washed with lysis buffer, eluted with 40mM glutathione in buffer U. The eluted Met32-bound SCFMet30/Met4 complex was incubated at 30 °C with ubiquitylation reaction mixture (250nM of E1, 4 μM Cdc34, 80μM of ubiquitin, 5 mM ATP). The concentration of SCFMet30 in the reactions was approximately 150nM. Alternatively (Figure 6D), SCFMet30 and SCFMet30/Met4R163K were eluted from glutathione beads and incubated with purified RGS6His-3HAMet32 (25nM) and ubiquitylation reaction mix at 30°C. Ubiquitylation reactions were analyzed by immunoblotting.
Supplementary Material
Acknowledgments
We are grateful to R. Deshaies, W. Harper, M. Petroski, D. Skowyra, and M. Tyers for expression constructs, antibodies, and advice on in vitro ubiquitylation. We thank H. Zhang for technical support, C. Papagiannis for comments on the manuscript, M. Tan and K. Hertel for help with the insect cell expression system, and the members of the Kaiser lab for helpful discussions. This work was supported by National Institutes of Health Grants GM66164 and GM66164AS1 (to P.K.). I.O. is a recipient of a National Institutes of Health Ruth Kirschstein Postdoctoral Fellowship T32 (CA-113265).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, et al. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol. 2010;28:738–742. doi: 10.1038/nbt.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell. 1998;92:291–294. doi: 10.1016/s0092-8674(00)80922-8. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofman K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 Connects Cell Cycle Regulators to the Ubiquitin Proteolysis Machinery through a Novel Motif, the F-Box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Barbey R, Baudouin-Cornu P, Lee TA, Rouillon A, Zarzov P, Tyers M, Thomas D. Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium. Embo J. 2005;24:521–532. doi: 10.1038/sj.emboj.7600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiseau PL, Thomas D. Multiple transcriptional activation complexes tether the yeast activator Met4 to DNA. Embo J. 1998;17:6327–6336. doi: 10.1093/emboj/17.21.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Deffenbaugh AE, Ford DA, Bailly E, Mathias N, Skowyra D. Destabilization of binding to cofactors and SCFMet30 is the rate-limiting regulatory step in degradation of polyubiquitinated Met4. Mol Cell. 2006;24:689–699. doi: 10.1016/j.molcel.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Fleming JA, Lightcap ES, Sadis S, Thoroddsen V, Bulawa CE, Blackman RK. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc Natl Acad Sci U S A. 2002;99:1461–1466. doi: 10.1073/pnas.032516399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick K, Ouni I, Wohlschlegel JA, Capati C, McDonald WH, Yates JR, Kaiser P. Proteolysis-independent regulation of the transcription factor Met4 by a single Lys 48-linked ubiquitin chain. Nat Cell Biol. 2004;6:634–641. doi: 10.1038/ncb1143. [DOI] [PubMed] [Google Scholar]
- Flick K, Raasi S, Zhang H, Yen JL, Kaiser P. A ubiquitin-interacting motif protects polyubiquitinated Met4 from degradation by the 26S proteasome. Nat Cell Biol. 2006;8:509–515. doi: 10.1038/ncb1402. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Nelson ZW, Gottschling DE. Degradation-mediated protein quality control in the nucleus. Cell. 2005;120:803–815. doi: 10.1016/j.cell.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Garriga J, Bhattacharya S, Calbo J, Marshall RM, Truongcao M, Haines DS, Grana X. CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2. Mol Cell Biol. 2003;23:5165–5173. doi: 10.1128/MCB.23.15.5165-5173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Gungor C, Taniguchi-Ishigaki N, Ma H, Drung A, Tursun B, Ostendorff HP, Bossenz M, Becker CG, Becker T, Bach I. Proteasomal selection of multiprotein complexes recruited by LIM homeodomain transcription factors. Proc Natl Acad Sci U S A. 2007;104:15000–15005. doi: 10.1073/pnas.0703738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology. Vol. 194. San Diego: Academic Press, Inc; 1991. [Google Scholar]
- Hao B, Zheng N, Schulman BA, Wu G, Miller JJ, Pagano M, Pavletich NP. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell. 2005;20:9–19. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Harper JW. Protein destruction: adapting roles for Cks proteins. Curr Biol. 2001;11:R431–435. doi: 10.1016/s0960-9822(01)00253-6. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Kaiser P, Flick K, Wittenberg C, Reed SI. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCF(Met30)-mediated inactivation of the transcription factor Met4. Cell. 2000;102:303–314. doi: 10.1016/s0092-8674(00)00036-2. [DOI] [PubMed] [Google Scholar]
- Kaiser P, Su NY, Yen JL, Ouni I, Flick K. The yeast ubiquitin ligase SCF-Met30: connecting environmental and intracellular conditions to cell division. Cell Div. 2006;1:16. doi: 10.1186/1747-1028-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P, Tagwerker C. Is This Protein Ubiquitinated? Methods Enzymol. 2005;399C:243–248. doi: 10.1016/S0076-6879(05)99016-2. [DOI] [PubMed] [Google Scholar]
- Kiernan RE, Emiliani S, Nakayama K, Castro A, Labbe JC, Lorca T, Nakayama Ki K, Benkirane M. Interaction between cyclin T1 and SCF(SKP2) targets CDK9 for ubiquitination and degradation by the proteasome. Mol Cell Biol. 2001;21:7956–7970. doi: 10.1128/MCB.21.23.7956-7970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Barbey R, Thomas D. Assembly of a bZIP-bHLH transcription activation complex: formation of the yeast Cbf1-Met4-Met28 complex is regulated through Met28 stimulation of Cbf1 DNA binding. Embo J. 1997;16:2441–2451. doi: 10.1093/emboj/16.9.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Cherest H, Surdin-Kerjan Y, Thomas D. A heteromeric complex containing the centromere binding factor 1 and two basic leucine zipper factors, Met4 and Met28, mediates the transcription activation of yeast sulfur metabolism. Embo J. 1996;15:2519–2529. [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Rouillon A, Lee T, Barbey R, Tyers M, Thomas D. Dual regulation of the met4 transcription factor by ubiquitin-dependent degradation and inhibition of promoter recruitment. Mol Cell. 2002;10:69–80. doi: 10.1016/s1097-2765(02)00561-0. [DOI] [PubMed] [Google Scholar]
- Lee TA, Jorgensen P, Bognar AL, Peyraud C, Thomas D, Tyers M. Systematic Dissection of Combinatorial Control by the Met4 Transcriptional Complex. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T. A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- Menant A, Baudouin-Cornu P, Peyraud C, Tyers M, Thomas D. Determinants of the ubiquitin-mediated degradation of the Met4 transcription factor. J Biol Chem. 2006;281:11744–11754. doi: 10.1074/jbc.M600037200. [DOI] [PubMed] [Google Scholar]
- Patton EE, Peyraud C, Rouillon A, Surdin KY, Tyers M, Thomas D. SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. Embo J. 2000;19:1613–1624. doi: 10.1093/emboj/19.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005a;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. In vitro reconstitution of SCF substrate ubiquitination with purified proteins. Methods Enzymol. 2005b;398:143–158. doi: 10.1016/S0076-6879(05)98013-0. [DOI] [PubMed] [Google Scholar]
- Reed SI, Hadwiger JA, Lorincz AT. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985;82:4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon A, Barbey R, Patton EE, Tyers M, Thomas D. Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCF(Met30)complex. Embo J. 2000;19:282–294. doi: 10.1093/emboj/19.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MM, Choi JY, Voelker DR. Phosphatidylserine transport to the mitochondria is regulated by ubiquitination. J Biol Chem. 2002;277:51033–51042. doi: 10.1074/jbc.M205301200. [DOI] [PubMed] [Google Scholar]
- Seufert W, Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate degradation of short-lived and abnormal proteins. EMBO J. 1990;9:543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- Su NY, Flick K, Kaiser P. The F-box protein Met30 is required for multiple steps in the budding yeast cell cycle. Mol Cell Biol. 2005;25:3875–3885. doi: 10.1128/MCB.25.10.3875-3885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su NY, Ouni I, Papagiannis CV, Kaiser P. A dominant suppressor mutation of the met30 cell cycle defect suggests regulation of the S. cerevisiae Met4 CBF1 transcription complex by Met32. J Biol Chem. 2008 doi: 10.1074/jbc.M708230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, Bronson R, Yaffe MB, Zhou J, Benjamin T. TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol. 2007;27:6383–6395. doi: 10.1128/MCB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, McDonald H, Yates JR, Deshaies RJ. Selective degradation of ubiquitinated sic1 by purified 26s proteasome yields active s phase cyclin-cdk. Mol Cell. 2001;8:439–448. doi: 10.1016/s1097-2765(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Trotter EW, Dawes IW, Grant CM. Coupling of the transcriptional regulation of glutathione biosynthesis to the availability of glutathione and methionine via the Met4 and Yap1 transcription factors. J Biol Chem. 2003;278:49920–49928. doi: 10.1074/jbc.M310156200. [DOI] [PubMed] [Google Scholar]
- Willems AR, Schwab M, Tyers M. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Wu CY, Roje S, Sandoval FJ, Bird AJ, Winge DR, Eide DJ. Repression of sulfate assimilation is an adaptive response of yeast to the oxidative stress of zinc deficiency. J Biol Chem. 2009;284:27544–27556. doi: 10.1074/jbc.M109.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Meng X, Cai Y, Liang H, Nagarajan L, Brandt SJ. Single-stranded DNA-binding proteins regulate the abundance of LIM domain and LIM domain-binding proteins. Genes Dev. 2007;21:942–955. doi: 10.1101/gad.1528507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HC, Elledge SJ. Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science. 2008;322:923–929. doi: 10.1126/science.1160462. [DOI] [PubMed] [Google Scholar]
- Yen JL, Su NY, Kaiser P. The Yeast Ubiquitin Ligase SCFMet30 Regulates Heavy Metal Response. Mol Biol Cell. 2005;16:1872–1882. doi: 10.1091/mbc.E04-12-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.