INTRODUCTION
Food allergy is common and increasing in prevalence amongst U.S. children(1). Despite increased awareness, food allergy remains the most common cause of anaphylaxis in U.S. emergency departments (2). Because no treatment is currently available, food-allergic patients must maintain strict dietary allergen avoidance and be prepared to treat reactions caused by accidental exposures. As a result, the quality of life of food-allergic patients and their families is significantly impaired (3). A disease-modifying intervention is greatly needed.
Hen’s egg is an important food allergen, affecting approximately 1–2% of U.S. children (4), and the prevalence is significantly higher in children with atopic dermatitis (AD) (5). In young children, egg sensitization is a known risk factor for later progression to allergic respiratory disease (6). Most egg allergens are susceptible to heat and gastric digestion, and perhaps as a result, egg-allergic children are commonly expected to outgrow it in early life (7). However, recent studies have reported persistence into the second decade (8). These patients tend to be distinguished by more severe clinical reactions and a robust IgE response, especially to the linear epitopes of the major allergen ovomucoid, which is resistant to digestion (8,9). These data suggest that multiple egg allergy phenotypes may exist, which may have important therapeutic implications.
Oral immunotherapy (OIT) is an experimental interventional strategy intended to establish oral tolerance in food-allergic patients. Most published OIT trials were nonrandomized and studied a uniform dose and duration of maintenance allergen given to all subjects. For example, we previously described partial desensitization in our uncontrolled, proof-of-concept egg OIT trial utilizing a 300 mg/day maintenance dose (10). Additional subjects have been enrolled (11), and here we report our updated experience in these previously unreported subjects. We hypothesized that further dose escalation would enhance OIT outcomes and implemented a conditional updosing strategy in which the maintenance dose is individually increased based on the subject’s egg white IgE (EW-IgE) level. We show that clinical tolerance developed in all six subjects completing this OIT protocol, along with immunologic changes which may be antigen-specific. Length of treatment and conditional dosing may be important variables in OIT protocols.
METHODS
Subject Recruitment and Selection
Egg-allergic subjects, ages 1 to 16 years, were recruited as part of the same ongoing trial previously reported (10,11) from the pediatric allergy and immunology clinics and surrounding offices at Duke University Medical Center. The Duke Institutional Review Board granted ethics approval. Written informed consent was obtained in accordance with ethics guidelines for research in children.
Subjects were included with a clinical history of reaction within 60 minutes of ingesting egg, a positive egg-white skin prick test (SPT), and an EW CAP-FEIA > 7 kU/L (or > 2 if less than 2 years of age). Subjects were excluded for history of severe anaphylaxis (i.e., hypotension) to egg, severe or poorly controlled asthma, or a medical condition preventing completion of a food challenge.
OIT Protocol
Subjects underwent an egg OIT protocol consisting of three phases: initial day escalation, buildup, and maintenance. The primary objective of the study was the development of clinical tolerance, defined as the successful completion of a double-blinded, placebo-controlled food challenge (DBPCFC) following a one-month cessation of OIT. Throughout the protocol, subjects were instructed to mix the OIT dose in a vehicle food and ingest it at home daily, remaining on an otherwise egg-free diet. Subjects kept a diary of any missed doses or adverse symptoms, and self-injectable epinephrine was provided. The study team was readily available at all times throughout the study, and parents were instructed to call with any concerns about illness or adverse events.
Initial Day Escalation
The initial day escalation occurred on the Duke Clinical Research Unit (DCRU). A 10 mg/mL solution of powdered egg white (Michael Foods, Minnetonka, MN) in distilled water was prepared for all doses < 25 mg. For doses ≥ 25 mg, powdered egg white was dispensed from individual preweighed containers. All doses were mixed with a vehicle food of the subject’s choice. After placement of an intravenous catheter, the escalation began at 0.1 mg. The dose was approximately doubled every 30 minutes until the highest tolerated single dose was determined (maximum 50 mg). If the subject had a mild reaction (i.e., oral pruritus), the previously tolerated dose was repeated before resuming the process. If significant symptoms developed, the escalation stopped and the reaction was treated. The highest tolerated single dose was used as the starting dose for the buildup phase.
Standard and Conditional Buildup Phase
Subjects returned to the DCRU for the initial buildup dose and biweekly for dose escalations. Doses were increased by 25 mg increments until 150 mg was reached, and then by 50 mg to 300 mg. The 300 mg dose was continued for four months and the EW-IgE was measured. If the EW-IgE remained > 2 kU/L, the subject underwent an open oral food challenge (OFC) at DCRU to assess desensitization. The following day, the dose was increased according to the highest tolerated dose during the OFC, to a maximum of 300 mg. Subjects were then continued on this dose for four months, and the EW-IgE was repeated. If > 2 kU/L, the dose was increased by 600 mg at the DCRU. For as long as the EW-IgE remained > 2 kU/L, this four month cycle of reassessment and 600 mg updosing continued, to a maximum of 3600 mg/day. Subjects were evaluated every four months while on continued maintenance dosing. When EW-IgE was < 2 kU/L, OIT was stopped and DBPCFC was performed the following day as the first step in tolerance assessment. If successful, the DBPCFC was repeated one month later off of OIT.
Oral Food Challenges
Prior to all food challenges, all subjects were asked to restrict use of antihistamines, β-agonists, theophylline, and montelukast. In order to assess desensitization after four months of maintenance OIT, all subjects underwent an OFC consisting of 4 doses (300 mg, 600 mg, 1200 mg, 1800 mg) of egg protein given every 30 minutes.
DBPCFCs were performed once the EW-IgE was < 2 kU/L to assess tolerance. Randomization and preparation of the challenge materials was performed by the dietitian or a laboratory representative to blind the provider and supervising investigator administering the challenge. The DBPCFC involved two separate challenges of 7 doses of egg or placebo given every 10–20 minutes in increasing amounts up to a total of 10 grams. All negative challenge results were confirmed by open challenge, when approximately twice the quantity of egg was given openly at meal or snack.
Egg-specific IgE, IgG, and IgG4
EW- and ovomucoid-specific IgE, IgG, and IgG4 levels were measured in serum samples using the ImmunoCAP 100 instrument (Phadia AB; Pharmacia, Inc, Uppsala, Sweden) according to the manufacturer’s instructions.
Analyses of cytokine production and regulatory T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from ~25 mL heparinized blood using Ficoll-based density separation (LymphoH; Atlanta Biologicals, Lawrenceville, Ga). For cytokine assays, PBMCs were distributed into 96-well flat-bottom plates at a concentration of 4 × 105 cells/well in triplicate and incubated with egg protein (200 mcg/mL), concanavalin A (40 mcg/mL; Sigma, St Louis, Mo), or medium alone (RPMI-1640 with 2 mmol/L L-glutamine, 25 mmol/L HEPES buffer containing 10% human AB serum, 100 IU/mL penicillin, and 100 mg/mL streptomycin; Mediatech, Manassas, Va). Cells were cultured at 37° C in 5% humidified CO2 for 48 (ConA) and 96 hours (egg). Culture supernatants were collected at each time point and analyzed by ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minn).
For flow cytometry, PBMCs (4 × 106 cells/well) were cultured in 24-well plates under the same stimulation conditions. After 6 days, cells were collected and stained with fluorescent monoclonal antibodies: anti-CD3–PerCP, CD4–fluorescein isothiocyanate, and CD25-phycoerythrin (BD Biosciences, San Jose CA). Isotype controls were included for each condition. 3-color events were detected in a FACSCalibur flow cytometer (Beckman-Coulter, Brea CA). At least 10,000 events were acquired for each experimental condition, and data were analyzed with FlowJo (Tree Star, Inc, Ashland OR).
Statistical Analysis
Means, medians, and variance were tabulated for all data and evaluated for normality. Pair-wise skin test, antibody, and cytokine data were analyzed using the nonparametric Wilcoxon signed-rank test. Simple linear regression compared trends of milk, peanut, and egg-specific IgE levels over time. All analyses were performed with Prism for Windows (Graphpad, La Jolla CA). p < 0.05 was considered significant.
RESULTS
Subjects
Eight subjects were enrolled in the study (Table I). The median age was 5 years (range 3–13y). Five subjects (62.5%) were boys. Seven subjects (87.5%) had comorbid atopic disease, primarily AD and other food allergies. For all subjects at baseline, the median and mean, respectively for egg-white SPT was 9 and 9.3mm (range 5 – 14 mm), for EW-IgE, 12.5 kU/L and 25 kU/L (range 0.7–127 kU/L), and for ovomucoid IgE, 8.6 kU/L and 25.3 kU/L (range 0.4 – 110).
Table 1.
Characteristics of the study population.
| Subject ID | Age/Gender | Comorbidities | Baseline PST (mm) |
Baseline IgE (kU/L) |
Length of OIT Treatment |
Highest Maintenance Dose (mg) |
|---|---|---|---|---|---|---|
| DE01 | 13 / M | probable EE | 9 | 6.03 | 26d - withdrew* | n/a |
| DE02 | 6 / M | none | 8 | 0.7 | 18mo | 300 |
| DE03 | 3 / M | AD | 9 | 11.2 | 182d - withdrew** | n/a |
| DE04 | 6 / F | AD, AR, asthma, FA (wheat, PN) | 14 | 127 | 50mo | 2400 |
| DE05 | 4 / F | AD, AR, FA (milk, PN) | 5 | 23.2 | 50mo | 2400 |
| DE06 | 6 / M | AD, asthma, FA (PN) | 10 | 13.8 | 38mo | 3600 |
| DE07 | 4 / M | AD, AR, asthma, FA (PN) | 5.5 | 3.7 | 19mo | 1200 |
| DE08 | 4 / F | AD, AR, FA (shrimp, PN) | 14 | 14.4 | 28mo | 2400 |
| Mean (SD) | 5.8 (3.2) | 9.3 (3.4) | 25 (41.8) | 33.8 (14.5) | 2050 (1145) | |
| Median | 5 | 9 | 12.5 | 33 | 2400 | |
stress exacerbated by comorbid psychological disorder
concern over worsening AD, complicated by nonadherence to standard therapy
EE, eosinophilic esophagitis; AD, atopic dermatitis; AR, allergic rhinitis, FA, food allergy; PN, peanut
AP, abdominal pain; N, nausea; V, vomiting; D, diarrhea; W, wheezing, U, urticaria
Subject DE01 was voluntarily withdrawn from the study for the worsening of unrelated pre-existing behavioral symptoms. Subject DE03 built up to 300 mg and received this maintenance dose for four months but was withdrawn from the study prior to challengedue to poor AD control and nonadherence to topical AD therapy‥ Six subjects (75%) successfully completed the protocol and will be discussed further.
Safety
Five of six (83%) developed allergic symptoms during the initial escalation day, prior to ingestion of 50 mg. In three subjects, these symptoms were dose-limiting. Four subjects (50%) developed abdominal pain, which was accompanied by vomiting and diarrhea in two. Two subjects developed urticaria which resolved with diphenhydramine. During the observation period after the 50 mg dose, one subject with asthma developed nasal congestion, sneezing, and wheezing, which resolved with albuterol.
During the buildup phase, three subjects developed transient oral pruritus at doses less than 100 mg, none of which required dose adjustment or treatment. Eight days after building up to 250 mg, subject DE08 unexpectedly developed swelling of her left eye and face within 15 minutes of her OIT dose, which resolved with diphenhydramine.
There were no adverse events associated with maintenance dosing. No subjects required unscheduled physician evaluation or epinephrine during the trial. One subject, DE08, reported an accidental ingestion while on 1800 mg, when she accidentally ate a sibling’s sandwich containing mayonnaise but did not develop symptoms.
OFC outcomes
The median time to reaching the 300 mg maintenance dose was 174 days (interquartile range [IQR] 125–177). Four months after reaching maintenance, 5/5 (100%) subjects with EW-IgE levels > 2 kU/L passed an OFC to 3900 mg total egg protein, and were considered desensitized. EW-IgE was undetectable in subject DE02, and he remained on 300 mg for 18 months without undergoing the initial desensitization challenge. After a median length of treatment of 33 months with a median maintenance dose of 2400 mg, 6/6 (100%) subjects passed the first DBPCFC. After decreasing early on, the EW-IgE levels in subjects 4,5, and 6 plateaued after years of maintenance. As a result, parents and investigators agreed to challenge these subjects at EW-IgE levels of 9.94, 4.83, and 3.95 kU/L, respectively. All subjects subsequently passed the second DBPCFC.
Egg white-specific skin prick testing and IgE and IgG4 levels
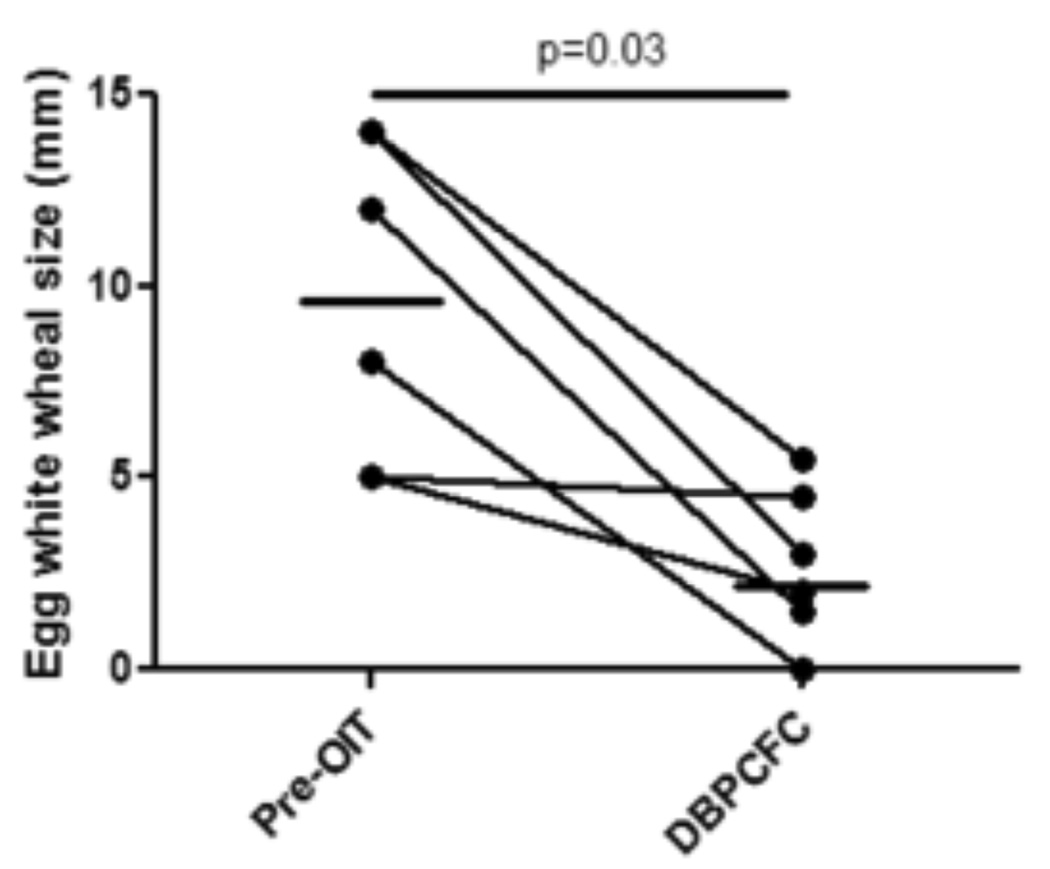
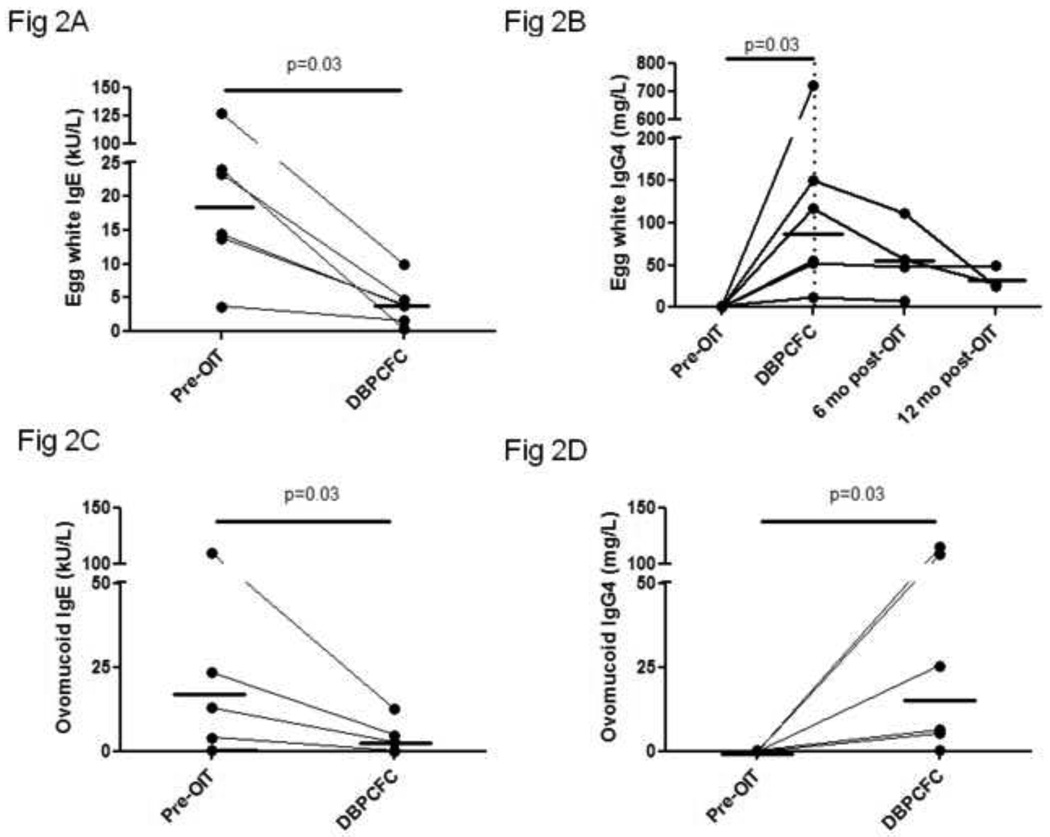
Median EW SPT wheal diameter and IgE decreased from baseline values of 10 mm and 18.8 kU/L to 2.5 mm and 3.9 kU/L, respectively, at the time of the tolerance challenge (Figures 1 and 2A) (p=0.03 for both). EW-IgG4 increased from a median of 0.65 mg/L at baseline to 86.15 mg/L (p=0.03) (Figure 2B). The EW-IgG4 dropped at 6 and 12 months after OIT was discontinued, but this was not statistically significant. Similar changes were seen over time in ovomucoid IgE and IgG4 levels (Figure 2C and 2D).
Figure 1. Egg OIT is associated with suppression of IgE responses in vivo.
Titrated skin prick testing was performed at the indicated timepoints using a commercially available egg white extract. Solid line, median. Dotted line, end of treatment.
Figure 2. Production of egg-white and ovomucoid specific IgE and IgG4 is decreased and increased, respectively, while on egg OIT.
Sera were collected at the indicated timepoints and IgE and IgG4 specific for egg white (A and B, respectively) and ovomucoid (C and D, respectively) were measured by CAP-FEIA. Solid line, median. Dotted line, end of treatment.
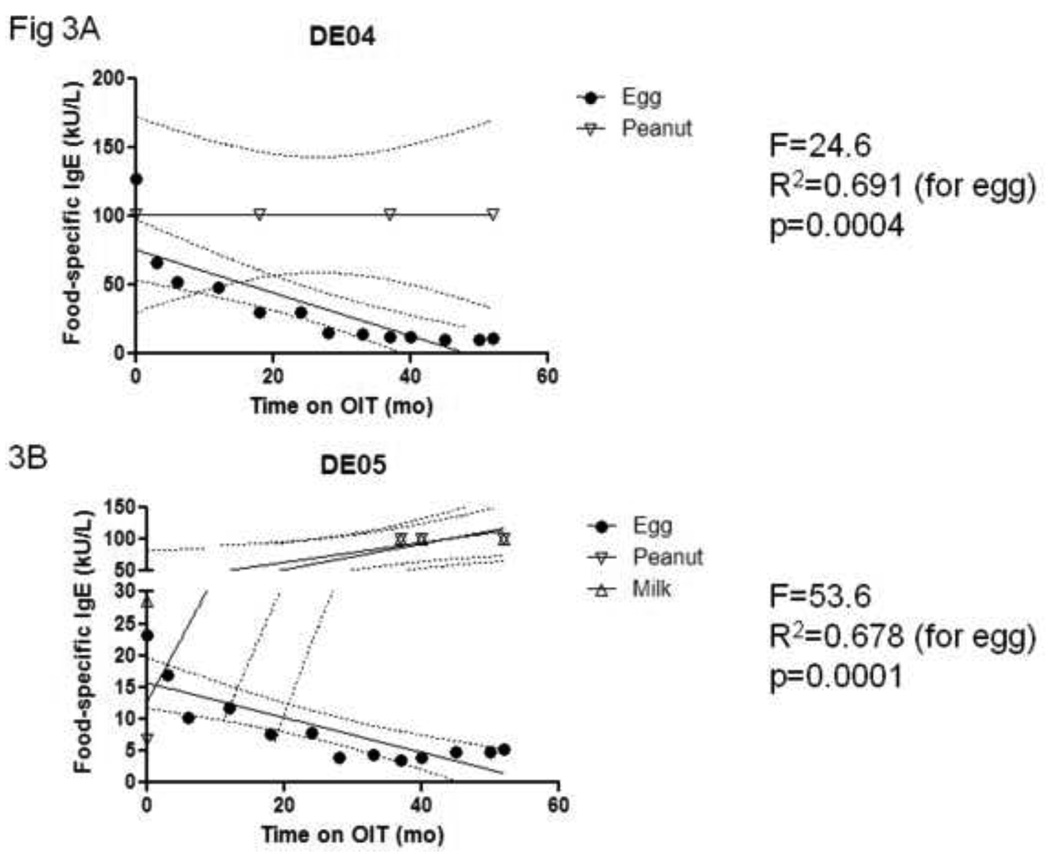
The impact of egg OIT on IgE levels may be allergen-specific. We examined the IgE responses to other foods in two subjects (DE04 and DE05) with comorbid peanut allergy, and peanut plus milk allergy, respectively. As shown in Figures 3A and 3B, only EW-specific IgE levels decreased while on egg OIT, while peanut- and milk-specific IgE levels remained unchanged.
Figure 3. The reduction in IgE production during egg OIT is allergen-specific.
Sera were collected from two subjects and IgE specific for egg and peanut (A), and egg, peanut, and milk (B) were measured by CAP-FEIA over time. Values >100 were transformed to 101. Solid line, regression line. Dashed line, 95% confidence interval for slope parameter.
Cytokines
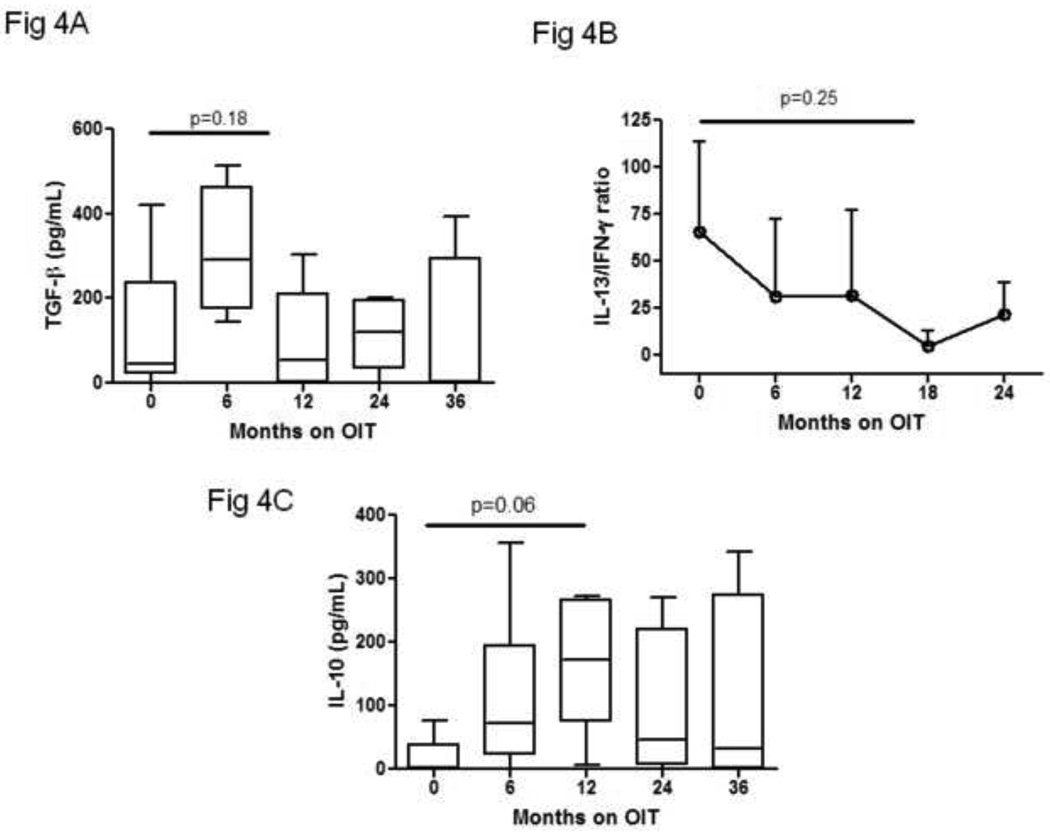
Median TGF-β levels were 44 pg/mL (IQR 22–237) at baseline and 291 pg/mL (IQR 175–463) at six months (p=0.18) (Figure 4A). IL-10 was generally undetectable at baseline (median 0 pg/mL; IQR 0–38.5), and at 12 months the median level was 172 pg/mL (IQR 73.75–267) (p = 0.06). The ratio of IL-13 to interferon-gamma was 65.7 at baseline and 5.1 at 18 months (P=0.25).
Figure 4. Transient changes in regulatory and pro-allergic cytokine production occur during egg OIT.
PBMCs were collected at the indicated timepoints and stimulated with egg allergen for 96 hours.
Supernatants were collected, frozen, and later analyzed for the production of TGF-β (A), IL-13 and IFN-γ (B), and IL-10 (C) by standard ELISA. Boxplots, median and interquartile range. Whiskers, 10th and 90th percentiles.
Regulatory T cells
We attempted to quantify egg-specific T cells which might have regulatory properties. These early studies used a flow cytometric approach to gate on CD4+CD25hi populations after egg stimulation in vitro. All subjects had detectable populations, but we were unable to detect a change over time (not shown).
DISCUSSION
Egg allergy is one of the most common allergic disorders of childhood, especially in children with AD, and predicts later allergic disease. We previously reported interim clinical results from a pilot egg OIT trial, which used a maintenance dose of 300 mg (10). After 24 months, four of seven (57%) subjects were considered to be desensitized based on increased OFC thresholds; two of these four tolerated egg after stopping OIT. Subject enrollment continued at both sites (11), and based on these findings, we revised our protocol. Here we report the impact of individualized dosing on the clinical and immunologic outcomes of different subjects. This trial, which has been ongoing for nearly 60 months, is the longest of its kind reported, and offers important insight about OIT protocols. Although preliminary data from OIT trials are promising, critical questions remain unanswered, such as the appropriate dose and length of treatment (12).
Most OIT studies, including our previous study, have treated all subjects with the same dose and for the same often relatively short duration. In contrast, the subjects in this study underwent conditional stepwise increases in an attempt to reciprocally drive EW-IgE levels below 2 kU/L before assessing tolerance. Interestingly, this was not possible in three subjects, in whom progressive dose increases had no further impact. Yet in contrast to our previous study, all six subjects who completed the protocol passed DBPCFCs on egg OIT and again one month after stopping treatment, indicating clinical tolerance to egg. All of these subjects have incorporated egg into their diets without difficulty. Importantly, the higher doses and longer treatment courses delivered in a monitored setting by experienced investigators were safe and well-tolerated. These results suggest that subjects may derive additional benefit from OIT with longer treatment and/or higher doses. These findings are consistent with Narisety et al., who enrolled partially desensitized children in an extended open-label dose escalation protocol after a short double-blinded milk OIT study (13). In these children, prolonged higher-dose treatment induced new immunologic changes and enhanced the desensitization effect.
Changes in antibody production observed in OIT subjects were consistent with other forms of allergen immunotherapy. The longitudinal trends in EW-IgE and IgG4 were inversely related to each other. The EW-IgG4 was undetectable in our study at baseline and increased over time before falling in two clinically tolerant subjects once OIT was discontinued. We demonstrate for the first time to our knowledge that OIT’s effect on IgE levels may be allergen-specific, since peanut and milk IgE did not change in two subjects cosensitized to these foods. Although a small sample size prevents definitive conclusions, these observations provide interesting trends for future study. Long term data from controlled studies, including detailed dietary histories and follow-up immunologic analyses, will be necessary to better understand OIT-induced antibody changes.
Trends in egg-induced cytokine production suggested transient production of the immunoregulatory cytokines IL-10 and TGF-β and a shift from Th2 to Th1, but these data were not statistically significant. However, they are similar to reports from allergen immunotherapy trials and are internally consistent, since IgG4 production has been correlated with IL-10 levels in this setting. We hypothesized that OIT would be associated with regulatory T cell development. All subjects did express an egg-specific CD4+CD25hi population, but we were unable to identify changes over time, presumably because we did not use a specific marker (i.e., FOXP3). No previous studies of egg allergy treatment have reported egg-specific cytokine production or the identification of egg-specific regulatory T cells (4,14–16). Clearly, more mechanistic study in a larger cohort is needed.
The small sample size and lack of a control group are limitations to our study, since many children will naturally outgrow egg allergy. However, our subjects were atopic, older, and had a history of convincing IgE-mediated symptoms after egg ingestion and average EW-IgE levels exceeding established thresholds for predictive clinical reactivity. Similar characteristics predicted persistent egg allergy in a study of over 850 children aged four to six years, in which median EW-IgE in persistent subjects was 7.6 kU/L, compared to 1.8 kU/L in those who went on to develop tolerance (8). Using the strictest definition of clinical tolerance, these authors calculated that 12% of patients naturally developed tolerance by age six and 37% by age ten, for a 25% acquisition rate over a four-year period. By comparison, baseline median values for age and EW-IgE in our study were five years and 12.5 kU/L, and ovomucoid-specific IgE, a known predictor of persistent allergy (9), was also elevated; and 100% of our six subjects developed tolerance at a median of 33 months. Taken together, these data suggest that our subjects were at risk for persistent egg allergy, and although they may have naturally outgrown it, the use of egg OIT with a progressive dosing strategy is likely to be faster and more effective than waiting for tolerance to develop naturally. Even if our subjects ultimately developed natural tolerance, OIT offers the substantial protection of clinical desensitization. A large, multicenter, double-blinded, placebo-controlled egg OIT trial is underway, which should have sufficient statistical power to address efficacy (clinicaltrials.gov, NCT00461097)
In summary, we report here our five-year experience with an original study of egg OIT. Utilizing an innovative, IgE-dependent dosing strategy safely induces favorable immunologic changes and may facilitate clinical egg tolerance. Such an individualized approach may be necessary to achieve a full therapeutic response in some patients.
ACKNOWLEDGMENTS
We appreciate the support provided by the DCRU staff and personnel. The project described was supported by Grant Number UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Source of Funding: NIH-NIAID 5R01 AI068074-03
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registry: www.clinicaltrials.gov, NCT00597558
Potential Conflicts of Interest: (Burks): Consultant – Actogenix, Intelliject, McNeil Nutritionals, Novartis; Minority Stockholder – Allertein, MastCell, Inc; Advisory Boards – Dannon; Expert Panel – Nutricia
| Study Design | Clinical Trial | Lab Assays | Data Analysis | Manuscript Preparation | |
|---|---|---|---|---|---|
| Vickery | X | X | X | ||
| Pons | X | X | X | X | |
| Kulis | X | X | X | ||
| Steele | X | X | X | ||
| Jones | X | X | X | X | |
| Burks | X | X | X | X | X |
REFERENCES
- 1.Branum AM, Lukacs SL. Food allergy among US children: Trends in prevalence and hospitalizations. National Center for Health Statistics Data Brief. 2008 No. 10. [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 3.Avery N, King R, Knight S, Hourihane JOB. Assessment of quality of life in children with peanut allergy. Pediatric Allergy and Immunology. 2003;14(5):378–382. doi: 10.1034/j.1399-3038.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008 Nov;122(5) doi: 10.1016/j.jaci.2008.09.007. 977–983.e1. Epub 2008 Oct 11. [DOI] [PubMed] [Google Scholar]
- 5.Burks A, James JM, Hiegel A, Wilson G, Wheeler J, Jones SM, et al. Atopic dermatitis and food hypersensitivity reactions. J Pediatrics. 1998 Jan;132(1):132–136. doi: 10.1016/s0022-3476(98)70498-6. [DOI] [PubMed] [Google Scholar]
- 6.Nickel R, Kulig M, Forster J, Bergmann R, Bauer CP, Lau S, et al. Sensitization to hen's egg at the age of twelve months is predictive for allergic sensitization to common indoor and outdoor allergens at the age of three years. J Allergy Clin Immunol. 1997;99(5):613–617. doi: 10.1016/s0091-6749(97)70021-6. [DOI] [PubMed] [Google Scholar]
- 7.Wood RA. The Natural History of Food Allergy. Pediatrics. 2003 Jun 1;111(6):1631–1637. [PubMed] [Google Scholar]
- 8.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120(6):1413–1417. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Jarvinen K, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen's egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62(7):758–765. doi: 10.1111/j.1398-9995.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119(1):199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Burks AW, Jones SM. Egg oral immunotherapy in nonanaphylactic children with egg allergy: Follow-up. J Allergy Clin Immunol. 2008 Jan;121(1):270–271. doi: 10.1016/j.jaci.2007.07.066. [DOI] [PubMed] [Google Scholar]
- 12.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. Journal of Allergy and Clinical Immunology. 2010 Jul;126(1):31–32. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, Burks AW, et al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow's milk allergy. Journal of Allergy and Clinical Immunology. 2009 Sep;124(3):610–612. doi: 10.1016/j.jaci.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62(11):1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 15.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17(3):459–465. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 16.Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Internat. 2010;59(1):1–9. doi: 10.2332/allergolint.09-OA-0107. [DOI] [PubMed] [Google Scholar]