Abstract
Purpose
To examine whether the relationship between cardiovascular disease risk factors and coronary artery calcification (CAC) is modified by race among those with diabetes.
Methods
Data were pooled data from three studies (Multi-Ethnic Study of Atherosclerosis, Family Heart Study, Diabetes Heart Study) for a total of 835 blacks and 1122 whites with diabetes. CAC was quantified by cardiac computed tomography and risk factors were obtained using standard methods. Regression models examined the relationship between risk factors and presence and quantity of CAC.
Results
The average age of the cohort was 60 years; 57% were women. Presence of CAC was lower in blacks compared to whites (odds ratio = 0.22 for men, 0.57 for women, p<0.01). HbA1c, duration of diabetes, LDL, smoking, and BMI were independently associated with presence of CAC; HDL, triglycerides and CRP were not. Race did not modify these associations. Adjustment for multiple risk factors did not explain the race disparity in CAC.
Conclusions
CAC was reduced in blacks compared to whites in persons with diabetes. This effect was most pronounced in men. The relationship between risk factors and CAC did not differ between races. Racial differences in CAC are likely due to unmeasured risk factors and/or genetic susceptibility.
Keywords: coronary artery disease, diabetes mellitus, epidemiology, African Americans, cohort studies
Coronary artery calcification (CAC) is less prevalent in blacks than whites (1–4), although this finding is not universal (5, 6). The underlying cause(s) of racial differences in CAC are not understood. Differences in the frequency of cardiovascular disease (CVD) risk factors or the strength of their relationship with CAC may play a role. These relationships have not been fully examined in persons with type 2 diabetes, in whom atherosclerosis is accelerated.
We previously reported different risk factor relationships between blacks and whites for LDL, HbA1c, and sex, with CAC (7). In each case, the strength of the association was greater in whites than in blacks. The present report extends these findings to a larger sample of blacks by pooling three cohorts that include large numbers of blacks in which measurement of CAC was obtained similarly. The cohorts include the Multi-Ethnic Study of Atherosclerosis (MESA), the Family Heart Study (FHS), and the Diabetes Heart Study (DHS). We hypothesize that risk factors for atherosclerosis among adults with diabetes are at least as frequent in blacks as in whites, but their relationship with CAC is weaker.
Methods
Data were pooled from three cohorts in which CAC was quantified by cardiac computed tomography (CT). Persons with diabetes who were free of clinical CVD were selected for this analysis (835 blacks and 1122 whites). Cross-sectional associations between CVD risk factors and CAC were analyzed and compared between blacks and whites.
Multi-Ethnic Study of Atherosclerosis (MESA)
MESA was initiated to investigate the prevalence, correlates, and progression of subclinical CVD (8). The cohort of 6814 persons aged 45–84 years was recruited in 2000–2002 from six urban sites using population-based methods with oversampling of blacks, Chinese and Hispanics. Recruitment was restricted to persons free of clinical CVD. The cohort includes participants with both previously diagnosed and newly diagnosed diabetes. All data for this report were collected at the baseline examination with the exception of HbA1c, collected at Exam 2. MESA contributed data for 332 blacks and 158 whites.
Family Heart Study (FHS)
FHS recruited and examined 5381 participants from 1245 families in four sites in 1994–1996 to identify and evaluate genetic and non-genetic determinants of coronary heart disease (CHD) (9). Half of the families were chosen at random and the other half on the basis of a higher than expected risk of CHD. In 2002–2004, a substudy of 3359 FHS participants aged 30–93 years was conducted in order to obtain cardiac CT scans for the measurement of CAC (10). For this CAC substudy, a fifth site was added in Birmingham, AL, in order to recruit blacks, who were inadequately represented in the original FHS. This site recruited individuals from the HyperGEN study of hypertensive sibships (11). The cohort includes participants with both previously diagnosed and newly diagnosed diabetes. For this analysis, we excluded persons with clinical CVD defined as myocardial infarction, stroke, and coronary artery bypass graft. FHS contributed data for 120 blacks and 209 whites.
Diabetes Heart Study (DHS)
The Diabetes Heart Study (DHS) is a family study of the genetics and epidemiology of CVD in 1125 siblings concordant for diabetes, conducted at a single site in Forsyth County, NC (7). Participants aged 30–86 years with a history of diabetes were recruited from the community and outpatient clinics and examined in 1999–2005. A companion study (the African American DHS) was initiated in 2007 and is recruiting 500 additional unrelated blacks to conduct admixture mapping for CAC (12). Participants with clinical CVD defined as myocardial infarction, stroke, and coronary artery bypass graft were excluded. The DHS studies contributed data for 383 blacks and 755 whites.
Variables
We selected only those variables that were obtained using similar techniques. CAC was measured in each study by non-contrast cardiac CT with ECG gating in late diastole based on a previously published protocol (13). Data are presented as Agatston score using 130 CT number threshold. Diabetes was defined either by fasting glucose of at least 7.0 mmol/l (126 mg/dl), self-reported previous physician diagnosis, or use of diabetes medication. The data set is comprised largely of persons with type 2 diabetes, although it likely that there are a small number of persons with type 1 diabetes included. Race and ethnicity were obtained by self-report. Risk factors were obtained using standard methods and include duration of and treatment for diabetes, HbA1c (not available in FHS), LDL, HDL, triglycerides, BMI, CRP, smoking status and pack-years, hypertension, and use of lipid-lowering medications.
Statistical Analysis
CAC was analyzed as both a binary variable (presence vs absence) and a continuous variable (quantity). For the continuous variable, the natural log of CAC + 1 was analyzed. To account for the correlations among the observations (FHS and DHS include related individuals), tests of significance were based on generalized estimating equations (GEE1) assuming exchangeable correlation and using the robust estimator of the variance. We used the identity link for quantity of CAC and the logit link for the presence of CAC. To test whether a putative risk factor was related to quantity of CAC, a GEE1 linear model was computed separately for each risk factor. Age, study, race, sex, and race-by-sex interaction (race X sex) were included in all models. The models also included an interaction term between the risk factor and race, to test whether the risk factor’s influence on CAC differed by race. To test whether a putative risk factor was related to presence of CAC, a GEE1 model using the logit link was computed separately for each risk factor following the steps outlined above for the linear model. Model 1 minimally adjusted for age, study, race, sex, and race X sex. A full model (Model 2) was fit by including all factors in Model 1 plus all risk factors.
Results
The average age of the pooled cohort was 60 years, 57% were women, and 43% were black (Table 1). Several CVD risk factors (LDL, CRP, smoking, BMI) and markers of diabetes severity (diabetes duration, HbA1c) were greater (or more frequent) in blacks than in whites, or did not differ between blacks and whites. Whites were more likely to be taking lipid lowering medications (p<0.0001). There were other instances in which risk factors favored blacks: blacks had lower triglycerides and higher HDL than whites (p<0.0001), less pack-years (p<0.01), and a lower prevalence of hypertension (p<0.0001). Prevalence of CAC, adjusted for age and study, was lower in blacks than in whites for both women and men (Table 2). Quantity of CAC was lower in black men than in white men, but did not differ between black and white women.
Table 1.
Characteristics of the Combined Cohort of Persons with Diabetes Adjusted for Age and Study, Mean (standard error) or Percent, and p-value for Black vs White
| Women | Men | |||
|---|---|---|---|---|
| Black (N=495) | White (N=618) | Black (N=340) | White (N=504) | |
| Age (yrs) | 59 (0.5) (<0.0001) | 62 (0.4) | 60 (0.6) (0.02) | 62 (0.4) |
| Study, N (%) | ||||
| DHS | 244 (49) | 441 (71) | 139 (41) | 314 (62) |
| MESA | 169 (34) | 63 (10) | 163 (48) | 95 (19) |
| FHS | 82 (17) (<0.0001) | 114 (18) | 38 (11) (<0.0001) | 95 (19) |
| Duration of diabetes (yrs) | 8.6 (0.4) (0.002) | 6.9 (0.4) | 8.3 (0.5) (0.01) | 7.0 (0.4) |
| HbA1c (%) * | 7.9 (0.1) (0.0003) | 7.4 (0.1) | 7.9 (0.1) (<0.0001) | 7.2 (0.1) |
| Currently treated for diabetes (%) | 85 (<0.0001) | 86 | 79 (<0.0001) | 82 |
| LDL (mg/dL) | 112.8 (1.6) (0.06) | 108.3 (1.5) | 106.6 (2.1) (0.03) | 101.2 (1.5) |
| HDL (mg/dL) | 53.3 (0.7) (<0.0001) | 47.5 (0.6) | 44.8 (0.7) (<0.0001) | 39.4 (0.5) |
| Triglycerides (mg/dL) | 120.1 (5.3) (<0.0001) | 203.7 (6.1) | 123.6 (6.9) (<0.0001) | 189.5 (7.7) |
| BMI (kg/m2) | 35.1 (0.3) (0.0001) | 33.3 (0.3) | 31.1 (0.3) (0.41) | 31.5 (0.3) |
| CRP (mg/L) | 9.7 (0.8) (0.001) | 6.4 (0.5) | 5.7 (0.5) (0.0003) | 3.7 (0.4) |
| Current smoking (%) | 16 (<0.0001) | 13 | 21 (0.004) | 18 |
| Past smoking (%) | 29 (0.001) | 28 | 47 (<0.0001) | 52 |
| Pack-years | 7.8 (0.8) (0.005) | 10.7 (1.0) | 16.0 (1.2) (<0.0001) | 25.2 (1.4) |
| Hypertension (%) | 80 (<0.0001) | 86 | 74 (<0.0001) | 77 |
| Lipid-lowering medication (%) | 39 (<0.0001) | 44 | 35 (<0.0001) | 41 |
Sample size reduced: HbA1c not available for FHS.
Table 2.
Prevalence and Quantity of Coronary Artery Calcified Plaque in the Combined Cohort of Persons with Diabetes, Adjusted for Age and Study, and p-value for Black vs White
| Women | Men | |||
|---|---|---|---|---|
| Black (N=492) | White (N=595) | Black (N=337) | White (N=488) | |
| Prevalence | ||||
| Percent > 0 (p-value) | 63 (<0.0001) | 74 | 76 (<0.0001) | 83 |
| Quantity | ||||
| Mean ± standard error (p-value) | 172 ± 21 (0.16) | 190 ± 25 | 288 ± 35 (<0.0001) | 573 ± 47 |
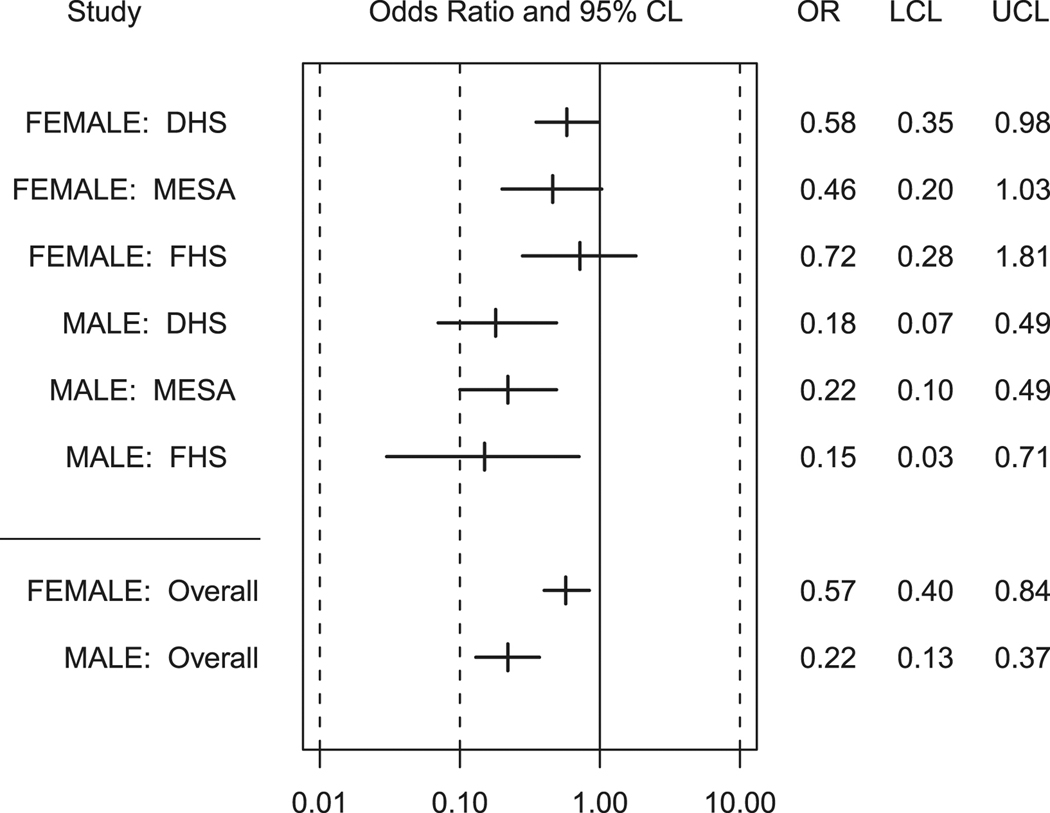
In order to develop a base model to test the risk factor − race interactions, we tested whether there were race interactions with the demographic variables (age, sex and study) separately for the linear and logistic models of CAC. In models including only age, study, race, and sex, we observed a significant race X sex interaction for both the logistic and linear models (p≤0.001). Race is a stronger risk factor for presence and quantity of CAC in men than in women. Odds of CAC presence in black men relative to white men are 0.22 (95% CI: 0.13, 0.37) and in black women relative to white women are 0.57 (95% CI: 0.40, 0.84) (Figure 1). Odds of CAC presence are consistently reduced in black men relative to white men in all three studies. Among women, a race X study interaction was observed (p=0.046), with odds of CAC presence in black women relative to white women ranging from 0.46 in MESA to 0.72 in FHS. A race X study interaction was not observed for quantity of CAC; quantity of CAC was significantly reduced in blacks relative to whites in all three studies (0.0001 < p < 0.05). No race X age interactions were observed.
Figure 1.
Odds and 95% confidence limits of Coronary Artery Calcified Plaque Presence for Blacks Relative to Whites with Diabetes, Overall and by Study, (Black Females Referenced to White Females, Black Males Referenced to White Males). OR=odds ratio; LCL=lower confidence limit; UCL=upper confidence limit
We next evaluated whether there were race X risk factor interactions, adjusting for age, sex, study, race, and race X sex. No race X risk factor interaction (other than sex) reached statistical significance (p<0.05) in either the linear or logistic models (Supplemental Tables). Thus, we present effect sizes of risk factors on CAC for the combined cohort of blacks and whites (Tables 3 and 4). Age, study, HbA1c, diabetes duration, LDL, pack-years, current smoking, hypertension, and BMI were significantly associated with presence of CAC (Table 3, Model 1). These factors were all independently associated with presence of CAC in the fully adjusted model with the exception of hypertension (Model 2). HDL, triglycerides and CRP were not associated with presence of CAC. Adjustment for multiple risk factors had only minimal impact on the strong race effect.
Table 3.
Risk Factors for Presence of Coronary Artery Calcified Plaque in the Combined Cohort of Persons with Diabetes
| MODEL 1 Adjusted for Age, Study, Race, Sex, and Race X Sex |
MODEL 2 Fully Adjusted Model * |
|||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence interval |
p-value | Odds Ratio | 95% Confidence interval |
p-value | |
| Age (yrs) For 10 years | 2.14 | [1.89,2.42] | <0.0001 | 2.26 | [1.94,2.64] | <0.0001 |
| Race X Sex (ref., white males) | Black female: 0.18 | [0.09,0.38] | <0.0001 | Black female: 0.16 | [0.07,0.39] | <0.0001 |
| Black male: 0.27 | [0.18,0.40] | Black male: 0.26 | [0.16,0.42] | |||
| White female: 0.30 | [0.21,0.44] | White female: 0.31 | [0.20,0.49] | |||
| Study (ref., MESA) | DHS: 3.07 | [2.31,4.07] | <0.0001 | DHS: 2.59 | [1.86,3.61] | <0.0001 |
| FHS: 2.53 | [1.75,3.65] | <0.0001 | FHS: 2.63 | [1.75,3.93] | <0.0001 | |
| HbA1c (%) † | 1.11 | [1.04,1.20] | 0.002 | 1.11 | [1.02,1.2] | 0.02 |
| Duration of diabetes (yrs) For 10 years | 1.28 | [1.09,1.50] | 0.001 | 1.36 | [1.15,1.61] | 0.0003 |
| LDL (mg/dl) For 20 mg/dl | 1.07 | [1.00,1.15] | 0.049 | 1.12 | [1.03,1.19] | 0.005 |
| HDL (mg/dl) For 15 mg/dl | 0.94 | [0.83,1.06] | 0.33 | 0.95 | [0.81,1.10] | 0.48 |
| TG (mg/dl) For 135 mg/dl | 1.06 | [0.94,1.19] | 0.34 | 0.95 | [0.73,1.23] | 0.68 |
| CRP ((mg/l)) For 10 mg/dl | 1.30 | [0.45,3.71] | 0.63 | 0.96 | [0.86,2.68] | 0.41 |
| Pack-years For 10 pack-yrs | 1.10 | [1.03,1.17] | 0.002 | 1.08 | [1.03,1.16] | 0.005 |
| Current smoker (ref., non-smoker) | 1.86 | [1.36,2.56] | <0.001 | 1.80 | [1.21,2.67] | 0.004 |
| Hypertension | 1.41 | [1.07,1.86] | 0.02 | 1.33 | [0.97,1.83] | 0.08 |
| BMI (kg/m2) | 1.02 | [1.00,1.04] | 0.02 | 1.02 | [1.00,1.25] | 0.04 |
Fully adjusted model includes age, study, race, sex, race X sex, duration of diabetes, LDL, HDL, TG, pack-years, current smoker, hypertension, CRP, and BMI.
Models of HbA1c are restricted to the MESA and DHS studies.
Table 4.
Risk Factors for Quantity of Coronary Artery Calcified Plaque in the Combined Cohort of Persons with Diabetes
| MODEL 1 Adjusted for Age, Study, Race, Sex, and Race X Sex |
MODEL 2 Fully Adjusted Model * |
|||||
|---|---|---|---|---|---|---|
| Difference in [log (CAC +1)] |
95% Confidence interval |
p-value | Difference in [log (CAC +1)] |
95% Confidence interval |
p-value | |
| Age (yrs) For 10 years | 1.09 | [0.98,1.20] | <0.0001 | 1.04 | [1.03,1.06] | <0.0001 |
| Race X Sex (ref., white males) | Black female: −2.15 | [−2.78,−1.51] | <0.0001 | Black female: −1.98 | [−2.05,−1.92] | <0.0001 |
| Black male: −1.25 | [−1.61,−0.89] | Black male: −1.21 | [−1.61,−0.81] | |||
| White female: −1.69 | [−1.96,−1.41] | White female: −1.41 | [−1.74,−1.08] | |||
| Study (ref., MESA) | DHS: 0.99 | [0.71,1.26] | <0.0001 | DHS: 0.64 | [0.33,0.95] | <0.0001 |
| FHS: 0.44 | [0.09,0.79] | 0.01 | FHS: 0.43 | [0.07,0.79] | 0.02 | |
| HbA1c (%) † | 0.07 | [0.008,0.14] | 0.03 | 0.06 | [−0.01,0.14] | 0.09 |
| Duration of diabetes (yrs) For 10 years | 0.36 | [0.22,0.51] | <0.0001 | 0.46 | [0.30,0.61] | <0.0001 |
| LDL (mg/dL) For 20 mg/dl | −0.02 | [−0.08,0.05] | 0.63 | 0.02 | [−0.05,0.09] | 0.83 |
| HDL (mg/dL) For 15 mg/dl | −0.07 | [−0.21,0.06] | 0.30 | −0.06 | [−0.21,0.09] | 0.45 |
| TG (mg/dL) For 135 mg/dl | 0.07 | [−0.03,0.17] | 0.16 | 0.01 | [−0.22,0.23] | 0.95 |
| CRP(mg/dL) For 10 mg/dl | −0.10 | [−1.06,0.86] | 0.83 | −0.04 | [−1.02,0.93] | 0.38 |
| Pack-years For 10 pack-yrs | 0.14 | [0.09,0.18] | <0.0001 | 0.10 | [0.05,0.16] | <0.0001 |
| Current smoker (ref., non-smoker) | 0.91 | [0.62,1.20] | <0.0001 | 0.73 | [0.39,1.08] | <0.0001 |
| Hypertension | 0.53 | [0.27,0.80] | <0.0001 | 0.42 | [0.13,0.72] | 0.005 |
| BMI (kg/m2) | 0.01 | [−0.00,0.03] | 0.12 | 0.01 | [−0.00,0.03] | 0.20 |
Fully adjusted model includes age, study, race, sex, race X sex, duration of diabetes, LDL, HDL, TG, pack-years, current smoker, hypertension, CRP, and BMI.
Models of HbA1c are restricted to the MESA and DHS studies.
Age, study, HbA1c, diabetes duration, pack-years, current smoking, and hypertension were significantly associated with quantity of CAC (Table 4). LDL and BMI were not associated with quantity of CAC as they were for presence of CAC. All of these factors remained associated with quantity of CAC in the fully adjusted model (Model 2) with the exception of HbA1c. Similar to the model fit for presence of CAC, HDL, triglycerides and CRP were not associated with quantity of CAC. Again, adjustment for multiple risk factors had only minimal impact on the strong race effect.
Discussion
To our knowledge, this is the largest cohort of blacks and whites with diabetes and a measure of CAC in whom racial differences in prevalence of (and risk factors for) CAC can be examined. We hypothesized that there would be a lower prevalence and quantity of CAC in blacks compared to whites, and that risk factors for atherosclerosis would be at least as frequent in blacks as whites, but their relationship with CAC would be weaker. Our study sample is unique in that we focused exclusively on persons with diabetes, individuals in whom atherosclerosis is accelerated. We confirmed the observation of a lower prevalence and quantity of CAC in blacks compared to whites, initially observed by others in non-diabetic cohorts (1, 2, 4), and in persons with diabetes in MESA (14). The differential was more pronounced in men than in women. The CVD risk profile, however, did not favor one racial group over the other, in contrast to our hypothesis that blacks would have a more deleterious (or similar) risk profile. Several risk factors for atherosclerosis and markers of diabetes severity were similar between blacks and whites, or greater (more frequent) in blacks. In contrast, blacks were less likely to be hypertensive, had lower triglycerides, higher HDL, and less pack-years than whites. Finally, apart from the observation that sex modified the association between race and CAC, we observed no instances of race modifying the association between risk factors or markers of diabetes severity and CAC. Conventional CVD risk factors did not explain the reduced presence and quantity of CAC in blacks compared to whites. This differential in CAC is likely to be accounted for by the presence of additional unmeasured risk factors and/or genetic susceptibility (15, 16).
The reduced burden of calcified atherosclerotic plaque in blacks relative to whites, observed in several previous non-diabetic cohorts, was confirmed in this cohort with type 2 diabetes. Thus, despite the higher than usual atherosclerotic risk conferred by diabetes, the race differential in CAC persists. This is an important observation, as CAC is a strong marker of increased risk of clinical cardiovascular events and mortality (17–19), even among persons with diabetes (20, 21). Furthermore, CAC appears to carry a similar prognostic value for incident coronary heart disease in blacks as in whites (17), although at nearly every level of CAC blacks have a considerably greater mortality risk than other race/ethnic groups (18). Thus, even though blacks have a reduced burden of CAC, they may not realize a reduced burden of overall disease or mortality.
We, like others, have observed a stronger race differential in CAC in men than in women. We detected a significant race X sex interaction for both presence and quantity of CAC. In men and women, blacks had a significantly reduced prevalence of calcified plaque relative to whites. Burden (quantity) of CAC did not differ significantly between black and white women, although there was a trend toward lower burden in black women. In a cohort of 16,560 men and women referred for CVD risk evaluation, black women had significantly higher odds of vascular calcification relative to non-black women, while black men had significantly lower odds (4). It is possible that referral biases explain this unusual finding. In a cohort of older adults, the reduced CAC score in blacks compared to whites persisted in men but not women with adjustment for risk factors (22). In a study comprised entirely of women, CAC scores did not differ between blacks and whites (23). It is not clear why the racial difference in CAC burden is more pronounced in men than in women. Possible explanations are differential participation by men and women in these studies (involving criteria related to CAC risk), as well as effects of sex hormones and hormone replacement therapy on CAC (24).
This is the largest report to examine whether risk factors for CAC in a cohort of persons with diabetes differs by race. We conducted this pooled analysis of three cohorts to more robustly and powerfully test our hypotheses relative to our original report (7). Based on this larger sample, we conclude that the associations between CVD risk factors and CAC do not differ by race, with the exception of sex. Only one other study has contrasted risk factor relationships for vascular calcium across racial groups. Age, smoking, hypertension, and diabetes were consistently associated with CAC in blacks and whites, while the relationships between BMI, LDL, and HDL were weaker in blacks (5). Limitations included the lack of formal statistical testing for interactions and an analysis that was restricted to presence/absence of CAC. We have previously identified albuminuria as a factor that interacts with race in its association with CAC (12). In that report focused on the DHS cohort alone, vascular calcium was associated with increased albumin-creatinine ratio in whites but not blacks (p=0.01 for race interaction). Nonetheless, adjustment for this factor did not eliminate the racial difference in vascular calcium. (We did not include albuminuria in this pooled analysis because we were focusing only on risk factors common to the three studies).
In this report, we observed that poor control of diabetes, as reflected in an increased HbA1c, and a longer duration of diabetes, were associated with increased prevalence and quantity of CAC. An increase of 1% in HbA1c increased the odds of CAC by 12% (p<0.0001), an effect that persisted in the fully adjusted model. Reaven and colleagues (25) reported on 309 veterans with diabetes and suboptimal glycemic control (HbA1c > 7.5%): advanced duration of disease but not HbA1c was associated with presence of CAC. Elkeles and colleagues (26) reported on 495 patients attending hospital diabetes clinics and free of CHD – the same findings were observed, i.e. association with duration of diabetes but not HbA1c. Our study provides new evidence that poorer control of diabetes as indicated by a higher HbA1c, in addition to advanced duration of disease, are independent correlates of CAC.
Additional risk factors associated with CAC in this study are consistent with findings observed in non-diabetic cohorts, including age, LDL, smoking, and hypertension (27). We did not observe an association between CRP and CAC. The literature linking CRP to CAC is mixed (28, 29). It may be more difficult to observe an association in our cohort as advanced levels of inflammation in diabetes may obscure a relationship of CRP with outcomes (30).
We pooled three epidemiologic cohorts in order to obtain a large sample of blacks with type 2 diabetes. Perhaps not surprisingly, the prevalence and quantity of CAC differed across these cohorts, with MESA having the lowest burden of disease and DHS having the greatest. The recruitment strategies differed considerably even though we attempted to create a homogeneous cohort by excluding persons with evidence of clinical CVD. MESA was population-based, excluded persons with known CVD, and was not focused on the recruitment of persons with diabetes. DHS recruited from outpatient clinics and community advertising, and was focused on families affected with type 2 diabetes. FHS over-sampled persons with increased family risk of CHD or hypertension. Nevertheless, we confirmed that the race differential in the prevalence and quantity of CAC persisted in all three of the sample cohorts, albeit with a non-significant effect on presence of CAC in the smaller FHS cohort. This consistency suggests that regardless of extent of disease, the race differential in CAC is present.
Our report does not resolve the question of why the burden of CAC is lower in blacks than whites. Arterial calcification appears to be the only subclinical marker of atherosclerosis with a lower burden in blacks. This lower burden is observed consistently across all vascular beds (31). In contrast, blacks are more likely to have peripheral arterial disease as indicated by a reduced ankle-brachial index (32), and more extensive carotid atherosclerosis of the common carotid artery as measured by ultrasonography (33; 34). Together, these findings suggest that a lower burden of arterial calcification in blacks is not due to their lower overall burden of atherosclerosis, nor to a preference for lesions to occur in a specific vascular bed, but to possible ethnic differences in the pathophysiology of the lesion (i.e., calcification).
The nature of the atherosclerotic lesion and overall composition of the arterial wall may differ in ways which alter the propensity to calcify, such as the relative proportions of inflammation to connective tissue content. Blacks are known to have a higher incidence of hypertension, which is associated with increased collagen and connective tissue accumulation in the artery (35), which in turn would decrease ankle-brachial index and increase carotid artery intimal-medial thickness. At the same wall thickness, greater connective tissue content would likely indicate a relatively lower burden of inflammation in the artery, and reductions in the inflammation/connective tissue ratio, which could reduce the likelihood of calcification.
Ethnic differences in calcium and vitamin D metabolism are also being explored in relation to vascular calcification. Blacks have higher levels of bone mineral density than whites (36), which is consistent with the “calcification paradox” (i.e., bone mineral density varies inversely with arterial calcification) (37). Blacks also have lower circulating concentrations of vitamin D than whites. Recently we observed that serum vitamin D levels were positively associated with calcified plaques in blacks (38), whereas vitamin D has been inversely associated with vascular calcification in previous studies in largely white populations. These ethnic differences have led us and others to pursue calcium and vitamin D metabolism as possible factors underlying ethnic differences in the propensity to develop calcified atherosclerotic plaque.
Finally, CAC may have a considerable genetic component. A recent report in 1417 MESA participants in whom individual ancestry was estimated from 199 ancestry informative markers provides provocative data on the genetic basis of subclinical CVD (15). A one standard deviation increase in European ancestry was associated with 8% greater odds of CAC in blacks (p=0.01). These findings provide strong support for admixture mapping of CAC in blacks, a project that is underway in our samples.
In conclusion, we confirm a reduced burden of calcified atherosclerotic plaque in the coronary arteries of blacks compared to whites. Our report extends this observation to persons with type 2 diabetes, a growing segment of the world population and a group for whom the risk of atherosclerosis is accelerated. Risk factors associated with CAC in this cohort included increased duration of diabetes, HbA1c, LDL, hypertension, BMI and smoking; the relationship between these risk factor and CAC did not differ between blacks and whites. Furthermore, adjustment for these and other factors does not eliminate the racial disparity in CAC. Continued efforts to understand the racial disparity in the burden of CAC must focus on novel risk factors and include genetic susceptibility.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of MESA, the DHS, and the FHS, for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The DHS investigators acknowledge study recruiters Carrie Smith and Cassandra Bethea, and CT image reader R. Caresse Hightower.
The Diabetes Heart Study (DHS) and the African American-Diabetes Heart Study were supported in part by the General Clinical Research Center of the Wake Forest University School of Medicine (M01-RR-07122), National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-071891) (BIF), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR-048797) (JJC), and National Heart, Lung, and Blood Institute (R01-HL-67348) (DWB). The Multi-Ethnic Study of Atherosclerosis (MESA) was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The Family Heart Study (FHS) was supported by the National Heart, Lung, and Blood Institute cooperative agreement grants U01-HL-67893 through U01-HL-67902. Support was also partially provided by the National Heart, Lung, and Blood Institute cooperative agreement grants U01-HL-56563 through U01-HL-56569.
Abbreviations
- BMI
body mass index
- CAC
coronary artery calcification
- CRP
C-reactive protein
- CT
computed tomography
- CVD
cardiovascular disease
- DHS
Diabetes Heart Study
- FHS
Family Heart Study
- GEE1
generalized estimating equations
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MESA
Multi-Ethnic Study of Atherosclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no relationships with industry to disclose.
References
- 1.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 2.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Hsu FC, Langefeld CD, Rich SS, Herrington DM, Carr JJ, Xu J, Bowden DW, Wagenknecht LE. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu St, Flores F, Blumenthal RS. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343–350. doi: 10.1016/j.atherosclerosis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 6.Bild DE, Folsom AR, Lowe LP, Sidney S, Kiefe C, Westfall AO, Zheng Z-J, Rumberger J. Prevalence and correlates of coronary calcification in black and white young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–857. doi: 10.1161/01.atv.21.5.852. [DOI] [PubMed] [Google Scholar]
- 7.Wagenknecht LE, Langefeld CD, Freedman BI, Carr JJ, Bowden DW. A comparison of risk factors for calcified atherosclerotic plaque in the coronary, carotid, and abdominal aortic arteries. The Diabetes Heart Study. Am J Epidemiol. 2007;166:340–347. doi: 10.1093/aje/kwm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 9.Higgins M, Province M, Heiss G, Eckfeldt J, Ellison RC, Folsom AR, Rao DC, Sprafka JM, Williams R. NHLBI Family Heart Study: objectives and design. Am J Epidemiol. 1996;143:1219–1228. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins PN, Ellison RC, Province MA, Pankow JS, Carr JJ, Arnett DK, Lewis CE, Heiss G, Hunt SC. Association of coronary artery calcified plaque with clinical coronary heart disease in the National Heart, Lung, and Blood Institute’s Family Heart Study. Am J Cardiol. 2006;97:1564–1569. doi: 10.1016/j.amjcard.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 12.Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, Xu J, Langefeld CD, Freedman BI. Ethnic differences in the relationship between albuminuria and calcified atherosclerotic plaque: the African American-diabetes heart study. Diab Care. 2010;33:131–138. doi: 10.2337/dc09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr JJ, Nelson JC, Wong ND, Nitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 14.Carnethon MR, Bertoni AG, Shea S, Greenland P, Ni H, Jacobs DR, Jr, Saad M, Liu K. Racial/ethnic differences in subclinical atherosclerosis among adults with diabetes. Diab Care. 2005;28:2768–2770. doi: 10.2337/diacare.28.11.2768. [DOI] [PubMed] [Google Scholar]
- 15.Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in African Americans and Hispanics from the Multi-Ethnic Study of Atherosclerosis (MESA) Circ Cardiov Genet. 2009;2:629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Lewis CE, Wagenknecht LE, Myers RH, Pankow JS, Hunt SC, North KE, Hixson JE, Jeffrey Carr J, Shimmin LC, Borecki I, Province MA. Genome-wide admixture mapping for coronary artery calcification in African Americans: the NHLBI family heart study. Genet Epidemiol. 2008;32:264–272. doi: 10.1002/gepi.20301. [DOI] [PubMed] [Google Scholar]
- 17.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 18.Nasir K, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Flores PR, Flores FR, Raggi P, Berman DS, Blumenthal RS, Budoff MJ. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953–960. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 19.LaMonte MJ, Fitzgerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 20.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, Humphries SE, Richmond W, Flather MD PREDICT Study Group. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
- 22.Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22:424–430. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 23.Khurana C, Rosenbaum CG, Howard GV, Adams-Campbell LL, Detrano RC, Klouj A, Hsia J. Coronary artery calcification in black women and white women. Am Heart J. 2003;145:724–729. doi: 10.1067/mhj.2003.99. [DOI] [PubMed] [Google Scholar]
- 24.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 25.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–385. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 26.Elkeles RS, Feher MD, Flather MD, Godsland IF, Nugara F, Richmond W, Rubens MB, Wang D PREDICT Study Group. The association of coronary calcium score and conventional cardiovascular risk factors in Type 2 diabetic subjects asymptomatic for coronary heart disease (The PREDICT Study) Diabet Med. 2004;21:1129–1134. doi: 10.1111/j.1464-5491.2004.01409.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoff JA, Daviglus ML, Chomka EV, et al. Conventional coronary artery disease risk factors and coronary artery calcium detected by electron beam tomography in 30,908 healthy individuals. Ann Epidemiol. 2003;13:163–169. doi: 10.1016/s1047-2797(02)00277-6. [DOI] [PubMed] [Google Scholar]
- 28.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Grundy SM, McGuire DK. Relationship between C-reactive protein and subclinical atherosclerosis. The Dallas Heart Study. Circ. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D’Agostino RB, Wilson PWF, O’Donnell CJ. C-reactive protein is associated with subclinical epidardial coronary calcification in men and women. The Framingham Heart Study. Circ. 2002;106:1189–1191. doi: 10.1161/01.cir.0000032135.98011.c4. [DOI] [PubMed] [Google Scholar]
- 30.Bowden DW, Lange LA, Langefeld CD, Brosnihan KB, Freedman BI, Carr JJ, Wagenknecht LE, Herrington DM. The relationship between C-reactive protein and subclinical cardiovascular disease in the Diabetes Heart Study (DHS) Am Heart J. 2005;150:1032–1038. doi: 10.1016/j.ahj.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O'Brien K, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;198:104–114. doi: 10.1016/j.atherosclerosis.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 33.D'Agostino RB, Jr, Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke. 1996;27:1744–1749. doi: 10.1161/01.str.27.10.1744. [DOI] [PubMed] [Google Scholar]
- 34.Howard G, Sharrett R, Heiss G, Evans GW, Chambless LE, Riley WA, Burke GL. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. Stroke. 1993;24:1297–1304. doi: 10.1161/01.str.24.9.1297. [DOI] [PubMed] [Google Scholar]
- 35.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension. Roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 36.Looker AC, Melton LJ, 3rd, Harris T, Borrud L, Shepherd J, McGowan J. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int. 2009;20:1141–1149. doi: 10.1007/s00198-008-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Freedman BI, Wagenknecht LE, Hairston KG, Bowden DW, Carr JJ, Hightower RC, Gordon EJ, Xu J, Langefeld CD, Divers J. Vitamin D, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–1083. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.