Abstract
The therapeutic usefulness of anticancer agents relies on their ability to exert maximal toxicity to cancer cells and minimal toxicity to normal cells. The difference between these two parameters defines the therapeutic index of the agent. Towards this end, much research has focused on the design of anticancer agents that have optimized potency against a variety of cancer cell types; however, much less effort is spent on the design of drugs that are minimally toxic to normal cells. We have previously described a concept for a novel drug delivery platform that relies on the propensity of drugs with optimal physicochemical properties to distribute differently in normal versus cancer cells due to differences in intracellular pH gradients. Specifically, we demonstrated in vitro that certain weakly basic anticancer agents had the propensity to distribute to intracellular locations in normal cells that prevent interaction with the drug target, and to intracellular locations in cancer cells that promote drug-target interactions. We refer to this concept broadly as intracellular distribution-based drug targeting. Here we will discuss current in vivo work from our laboratory that examined the role of lysosome pH on the intracellular distribution and toxicity of inhibitors of the Hsp90 molecular chaperone in mice.
Keywords: Intracellular, Drug delivery, Drug targeting, Lysosomes, Anticancer, Heat shock proteins
How a drug distributes and localizes within a cell is a fundamentally important variable in drug effectiveness. Drug targets typically have well-defined intracellular localization sites. In order for a drug to exert its action it must not only enter cells but it must also sufficiently concentrate in the same intracellular compartment that houses its target. For many new and traditional anticancer agents these targets are localized either in the cell cytosol, for example heat shock proteins (1–2) and microtubules (3) or in the nucleus, i.e. DNA (4–5), and topoisomerases (6–7).
We and others have studied how structural and physicochemical properties of drugs influence their intracellular distribution (8–11). Relevant to this work, we have shown that many weakly basic molecules are excellent substrates for extensive sequestration in acidic lysosomes according to an ion trapping-type mechanism (8–9, 12). Briefly stated, when in the relatively neutral cell cytosol, weakly basic molecules with appropriate pKa values will exist to a significant extent in their un-ionized, membrane-permeable form. Upon crossing lipid bilayers of organelles with very acidic luminal pH such as lysosomes, the drug now exists almost exclusively in its ionized, membrane-impermeable form, which cannot readily diffuse out of the organelle. This change in ionization state lowers the concentration of the un-ionized species in the lumen of the organelle, which subsequently drives further drug accumulation from the cytosol. Such substrates for sequestration in lysosomes are typically referred to as lysosomotropic, or acidotropic compounds.
The extent of lysosomal sequestration of weakly basic drugs is a relevant therapeutic consideration. In some instances lysosomal sequestration can account for nearly 100% of the total drug accumulation within a cell (13–14). Normal cells typically have low lysosomal pH values around 4.0, and can theoretically concentrate up to 1000-fold higher concentration of drug compared to the cytosol (15–16). Despite being relatively low, the concentration of drug in the cytosol is in pseudo-equilibrium with concentrations in the lysosome. The theoretical lysosome-to-cytosol concentration ratio is dictated by both the pKa of the drug and the lysosome-to-cytosol pH gradient (9, 12, 16). Under these circumstances small shifts in lysosomal pH can profoundly influence drug concentrations in the cytosol, where many drug targets are localized. This is also important for drugs that have nuclear targets since the nuclear envelope contains numerous pore complexes that allow for free diffusion of small, low-molecular weight molecules to and from the cytosol (17).
Interestingly, we and others have shown that some cancer cell lines have defective acidification of lysosomes (18–21), resulting in a reduction of lysosome-to-cytosol pH gradients. Consistent with ion trapping theory, this results in a reduced capacity for lysosomal sequestration in these types of cancer cells relative to normal cells. Consequently, the cytosolic concentration of lysosomotropic drugs in cancer cells increases, thus allowing a greater amount of drug to interact with cytosolic and/or nuclear targets than is the case in normal cells with low lysosomal pH. It is the resulting change in drug distribution between normal and cancer cells that we propose can provide the basis for intracellular distribution-based (IDB) drug targeting.
Traditional drug targeting approaches generally utilize strategies that facilitate drug accumulation in cancer cells while limiting accumulation in normal cells (22–24). This approach is fundamentally different from the IDB drug targeting approach, which assumes drug accumulates equally in normal versus cancer cells. The IDB approach then exploits drug distribution differences to enhance drug activity in cancer cells.
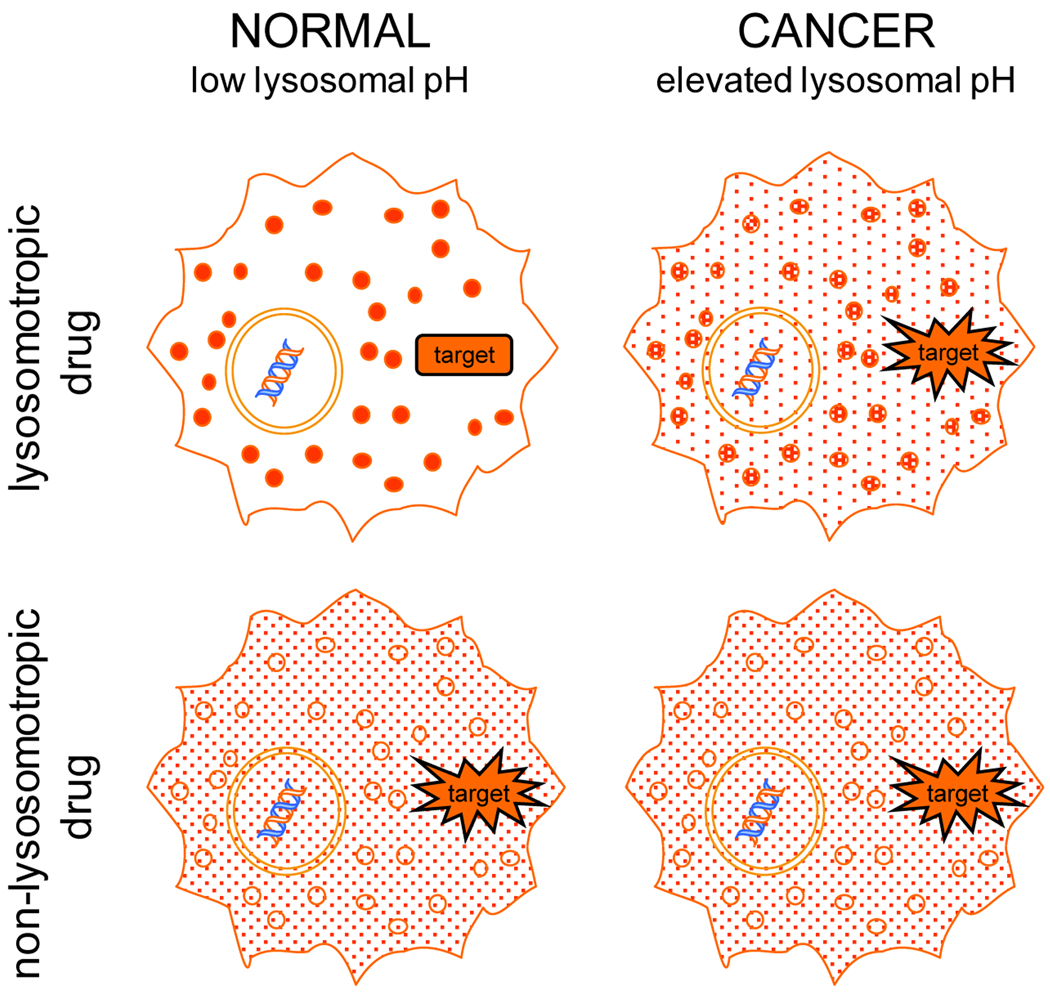
In order to conduct initial proof-of-concept evaluation of the aforementioned IDB drug targeting platform, we tested inhibitors of the molecular chaperone Hsp90, with or without lysosomotropic properties. Hsp90 inhibitors were an attractive choice since they have cytosolic targets; therefore, their activity should be responsive to the degree of lysosomal sequestration, or lack thereof. The inhibitor geldanamycin (GDA) and its structural analogs were particularly well-suited for our evaluations since GDA is neutral and therefore non lysosomotropic, yet is amenable to modification at the 17-position to create analogs with lysosomotropic properties. Most importantly, these modifications have been shown to have little or no impact on Hsp90 binding affinity (25). Consequently, much of our work focused on comparative studies using GDA and its analog, 17-DMAG. 17-DMAG is weakly basic with a pKa of approximately 7.6 (12), which makes it an ideal candidate for sequestration in lysosomes through ion trapping. Accordingly, the degree of 17-DMAG interaction with cytosolic Hsp90 should be sensitive to changes in lysosomal pH (i.e., greater interaction in cancer-like cells with elevated lysosomal pH and reduced interactions in cells with normal lysosomal pH). Conversely, the drug target interaction and activity of GDA should be insensitive to changes in lysosomal pH considering that it is not lysosomotropic. An illustrative overview of the IDB drug targeting platform is presented in Figure 1.
Figure 1. Diagrammatic overview of the intracellular distribution-based (IDB) drug targeting platform.
IDB drug targeting capitalizes on differences in intracellular distribution behavior that exist for lysosomotropic drugs in cells with low (normal) and elevated lysosomal pH (cancer). Drugs (represented as red dots) with lysosomotropic properties will be extensively sequestered in lysosomes of normal cells and will have relatively little interaction with cytosolic targets (top left cell). The same lysosomotropic drug will localize differently in cancer cells with elevated lysosomal pH (top right cell). Specifically, the drug concentration in the lysosomes of cancer cells will be reduced and the concentration in the cytosol will concomitantly increase. The increase in cytosolic levels of the drug allows for greater interaction with targets and an increased therapeutic response. Anticancer drugs without lysosomotropic properties will not differentially localize in normal and cancer cells regardless of lysosomal pH status (lower cells) and drug-to-drug target interactions will not be affected.
We have previously published studies evaluating this IDB drug targeting platform in vitro using cultured cells with low or elevated lysosome pH (12). Quantitative evaluations of lysosome-to-cytosol concentration ratios of neutral and weakly basic inhibitors demonstrated that lysosome-to-cytosol concentration ratios for lysosomotropic inhibitors decreased in cells with elevated lysosomal pH (i.e., cancer-like cells). Alternatively, the lysosome-to-cytosol concentration ratio for the non-lysosomotropic GDA was low (near 1) and was not influenced by lysosomal pH. Consistent with these quantitative observations, we found that 17-DMAG was much more toxic (lower IC50) to cells with elevated lysosomal pH compared to cells with normal, low lysosomal pH. Collectively, these experiments suggest that elevations in lysosomal pH cause an intracellular redistribution of weakly basic drugs that increase selectivity against cancer cells (with higher lysosomal pH).
The previous studies suggest that design or modifications of cancer drugs to impart lysosomotropic properties should be beneficial in promoting IDB drug selectivity. However, a significant concern remained regarding whether or not purposefully targeting toxic anticancer agents to lysosomes imparted a degree of safety in in vivo applications. In the current study, we specifically tested whether sequestration of anticancer drugs in lysosomes can reduce drug-induced toxicity in vivo. Our hypothesis predicts that control mice (normal lysosomal pH) will have a high degree of sequestration of a lysosomotropic drug, thus limiting the exposure to extra-lysosomal targets and the drug will therefore be relatively non-toxic. This model also predicts that in experimental mice (with elevated lysosomal pH), lysosomal sequestration will be reduced, thus increasing both cytosolic drug-target interactions and toxicity.
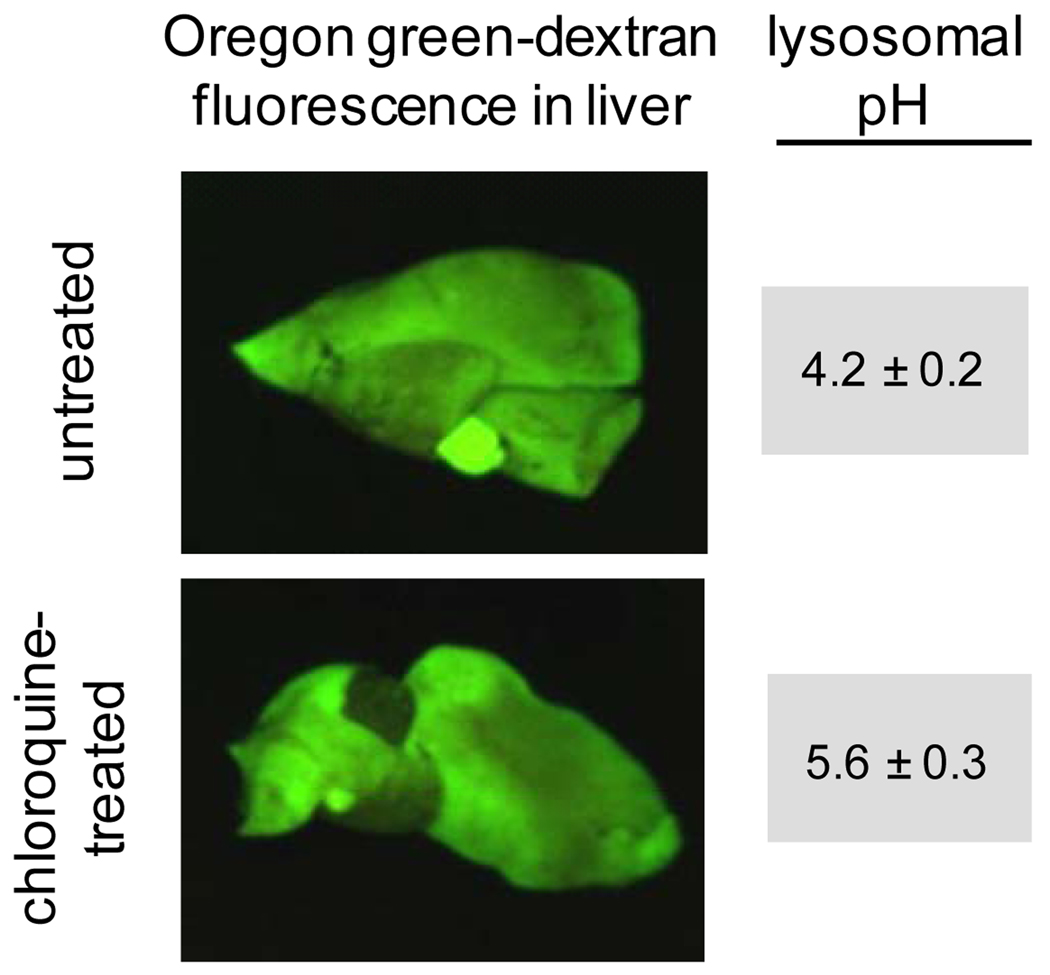
To directly test our hypothesis in mice we utilized a novel approach to increase lysosomal pH in vivo using the anti-malarial drug, chloroquine. Cell culture based methods to raise lysosomal pH, such as targeting the vacuolar-H+-ATPase with inhibitors such as concanamycin A (26), are effective, but their safety in vivo have not been established. Alternatively, chloroquine has been shown to increase lysosomal pH in cultured cells (27), and is known to be well tolerated in mice and humans (28). Therefore, to induce changes in lysosomal pH we dosed mice with CQ at 50 mg/kg/day for 5 days. Our method to assess lysosomal pH in vivo utilizes Oregon Green-dextran, which is a pH sensitive fluorescent probe used routinely to determine lysosomal pH in cultured cells (29). Mehvar and colleagues have shown that dextran polymers of the size used in this study (70 kD) are specifically localized within the liver immediately after administration and remain there virtually unchanged for up to 48 hours (30). We reasoned that sustained localization of the dextran polymers in the vicinity of the hepatocyte would allow for them to be endocytosed and reach terminal lysosomes. Mice were therefore dosed with Oregon Green dextran and 6 hours after injection livers were harvested for determination of lysosomal pH. As shown in Figure 2, the Oregon Green fluorescence was associated with the liver in both untreated and CQ treated mice. Subsequent analysis in isolated hepatocytes confirmed that the dextran indeed localized to the lysosomes (31). The lysosomal pH of CQ treated mice was found to be significantly higher than in untreated control mice (Figure 2). These novel methods for modulating and evaluating lysosomal pH in vivo and the finding that CQ can significantly raise lysosomal pH after 5 days of treatment is noteworthy. The dose of CQ used in our studies was approximately 5 times higher than standard therapeutic doses. Accordingly, it is not known if typical therapeutic doses of CQ used in humans can elevate lysosomal pH and cause changes in intracellular distribution of co-administered weakly basic drugs.
Figure 2. Oregon Green dextran (70 kD) localizes extensively in livers of CQ-treated and untreated mice.
Mice were dosed with 50 mg/kg/day chloroquine i.p. (or with normal saline vehicle control) for 5 days prior to a tail vein injection of 0.5 mg Oregon Green-labeled dextran. To visualize dextran localization, livers were extracted and imaged using the Maestro In Vivo Imaging system. Lysosomal pH of liver cells was found to be significantly elevated in mice treated with CQ. Lysosomal pH values were obtained by calibrating intracellular pH to known values using the ionophores nigericin (10µM) and monensin (20µM). The microscopically determined lysosomal pH values obtained from mice livers, with or without CQ treatment, are shown (pH values are mean ± SD, n=3).
Using mice with normal or elevated lysosomal pH, we evaluated the effects of lysosomal pH on drug-induced toxicity. Consistent with our hypothesis, our results indicate that the weakly basic Hsp90 inhibitor 17-DMAG was significantly more toxic to CQ-treated mice (elevated lysosomal pH) compared to control mice (normal lysosomal pH). Importantly, we demonstrated that the toxicity of a non-lysosomotropic Hsp90 inhibitor (GDA) to mice was not influenced by the lysosomal pH status of mice. This finding suggests that the CQ treatment did not generally enhance the toxicity of this class of inhibitors, but that enhanced toxicity was limited to inhibitors with lysosomotropic properties. In a control experiment, we demonstrated that CQ treatment did not significantly alter the pharmacokinetics of either GDA or 17-DMAG. Specifically, we showed that tissue-to-plasma concentration ratios for the inhibitors were not significantly influenced by the CQ treatment. This control experiment is particularly important because it strongly supports the conclusion that the increased toxicity of 17-DMAG in mice treated with CQ was due to an intracellular redistribution of the drug from lysosomes to cytosol.
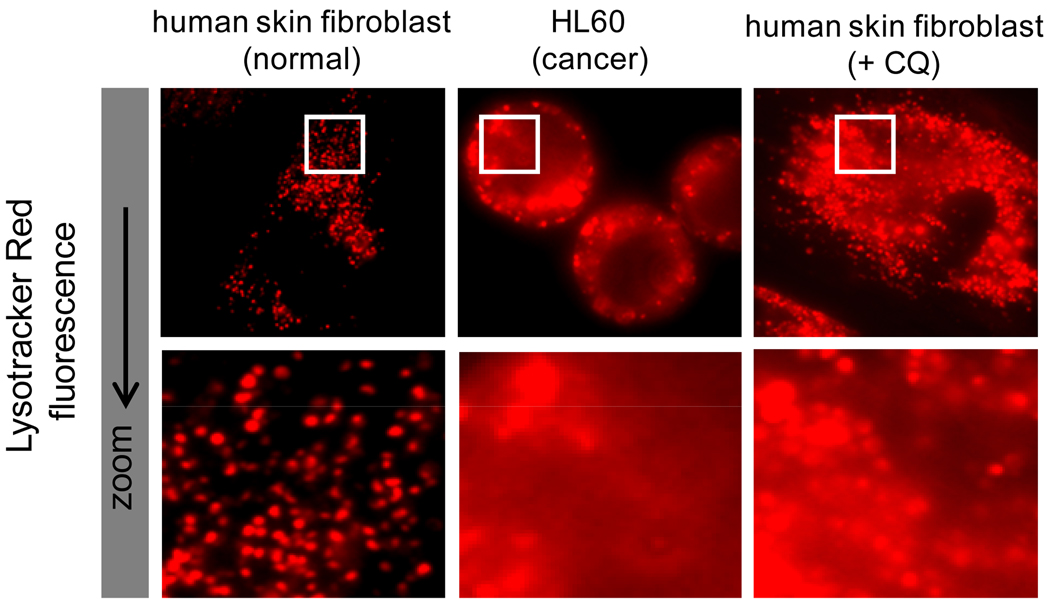
Collectively, this work illustrates, in an in vivo system, the influence of intracellular distribution of drugs on their ability to interact with intended targets and elicit a therapeutic response. Because 17-DMAG is not fluorescent, it is not possible to directly view the predicted changes in intracellular distribution that are likely occurring in our studies (as depicted in Figure 1). However, we propose that, in principle, other fluorescent weakly basic amines should have a similar intracellular distribution based on ion trapping principles. As described previously, the pKa value of a membrane permeable weakly basic molecule is a known predictor of the degree of lysosomal sequestration (9). Considering this, we evaluated the intracellular distribution of Lysotracker Red (LTR) which has a pKa value nearly identical to 17-DMAG (7.5 versus 7.6, respectively), and should therefore have similar pH-dependent changes in intracellular distribution. Since the fluorescence of LTR make it readily detectable using fluorescence microscopy, we evaluated the intracellular distribution of LTR in cells grown in culture with or without CQ treatment, analogous to what was done in our current, in vivo, work. In normal human fibroblasts with low lysosomal pH, LTR is almost exclusively localized in acidic lysosomes, with very little accumulation in the cytosol (see Figure 3). We pretreated the same human fibroblasts with CQ in an attempt to mimic the situation with CQ-pretreated mice. In these cells the LTR is still localized to lysosomes; however, the degree of sequestration is reduced and LTR has noticeably redistributed to the cytosol to a greater extent than in control cells. This is particularly evident under high magnification (see Figure 3). This observation is consistent with the implication that in CQ-pretreated mice, 17-DMAG distributes to a greater extent in the cytosol, which promotes interactions with cytosolic Hsp90 and therefore greater drug-induced toxicity is observed.
Figure 3. The lysosomotropic fluorophore Lysotracker Red (LTR) has enhanced cytosolic localization in cells with elevated lysosomal pH (cancer cells and CQ-treated normal cells) relative to untreated normal cells.
Normal fibroblasts have a low lysosomal pH (4.2) and localize the LTR almost exclusively in punctate compartments, which are presumed to be lysosomes (or other very low pH compartments). The human leukemic HL60 cell line has elevated lysosomal pH (6.5) and therefore would have reduced capacity for LTR sequestration. Consistent with this, the LTR in HL60 cells shows considerably greater diffuse cytosolic fluorescence. Similar to the HL60 cells, normal fibroblasts pretreated with CQ have elevated lysosomal pH. These CQ-treated cells have enhanced cytosolic fluorescence, very similar to cancer cells with elevated lysosomal pH. All cells shown have been incubated identically with Lysotracker Red (100nm for 30 minutes). Cells treated with CQ were incubated with 100µM CQ for 30 min prior to incubation with LTR. The cells were washed 3 times with PBS prior to viewing on an upright epifluorescence microscope with identical lamp power and exposure time.
As previously discussed, lysosomal pH has been shown to be abnormally elevated in certain cancer cell types. To visualize the impact of this abnormal acidification on the distribution of a lysosomotropic compound, we incubated HL60 human leukemic cells that have elevated lysosomal pH (without CQ treatment) with LTR (Figure 3). These cells appear to have a higher degree of cytosolic LTR fluorescence than normal fibroblasts and appear similar to CQ-pretreated cells evaluated in this work. We propose that these differences in intracellular distribution may be, at least partially, responsible for the observed differences in drug selectivity against cancer cells with elevated lysosomal pH. It is undeniable that intrinsic differential selectivity of a cancer drug relies on key biochemical and/or proliferation differences that exist between normal and transformed cells (32–33). However, our results suggest that differences in intracellular distribution between normal and cancer cells may provide an additional degree of selectivity.
On the whole, the IDB anticancer drug selectivity platform described here would suggest that weakly basic anticancer agents with lysosomotropic potential might preferentially exert toxic effects toward cancer cells as a result of favorable differences in intracellular distribution between normal cells and cancer cells with elevated lysosomal pH. A large number of successful anticancer agents already possess some degree of lysosomotropic potential. It is tempting to speculate that cancer drugs with fully optimized lysosomotropic properties could have even further improved differential selectivity. Accordingly, future studies in this area could lead to the development of new anticancer agents that are rationally designed to exploit differences in intracellular pH gradients between normal and cancer cells. It is important to realize that such drugs would have improved therapeutic index not because of improved potency against cancer cells, but instead because of reduced toxicity toward normal cells.
Acknowledgements
We thank Nancy Schwarting for assistance in animal dosing procedures. This work was supported by the National Institutes of Health (Grant No. RO1 CA106655) and the Kansas IDeA Network of Biomedical Research Excellence Award to J.P.K
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Ciocca DR, Clark GM, Tandon AK, Fuqua SAW, Welch WJ, McGuire WL. Heat shock protein Hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 2.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 4.Issa JJ. DNA methylation as a therapeutic target in cancer. Clin Cancer Res. 2007;13:1634–1637. doi: 10.1158/1078-0432.CCR-06-2076. [DOI] [PubMed] [Google Scholar]
- 5.Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002 Feb;:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- 6.Fortune JM, Velea L, Graves DE, Utsugi T, Yamada Y, Osheroff N. DNA topoisomerases as targets for the anticancer drug TAS-103: DNA interactions and topoisomerase catalytic inhibition. Biochemistry. 1999;38:15580–15586. doi: 10.1021/bi991792g. [DOI] [PubMed] [Google Scholar]
- 7.Nitiss J, Wang JC. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci USA. 1988;85:7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvvuri M, Gong Y, Chatterji D, Krise JP. Weak base permeability characteristics influence the intracellular sequestration site in the multidrug-resistant human leukemic cell line HL-60. J Biol Chem. 2004;279:32367–32372. doi: 10.1074/jbc.M400735200. [DOI] [PubMed] [Google Scholar]
- 9.Duvvuri M, Konkar S, Funk RS, Krise JM, Krise JP. A Chemical strategy to manipulate the intracellular localization of drugs in resistant cancer cells. Biochemistry. 2005;44:15743–15749. doi: 10.1021/bi051759w. [DOI] [PubMed] [Google Scholar]
- 10.Lansiaux A, Tanious F, Mishal Z, et al. Distribution of furamidine analogues in tumor cells: targeting of the nucleus or mitochondria depending on the amidine substitution. Cancer Res. 2002;62:7219–7229. [PubMed] [Google Scholar]
- 11.Smith RAJ, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvvuri M, Konkar S, Hong KH, Blagg BSJ, Krise JP. A new approach for enhancing differential selectivity of drugs to cancer cells. ACS Chem Biol. 2006;1:309–315. doi: 10.1021/cb6001202. [DOI] [PubMed] [Google Scholar]
- 13.Bulychev A, Trouet A, Tulkens P. Uptake and intracellular distribution of neutral red in cultured fibroblasts. Expl Cell Res. 1978;115:343–355. doi: 10.1016/0014-4827(78)90288-4. [DOI] [PubMed] [Google Scholar]
- 14.Duvvuri M, Krise JP. A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than pH-Partitioning theory would predict. Mol Pharm. 2005;2:440–448. doi: 10.1021/mp050043s. [DOI] [PubMed] [Google Scholar]
- 15.Yang WCT, Strasser FF, Pomerat CM. Mechanism of drug-induced vacuolization in tissue culture. Exp Cell Res. 1965;38:495–506. doi: 10.1016/0014-4827(65)90373-3. [DOI] [PubMed] [Google Scholar]
- 16.de Duve C, de Barsy T, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 17.Tonini R, Grohovaz F, Laporta CAM, Mazzanti M. Gating mechanism of the nuclear pore complex channel in isolated neonatal and adult mouse liver nuclei. FASEB J. 1999;13:1395–1403. doi: 10.1096/fasebj.13.11.1395. [DOI] [PubMed] [Google Scholar]
- 18.Altan N, Chen Y, Schindler M, Simon SM. Defective acidification in human breast tumor cells and implications for chemotherapy. J Exp Med. 1998;187:1583–1598. doi: 10.1084/jem.187.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Y, Duvvuri M, Krise JP. Separate roles for the Golgi apparatus and lysosomes in the sequestration of drugs in the multi-drug resistant human leukemic cell line HL-60. J Biol Chem. 2003;278:50234–50239. doi: 10.1074/jbc.M306606200. [DOI] [PubMed] [Google Scholar]
- 20.Kokkonen N, Rivinoja A, Kauppila A, Suokas M, Kellokumpu I, Kellokumpu S. Defective acidification of intracellular organelles results in aberrant secretion of cathepsin D in cancer cells. J Biol Chem. 2004;279:39982–39988. doi: 10.1074/jbc.M406698200. [DOI] [PubMed] [Google Scholar]
- 21.Schindler M, Grabski S, Hoff E, Simon SM. Defective pH regulation of acidic compartments in human breast cancer cells (MCF-7) is normalized in adriamycin-resistant cells (MCF-7adr) Biochemistry. 1996;35:2811–2817. doi: 10.1021/bi952234e. [DOI] [PubMed] [Google Scholar]
- 22.Houshmand P, Zlotnik A. Targeting tumor cells. Curr Opin Cell Biol. 2003;15:640–644. doi: 10.1016/s0955-0674(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kataoka K. Selective delivery of adiramycin to a solid tumor using a polymeric micelle carrier system. J Drug Target. 1999;7:171–186. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- 24.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 25.Tian Z, Liu Y, Zhang D, et al. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem. 2004;12:5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 26.Temesvari LA, Rodriguez-Paris JM, Bush JM, Zhang L, Cardelli JA. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. J Cell Sci. 1996;109:1479–1495. doi: 10.1242/jcs.109.6.1479. [DOI] [PubMed] [Google Scholar]
- 27.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chico RM, Pittrof R, Greenwood B, Chandramohan D. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar J. 2008;7:255. doi: 10.1186/1475-2875-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 30.Mehvar R, Robinson MA, Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci. 1994;83:1495–1499. doi: 10.1002/jps.2600831024. [DOI] [PubMed] [Google Scholar]
- 31.Ndolo RA, Forrest ML, Krise JP. The role of lysosomes in limiting drug toxicity in mice. J Pharmacol Exp Ther. 2010;333:120–128. doi: 10.1124/jpet.109.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutcher JP, Novik Y, O'Boyle K, Marcoullis G, Secco C, Wiernik PH. 20th-century advances in drug therapy in oncology--Part I. J Clin Pharmacol. 2000;40:1007–1024. doi: 10.1177/00912700022009620. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Pouyssegur J. Tumor Cell Metabolism: Cancer's Achilles' Heel. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]