Abstract
Fibroblast growth factor 2 (basic FGF or FGF2) has been shown to affect growth and differentiation in some tissues and to be required for cardiac hypertrophy in vivo. FGF2 has been shown in vitro to signal through the mitogen-activated protein kinase (MAPK) to affect cell survival and growth. To ascertain the role of FGF2 in cardiac hypertrophy, wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic mice were treated with isoproterenol or saline via subcutaneous mini-osmotic pump implants to induce a hypertrophic response to β-adrenergic stimulation. Fgf2 knockout hearts are protected from isoproterenol-induced cardiac hypertrophy; whereas, FGF2 transgenic hearts show exacerbated cardiac hypertrophy as assessed by heart weight-to-body weight ratios and myocyte cross-sectional area. Echocardiography reveals significantly decreased fractional shortening in isoproterenol-treated FGF2 transgenic mice but not in Fgf2 knockout mice suggesting that FGF2 mediates the maladaptive cardiac dysfunction seen in cardiac hypertrophy induced by isoproterenol. Western blot analysis also reveals alterations in MAPK signaling in Fgf2 knockout and FGF2 transgenic hearts subjected to isoproterenol treatment, suggesting that this cascade mediates FGF2's pro-hypertrophic effect. Pharmacologic inhibition of extracellular signal-regulated kinase (ERK) signaling results in an attenuated hypertrophic response in isoproterenol-treated FGF2 transgenic mice, but this response is not seen with p38 mitogen-activated protein kinase (p38) pathway inhibition, suggesting that FGF2 activation of ERK but not p38 is necessary for FGF2's role in the mediation of cardiac hypertrophy.
Keywords: Fibroblast growth factor, FGF2, Basic FGF, Cardiac hypertrophy, Extracellular regulated kinase, ERK, Mitogen associated protein kinase, MAPK, Isoproterenol
Introduction
Multiple stressful stimuli result in adaptation of the heart through cardiomyocyte hypertrophy. The cardiac hypertrophic response can occur during both physiologic states such as exercise or pregnancy or pathologic states such as chronic hypertension, hypervolemia, abnormalities in sarcomeric proteins, or excess catecholamine stimulation. The pathologic hypertrophic response is characterized by increased cardiac mass resulting from both increased cardiomyocyte size and hyperplasia of cardiac fibroblasts resulting in remodeling of the architecture of the heart which ultimately leads to cardiac dysfunction, dilatation, and heart failure.
Growth factors, including those of the FGF1 family, have been shown to be involved in the progression of cardiac hypertrophy to a variety of stimuli. Several lines of evidence have suggested a role for a member of this protein family, FGF2, in the cardiac hypertrophic response. The addition of FGF2 to cardiomyocytes in vitro was shown to alter contractile protein expression from adult to fetal isoforms (1) which has been correlated with pressure overload-induced cardiac hypertrophy (2–5) and to stimulate protein synthesis and increase myocyte size (6). In addition, paced cardiomyocytes released FGF2 (7), evoking a hypertrophic response (6). FGF2 has also been shown to play a role in the development of hypertrophy in vivo. Our laboratory has previously shown a significantly reduced cardiac hypertrophic response in FGF2-deficient mice in response to transverse aortic coarctation, a model of pressure overload-induced hypertrophy (8). Renal artery banding leading to elevated angiotensin II levels and hypertension failed to induce a full hypertrophic response in another FGF2-deficient mouse model (9).
In order to fully understand the role of FGF2 in the progression of cardiac hypertrophy, it is necessary to elucidate the signaling mechanisms which mediate FGF2’s pro-hypertrophic effect. FGF2 has been shown to activate multiple signal transduction cascades including members of the MAPK family (10). Both ERK and p38 have been correlated with cardiac hypertrophy in a variety of models, but their necessity to FGF2-mediated hypertrophy through β-adrenergic stimulation remains unknown.
We have utilized both mice deficient of FGF2 and mice with a cardiac-specific overexpression of all human isoforms of FGF2 in order to definitively ascertain the role of endogenously expressed FGF2 in isoproterenol-induced hypertrophy. Our results demonstrate that ablation of the Fgf2 gene results in protection from isoproterenol-induced cardiac hypertrophy while overexpression of FGF2 exacerbates hypertrophy due to β-adrenergic stimulation. Echocardiographic data reveal that FGF2 may mediate the maladaptive cardiac dysfunction seen in cardiac hypertrophy. In addition, the present study shows alterations in ERK-mediated MAPK signaling in isoproterenol-treated animals lacking or overexpressing FGF2. Finally, the use of pharmacological inhibitors conclusively demonstrates that FGF2 activation of ERK but not p38 mediates isoproterenol-induced hypertrophy, in vivo.
Materials and Methods
Mice
Mice were housed in a pathogen-free facility and handled in accordance with standard use protocols, animal welfare regulations, and the NIH Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Mice with a targeted ablation of the Fgf2 gene (Fgf2 knockout) maintained on a mixed background of 50% 129 and 50% Black Swiss were generated as previously described. Mice with a cardiac-specific overexpression of all four isoforms of human FGF2 (FGF2 transgenic) were generated as previously described and maintained on a FVBN background. Wildtype (Wt), Fgf2 knockout (Fgf2 KO), non-transgenic (NTg), and FGF2 transgenic (FGF2 Tg) mice at 10 to 12 weeks of age were randomly assigned to the present study – 2 day isoproterenol or saline treatment (23 Wt, 21 Fgf2 KO, 19 NTg and 20 FGF2 Tg), 14 day isoproterenol or saline treatment (28 Wt, 34 Fgf2 KO, 17 NTg and 14 FGF2 Tg), UO126 studies (8 NTg and 11 FGF2 Tg), and SB203580 studies (8 NTg and 8 FGF2 Tg). Two independently derived FGF2 transgenic lines were used in all experiments to ensure that results were not due to random transgene insertion affecting other genetic loci. Since similar results were obtained in both FGF2 transgenic lines, all figures depict combined data from both transgenic lines.
Mouse Model of Cardiac Hypertrophy
To induce a cardiac hypertrophic response, wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic mice were subjected to either saline or isoproterenol treatment via Alzet mini-osmotic pump implants (DURECT Corporation). Briefly, mice were anesthetized intraperitoneally with 2.5% Avertin (200 mg/kg). Pumps loaded with either saline or isoproterenol (60 mg/kg/day, Sigma Aldrich) were placed subcutaneously through a midscapular incision which was then closed with 5.0 Ticron suture (United States Surgical). The contents of the mini-osmoticpump were delivered into the local subcutaneous space at a rateof 0.25 μl/hour for either 2 or 14 days. Mice were then monitored daily and sacrificed on either day 2 or day 14.
Echocardiography
Echocardiography was performed preoperatively and following 14 days isoproterenol or saline treatment in wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic mice. Mice under isoflurane anesthesia were placed in a supine position, and a warm layer of ultrasonic gel was applied over the left hemithorax. Using a Sonos 5500 Ultrasound System(Hewlett-Packard, Andover, Massachusetts, USA) equipped with both 15- and 12-MHz transducers, the following measurements were obtained: septal wall thickness (SWT) in diastole and systole, LV end-diastolic and systolic chamber size, LV posterior wall thickness (PWT) in diastole and systole, and R-R interval. M-mode measurements were made from original tracings, as suggested by the American Society of Echocardiography (11). LV mass was calculated using a corrected cube formula (12). LV mass = {1.04[(SWT + PWT + LVED)3 – LVED3] x 0.8} where SWT is septal wall thickness, PWT is posterior wall thickness, and LVED is LV end-diastolic chamber size. In addition, cardiac function, measured as percent fractional shortening, was determined using the parameters of LVED and LV end-systole (LVES). Cardiac function = [(LVED – LVES)/LVED] x 100.
Myocyte Cross Sectional Area and Histological Examination
Saline and isoproterenol-treated wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic hearts were fixed in 4% formalin and transferred to 70% ethanol the next day. Hearts were paraffin-embedded and then sectioned longitudinally (5–10 μm). Sections were then deparaffinized, rehydrated, and stained with fluorescent TRITC-labeled wheat germ agglutinin (1:4 dilution, Sigma Aldrich). Fluorescence was visualized using a Nikon Eclipse TE 2000-U microscope, and digital images were captured and analyzed with Image Pro-Plus 5.0 software. Approximately 150 cells per heart were measured to determine the average myocyte cross sectional area for each heart. In addition, hematoxylin/eosin and Masson's trichrome stain were used for histological and pathological examination of hearts.
MAPK Pathway Inhibition
UO126 (1,4-diamino-2,3-dicyano-1, 4-bis[2-aminophenylthio]butadiene, Calbiochem), an inhibitor of MEK1/2, upstream kinases of ERK activation (13), or SB203580 hydrochloride (4-(4-fluorophenyl)-2-(4-methyl-sulfinylphenyl)-5-(4-pyridyl)-1H-imidazole, Calbiochem), a p38 inhibitor (14), was administered in conjunction with either saline or isoproterenol in order to determine the necessity of ERK or p38 pathway activation in FGF2-mediated cardiac hypertrophy. UO126 (175 μg/kg/day) or SB203580 (1 mg/kg/day) were infused with either saline or isoproterenol via mini-osmotic pumps as described in “Mouse Model of Cardiac Hypertrophy” section. These drug doses were chosen based on the Ki of each inhibitor and a preliminary dose response effect of inhibition of ERK or p38 activation, respectively.
Western Blot Analysis of MAPK Phosphorylation
Wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic hearts subjected to either saline or isoproterenol treatment for 2 days or 14 days were analyzed to determine if alterations in MAPK phosphorylation occur in response to β-adrenergic stimulation and/or alterations in FGF2 levels. In addition, non-transgenic and FGF2 transgenic hearts subjected for 2 days to UO126 or SB203580 treatment in the presence or absence or isoproterenol were analyzed to determine the efficacy of UO126 and SB203580 in ERK or p38 pathway inhibition. Hearts were snap frozen in liquid nitrogen and homogenized in buffer containing 25mmol/L Hepes, 150mmol/L NaCl, 1% Triton X-100, 5mmol/L EDTA, 1% glycerol, and various protease and phosphatase inhibitors (Roche complete mini EDTA-free protease inhibitor cocktail, PMSF, okadaic acid). Homogenates were centrifuged at 3000X g to remove cell debris, and 100μg of each supernatant was subjected to SDS-PAGE. Western blot analysis was performed using phospho-specific ERK and p38 antibodies (1:1000 dilution, Cell Signaling). Equal protein loading was assessed via Ponceau S (Sigma Aldrich) staining of total protein content and Western blot analysis with antibodies to total ERK (1:1000 dilution, BD Transduction Labs) and total p38 (1:1000 dilution, Santa Cruz Technologies). Densitometry of protein bands was performed using a Fluorchem 8800 gel imager (Alpha Innotech).
Statistical Analysis
All values are expressed as mean ± SEM. All data was compared using a Student’s t-test. Statistical significance was determined by a p<0.05.
Results and Discussion
To ascertain the role of FGF2 in the cardiac hypertrophic response, genetically modified mice were utilized containing either a targeted ablation of the Fgf2 gene (Fgf2 knockout) or cardiac-specific overexpression of all human FGF2 isoforms (FGF2 transgenic). The generation of these mice has been previously described (15, 16). These mice are viable and fertile and have normal cardiac development. Heart weight-to-body weight ratios showed no signs of spontaneous hypertrophy in either Fgf2 knockout or FGF2 transgenic mice, supporting previously described findings by our laboratory (15). Also, no differences were found in heart weight-to-body weight ratio in saline-treated Fgf2 knockout or FGF2 transgenic hearts, confirming no spontaneous cardiac hypertrophy as a result of manipulation of endogenous FGF2 expression (Figure 1). No basal differences were observed in any echocardiographic parameter, including morphometric and functional measures, between wildtype and Fgf2 knockout mice or non-transgenic and FGF2 transgenic mice (Table 1).
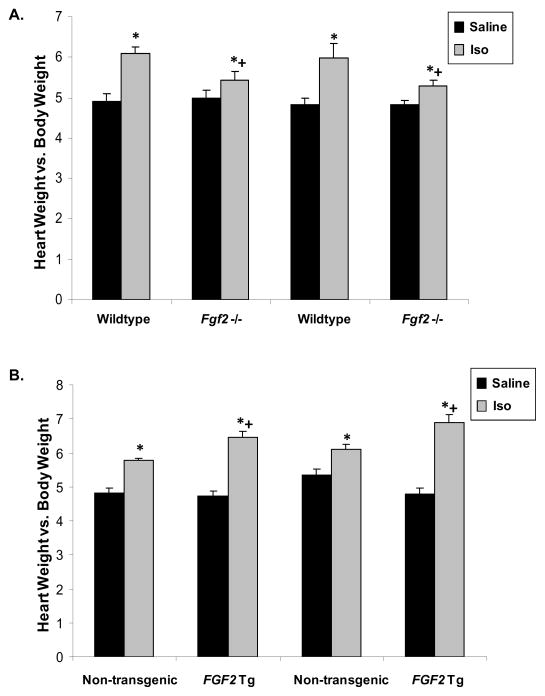
Figure 1. Heart weight-to-body weight ratios following treatment with saline or isoproterenol for either 2 or 14 days.
(A) Treatment with isoproterenol significantly increased heart weight-to-body weight ratios in wildtype mice after both 2 and 14 days. This response was significantly attenuated in Fgf2 knockout (-/-) animals. (B) Isoproterenol-treated non-transgenic animals exhibited increased heart weight-to-body weight ratios compared to saline-treated non-transgenic animals. Isoproterenol-treated FGF2 transgenic (Tg) animals showed an even greater increase in heart weight-to-body weight ratios compared to non-transgenic controls. *p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, n=8–14 for each group.
Table 1. Echocardiographic measures in wildtype, Fgf2 knockout (−/ −), non-transgenic, and FGF2 transgenic (Tg) mice at 10–12 weeks of age.
No significant differences were observed between genotypes for any basal echocardiographic parameter measured. LVED, left ventricular end-diastole (mm). LVES, left ventricular end-systole (mm). SWT septal wall thickness at end-diastole (mm). PWT, posterior wall thickness at end diastole (mm). LV mass, calculated left ventricular mass. HR, heart rate (beats/min). % FS, percent fractional shortening. n=13–25 for each group
| LVED | LVES | SWT | PWT | Mass | HR | %FS | |
|---|---|---|---|---|---|---|---|
| WildtypT | 3.75 ± 0.09 | 2.25 ± 0.09 | 0.74 ± 0.03 | 0.72 ± 0.02 | 76 ± 4.8 | 451 ± 21 | 40 ± 1.4 |
| Fgf2 −/ − | 3.77 ± 0.16 | 2.41 ± 0.17 | 0.76 ± 0.03 | 0.77 ± 0.03 | 81 ± 5.6 | 488 ± 26 | 37 ± 1.7 |
| Non-transgenic | 3.56 ± 0.04 | 2.24 ± 0.04 | 0.70 ± 0.02 | 0.73 ± 0.02 | 84 ± 2.8 | 423 ± 12 | 37 ± 0.6 |
| FGF2 Tg | 3.49 ± 0.04 | 2.17 ± 0.04 | 0.68 ± 0.01 | 0.73 ± 0.02 | 80 ± 2.4 | 421 ± 17 | 38 ± 0.7 |
The cardiac hypertrophic response was evaluated in wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic mice following infusion of saline or isoproterenol via subcutaneous mini-osmotic pump implants. Two time points were studied including an early (2 days) and a later (14 days) hypertrophic response. Heart weight-to-body weight ratios indicate a marked hypertrophic response in isoproterenol-treated wildtype animals at both 2 days and 14 days (p<0.05, Figure 1A). During both of these time points, ablation of the Fgf2 gene resulted in attenuation but not complete abrogation of the hypertrophic response to isoproterenol (p<0.05, Figure 1A). In contrast, overexpression of FGF2 in the heart resulted in an exacerbation of the hypertrophic response to isoproterenol as reflected by a significantly increased heart weight-to-body weight ratio compared to isoproterenol-treated non-transgenic animals (p<0.05, Figure 1B). To ensure that alterations in heart weight-to-body weight ratios were not due to differences in weight gain or loss following surgical manipulation, heart weight-to-tibia length ratios were also calculated showing the same reduction in hypertrophic response in Fgf2 knockout animals and exacerbation of cardiac hypertrophy in FGF2 transgenic mice (data not shown).
Increases in heart weight-to-body weight ratios are indicative of a hypertrophic response, but could also result from hyperplasia of the myocardium or significant expansion of cardiac connective tissue leading to fibrosis. To confirm that alterations in heart weight-to-body weight ratios were due to hypertrophy of cardiomyocytes, histological examination of wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic hearts subjected to saline or isoproterenol treatment for 14 days was undertaken. Wildtype hearts subjected to isoproterenol treatment showed a significantly increased cardiomyocyte size compared to saline-treated wildtype animals (p<0.05, Figure. 2A and B).Fgf2 knockout animals also showed an increase in cardiomyocyte size upon isoproterenol stimulation, but this response was significantly attenuated compared to wildtype animals (p<0.05, Figure. 2A and B), suggesting that FGF2 partially mediates the isoproterenol-induced cardiac hypertrophy. In contrast, isoproterenol-treated FGF2 transgenic mice showed a markedly increased change in cardiomyocyte size compared to non-transgenic animals (p<0.05, Figure 2C and D). In addition, isoproterenol-treated FGF2 transgenic hearts also exhibited many fibrotic lesions. These data are consistent with gravimetric examination of the hearts and demonstrates a role for FGF2 in the in vivo cardiac hypertrophic response to β-adrenergic stimulation.
Figure 2.
Representative images of hearts from (A) wildtype and Fgf2 knockout (-/-) mice and (C) non-transgenic and FGF2 transgenic (Tg) mice after 14 days of treatment with saline or isoproterenol. Wildtype but not Fgf2-/- hearts treated with isoproterenol show an increase in myocyte size compared to saline-treated hearts. FGF2 Tg hearts show an exacerbation of the cardiomyocyte hypertrophic response compared to non-transgenic animals and an increased amount of fibrosis following isoproterenol-treatment. Images acquired at 40X. Scale bar = 20 μm. Quantitation of cardiomyocyte cross-sectional area following 14 days of saline or isoproterenol treatment in (B) wildtype and Fgf2-/- mice and (D) non-transgenic and FGF2 Tg hearts. Wildtype hearts treated with isoproterenol show a significant increase in cardiomyocyte size compared to saline-treated wildtype hearts. This hypertrophic response is significantly attenuated in isoproterenol-treated Fgf2-/- hearts whereas isoproterenol-treated FGF2 Tg hearts show a significantly increased cardiomyocyte size compared to non-transgenic controls. *p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, n=4–5 for each group.
Echocardiographic analysis of wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic mice subjected to 14 days of saline or isoproterenol treatment was performed. Calculated left ventricular mass, which was normalized to tibia length, was significantly increased in isoproterenol-treated wildtype animals, but not in Fgf2 knockout mice (p<0.05, Figure 3A). In contrast, isoproterenol-treated FGF2 transgenic mice exhibited a significantly increased LV mass, as measured by echo, compared to isoproterenol-treated non-transgenic controls. Functional measurements depicted as percent fractional shortening revealed a small but significant decrease in function in isoproterenol-treated wildtype animals (p<0.05, Figure 3B); whereas, this decrease did not occur in isoproterenol-treated Fgf2 knockout animals. FGF2 transgenic animals showed a significant decrease in percent fractional shortening following isoproterenol treatment which was not observed in non-transgenic mice (p<0.05, Figure 3B).
Figure 3.
(A) Echocardiographic analysis of calculated left ventricular mass normalized to tibia length ratio for wildtype, Fgf2 knockout (-/-), non-transgenic, and FGF2 transgenic (Tg) mice subjected to 14 days of saline or isoproterenol treatment. Isoproterenol-treated wildtype mice showed significantly increased LV mass compared to saline-treated wildtype animals; whereas, this response was not seen in Fgf2-/- mice. In contrast, isoproterenol-treated FGF2 Tg mice showed a significantly increased LV mass compared to isoproterenol-treated non-transgenic mice. (B) Percent (%) fractional shortening of wildtype, Fgf2-/-, non-transgenic, and FGF2 Tg mice subjected to 14 days of saline or isoproterenol treatment. Wildtype mice with isoproterenol-induced hypertrophy showed a significant decrease in % fractional shortening; whereas, this decrease was not seen in Fgf2-/- mice treated with isoproterenol. FGF2 Tg hearts showed significantly decreased % fractional shortening compared to non-transgenic controls following isoproterenol stimulation. *p<0.05 vs. saline treatment n=4–16 for each group.
This study has demonstrated a role for FGF2 in isoproterenol-induced cardiac hypertrophy. Isoproterenol stimulation resulted in increased heart weight-to-body weight ratios (Figure. 1A) and increased cardiomyocyte size (Figure 2A and B) in wildtype mice. This response was significantly attenuated in Fgf2 knockout mice. These data are consistent with previous reports of the necessity of FGF2 for a full hypertrophic response to either pressure-overload (8) or renal artery banding (9). FGF2 deficiency did not, however, fully abrogate isoproterenol-induced cardiac hypertrophy as isoproterenol-treated Fgf2 knockout mice still showed a significantly increased heart weight-to-body weight ratio and increased myocyte cross-sectional area compared to saline-treated Fgf2 knockout mice. This reduced response is likely due to the remaining presence of multiple other proteins in Fgf2 knockout mice which may be able to partially mediate isoproterenol-induced cardiac hypertrophy such as other FGF protein family members and other growth factors including members of the transforming growth factor family.
In contrast, cardiac-specific overexpression of FGF2 resulted in an exacerbation of isoproterenol-induced hypertrophy as reflected by significantly increased heart weight-to-body weight ratios (Figure. 1B) and cardiomyocyte cross-sectional area (Figure. 2C and D) in isoproterenol-treated FGF2 transgenic mice compared to isoproterenol-treated non-transgenic animals. These data are the first definitive evidence of an exacerbation of cardiac hypertrophy as a result of FGF2 overexpression in vivo. One previous study had reported increased heart weight-to-body weight ratios in isoproterenol-treated animals with an overexpression of solely the low molecular weight isoform of FGF2 (17); however, this increase was not evident until four weeks after isoproterenol exposure and was attributed primarily to differences in the amount of fibrosis in the low molecular weight isoform FGF2 transgenic mice. The present study also notes increased fibrosis in our cardiac-specific FGF2 transgenic mouse lines but has unequivocally demonstrated increased myocyte cross-sectional area in isoproterenol-treated FGF2 transgenic mice indicative of an increased hypertrophic response.
Isoproterenol acts directly on the β1-adrenergic receptors in the myocardium activating several pathways in cardiomyocytes that are implicated in the hypertrophic response (18, 19). Multiple mechanisms exist which may explain how isoproterenol-induced cardiac hypertrophy may be affected by alteration in FGF2 signaling. For example, β-adrenergic stimulation has been shown to increase FGF2 expression in adipocyte cell culture through increased FGF2 mRNA transcription (20). In addition, FGF2 may be released into the extracellular milieu following isoproterenol stimulation so that FGF2 can activate FGF receptors in an autocrine or paracrine manner. FGF2 does not contain a signal sequence for secretion, and yet, FGF2 has been shown to be present in the extracellular matrix (21) and to bind high-affinity extracellular FGF receptors (22). One possibility for the mechanism of FGF2 release to the extracellular environment is through transient, survivable disruption, or wounding, of the plasma membrane. It has been shown that this cell membrane wounding occurs in response to β-adrenergic stimulation of the heart resulting in increased FGF2 release (7).
FGF2’s pro-hypertrophic effects may be mediated through alterations of multiple signaling cascades including the mitogen activated protein kinase cascade (MAPK). FGF2 has been shown to activate FGF receptor tyrosine kinases which leads to the activation of multiple signaling molecules ultimately resulting in activation of the RAS/MAPK signaling cascade (23, 24). FGF2 has been shown to stimulate ERK activation both in cardiomyocytes in vitro (25) and in whole heart (26, 27). To determine the MAPK signaling alterations which might mediate FGF2’s role in the cardiac hypertrophic response, Western blot analysis of MAPK phosphorylation was undertaken in wildtype, Fgf2 knockout, non-transgenic, and FGF2 transgenic hearts subjected to either 2 days or 14 days of isoproterenol or saline treatment. Isoproterenol treatment for 2 days resulted in significantly increased levels of ERK phosphorylation in wildtype animals, but this response was significantly abrogated in Fgf2 knockout mice (p<0.05, Figure. 4A). Differences in ERK phosphorylation were diminished by 14 days of isoproterenol or saline treatment in these two groups. FGF2 transgenic hearts, however, showed significantly increased levels of ERK phosphorylation in response to 2 days of isoproterenol treatment in comparison to non-transgenic hearts (p<0.05, Figure. 4B). By 14 days, ERK activation returned to similar levels as non-transgenic hearts (Figure. 4B). These data are consistent with other reports of correlations between ERK activity and the progression of cardiac hypertrophy resulting from pressure overload (due to aortic banding) (28, 29) or volume overload (30). Interestingly, there was a significant increase in ERK activation in isoproterenol-treated wildtype hearts which was not observed in isoproterenol-treated non-transgenic hearts (Figure 4A and 4B). This difference may be due to the fact that these mice are from different genetic strains. In this study, wildtype mice are bred on a 50% 129 and 50% Black Swiss background whereas the non-transgenic mice have a FVBN background. Supporting this idea, strain-specific differences in the cardiac response to isoproterenol has been observed in multiple studies (31, 32). No differences were observed in p38 phosphorylation following either 2 days or 14 days of treatment with saline and isoproterenol in wildtype or Fgf2 knockout hearts (Figure 5A). Similarly, no differences in p38 phosphorylation were observed in non-transgenic or FGF2 transgenic hearts (Figure 5B). These data suggest a role for ERK phosphorylation downstream of FGF2 but not p38 phosphorylation in the mediation of isoproterenol-induced cardiac hypertrophy. FGF2 has been shown in various tissues under various stimuli to either activate or inhibit p38. Activation of p38 has been observed in models of cardiac hypertrophy including hypertrophy induced by either angiotensin II (9) or chronic hypertension (33). In the present study, however, no significant differences were observed in response to either isoproterenol stimulation or FGF2 ablation or overexpression (Figure 5). Since only two time points (2 and 14 days) were observed, it is possible that alterations in the p38 pathway may occur at other time points.
Figure 4. ERK phosphorylation after 2 or 14 days of saline or isoproterenol treatment in wildtype, Fgf2 knockout (-/-), non-transgenic, and FGF2 transgenic (Tg) mouse hearts.
(A) At 2 days, there is a significant increase in ERK phosphorylation in isoproterenol-treated wildtype hearts. This response is abrogated in isoproterenol-treated Fgf2-/- hearts, suggesting a role for activation of ERK by FGF2 in the progression of isoproterenol-induced cardiac hypertrophy. This early response is not seen after 14 days of isoproterenol treatment. (B) Isoproterenol-treated FGF2 Tg hearts showed significantly increased ERK phosphorylation in comparison to non-transgenic controls after 2 days but not 14 days of isoproterenol treatment. *p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, n=4–10 for each group.
Figure 5.
p38 phosphorylation after 2 or 14 days of saline or isoproterenol treatment in (A) wildtype and Fgf2 knockout (-/-) mouse hearts and (B) non-transgenic and FGF2 transgenic (Tg) mouse hearts. There is no significant difference in the phosphorylation state of p38 in any treatment group suggesting p38 is not activated at these time points in this model of isoproterenol-induced hypertrophy. *p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, n=4–10 for each group.
Molecular alterations in MAPK activation may suggest a role of these kinases in FGF2-induced cardiac hypertrophy, but do not definitively identify their importance in the hypertrophic response. For conclusive evidence of the role of ERK or p38 activation in FGF2-mediated cardiac hypertrophy, inhibitors of the ERK or p38 signaling pathway were utilized in FGF2 transgenic and non-transgenic mice. UO126, an inhibitor of MEK1/2 (upstream kinases of ERK), and SB203580, a p38 inhibitor, were administered in conjunction with saline or isoproterenol in non-transgenic and FGF2 transgenic mice for 2 days since signaling alterations in the MAPK cascade had been observed at this time point. Western blot analysis of ERK and p38 phosphorylation was performed in order to determine the efficacy of UO126 and SB203580 in ERK and p38 pathway inhibition, respectively. The specificity of UO126 for inhibition of the MEK-ERK signaling cascade has been well-established (13), and inhibition of ERK phosphorylation by UO126 was confirmed in that UO126 treatment significantly reduced ERK phosphorylation in isoproterenol-treated FGF2 transgenic hearts similar to non-transgenic levels (p<0.05, Figure 6A). Following treatment with the ERK pathway inhibitor, UO126, heart weight-to-body weight ratios of isoproterenol-treated FGF2 transgenic mice were significantly reduced (p<0.05, Figure 7A) similar to the level of isoproterenol-treated non-transgenic mice suggesting the necessity of the ERK activation in FGF2’s pro-hypertrophic effect. SB203580 treatment significantly reduced p38 phosphorylation in isoproterenol-treated non-transgenic and FGF2 transgenic hearts (p<0.05, Figure 6B). In contrast, co-administration of isoproterenol and SB203580 resulted in increases in heart weight-to-body weight ratio in non-transgenic and FGF2 transgenic mice similar to that seen with isoproterenol-treatment alone (Figure 7A). These data suggest the p38 pathway does not play a role downstream of FGF2 in isoproterenol-induced cardiac hypertrophy. To confirm the gravimetric results, cardiomyocyte cross-sectional area was measured using a wheat germ agglutinin stain of sarcolemmal-associated saccarrides. Consistent with gravimetric analysis, UO126 treatment significantly reduced isoproterenol-treated FGF2 transgenic cardiomyocyte size to non-transgenic levels (p<0.05, Figure 7B), while SB023580 treatment had no effect on the increase in cardiomyocyte size seen in isoproterenol-treated FGF2 transgenic hearts (Figure 7B) In summary, these data indicate that the doses of UO126 and SB203580 used in these experiments were able to inhibit ERK and p38 activity, respectively, and more importantly, unequivocally demonstrate the need for ERK, but not p38 activation, in mediating FGF2’s effect in the hypertrophic response to isoproterenol.
Figure 6. MAPK phosphorylation in non-transgenic and FGF2 transgenic (Tg) mouse hearts treated for 2 days with saline or isoproterenol in the presence or absence of ERK or p38 pathway inhibition.
(A) ERK phosphorylation is significantly increased in isoproterenol-treated FGF2 Tg hearts compared to isoproterenol-treated non-transgenic hearts. UO126 treatment significantly reduced the amount of ERK phosphorylation in isoproterenol-treated FGF2 Tg hearts. (B) SB203580-treatment significantly reduced p38 phosphorylation in isoproterenol-treated non-transgenic and FGF2 Tg mouse hearts. p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, # p<0.05 vs. no ERK or p38 pathway inhibitor, n=4–10 for each group.
Figure 7. ERK pathway inhibition with UO126 and p38 pathway inhibition with SB203580 in isoproterenol-induced cardiac hypertrophy.
(A) Heart weight-to-body weight ratios and B) cardiomyocyte cross-sectional area for non-transgenic and FGF2 transgenic (Tg) mice treated for 2 days with saline or isoproterenol in the presence or absence of ERK or p38 pathway inhibition. ERK pathway inhibition resulted in a significantly reduced heart weight-to-body weight ratio (A) and cardiomyocyte size (B) in isoproterenol-treated FGF2 Tg mice similar to the level of isoproterenol-treated non-transgenic animals suggesting ERK activation downstream of FGF2 in the progression of isoproterenol-induced cardiac hypertrophy. Treatment with SB203580, a p38 inhibitor, did not significantly alter either heart weight-to-body weight ratios or myocyte cross-sectional area suggesting it is not involved in the progression of isoproterenol-induced cardiac hypertrophy. *p<0.05 vs. saline treatment, +p<0.05 vs. wildtype, # p<0.05 vs. no ERK or p38 pathway inhibitor, n=4–10 for each group.
These data suggest that in vivo, FGF2 activation of ERK mediates the progression of isoproterenol-induced cardiac hypertrophy. Other studies have suggested a role for ERK activation in the progression of cardiac hypertrophy. In vitro, ERK pathway inhibition has been shown to reverse cardiomyocyte hypertrophy resulting from G protein coupled receptor agonists such as endothelin-1 and phenylephrine (34). In addition, expression of a phosphatase (MKP-1) which blocks activation of all MAPK family members including ERK, prevented catecholamine-induced hypertrophy of primary myocyte cultures (35). In vivo, transgenic mice overexpressing MEK1, a MAPK kinase family member directly upstream of ERK phosphorylation, exhibit concentric hypertrophy (36). The present study, however, provides the first direct in vivo evidence of the necessity of ERK pathway activation for cardiac hypertrophy mediated through FGF2. This study also suggests that ERK activation may be downstream of FGF2 activation by isoproterenol stimulation. Wildtype hearts treated with isoproterenol show significantly increased ERK activation whereas Fgf2 knockout hearts subjected to isoproterenol treatment show no difference in ERK activation compared to saline-treated controls (Figure 4A) suggesting the necessity of FGF2 for ERK activation downstream of isoproterenol stimulation in this model. Future studies which delineate the mechanisms which mediate ERK activation by FGF2 in the context of isoproterenol stimulation will be needed including investigation of the necessity of FGF receptor activation using FGF receptor inhibitors or FGF receptor knockout mice in this model.
In the present study, administration of SB203580, to isoproterenol-treated non-transgenic and FGF2 transgenic animals did not significantly affect either heart weight-to-body weight ratios (Figure 7A) or cardiomyocyte size (Figure 7B). Other investigators, however, have seen an effect of p38 inhibition in hypertrophy progression. For example, inhibition of p38 in a hypertensive rat model resulted in reduced left ventricular hypertrophy and cardiac dysfunction (33). SB203580 has been shown to inhibit the catalytic activity of p38 by occupying the ATP-binding pocket without effecting MKK-dependent phosphorylation of p38 (37). In the present study, a decreased level of p38 phosphorylation was observed in isoproterenol-treated non-transgenic and FGF2 transgenic hearts subjected to SB203580. These data suggest an autophosphorylation mechanism of p38 in our hypertrophy model. Recent studies have shown MKK-independent, SB203580-sensitive phosphorylation of p38 resulting from intramolecular p38 autophosphorylation (38, 39). This type of autophosphorylation seems to be tissue- and stimulus-specific, but it has been shown to occur in the heart (40). This autophosphorylation of p38, however, does not seem necessary for FGF2-mediated cardiac hypertrophy as p38 inhibition had no significant effect on the exacerbation of isoproterenol-induced cardiac hypertrophy in FGF2 transgenic mice (Figure 7A and B).
Our data has shown the necessity of FGF2 activation of ERK in the full hypertrophic response of cardiomyocytes to isoproterenol. The substrates that mediate the effects of ERK downstream of FGF2 in cardiac hypertrophy are currently unknown but several possibilities exist. ERK phosphorylates many regulatory proteins which can result in the control of transcription, translation, and cytoskeletal rearrangement (41). ERK has been shown to activate p70 S6 kinase 1 and 2 which results in increased translational efficiency contributing to protein accumulation during the hypertrophic response in cardiac myocytes (42, 43). Upon activation, ERK is also known to translocate to the nucleus and interact with several transcription factors to affect gene transcription including ELK-1, ETS1, SAP1a, c-MYC, and STAT factors (44). In the heart, ERK activates the cardiac-enriched transcription factor GATA4 (45, 46) which regulates many cardiac-expressed structural genes and hypertrophy responsive genes (47). In addition, ERK has been shown to activate multiple other signaling molecules including MAPKAP kinase 1 (48), phospholipase A2 (49), and p90 ribosomal S6 kinase (50).
This study has demonstrated that FGF2 mediates the cardiac hypertrophic response to isoproterenol through activation of the ERK pathway. It is possible that other signaling cascades may also mediate this effect. FGF2 has also been shown to signal through the protein kinase C, nitric oxide synthase, and PI3K pathways (51–54). These additional pathways may participate in FGF2-mediated cardiac hypertrophy, but this topic is beyond the scope of the current manuscript and will be the subject of future studies.
In summary, the present study has unequivocally demonstrated a role for FGF2 in the mediation of isoproterenol-induced cardiac hypertrophy in vivo. Ablation of the FGF2 gene provided protection from cardiac hypertrophy; whereas, cardiac-specific overexpression of FGF2 resulted in an exacerbation of cardiac hypertrophy due to β-adrenergic stimulation. We then provided the first conclusive, in vivo evidence of the necessity of ERK but not p38 activation downstream of FGF2 in the mediation of cardiac hypertrophy. These data may aid in the development of preventative strategies for patients at risk for cardiac hypertrophy by identifying novel therapeutic targets.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL70174 and HD26471) and the Center for Environmental Genetics (ES06096) awarded to T. Doetschman and grants from the American Heart Association (SDG 23004N), the Pharmaceutical Research and Manufacturers of America (Research Starter Grant), and NIH/NHLBI R01 (HL075633) awarded to Jo El J. Schultz. The investigators acknowledge S. Pawlowski, C. York, M. Bender, and A. Whittaker for their excellent animal husbandry work, and D. Porter for mouse genotyping.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Parker TG, Packer SE, Schneider MD. Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990;85:507–14. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lompre AM, Mercadier JJ, Schwartz K. Changes in gene expression during cardiac growth. Int Rev Cytol. 1991;124:137–86. doi: 10.1016/s0074-7696(08)61526-0. [DOI] [PubMed] [Google Scholar]
- 3.Izumo S, Lompre AM, Matsuoka R, et al. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987;79:970–7. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz K, de la Bastie D, Bouveret P, Oliviero P, Alonso S, Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986;59:551–5. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld JR, Vasser M, Jhurani P, et al. Distinct molecular phenotypes in murine cardiac muscle development, growth, and hypertrophy. J Mol Cell Cardiol. 1998;30:2269–80. doi: 10.1006/jmcc.1998.0787. [DOI] [PubMed] [Google Scholar]
- 6.Kaye D, Pimental D, Prasad S, et al. Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest. 1996;97:281–91. doi: 10.1172/JCI118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circulation Research. 1995;76:927–34. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 8.Schultz JJ, Witt SA, Nieman ML, et al. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–19. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellieux C, Foletti A, Peduto G, et al. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J Clin Invest. 2001;108:1843–51. doi: 10.1172/JCI13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 11.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 14.Cuenda A, Rouse J, Doza YN, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–33. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 15.House SL, Bolte C, Zhou M, et al. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation. 2003;108:3140–8. doi: 10.1161/01.CIR.0000105723.91637.1C. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Sutliff RL, Paul RJ, et al. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–7. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meij JT, Sheikh F, Jimenez SK, Nickerson PW, Kardami E, Cattini PA. Exacerbation of myocardial injury in transgenic mice overexpressing FGF-2 is T cell dependent. Am J Physiol Heart Circ Physiol. 2002;282:H547–H555. doi: 10.1152/ajpheart.01019.2000. [DOI] [PubMed] [Google Scholar]
- 18.Molkentin JD, Dorn IG. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. 391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 19.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H, Sato N, Kizaki T, et al. Norepinephrine stimulates the expression of fibroblast growth factor 2 in rat brown adipocyte primary culture. Cell Growth Differ. 1995;6:1457–62. [PubMed] [Google Scholar]
- 21.DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989;244:688–90. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- 22.Safran A, Avivi A, Orr-Urtereger A, et al. The murine flg gene encodes a receptor for fibroblast growth factor. Oncogene. 1990;5:635–43. [PubMed] [Google Scholar]
- 23.Kouhara H, Hadari YR, Spivak-Kroizman T, et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang JK, Xu H, Li HC, Goldfarb M. Broadly expressed SNT-like proteins link FGF receptor stimulation to activators of Ras. Oncogene. 1996;13:721–9. [PubMed] [Google Scholar]
- 25.Liu L, Pasumarthi KB, Padua RR, et al. Adult cardiomyocytes express functional high-affinity receptors for basic fibroblast growth factor. American Journal of Physiology. 1995;268(Pt 2):H1927–38. doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- 26.House SL, Branch K, Newman G, Doetschman T, Schultz JJ. Cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2 is mediated by the MAPK cascade. Am J Physiol Heart Circ Physiol. 2005;289:H2167–H2175. doi: 10.1152/ajpheart.00392.2005. [DOI] [PubMed] [Google Scholar]
- 27.Padua RR, Merle PL, Doble BW, et al. FGF-2-induced negative inotropism and cardioprotection are inhibited by chelerythrine: involvement of sarcolemmal calcium-independent protein kinase C. JMol Cell Cardiol. 1998;30:2695–709. doi: 10.1006/jmcc.1998.0832. [DOI] [PubMed] [Google Scholar]
- 28.Rapacciuolo A, Esposito G, Prasad SV, Rockman HA. G protein-coupled receptor signalling in in vivo cardiac overload. Acta Physiol Scand. 2001;173:51–7. doi: 10.1046/j.1365-201X.2001.00884.x. [DOI] [PubMed] [Google Scholar]
- 29.Takeishi Y, Huang Q, Abe J, Glassman M, et al. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stretch. J Mol Cell Cardiol. 2001;33:1637–48. doi: 10.1006/jmcc.2001.1427. [DOI] [PubMed] [Google Scholar]
- 30.Lazou A, Sugden PH, Clerk A. Activation of mitogen-activated protein kinases (p38-MAPKs, SAPKs/JNKs and ERKs) by the G-protein-coupled receptor agonist phenylephrine in the perfused rat heart. Biochem J. 1998;332:459–65. doi: 10.1042/bj3320459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faulx MD, Ernsberger P, Vatner D, et al. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol. 2005;289:H30–H36. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- 32.Berthonneche C, Peter B, Schupfer F, et al. Cardiovascular response to beta-adrenergic blockade or activation in 23 inbred mouse strains. PLoS One. 2009;4:e6610. doi: 10.1371/journal.pone.0006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behr TM, Nerurkar SS, Nelson AH, et al. Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation. 2001;104:1292–8. doi: 10.1161/hc3601.094275. [DOI] [PubMed] [Google Scholar]
- 34.Yue TL, Gu JL, Wang C, et al. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 35.Bueno OF, De Windt LJ, Lim HW, et al. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88:88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 36.Bueno OF, De Windt LJ, Tymitz KM, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gum RJ, McLaughlin MM, Kumar S, et al. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J Biol Chem. 1998;273:15605–10. doi: 10.1074/jbc.273.25.15605. [DOI] [PubMed] [Google Scholar]
- 38.Ge B, Gram H, Di Padova F, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–4. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 39.Tanno M, Gorog DA, Bellahcene M, Cao X, Quinlan RA, Marber MS. Tumor necrosis factor-induced protection of the murine heart is independent of p38-MAPK activation. J Mol Cell Cardiol. 2003;35:1523–7. doi: 10.1016/j.yjmcc.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Maulik N, Yoshida T, Zu YL, Sato M, Banerjee A, Das DK. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. Am J Physiol. 1998;275:H1857–H1864. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- 41.Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci U S A. 1995;92:8881–5. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iijima Y, Laser M, Shiraishi H, et al. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277:23065–75. doi: 10.1074/jbc.M200328200. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Gout I, Proud CG. Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK-dependent activation of S6K2 in cardiomyocytes. J Biol Chem. 2001;276:32670–7. doi: 10.1074/jbc.M102776200. [DOI] [PubMed] [Google Scholar]
- 44.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 45.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245–53. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto T, Hasegawa K, Kaburagi S, et al. Phosphorylation of GATA-4 is involved in alpha 1-adrenergic agonist-responsive transcription of the endothelin-1 gene in cardiac myocytes. J Biol Chem. 2000;275:13721–6. doi: 10.1074/jbc.275.18.13721. [DOI] [PubMed] [Google Scholar]
- 47.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–52. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3441–5. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–78. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 50.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 51.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 52.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.House SL, Melhorn SJ, Newman G, Doetschman T, Schultz JJ. The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2. Am J Physiol Heart Circ Physiol. 2007;293:H354–H365. doi: 10.1152/ajpheart.00804.2006. [DOI] [PubMed] [Google Scholar]
- 54.Carpenter G, Porter D, House SL, Liao S, Newman G, Schultz JJ. Inducible nitric oxide synthase (iNOS), not neuronal NOS (nNOS) in cardioprotection by fibroblast growth factor-2 (FGF-2) J Mol Cell Cardiol. 2007;42:S187. [Google Scholar]