Abstract
Lung function detection in mice is currently most accurately measured by invasive techniques, which are costly, labor intensive, and terminal. This limits their use for large-scale or longitudinal studies. Noninvasive assays are often used instead, but their accuracy for measuring lung function parameters such as resistance and elastance has been questioned in studies involving small numbers of mouse strains. Here we compared parameters detected by two different methods using 29 inbred mouse strains: enhanced pause (Penh), detected by unrestrained plethysmography, and central airway resistance and lung elastance, detected by a forced oscillation technique. We further tested whether the phenotypic variations were determined by the same genomic location in genome-wide association studies using a linear mixed model algorithm. Penh, resistance, and elastance were measured in nonexposed mice or mice exposed to saline and increasing doses of aerosolized methacholine. Because Penh differed from airway resistance in several strains and because the peak genetic associations found for Penh, resistance, or elastance were located at different genomic regions, we conclude that using Penh as an indicator for lung function changes in high-throughput genetic studies (i.e., genome-wide association studies or quantitative trait locus studies) measures something fundamentally different than airway resistance and lung elastance.
Keywords: lung function, genomics, airway resistance, genome-wide association
methods to measure lung function in animal models of airway disease have been controversial (15). In an attempt to obviate the need for invasive measurements of resistance and elastance, a noninvasive method (unrestrained plethysmography, UP) was proposed by Buxco Research Systems (Wilmington, NC). UP measures pressure changes due to respiratory efforts over time in a chamber, thereby allowing detection of respiratory frequency, tidal volume (Vt), and inspiratory and expiratory times (Ti and Te, respectively) (13, 20). These data are used to derive the empirical measure, referred to as enhanced pause (Penh), which arises from the box pressure variations over time that are caused by an animal's chest movement and by humidification and warming of the respired air (19). Advantages of this method are its simplicity, noninvasiveness (allowing repetitive studies), and labor efficiency. These features make it especially desirable for genetic studies, which require the phenotyping of large numbers of mice [e.g., quantitative trait locus (QTL) studies or genome-wide association studies]. However, these advantages are only useful if Penh is a reliable metric that reflects lung functions (e.g., airway resistance, elastance).
Alternatively, airway resistance can be measured in anesthetized mice using the forced oscillation technique (FOT) with a computer-controlled piston ventilator (e.g., flexiVent by SCIREQ, Montreal, Quebec, Canada) and then fitting a constant phase model to the data (3, 14). The advantage of this method is the precise detection of lung function parameters (e.g., airway resistance, elastance) within the lung. However, it is more labor intensive, which makes it less desirable for screening large numbers of animals. In addition, the method as often used is terminal (3, 13), although with modern mouse intubation procedures it need not be (21).
The usefulness of Penh as an indicator of resistance or elastance has been vigorously debated. More than a decade ago, Hamelmann et al. (13) presented correlations between Penh, lung resistance, and intrapleural pressure, and Dohi et al. (9) argued that Penh is an indicator of allergen-induced bronchoconstriction. Other authors, however, have presented evidence against the usefulness of Penh as a measure of airway resistance by reporting 1) theoretical arguments showing that Penh is more a measure of gas conditioning than a measure of airway function (20, 25) and 2) experimental data showing a lack of correlation between Penh and invasively detected lung function parameters (2, 8, 12, 27, 28). So far, all comparisons between Penh and invasively detected lung functions are based on only a few common inbred mouse strains, which may not be representative of mouse airway physiology in general.
Recently, we showed that invasively detected airway resistance [in particular, central airway resistance (Rn)] varies among 36 mouse strains and that those variations can be used in quantitative genetic studies to identify the genomic locations that are associated with the phenotypic variations among the strains (17). In the past, regions within the genome that are associated with variations in both Penh and invasively detected airway resistance were detected using QTL studies. A summary of the QTL studies for airway functions, found elsewhere (17), showed that 1) only eight QTL studies have been performed thus far and 2) only two of these eight studies can be used to compare the QTL results for Penh and airway resistance (1, 6). Interestingly, these two QTL studies showed common genomic regions at the distal end of chromosome (Chr) 2, indicating that common genetic variants may drive the biological processes that determine Penh and airway resistance. Nevertheless, those findings have not been repeated in any of the other QTL studies, and the lack of other common genomic regions casts doubt on the possibility that Penh and airway resistance share the same underlying genetics.
Here we aimed to combine comparisons of the physiological parameters and underlying genomic variations between Penh and Rn and Penh and lung elastance (H) among 29 inbred strains in an unbiased genome-wide approach. We hypothesize that if variations in Penh, Rn, and H originate from different physiological mechanisms within the lung, they will be driven by different genomic variations.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained at The Jackson Laboratory on a 12 h light/12 h dark cycle. We studied female and male mice of the following 29 strains: 129S1/SvImJ, A/J, AKR/J, BALB/cByJ, BALB/cJ, BTBR T+ tf/J, BUB/BnJ, C3H/HeJ, C57BL/10J, C57BL/6J, C57BLKS/J, C57BR/cdJ, C57L/J, CBA/J, CE/J, DBA/1J, DBA/2J, FVB/NJ, I/LnJ, KK/HlJ, LP/J, NOD/LtJ, NON/LtJ, NZW/LacJ, PL/J, RIIIS/J, SJL/J, SM/J, and SWR/J. For each strain, female (n = 7–14) and male (n = 8–21) mice were measured with UP at The Jackson Laboratory. Another set of female (n = 6–10) and male (n = 6–12) mice, which were different individual animals of the same strains used at The Jackson Laboratory, were shipped to the University of Pittsburgh for invasive lung function analysis using the FOT. Invasive and noninvasive lung function measures were obtained at 9–12 wk of age. Animal protocols were reviewed and approved by the Animal Care and Use Committees at The Jackson Laboratory and the University of Pittsburgh.
In both animal rooms at The Jackson Laboratory and the University of Pittsburgh, mice were housed in groups of five mice in pressurized and individually ventilated cages containing pine shaving bedding and topped with a polyester filter; the cage types did differ: while cages by Thoren Caging System (Hazleton, PA) were used at The Jackson Laboratory, cages by Allentown (Allentown, NY) were used at the University of Pittsburgh. Although the diets were chow at both institutions, they did differ with LabDiet 5K52 (PMI Nutritional International, Bentwood, MO) used at The Jackson Laboratory and Prolab 5P76 Isopro 3000 (PMI Nutritional International) at the University of Pittsburgh. The animal room at The Jackson Laboratory was specific pathogen free and was regularly monitored for and was free of viruses, bacterial species, Mycoplasma spp., and parasites, as described at http://jaxmice.jax.org/. The vivarium at the University of Pittsburgh was inspected four times a year for viruses and parasites and found to be free except for one virus (murine norovirus) detected in a different room of the vivarium. The mice from The Jackson Laboratory were at the University of Pittsburgh for a maximum of 3 wk before measurements were taken, so it is unlikely that the health status differed between the two groups. Finally, the ages of the mice being tested by the two procedures were the same, 9–12 wk.
Noninvasive measurement of lung functions using UP.
Mice were placed from the home cage into a plethysmograph chamber (Buxco Research Systems, Wilmington, NC), and inspiratory and expiratory pressures (Pi and Pe, respectively) as well as Ti and Te were measured. Those parameters were used to calculate Penh according to the equation: Penh = (Pi/Pe) * [(Te-Tr)/Tr], in which Pi is the chamber pressure change during inspiration, Pe is the chamber pressure change during expiration, Te is the expiratory time, and Tr is the relaxation time (i.e., the time interval that includes 63% of the integrated expiratory pressure signal). We also calculated additional UP parameters for each mouse: Ti + Te (Ttot), Ti/Ttot, Vt/Ti, Vt/Ttot, and Vt/Te as it was previously reported (2).
Each mouse was subjected to our standard protocol, which consisted of the following procedures: 1) acclimatization within the plethysmograph chamber for at least 15 min, 2) baseline measurements collected as six 30 s data logs, and 3) inhalation challenge measurements in response to aerosolized saline or increasing concentrations of methacholine (5, 10, 20 mg/ml methacholine resolved in saline) collected as eight to ten 30 s data logs. To ensure that each mouse received the same methacholine exposure, we tested the efficiency of the nebulizer at the beginning of each test week and adjusted the time of exposure to deliver the same methacholine concentration to each animal. When the nebulizers were new, the time was generally 2.5 min, but as the nebulizers got older and became less efficient, the time was often extended. When the time required was >3.5 min to achieve the same methacholine dose, the nebulizer was discarded and replace by a new nebulizer.
Invasive measurements of lung function using FOT.
Mice measured with the invasive FOT were age-matched, but individually different, mice from the same 29 mouse strains that were used for noninvasive UP. Mice were shipped to the University of Pittsburgh, where they were allowed to acclimatize for at least 7 days and no longer than 3 wk. Invasive lung function phenotyping was performed as follows. Mice were anesthetized with 60 mg/kg pentobarbital sodium ip (Ovation Pharmaceuticals, Deerfield, IL), a tracheostomy was performed, and mice were attached to a computer-controlled piston ventilator (flexiVent system). The immediate (baseline) ventilation for each mouse consisted of a Vt of 10 ml/kg at 120 breaths/min with 0 cmH2O positive end-expiratory pressure. Following 5 min of stabilization one deep lung inflation (DI) was applied to ensure uniform lung recruitment and to normalize to the same volume history for each mouse. This DI procedure consisted of a constant airflow with a pressure limited to 30 cmH2O over 3 s followed by a passive exhalation. Baseline impedance measurements were then obtained in triplicate during regular sinusoidal ventilation. Following those first baseline measurements animals were maintained under baseline ventilation for another 3 min, after which a second set of baseline impedance measurements were taken without preceding DI. This second set of baseline measurements was used to calculate the average baseline values per strain. Saline or methacholine challenges (1, 3, 10, and 30 mg/ml methacholine) were delivered by channeling inspiratory flow from the ventilator through an ultrasonic nebulizer (Aeroneb Lab Nebulizer System by Aerogen, Galway, Ireland). During aerosol challenge the ventilator was adjusted to deliver 40 ml/kg at 30 breaths/min. After challenge the ventilator settings were readjusted to deliver a Vt of 10 ml/kg at 120 breaths/min and impedance measurements were taken. After measurements were taken one DI was applied to reopen the lungs for the next aerosol challenge. Impedance measurements were taken every 5 s within the period of 30–60 s after each aerosol challenge. Therefore, for each aerosol challenge 6–12 impedance measurements were reported, of which we calculated the mean of the three highest Rn values.
The impedance is derived from the signals detected by the ventilator piston volume displacement (i.e., flow) and cylinder pressure (i.e., pressure), which is obtained during 8 s oscillatory volume perturbations (i.e., Prime-8 perturbations). The perturbations consisted of sine waves with frequencies ranging between 0.5 and 19.75 Hz. The impedance was then fitted to a constant-phase model of the viscoelastic lung, from which the parameters Rn (i.e., central airway resistance) and H (i.e., lung elastance) were obtained. Because the major objective of the experiments was to compare Rn and Penh, we mainly focused on the detection of Rn changes due to methacholine exposure. Therefore, H data were only collected at the Rn peak response and not at the later occurring H peak response.
Calculation of the slope measures for Penh, Rn, and H.
For all measures, Penh, Rn, and H, we determined the strength of their increase (expressed as Penh_slope, Rn_slope, and H_slope, respectively) caused by consecutive increasing methacholine exposure. For each animal Penh_slope, Rn_slope, and H_slope were derived as follows: 1) we calculated the average Penh, Rn, and H response for each animal at each dose (i.e., individual value), 2) we log-transformed each dose and each individual value because the raw data together were not normally distributed, and 3) we fitted a linear curve to the data of log-transformed methacholine concentrations (5, 10, and 20 mg/ml for Penh and 1, 3, 10, and 30 mg/ml for Rn and H) vs. log-transformed individual values. The increase of this curve is equivalent to the slope value for Penh, Rn, and H for each mouse. The strain values were derived by calculating the average of all individual Penh, Rn, and H slope values. Although different methacholine doses were used for the UP and the FOT, the ranges of the methacholine doses used were similar between the studies, and, therefore, we studied the same part of the dose-response curves that is descriptive for airway responsiveness in the mouse. All individual values as well as the slope data for Penh, Rn, and H are reported in the supplemental data on the Mouse Phenome Database (MPD; accession number: 351).
Statistical analysis.
Normal distribution of the individual Penh_slope, Rn_slope, and H_slope values was assessed with the Lilliefors test for normality (adaptation of Kolmogorov-Smirnov test for normality) and by examining the Q-Q plots. Because the data of all three traits were normally distributed, the raw data were used for generation of bar graphs for which the mean ± SE for each strain, sex, and trait combination was calculated. To compare Penh_slope to Rn_slope or H_slope we ensured that all three traits were brought onto the same scale by calculating the standardized ranks [i.e., individual data point (rank)/total number of data points for each trait and each strain] for each individual slope measure within each strain. We used the Wilcoxon rank sum test to compare the standardized ranks between Penh_slope and Rn_slope or H_slope as well as between female and male mice of each strain. We also used the median standardized ranks for each strain to obtain the correlation between the traits. Statistical significance was reached at a P value of <0.05. All statistical analyses were performed in R programming language.
Genome-wide association mapping.
Association mapping for Penh_slope, Rn_slope, and H_slope for 29 inbred mouse strains was performed using the free R add-on package EMMA (Efficient Mixed Models Association; http://mouse.cs.ucla.edu/emma), which uses a linear mixed model algorithm to control for population structure and genetic relatedness (16). We used the strain mean as phenotype input and carried out the analysis for females and males separately using a panel of 623,124 single nucleotide polymorphisms (SNPs) from the Mouse Diversity Genotyping Array, a high-density mouse genotyping array that captures the known genetic variation present in the laboratory mouse (29). Noninformative SNPs (i.e., SNPs that were not polymorphic between the strains used in our study) were removed from this data set, which resulted in a total of 334,659 informative SNPs. Each SNP was evaluated individually, and a P value was recorded as the strength of the genotype-phenotype association. All P values were transformed using −log10(P value) in the scan plots (score). For this manuscript we focused on associations with a P value of <10−5. Annotation and information regarding probe performance can be obtained from the Center for Genome Dynamics website (http://cgd.jax.org//tools/diversityarray.shtml).
RESULTS
Distribution of Penh detected by UP.
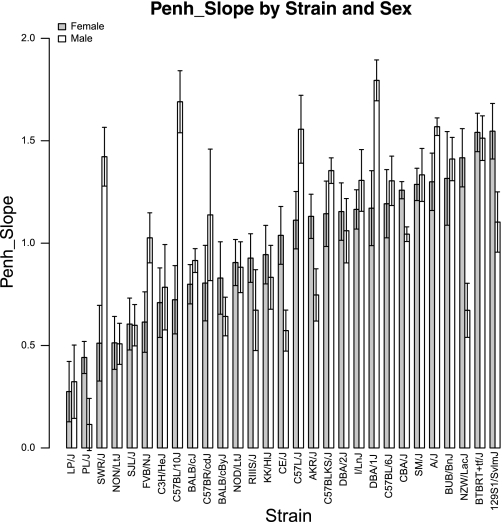
We observed wide variations of the Penh_slope measures among the analyzed strains (Fig. 1). Between the strains with the lowest and the highest mean Penh_slope measure, we observed a 5.6-fold increase for female and a 15.7-fold increase for male mice. On the individual strain level, the differences between female and male mice were significant for the strains SWR/J (P = 0.002), CBA/J (P = 0.003), NZW/LacJ (P = 0.003), C57BL/10J (P = 0.004), DBA/1J (P = 0.009), CE/J (P = 0.01), AKR/J (P = 0.04), and PL/J (P = 0.05) (Table 1). All strain means ± SE of individual exposure doses and slope values of Penh and additional UP parameters (i.e., Ti, Te, Ttot, Ti/Ttot, Vt, Vt/Ti, Vt/Ttot, and Vt/Te) are reported on the MPD (accession number: 351).
Fig. 1.
Distribution of Penh_slope among 29 mouse strains for female and male mice. Strains are sorted by increasing average strain values in female mice. Penh, enhance pause.
Table 1.
Penh_slope, Rn_slope, and H_slope of strains in which males and females show significant differences between strain means
| Method | Strain | Males (mean ± SE) | Females (mean ± SE) | P Value* |
|---|---|---|---|---|
| Penh_slope | AKR/J | 0.7 ± 0.1 | 1.1 ± 0.1 | 0.0379 |
| C57BL/10J | 1.7 ± 0.2 | 0.7 ± 0.2 | 0.0037 | |
| CBA/J | 1.0 ± 0.04 | 1.3 ± 0.04 | 0.0029 | |
| CE/J | 0.6 ± 0.1 | 1.0 ± 0.1 | 0.0111 | |
| DBA/1J | 1.8 ± 0.1 | 1.2 ± 0.2 | 0.0085 | |
| NZW/LacJ | 0.7 ± 0.1 | 1.4 ± 0.1 | 0.0029 | |
| PL/J | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.0499 | |
| SWR/J | 1.4 ± 0.1 | 0.5 ± 0.2 | 0.0025 | |
| Rn_slope | BALB/cJ | 0.4 ± 0.03 | 0.3 ± 0.03 | 0.0266 |
| C57BL/10J | 0.5 ± 0.02 | 0.3 ± 0.02 | <0.0001 | |
| CBA/J | 0.4 ± 0.03 | 0.2 ± 0.03 | 0.0085 | |
| FVB/NJ | 0.5 ± 0.03 | 0.3 ± 0.03 | 0.0003 | |
| LP/J | 0.5 ± 0.02 | 0.4 ± 0.02 | <0.0001 | |
| SJL/J | 0.4 ± 0.03 | 0.2 ± 0.02 | 0.001 | |
| H_slope | A/J | 0.5 ± 0.05 | 0.3 ± 0.05 | 0.0133 |
| C3H/HeJ | 0.1 ± 0.02 | 0.2 ± 0.02 | 0.035 | |
| C57BL/6J | 0.2 ± 0.03 | 0.06 ± 0.02 | 0.0037 | |
| DBA/2J | 0.3 ± 0.04 | 0.1 ± 0.03 | 0.0147 | |
| FVB/NJ | 0.2 ± 0.03 | 0.1 ± 0.03 | 0.0185 | |
| LP/J | 0.2 ± 0.03 | 0.04 ± 0.01 | <0.0001 | |
| NOD/LtJ | 0.2 ± 0.02 | 0.2 ± 0.03 | 0.0465 | |
| PL/J | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.0274 | |
| SJL/J | 0.2 ± 0.02 | 0.06 ± 0.01 | 0.0002 | |
| SWR/J | 0.2 ± 0.02 | 0.08 ± 0.01 | 0.0018 |
Standardized ranks were used for the comparison between males and females. Penh, enhance pause; Rn, airway resistance; H, elastance.
Distribution of Rn and H detected by FOT.
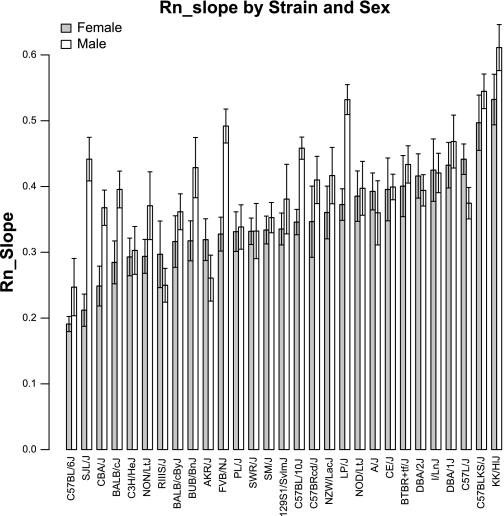
We observed wide variations of the Rn_slope measures among the analyzed strains (Fig. 2). Between the strains with the lowest and the highest mean Rn_slope measure, we observed a 2.8-fold increase for female and a 2.5-fold increase for male mice. On the individual strain level, the differences between female and male mice were significant for the strains C57BL/10J (P < 0.001), LP/J (P < 0.001), FVB/J (P < 0.001), SJL/J (P < 0.001), CBA/J (P = 0.009) and BALB/cJ (P = 0.03) (Table 1).
Fig. 2.
Distribution of Rn_slope among 29 mouse strains for female and male mice. Strains are sorted by increasing average strain values in female mice. Rn, airway resistance.
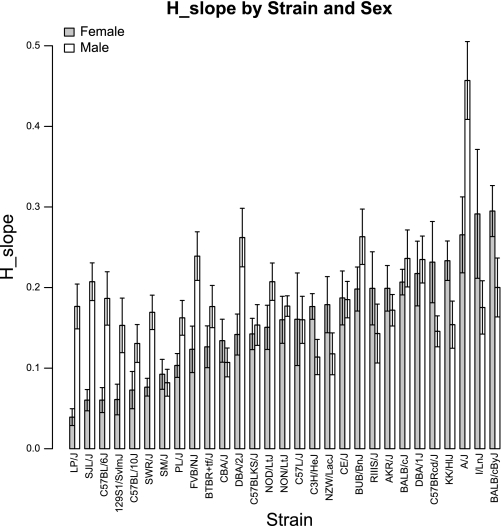
We also observed substantial variations of H_slope among the 29 strains (Fig. 3). The strains on the two ends of the H_slope spectrum showed a 7.5-fold difference for female and a 5.6-fold difference for male mice. On the individual strain level, measurements between female and male mice were significantly different for the strains LP/J (P < 0.001), SJL/J (P < 0.001), SWR/J (P = 0.002), C57BL/6J (P = 0.004), A/J (P = 0.01), DBA/2J (P = 0.01), FVB/NJ (P = 0.02), PL/J (P = 0.03), C3H/HeJ (P = 0.03), and NOD/LtJ (P = 0.05) (Table 1).
Fig. 3.
Distribution of H_slope among 29 mouse strains for female and male mice. Strains are sorted by increasing average strain values in female mice. H, elastance.
All strain means ± SE of individual exposure doses and slope values of Rn and H are also publically available on the MPD (accession number: 351).
Intrastrain distributions for Penh, Rn, and H.
Intrastrain variations for Penh_slope in each strain ranged from SE = 0.04 for the strain CBA/J to SE = 0.23 for the strain BUB/BnJ (i.e., 5.75-fold) for females and from SE = 0.04 for the strain CBA/J to SE = 0.23 for the strain C57BR/cdJ (i.e., 8-fold) for males. Intrastrain variations for Rn_slope in each strain ranged from SE = 0.01 for the strain C57BL/6J to SE = 0.05 for the strain C57BR/cdJ (i.e., 5-fold) for females and from SE = 0.02 for the strain C57BL/10J to SE = 0.05 for the strain 129S1/SvlmJ (i.e., 2.5-fold) for males. Intrastrain variations for H_slope in each strain ranged from SE = 0.01 for the strain LP/J to SE = 0.08 for the strain I/LnJ (i.e., 8-fold) for females and from SE = 0.01 for the strain NON/LtJ to SE = 0.05 for the strain A/J (i.e., 2.5-fold) for males.
Comparison of Penh and Rn measures.
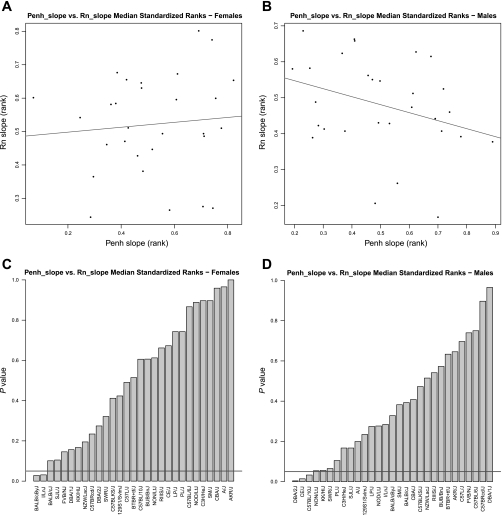
Across strains, the ranks of Penh_slope and Rn_slope were not significantly correlated for females (Rho = 0.12, P = 0.54) or males (Rho = −0.35, P = 0.07) as shown in Fig. 4, A and B, respectively. On the individual strain level, Penh_slope and Rn_slope were significantly different for the strains BALB/cByJ (P = 0.03) and I/LnJ (P = 0.03) among female mice and for the strains DBA/2J (P = 0.004), CE/J (P = 0.01), and C57BL/10J (P = 0.03) among male mice (Fig. 4, C and D, respectively).
Fig. 4.
Comparison of the median standardized ranks for Penh_slope and Rn_slope among 29 strains. There is no significant correlation between strain ranks of Penh_slope and Rn_slope for female (A, Rho = 0.12, P = 0.54) and male (B, Rho = −0.35, P = 0.07) mice. In A and B, the line indicates the line of best fit. P-value distributions of the differences in the strain ranks between Penh_slope and Rn_slope are shown for female (C) and male (D) mice. In C and D, the horizontal line indicates the threshold at P = 0.05. Strains below the line showed significant differences between the 2 parameters.
Comparison of Penh and H measures.
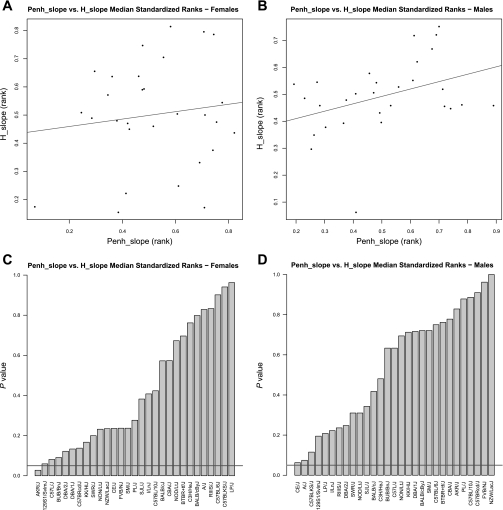
Across strains, the ranks of Penh_slope and H_slope were not significantly correlated for females (Rho = 0.02, P = 0.94) or males (r = 0.31, P = 0.1) as shown in Fig. 5, A and B, respectively. On the individual strain level, Penh_slope and H_slope were significantly different only for the strain AKR/J (P = 0.03) among female mice (Fig. 5, C and D).
Fig. 5.
Comparison of the median standardized ranks for Penh_slope and H_slope among 29 strains. There is no significant correlation between strain ranks of Penh_slope and H_slope for female (A, Rho = 0.02, P = 0.94) and male (B, Rho = 0.31, P = 0.1) mice. In A and B, the line indicates the line of best fit. P-value distributions of the differences in the strain ranks between Penh_slope and H_slope are shown for female (C) and male (D) mice. In C and D, the horizontal line indicates the threshold at P = 0.05. Strains below the line showed significant differences between the 2 parameters.
Genome-wide association mapping for Penh and Rn.
Genome-wide association mapping for females and males for both phenotypes, Penh_slope and Rn_slope, was performed by using the free R add-on package EMMA (http://mouse.cs.ucla.edu/emma), which uses a linear mixed model algorithm to control for population structure and genetic relatedness (16). We chose to use EMMA because it corrects for population structure and shared ancestry. This reduces false-positive associations and increases the power compared with previous methods such as single SNP or haplotype association mapping.
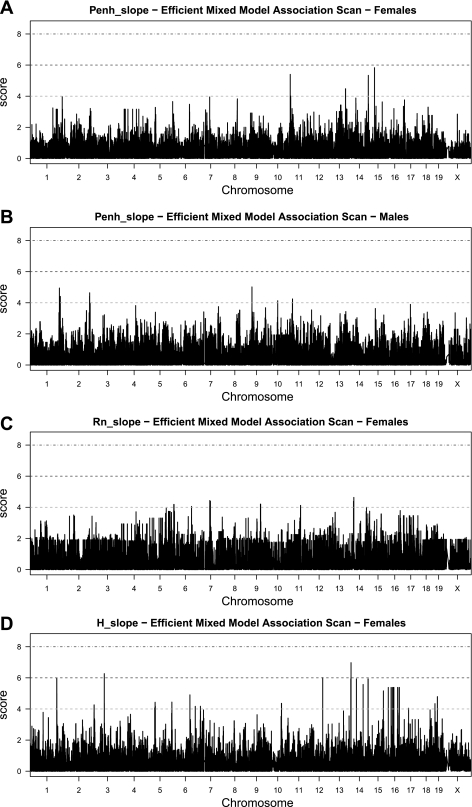
For Penh_slope we found the strongest associations (P < 10−5) on Chr 11 [at ∼12 million base pairs (Mb)], Chr 13 (at ∼97 Mb), Chr 14 (at ∼118 Mb) and Chr 15 (at ∼15 Mb) for females, and on Chr 1 (at ∼173 and 178 Mb), Chr 2 (at ∼155 Mb), Chr 9 (at ∼37 Mb), Chr 10 (at ∼65 Mb), and Chr 11 (at ∼25 Mb) for males (Fig. 6, A and B, respectively). For Rn_slope we observed the strongest associations on Chr 6 (at ∼10 and 112 Mb), Chr 7 (at ∼73 Mb), Chr 9 (at ∼86 Mb), Chr 11 (at ∼72 Mb), and Chr 14 (at ∼32 Mb) for females (Fig. 6C). Although both Penh_slope and Rn_slope mapped to common chromosomes (Chr 11 and Chr 14) in female mice, the peak associations were >57 Mb apart on Chr 11 and >84 Mb apart on Chr 14 (Table 2).
Fig. 6.
Genome-wide associations for Penh_slope in female (A) and male (B) mice, Rn_slope in female (C) mice and H_slope in female (D) mice. The x-axis shows the chromosomal location in million base pairs (Mb), and the y-axis shows the strength of the association as score [score = −log10(P value)].
Table 2.
Locations and characteristics of the strongest association peaks (P < 10−5) from genome-wide association studies for male and female Penh_slope, female Rn_slope, and female H_slope
| Phenotype | Sex | Chr | Approx. Position, Mb | pSNPs at P < 10−5, n | MAF | Gene Downstream | Gene Within | Gene Upstream |
|---|---|---|---|---|---|---|---|---|
| Penh_slope | M | 1 | 173.123–173.123 | 1 | 0.45 | Ppox | Usp21 | Ufc1 |
| 178.428–178.429 | 1 | 0.45 | Pld5 | Cep170 | ||||
| 154.792–154.792 | 1 | 0.48 | Ahcy | Itch | ||||
| 2 | 154.839–154.933 | 10 | 0.48 | Ahcy | Dynlrb1 | |||
| 154.937–154.946 | 3 | 0.48 | Cdc91l1 | Dynlrb1 | Map1lc3a | |||
| 154.969–155.036 | 11 | 0.48 | Map1lc3a | Cdc91l1 | ||||
| 155.053–155.053 | 1 | 0.48 | Map1lc3a | Trp53inp2 | ||||
| 155.384–155.385 | 1 | 0.48 | Trpc4ap | Edem2 | ||||
| 9 | 37.156–37.156 | 1 | 0.28 | NP_780398.2 | Robo4 | Q9Z2I4-2 | ||
| 37.186–27.222 | 4 | 0.28 | Q9Z2I4-2 | BC024479 | ||||
| 10 | 65.473–65.509 | 4 | 0.31 | Lrrtm3 | Reep3 | |||
| 11 | 24.761–24.829 | 3 | 0.31 | Bcl11a | Fancl | |||
| F | 11 | 11.655–11.664 | 2 | 0.31 | 4930415F15Rik | NM_001025597.1 | Fignl1 | |
| 13.956–13.991 | 2 | 0.41 | Cobl | BC027127 | ||||
| 13 | 97.03–97.035 | 3 | 0.28 | F2rl2 | Sv2c | |||
| 97.315–97.316 | 1 | 0.28 | Sv2c | 1200014M14Rik | ||||
| 14 | 118.048–118.058 | 6 | 0.24 | Cldn10 | Dzip1 | Djc3 | ||
| 118.058–118.106 | 7 | 0.24 | Dzip1 | Ugcgl2 | ||||
| 118.119–118.188 | 12 | 0.31 | Djc3 | Ugcgl2 | Hs6 st3 | |||
| 118.247–118.248 | 1 | 0.24 | Ugcgl2 | Hs6 st3 | ||||
| 15 | 32.234–32.234 | 1 | 0.14 | Tas2r119 | Sema5a | Sdc2 | ||
| Rn_slope | F | 6 | 9.055–9.155 | 9 | 0.41 | Ica1 | Nxph1 | |
| 9.750–10.405 | 17 | 0.41 | Nxph1 | Ndufa4 | ||||
| 112.497–112.551 | 5 | 0.31 | Oxtr | Rad18 | ||||
| 7 | 72,384–72.529 | 2 | 0.31 | Gm489 | 2310037I24Rik | |||
| 72.862–72.926 | 2 | 0.31 | 2310037I24Rik | Rgma | ||||
| 73.364–73.365 | 2 | 0.24 | Rgma | St8 sia2 | ||||
| 76.051–76.052 | 1 | 0.24 | Klhl25 | NP_766490.1 | ||||
| 9 | 85.708–85.712 | 2 | 0.48 | Tpbg | 2610018I03Rik | |||
| 86.901–86.902 | 1 | 0.48 | Cyb5r4 | XP_993588.1 | ||||
| 11 | 71.782–71.782 | 1 | 0.41 | BC030477 | Aipl1 | |||
| 71.872–71.945 | 4 | 0.41 | 6720460F02Rik | A330068P14Rik | 4933427D14Rik | |||
| 71.977–71.978 | 1 | 0.41 | A330068P14Rik | 4933427D14Rik | Txnl5 | |||
| 72.030–72.030 | 1 | 0.41 | Txnl5 | Med31 | 4930563E22Rik | |||
| 72.037–72.044 | 3 | 0.41 | 4930563E22Rik | Slc13a5 | ||||
| 14 | 31.943–32.034 | 8 | 0.34 | Lrrc18 | Arhgap22 | |||
| 33.789–33.789 | 1 | 0.24 | Wapal | Gcap14 | ||||
| H_slope | F | 1 | 157.656–157.830 | 33 | 0.24 | Qscn6/Tor1aip1 | Tor1aip1/ Ifrg15 | Tor1aip1/Ifrg15 |
| 3 | 617.852–617.961 | 2 | 0.31 | Rap2b | XP_902753.1 | |||
| 12 | 82.253–82.253 | 2 | 0.28 | Smoc1 | NP_001034084.1 | |||
| 14 | 17.330–17.331 | 1 | 0.34 | Ube2e1 | Ube2e2 | |||
| 15 | 84.071–84.072 | 1 | 0.45 | Samm50 | Parvb | Parvg | ||
| 16 | 12.219–12.220 | 1 | 0.03 | C530044N13Rik | Ercc4 | |||
| 28.529–28.530 | 1 | 0.03 | Fgf12 | 1600021P15Rik | ||||
| 35.556–35.557 | 1 | 0.03 | Pdia5 | Sema5b | Dirc2 | |||
| 42.097–42.098 | 1 | 0.03 | Lsamp | Gap43 | ||||
| 44.703–44.704 | 1 | 0.03 | Cd200r1 | Cd200r1 | Cd200r1 | |||
| 66.713–66.714 | 1 | 0.03 | Champ2b | Igsf4d | Gbe1 | |||
| 68.053–68.054 | 1 | 0.03 | Igsf4d | Gbe1 | ||||
| 76.776–76.777 | 1 | 0.03 | Nrip1 | Usp25 |
Genes were isolated by a Biomart search of the peak regions; genes that were directly hit by SNPs (Gene within) and those that were identified to be adjacent upstream or downstream genes (Gene upstream and Gene downstream, respectively) were reported. MAF, minor allele frequency; F, female; M, male; SNP, single nucleotide polymorphism; pSNPs, polymorphic SNPs.
For H_slope we found the strongest associations on Chr 1 (at ∼158 Mb), Chr 3 (at ∼62 Mb), Chr 12 (at ∼82 Mb), Chr 14 (at ∼17 Mb), Chr 15 (at ∼84 Mb), and Chr 16 (at ∼12 Mb) for females (Fig. 6D). H_slope and Penh_slope mapped to the same chromosomes (Chr 14 and Chr 15) in female mice, but the peaks were >101 Mb apart on Chr 14 and >69 Mb apart on Chr 15 (Table 2). H_slope and Rn_slope both mapped to Chr 14, and the peak associations were located 15 Mb apart. There were no outstanding associations observed on any chromosomes for Rn_slope and H_slope of male mice.
DISCUSSION
Here we present two strain surveys with the aim of comparing Penh (detected by UP) with Rn and H (detected by FOT) across multiple strains and in genome-wide association studies. With this work we aimed to identify the genomic basis of Penh, Rn, and H and hypothesized that if variations in Penh, Rn, and H originate from different physiological mechanisms within the lung, they will be driven by different genomic variations. Claims that Penh is not a valid measure of Rn or H have been based on theoretical derivations (20, 25) as well as physiological studies but in only a few strains (2, 8, 12, 27, 28). In particular, a previous study by Lundblad et al. (20) showed that UP measures primarily gas conditioning due to heating and humidification of the inhaled air and that the box pressure changes only relate to airway resistance under conditions of severely constricted airways. Also, UP does not measure transpulmonary pressure, which is the essential pressure necessary to measure resistance and elastance. In our survey of 29 inbred strains, we subjected mice to increasing doses of methacholine inhalation challenges and measured the strength of the increase (slope) in Penh, Rn, and H (Figs. 1, 2, and 3, respectively). We showed that although Penh_slope and Rn_slope as well as Penh_slope and H_slope are significantly different in only a few strains (Fig. 4C and 4D as well as Fig. 5C and 5D, respectively), Penh_slope and Rn_slope as well as Penh_slope and H_slope are not significantly correlated across all the 29 strains (Fig. 4A and 4B as well as Fig. 5A and 5B, respectively). And although Penh_slope, Rn_slope, and H_slope were derived from dose-response curves that used different doses of methacholine, all doses were kept in the same range of exposure (1–30 mg/ml). In addition, we found that different genomic regions are responsible for the phenotypic variations in Penh_slope, Rn_slope, and H_slope (Fig. 6 and Table 2). Therefore, in addition to previous theoretical and physiological studies, we provide another line of evidence that Penh may not be a valid indicator of changes in airway resistance or lung elastance even in high-throughput mouse studies.
Our genome-wide association studies identified different genetic loci for Penh_slope and Rn_slope, with no overlap between the scans (Fig. 6, Table 2). If Penh had detected lung functions somehow related to Rn, one would expect at least some overlap between the underlying genetic loci. Nevertheless, our findings of the location of the genomic loci are in agreement with those reported in the literature, in which investigators studied the genetic basis for both Penh and invasively detected airway resistance in QTL studies. A literature search (17) showed that QTLs identified for Penh (Penh-QTLs) (1, 30) differed from the QTLs identified for airway resistance (Raw-QTLs) (6, 7, 10, 11, 26). Penh-QTLs found by Zhang et al. (30) were located on Chr 9 (at ∼37 Mb), Chr 10 (at ∼82 Mb), Chr 11 (at ∼90 Mb), and Chr 17 (at ∼16 Mb), and those found by Ackerman et al. (1) were located on Chr 2 (between ∼142 and 179 Mb) and Chr 6 (between ∼4 and 87 Mb). In our study, we were able to repeat two of those Penh-QTLs with the Penh_slope association peaks on Chr 2 at 155 Mb and Chr 9 at 37 Mb in male mice.
In contrast to Penh-QTLs, Raw-QTLs were identified by Ewart et al. (10, 11) on Chr 2 (between ∼70 or 115 and 130 Mb) and Chr 6 (between ∼9 and 20 Mb as well as 34 and 49 Mb), by Nicolaides et al. (26) on Chr 13 (at ∼53 Mb), and by De Sanctis et al. (6, 7) on Chr 2 (at ∼158 Mb), Chr 6 (at ∼115 Mb), Chr 7 (at ∼37 Mb), Chr 15 (at ∼84 Mb), and Chr 17 (at ∼12 Mb). In our genome-wide association scans for Rn_slope, we were able to repeat two Raw-QTLs with two association peaks on Chr 6, one at 10 Mb and the other at 112 Mb.
Ackerman et al. (1) and De Sanctis et al. (6) found a Penh-QTL and Raw-QTL, respectively, at similar genomic locations on distal Chr 2 in male mice. However, we were only able to repeat those findings with our genome-wide association studies for Penh_slope, but not for Rn_slope in male mice. It is most likely that the missing overlap between the previous Raw-QTL and our Rn_slope associations on distal Chr 2 is due to the different invasive detection methods.
The lack of other overlaps between previous QTL studies and our genome-wide association studies may be explained by differences in 1) the genotype information, 2) phenotypic variations due to animal and environmental changes, and 3) differences in the scanning algorithms. First, the genotype information in QTL studies use less genetic variability (only two strains for crosses vs. 29 strains in our surveys) and a smaller marker coverage compared with the high-density SNP set in our genome-wide association studies. Second, the phenotypic variations could have been due to age and sex differences, differences in the sensitization/challenge methods (naïve vs. allergen-challenged mice), or differences in the presentation of airway responsiveness (slope vs. individual methacholine doses). Another possible but less likely factor that could have changed the phenotype is environmental stress due to the shipment of mice from The Jackson Laboratory to the University of Pittsburgh. To minimize the potential stress from relocating the mice to the University of Pittsburgh, we allowed that each mouse received an acclimatization period of at least 7 days and similar husbandry conditions at the Pittsburgh vivarium as provided at The Jackson Laboratory. Finally, although the current scanning algorithms for our genome-wide association studies are more powerful and less susceptible to false positives than previous single SNP or haplotype association methods, the lack of overlaps could also be due to the detection of some false positives. This possibility cannot yet be excluded and warrants further verification with, for example, QTL studies as suggested by a previous study (22).
The difference in the locations of the genomic variations that cause phenotypic variability in Penh and Rn supports the assumption that they are caused by different mechanisms within the respiratory system. We isolated the genes that underlie our association peaks by searching for the location of the peak SNPs on the Biomart annotation database (http://www.biomart.org) and by reporting those genes that directly overlapped and those that were located adjacent upstream and downstream of the SNPs (Table 2). Although the functional testing of these genes is not within the aim of this study, it breaks the ground for further investigations. As a result of our genome-wide association studies, we conclude that the attempt to study the genetics of resistance by measuring Penh will ultimately lead to erroneous conclusions.
We did not identify any common genomic regions or genes between Penh and H (Fig. 6 and Table 2). A lack in common genomic regions between Penh and H could have been caused by the collection of the H data at the Rn peak and not at the later occurring H peak, which could have also compromised a comparison between methacholine doses and mouse strains for the H parameter. The genomic locations that determine the H phenotype will have to be verified in the future with data collected at the H peak. Nevertheless, to measure lung function parameters accurately, such as Rn and H, transpulmonary pressure has to be detected, which is given by FOT, but not by UP. Conclusively, the use of Penh as measure of airway and peripheral lung parameters is not reliable.
In the past, several studies have examined the differences in lung responsiveness between mouse strains as part of physiological as well as genetic studies. Most of those studies agree about which strains are more responsive then others. For example, the strain A/J is generally accepted to be a highly responsive strain, whereas the strains C57BL/6J and C3H/HeJ are often considered resistant strains (1, 6, 7, 10, 11). Both of our strain surveys for Penh and Rn are consistent with those findings. Other studies, however, disagree in their findings about which strains are more responsive than others. For example, whereas McIntire et al. (23) showed that the strain BALB/cByJ is more responsive than the strains DBA/2J; other investigators presented the opposite (18). Our strain surveys for Penh and Rn showed that DBA/2J is more responsive than BALB/cByJ. One explanation for this discrepancy may be the different ways of presenting airway responsiveness data (slope of the increase vs. single dose exposure). Alternatively, DBA/2J and BALB/cByJ are strains with an intermediate response and therefore may be dependent on the within-strain variation. Despite those discrepancies, we were able to present a spectrum of novel strains that are more or less resistant than the classically studied strains by studying several additional strains that have not been investigated in airway responsiveness studies thus far. Therefore, our strain surveys open the opportunity for choosing novel strains with which the field's understanding of the pathophysiology and genetics underlying the mechanisms of airway responsiveness can be enhanced.
We found that for most strains the median standardized ranks for Penh_slope and Rn_slope or H_slope were not significantly different (Fig. 4 or 5, respectively). As argued previously, a lack of significant difference or the existence of a correlation between Penh and any other lung function parameter should not immediately lead to the ultimate conclusion that Penh can be used as a surrogate for those airway function measures unless the causality behind the relatedness can be explained otherwise (20, 25). In our study we showed that the correlations among the strains as well as the genetics underlying the strain variations did not support the argument that Penh and Rn or H are interchangeable parameters. Because Penh is derived from pressure/time changes that are measured within the plethysmograph chamber and not within the airways, environmental factors such as chamber temperature and humidity, mouse behavior such as sniffing or exploratory movements, and strain differences in anatomy such as upper or lower airways or chest wall are likely to influence the measurements. In contrast, Rn and H are derived from measuring the pressure and flow within the transtracheal tube in anesthetized mice, which allows the localized detection of changes that are mainly due to airway properties and less likely due to environmental, behavioral, or anatomical influences. Finally, the intrastrain variability of Penh was greater than those of Rn and H, supporting the assumption that the measurement of Penh as lung function parameter is less accurate for each strain than those for Rn and H.
We observed differences between female and male mice for Penh and Rn measures (Fig. 1 and 2, respectively). Sex-specific differences in airway functions have been reported previously (5). In studies that investigated fewer strains, it seemed that male mice showed higher airway resistance than females. However, here we showed that the sex with the greater airway resistance was strain dependent.
Finally, the authors are aware that the comparisons between Penh and either Rn or H do not complete the range of possible comparisons between parameters that can be obtained by FOT and UP. Several more opportunities for comparisons have been described elsewhere (2). All raw data in this study needed for subsequent analyses can be found at the MPD website (accession number: 351).
In summary, we presented two strain surveys of 29 mouse strains detecting either Penh with UP or Rn and H with FOT. We showed that these phenotypes are caused by different underlying genetic variations, illustrating that changes in Penh, Rn, and H are not originated by the same mechanics within the lung as shown in the past (20). The verification of the novel association peaks will be necessary in subsequent QTL studies. Our data lead to the conclusion that Penh is not a reliable indicator of invasively detected lung function parameters (e.g., resistance and elastance) even in high-throughput mouse studies. Nevertheless, we are not able to argue that Penh is not useful as an indicator of other parameters, such as the breathing pattern (2). Although our conclusion negating Penh for measuring lung function leaves the field with no labor-efficient solution for high-throughput genetic studies, alternative approaches for UP are currently under investigation (4, 24). We trust that a successful approach will emerge and provide a way to accurately, efficiently, and noninvasively measure lung function in long-term studies.
GRANTS
This work was supported by National Institutes of Health Grants HL-66611 and HL-83069 as well as by the Cancer Core Grant CA-34196 to The Jackson Laboratory and the University of Pittsburgh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Joanne Currer for help in preparing the manuscript and Jesse Hammer for assistance in preparing the graphics.
Current address for A. Berndt: Univ. of Pittsburgh, Pittsburgh, PA.
REFERENCES
- 1. Ackerman KG, Huang H, Grasemann H, Puma C, Singer JB, Hill AE, Lander E, Nadeau JH, Churchill GA, Drazen JM, Beier DR. Interacting genetic loci cause airway hyperresponsiveness. Physiol Genomics 21: 105–111, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 97: 286–292, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 94: 1297–1306, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bates JH, Thompson-Figueroa J, Lundblad LK, Irvin CG. Unrestrained video-assisted plethysmography: a noninvasive method for assessment of lung mechanical function in small animals. J Appl Physiol 104: 253–261, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chang HY, Mitzner W. Sex differences in mouse models of asthma. Can J Physiol Pharmacol 85: 1226–1235, 2007 [DOI] [PubMed] [Google Scholar]
- 6. De Sanctis GT, Merchant M, Beier DR, Dredge RD, Grobholz JK, Martin TR, Lander ES, Drazen JM. Quantitative locus analysis of airway hyperresponsiveness in A/J and C57BL/6J mice. Nat Genet 11: 150–154, 1995 [DOI] [PubMed] [Google Scholar]
- 7. De Sanctis GT, Singer JB, Jiao A, Yandava CN, Lee YH, Haynes TC, Lander ES, Beier DR, Drazen JM. Quantitative trait locus mapping of airway responsiveness to chromosomes 6 and 7 in inbred mice. Am J Physiol Lung Cell Mol Physiol 277: L1118–L1123, 1999 [DOI] [PubMed] [Google Scholar]
- 8. DeLorme MP, Moss OR. Pulmonary function assessment by whole-body plethysmography in restrained versus unrestrained mice. J Pharmacol Toxicol Methods 47: 1–10, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Dohi M, Tsukamoto S, Nagahori T, Shinagawa K, Saitoh K, Tanaka Y, Kobayashi S, Tanaka R, To Y, Yamamoto K. Noninvasive system for evaluating the allergen-specific airway response in a murine model of asthma. Lab Invest 79: 1559–1571, 1999 [PubMed] [Google Scholar]
- 10. Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol 23: 537–545, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Ewart SL, Mitzner W, DiSilvestre DA, Meyers DA, Levitt RC. Airway hyperresponsiveness to acetylcholine: segregation analysis and evidence for linkage to murine chromosome 6. Am J Respir Cell Mol Biol 14: 487–495, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Flandre TD, Leroy PL, Desmecht DJ. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J Appl Physiol 94: 1129–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 156: 766–775, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res 4: 4, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics 178: 1709–1723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leme AS, Berndt A, Williams LK, Tsaih SW, Szatkiewicz JP, Verdugo R, Paigen B, Shapiro SD. A survey of airway responsiveness in 36 inbred mouse strains facilitates gene mapping studies and identification of quantitative trait loci. Mol Genet Genomics 283: 317–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levitt RC, Mitzner W. Autosomal recessive inheritance of airway hyperreactivity to 5-hydroxytryptamine. J Appl Physiol 67: 1125–1132, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Lomask M. Further exploration of the Penh parameter. Exp Toxicol Pathol 57, Suppl 2: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol 93: 1198–1207, 2002 [DOI] [PubMed] [Google Scholar]
- 21. MacDonald KD, Chang HY, Mitzner W. An improved simple method of mouse lung intubation. J Appl Physiol 106: 984–987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manenti G, Galvan A, Pettinicchio A, Trincucci G, Spada E, Zolin A, Milani S, Gonzalez-Neira A, Dragani TA. Mouse genome-wide association mapping needs linkage analysis to avoid false-positive loci. PLoS Genet 5: e1000331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol 2: 1109–1116, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Mitzner W. Why can't mice just learn to pant? J Appl Physiol 105: 402, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Mitzner W, Tankersley C. Interpreting Penh in mice. J Appl Physiol 94: 828–831, author reply 831–822, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci USA 94: 13175–13180, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pauluhn J. Comparative analysis of pulmonary irritation by measurements of Penh and protein in bronchoalveolar lavage fluid in brown Norway rats and Wistar rats exposed to irritant aerosols. Inhal Toxicol 16: 159–175, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Petak F, Habre W, Donati YR, Hantos Z, Barazzone-Argiroffo C. Hyperoxia-induced changes in mouse lung mechanics: forced oscillations vs barometric plethysmography. J Appl Physiol 90: 2221–2230, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Yang H, Ding Y, Hutchins LN, Szatkiewicz J, Bell TA, Paigen BJ, Graber JH, de Villena FP, Churchill GA. A customized and versatile high-density genotyping array for the mouse. Nat Methods 6: 663–666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Lefort J, Kearsey V, Lapa e Silva JR, Cookson WO, Vargaftig BB. A genome-wide screen for asthma-associated quantitative trait loci in a mouse model of allergic asthma. Hum Mol Genet 8: 601–605, 1999 [DOI] [PubMed] [Google Scholar]