Abstract
Dextran sodium sulfate (DSS)-induced colitis is widely used to study pathological mechanisms and potential treatments of inflammatory bowel disease. Because temporal changes in genome expression profiles remain unknown in this model, we performed whole genome expression profile analysis during the development of DSS colitis in comparison with ulcerative colitis (UC) specimens to identify novel and common responses during disease. Colon tissue from DSS-treated mice was collected at days 0, 2, 4, and 6. Half of each specimen was used for histopathological analysis and half for Affymetrix whole genome expression profiling and qRT-PCR validation. Genesifter and Ingenuity software analysis was used to identify differentially expressed genes and perform interactive network analysis. Identified DSS-associated genes in mice were also compared with UC patient data. We identified 1,609 genes that were significantly altered during DSS colitis; the majority were functionally related to inflammation, angiogenesis, metabolism, biological adhesion, cellular growth and proliferation, and cell-to-cell signaling responses. Five hundred and one genes were progressively upregulated, while one hundred seventy-three genes were progressively downregulated. Changes in gene expression were validated in a subset of 33 genes by qRT-PCR, with r2 = 0.925. Ingenuity gene interaction network analysis revealed novel relationships among antigen presentation, cell morphology, and other biological functions in the DSS mouse. Finally, DSS colitis gene array data were compared with UC patient array data: 152 genes were similarly upregulated, and 22 genes were downregulated. Temporal genomewide expression profile analysis of DSS-induced colitis revealed novel associations with various immune responses and tissue remodeling events such as angiogenesis similar to those in UC patients. This study provides a comprehensive view of DSS colitis changes in colon gene expression and identifies common molecules with clinical specimens that are interesting targets for further investigation.
Keywords: inflammation, blood vessel, dextran sodium sulfate, inflammatory bowel disease
inflammatory bowel diseases (IBDs) are a group of chronic inflammatory disorders of the gastrointestinal tract resulting in abdominal pain, fever, diarrhea with blood and mucus, and increased risk of colon cancer. There are two primary types of IBD, Crohn's disease (CD) and ulcerative colitis (UC), with other less frequent and less understood secondary classifications. It is estimated that more than 1 million patients in the US alone suffer from IBD (31), and the incidence rates among Hispanics and Asians have recently increased (25). As such, there is a great need to better understand specific pathophysiological mechanisms of the disease.
Currently, infliximab and adalimumab (anti-TNF-α) are the newest approved therapeutic agents for colitis; however, side effects such as serious infection and immune depression can often occur, as well as a loss of therapeutic benefit over time. Other biological reagents that target inflammatory molecules have also been examined but were stopped at Phase II/III of investigation, such as IL-10, certolizumab pegol, and ISIS 2302 (5), suggesting that targeting single molecules to treat this multifactorial disease may be insufficient and that targeting more than one molecule might be more beneficial. Thus a comprehensive knowledge base of genomewide changes in gene expression in experimental colitis and human IBD is necessary.
Animal models of colitis serve as useful tools to study possible pathophysiological mechanisms of IBD. Although several animal models have been established, no single model can emulate every aspect of IBD disease (43). The dextran sodium sulfate (DSS) model is a well-established experimental colitis model that emulates numerous clinical and histopathological features of UC (48). Moreover, the DSS colitis model is widely used because of its convenient induction of intestinal inflammation (DSS dissolved in drinking water), lower mortality rate, and high reproducibility. As a physical agent, it is believed that DSS disrupts the epithelial cell barrier to promote immune cell exposure to normal mucosal microflora (1). However, we have previously reported that DSS-induced colitis is highly dependent on increased leukocyte recruitment responses as well as production of inflammatory mediators, which all contribute to tissue damage in DSS colitis (1, 10, 42).
Previous studies of clinical specimens have identified distinct gene expression profiles associated with increased IBD activity (47); however, no reports exist regarding the temporal nature of colon tissue genome expression profiles during the development of DSS experimental colitis compared with human specimens. Here we examined colon tissue of DSS colitis mice at days 0, 2, 4, and 6, using Affymetrix genomewide profiling analysis and quantitative real-time PCR (qRT-PCR) techniques coupled with histopathological analysis of disease progression compared with human UC specimens. Interestingly, we found that numerous genes are significantly altered in a temporal manner during the progression of acute DSS colitis. To our knowledge, this is the first report of a comprehensive genomewide expression profile analysis of DSS-mediated colitis over time that is directly compared with human UC gene array expression data. Numerous differences were identified that involve increased inflammatory gene expression along with concomitant downregulation of genes involved in tissue homeostasis and immune regulation. Finally, we found a greater number of genes similarly expressed between the DSS colitis model and UC tissue specimens than previously reported with smaller, annotated arrays, suggesting that the combination of global expression profiling and refined network analysis reveals previously unknown target molecules of IBD.
MATERIALS AND METHODS
DSS-induced colitis mouse model.
C57BL/6J mice aged 12–14 wk were used for these studies and were housed in specific pathogen-free conditions according to Institutional Animal Care and Use Committee (IACUC) regulations and the National Research Council's Guide for the Care and Use of Laboratory Animals. The Louisiana State University Health Sciences Center IACUC reviewed and approved all animal protocols. A 3% DSS solution was administered in the drinking water. Clinical signs of colitis were measured after mice drank DSS water at days 0, 2, 4, and 6. Water consumption was measured daily to ensure that equivalent amounts of DSS were consumed as we previously reported (1, 10). A disease activity index (DAI) was calculated from measurements of weight loss, occult blood positivity, stool consistency, and rectal bleeding daily from all time cohorts as we have reported (1, 10). Mice were killed and the colon tissue between cecum and anus was collected at respective time points. The tissue was longitudinally cut in half and rolled in a Swiss roll fashion with one half of each sample preserved in RNAlater reagent for RNA isolation and the other half used for histopathological analysis. Mice killed at day 0 (n = 5) before administration of DSS were used as controls; six mice were killed at days 2, 4, and 6 for experimental analysis.
Histopathology assessment of DSS-induced colitis.
Five-micrometer-thick sections were cut and stained with hematoxylin and eosin (H & E) and scored in a double-blind manner. Tissue sections were scored for the degree of inflammation, tissue injury, crypt damage, and percentage of tissue involvement, thus yielding a total histopathology score as we previously reported (11). Severity of inflammation was scored 0 to 3 for none, slight, moderate, and severe. Depth of injury was scored 0 to 3 for none, mucosal, mucosal and submucosal, and transmural. Crypt damage was scored 0 to 4 for none, basal one-third damaged, basal two-thirds damaged, only surface epithelium intact, and entire crypt epithelium lost. Next, the score of each parameter was multiplied by the percentage of tissue involvement (X1 0–25%, X2 25–50%, X3 50–75%, and X4 75–100%). Summation of these values for each sample animal results in a composite histopathological score.
Expression analyses using oligonucleotide arrays.
Total RNA was isolated from the entire colon with Qiagen RNeasy isolation kits. RNA integrity was assessed by electrophoresis on the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Double-stranded cDNA was synthesized from ∼5 μg of total RNA with a SuperScript cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) in combination with a T7-(dT)24 primer (Proligo, Boulder, CO). Biotinylated cRNA was transcribed in vitro with GeneChip Expression 3′-Amplification reagents (Affymetrix, Santa Clara, CA) and purified with the GeneChip Sample Cleanup Module (Affymetrix). Twenty micrograms of purified cRNA was fragmented by incubation in fragmentation buffer (in mM: 200 Tris-acetate, pH 8.1, 500 potassium acetate, 150 magnesium acetate) at 94°C for 35 min and chilled on ice. Ten micrograms of fragmented biotin-labeled cRNA was hybridized to the Mouse Genome 430 2.0 Array (Affymetrix), which provides the most comprehensive annotated coverage of the mouse genome, integrating over 39,000 transcripts and variants from over 34,000 well-characterized mouse genes. Arrays were incubated for 16 h at 45°C with constant rotation (60 rpm). The arrays were washed and then stained for 10 min at 25°C with 10 μg/ml streptavidin-R phycoerythrin (Vector Laboratories, Burlingame, CA) followed by 3 μg/ml biotinylated goat anti-streptavidin antibody (Vector Laboratories) for 10 min at 25°C. Arrays were then stained once again with streptavidin-R phycoerythrin for 10 min at 25°C. After washing and staining, the arrays were scanned with a GeneChip Scanner 3000. Four probe sets were used to verify hybridization quality control: AFFX-BIOB, AFFX-BIOC, AFFFX-BIOD, and AFFX-CRE. Pixel intensities were measured, expression signals were analyzed, and features were extracted with the commercial software package GeneChip Operating Software 1.4 (Affymetrix).
Microarray data normalization and analysis.
Arrays were globally scaled to a target intensity value of 500 in order to compare individual animals. A single gene chip was used for each animal specimen for each time point, for a total of six individual specimens per time point. No specimens were pooled in any of these studies. The absolute call (present, marginal, absent) of each gene expression in each sample as well as the direction of change and fold change of gene expressions between samples were identified with GeneChip Operating Software 1.4 (Affymetrix). All microarray data conform to MIAME standards and were uploaded in the NCBI Gene Expression Omnibus (GEO) database (accession number GSE22307) for public access.
Resulting data were log transformed and uploaded into the Genesifter program (www.genesifter.com). Differences in gene expression were identified by using a minimal threshold value of a twofold change with no maximal threshold value. One-way ANOVA was performed against day 0 coupled with the Benjamini and Hochberg posttest to diminish false discovery rates. All expression data are reported as log change in gene expression. To visualize and explore the molecular interaction networks of the differentially expressed genes, the subsequent data were uploaded into Ingenuity software (www.ingenuity.com) to organize the differentially expressed genes into networks based on the Ingenuity Knowledge Database (IKB), an extensive, manually created database of functional direct and indirect interactions between genes from peer-reviewed publications (8, 10).
Quantitative real-time PCR analysis of DSS-induced colitis.
Real-time PCR validation was carried out by measuring 33 genes that were either progressively up- or downregulated. Reverse transcription was carried out by using 1 μg of total RNA from each sample. Primers were designed with Beacon Designer software. The cDNA sequence of selected genes was downloaded from the NCBI Entrez database, after BLAST search for homology sequences in mouse and template structure search (folding temperature is 55.0°C) that avoid template secondary structure and avoid cross homology. Primers were 18–24 bp long, with PCR product length 75–200 bp. Primers were synthesized by Integrated DNA Technologies, and primer sequences are reported in Table 1. PCR products were verified by sequence analysis. A 40-fold dilution of the cDNA products was used as a template to perform qRT-PCR analysis with SYBR Green PCR master mix. After 10 min of denaturation of cDNA template at 95°C, the cycling condition of 40 cycles was denaturing at 95°C for 15 s, annealing at 55°C for 30 s, and elongation at 72°C for 30 s. All reactions were performed in triplicate. Dissociation curve analysis was done after each run to confirm the primer specificity. GAPDH was used as the internal control gene for all reactions. The threshold cycle (Ct) formula was used to calculate changes in gene expression. Statistical analysis was performed by one-way ANOVA with day 0 as control. Bonferroni's posttest was used to determine which groups significantly differed from each other.
Table 1.
Primer sequences used for quantitative real-time PCR analysis
| Gene Name | Accession No. | Sense Primer | Antisense Primer |
|---|---|---|---|
| Adamts1 | D67076 | TGGCTCACTGCTTCTACTC | ACGCACACCTTCACAGAG |
| C1qa | NM_007572 | AGGGCGTGAAAGGCAATC | CTTGGAAGTTGAAGTAATAGAAGC |
| C3 | K02782 | CCATCAAGATTCCAGCCAGTAAGG | GCTTTCTCCACCACCGTTTCC |
| C4bp | NM_007576 | GCTGCCAGGAAGGATTTATC | GGATCACAGGTATAGGAGATTC |
| Cav1 | AB029929 | TTACCGCTTGTTGTCTACG | AGAGTGGATCGCAGAAGG |
| CcR2 | BB148128 | ACCTGGTGCTTGATGGAGAAG | CGGAAGAAATTGATGTCACTTGTG |
| Ccr5 | D83648 | GAGGTGAGACATCCGTTCC | GGTGCTGACATACCATAATCG |
| CD40 | BB220422 | TGAGGACTGCTTGCTGAC | TGGTAGGTATCACTGTGGAC |
| Clip4 | BM217861 | AAGCAAAACACAAAAGAAAACACC | CTGTAGTGAGTCTGTAGTTCTTCC |
| Crem | AI467599 | ATTGGCAATAGAGCAAGC | GCAGCATCCTCATAATGG |
| Csf2rb | NM_007780 | GCAGACATAGAATGTGGCAGATC | TTGGAAAGTATTGACAGGCTGATG |
| Ednra | AW558570 | GTCATCACTGTAGGAGAAACTG | AGTATGCCTGAGACTTCCAG |
| Ereg | NM_007950 | CGTTGCGTTGACAGTGATTC | TCCTTGTCCGTAACTTGATGG |
| GAPDH | NM_008084 | GCCTTCCGTGTTCCTACC | CTTCACCACCTTCTTGATGTC |
| Gbp2 | BE197524 | TCAAGACATGTTGTCACAGTGG | ATCACAGAACAGGCAACTTTTAAC |
| Hnf1b | AB008174 | CAGACGCACAACCTGAAC | CGCACTCCTGACATCTTG |
| Hnf4a | NM_008261 | CGTGGGTAGGGGAGAATG | AAACTCCAGGGTGGTGTAG |
| ID1 | U43884 | CATCCCTTATCTCGCTCTG | TCCTCTTGCCTCCTGAAG |
| Ifitm6 | BB193024 | CCTACATCTACTCGGTGAAG | TCAGAATCTTGGCGGTTG |
| Ifng | K00083 | TTACTTCACTGACCAATAAG | TACTACCTGACACATTCG |
| igtp | NM_018738 | ACAGACATAGACCTTCATACTC | TGGCAACTTTGTGACTAAATC |
| IL33 | NM_133775 | ACTACGCTACTATGAGTCTC | TGTGAAGGACGAAGAAGG |
| Lgi2 | BE947711 | GTGGTTATCTTTGAAGGAGAAG | TGCGTTGGAACAGTAAGC |
| ligp1 | BM239828 | AATGTAAATATGCTGCTACTTAG | TTTGGGAAATTATTTTGCTTATG |
| Map3k8 | NM_007746 | CTGCCTGGACTGCTGAACTC | GCTCATACACTCCTGGCTCTTC |
| Mmp13 | NM_008607 | CATGCTTCCTGATGATGAC | CCTCGGAGACTGGTAATG |
| Mmp3 | NM_010809 | GTGTGGTGTTCCTGATGTTG | CACTCTGTCTTGGCAAATCC |
| Mpeg1 | L20315 | TGAATGCTTAGGGCTGGTATATG | CCTGGTAGGACCTCCAAGAC |
| Ptk6 | NM_009184 | AAGCCAGGAGCAGACTATG | CAGACAGATTGGAGAAGGATAC |
| Pyhin1 | BM241008 | AAGGAAGCAGGACTGAGTGGTTG | GGATGATGGAAGTGGAGGTCTACG |
| TNF | NM_013693 | GTTCTGTCCCTTTCACTCAC | TGCCTCTTCTGCCAGTTC |
| Trpm6 | BC022929 | CCACCGTCTTCCTTCATCATC | ACTCCCTTTCAAGAACACACTG |
| VEGF-A | U50279 | TTACTGCTGTACCTCCAC | GAAGATGTACTCTATCTCGTC |
Comparison of mouse DSS and ulcerative colitis microarray data.
UC gene expression data were obtained from Wu et al. (47), who used the Affymetrix human genome U133 Plus 2.0 array, to compare identified genes against our murine DSS gene array data. The Affymetrix human genome U133 Plus 2.0 array is annotated similarly to the Affymetrix mouse genome 430 2.0 array and includes over 38,500 unique transcripts. Human UC gene array data were downloaded at http://www.gastroenterology.org (doi: 10.1053/j.gastro.2008.07.068). These experiments compared pooled RNA samples from UC patients (n = 6) with normal healthy control subjects (n = 5).
RESULTS
DSS increases colitis parameters progressively over time.
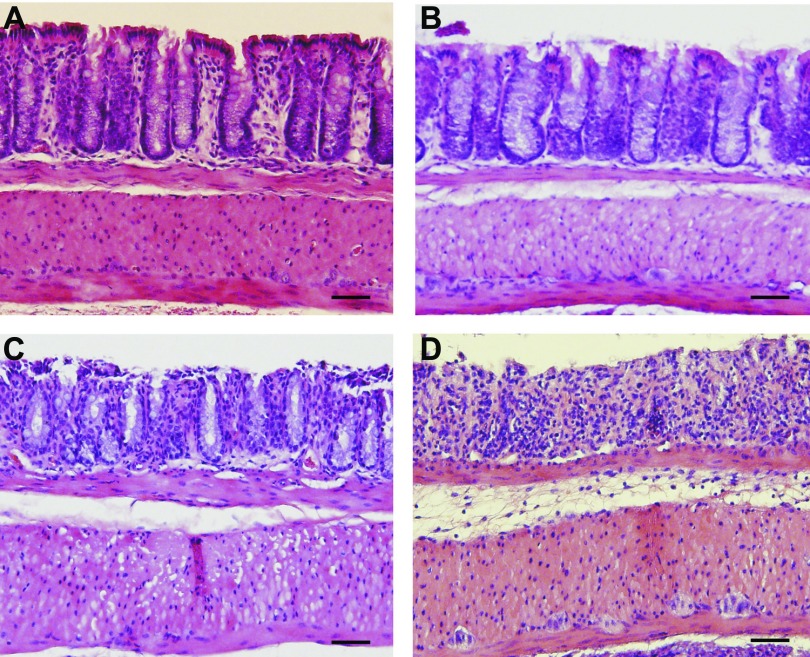
Previous studies from our group (1) and others (34, 36) indicate that DSS colitis pathology develops progressively over time. Therefore, in order to better understand temporal changes in genome expression profiles, 3% DSS-mediated tissue histopathology was evaluated and confirmed at days 0, 2, 4, and 6. Figure 1 shows representative photomicrographs of DSS-mediated colitis histopathology over time. Figure 1A illustrates normal colon histomorphology at day 0, whereas Fig. 1, B–D, demonstrate DSS-mediated histological changes at days 2, 4, and 6, respectively. These findings are consistent with our and others' previous results regarding DSS-mediated tissue histopathology showing progressive increases in leukocyte infiltrates, focal crypt lesions, goblet cell losses, and tissue edema.
Fig. 1.
Dextran sodium sulfate (DSS)-mediated tissue histopathology over time. A–D: DSS-mediated histological changes illustrated by hematoxylin and eosin staining of tissue harvested at days 0, 2, 4, and 6, respectively. Histopathological changes progressively increase over time including epithelial erosion, leukocyte infiltration, crypt damage, and edema formation. Magnification is ×200. Scale bars, 100 μm.
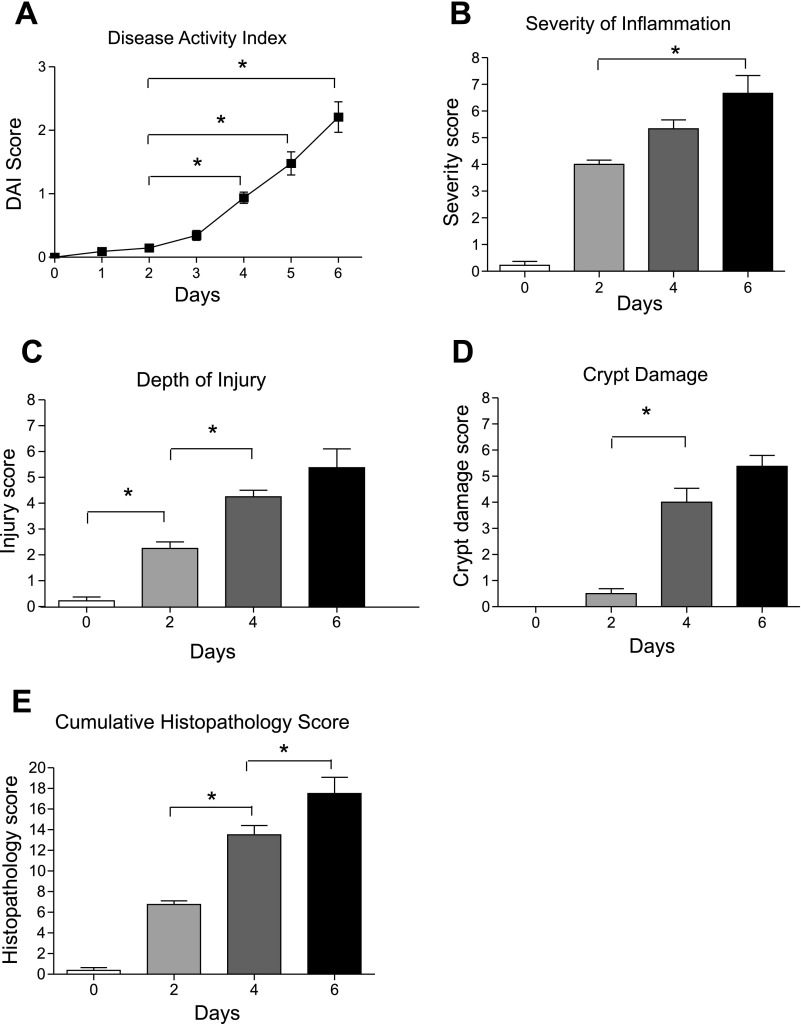
Additional disease parameters were also evaluated in a temporal manner during 3% DSS colitis. Figure 2A shows that DSS significantly increases the DAI, a combination of weight loss, stool consistency, occult blood positivity, and gross rectal bleeding, over time. Importantly, the earliest significant inflection of DAI was seen between days 2 and 4. Figure 2, B–D, report the severity of inflammation, depth of injury, and crypt damage scores, respectively. Again, these various histopathological parameters progressively increase over time. From these data it is clear that inflammatory infiltrates occur early in this model (within 2 days) and precede tissue injury and crypt loss, which become more prominent starting at day 4. Figure 2E illustrates the aggregate cumulative histopathology score, which encompasses scores from Fig. 2, B–D, and weights them according to the percentage of the colon cross-sectional area involved. These data clearly demonstrate that between days 2 and 4 colon histomorphological changes are most dramatic, suggesting that this time interval could delineate a breakpoint for rapid and severe progression of DSS colitis histopathology.
Fig. 2.
Quantitative evaluation of DSS clinical activity and tissue histopathology. A: clinical disease activity index (DAI) score over time during 3% DSS administration in drinking water. Several clinical indexes of disease were monitored, including weight loss, stool consistency, occult blood positivity, and gross rectal bleeding, to calculate a cumulative DAI score. B: severity of mucosal and submucosal inflammation elicited by 3% DSS over time. C: depth of mucosal tissue injury over time with 3% DSS treatment. D: crypt damage score in response to DSS treatment at discrete time points. E: cumulative histopathology score, which combines the 3 scores shown in B–D and weights them based on % area of colon tissue involvement as explained in materials and methods. *P < 0.05 vs. day 0 (n = 5) by 1-way ANOVA with Bonferroni posttest; n = 6 animals per time cohort at days 2, 4, and 6.
DSS colitis stimulates temporal changes in global gene expression.
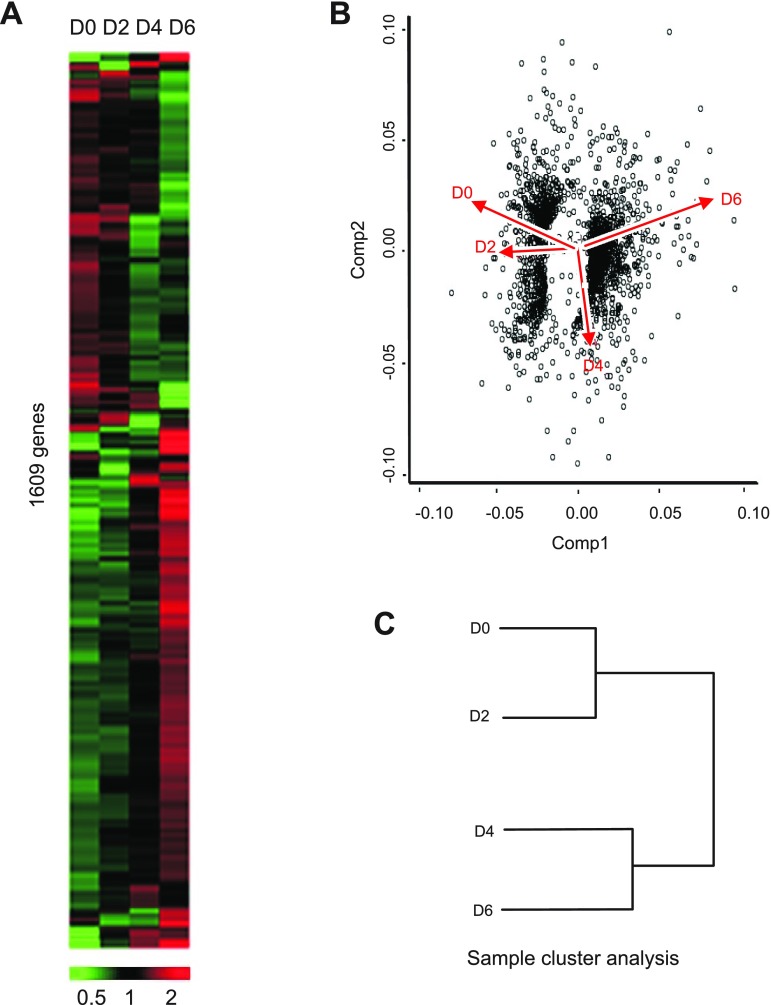
Individual colon total RNA specimens from respective time points were next hybridized to individual Mouse 430 2.0A Affymetrix gene chips to evaluate global changes in gene expression. This Affymetrix array was chosen for these studies as it contains one of the most highly annotated and complete probe sets representing the entire mouse genome. Figure 3A demonstrates that expression levels of 1,609 different genes represented by 1,873 probe sets were identified by Genesifter program analysis. From this heat map signature, it is quite clear that genes that are initially abundant are progressively downregulated and that genes that are initially less expressed become progressively upregulated. Principal component analysis (PCA) was next used to visualize the overall response of gene expression changes during the DSS colitis model (Fig. 3B). Component 1 represents the actual change in gene expression profiles, while component 2 illustrates the trend of change in gene expression. PCA revealed that data from early time points (i.e., days 0 and 2) had a clear downward trend in gene expression; however, gene expression profiles at day 4 had a clear break in expression pattern that was significantly increased at day 6. Likewise, sample cluster analysis also showed that gene expression profiles at day 0 and day 2 were much alike, while day 4 and day 6 were classed into a similar cluster group. These results suggest that key changes in gene expression occur between days 2 and 4 during DSS colitis, corroborating the above described temporal histopathological characteristics of the model.
Fig. 3.
Genesifter analysis of DSS-mediated temporal changes in gene expression. A: Genesifter program heat map analysis of Affymetrix mouse gene array analysis performed from DSS-treated colon tissue at different time points: 1,609 genes were identified that significantly changed over the experimental time course at days 0, 2, 4, and 6. B: principal component analysis (PCA) of the gene expression pattern using eigenvalues of covariance. Component 1 (Comp 1) represents the actual change in gene expression profiles, while component 2 (Comp 2) illustrates the trend of change in gene expression. x- and y-Axis values are arbitrary units for eigenvalues and gene expression represented by dots. C: sample cluster analysis of the 4 DSS time point samples, showing that changes in gene expression can be divided into 2 groups, day 0 and day 2 in 1 cluster and day 4 and day 6 in a second cluster.
Distribution of gene expression profile changes during DSS colitis.
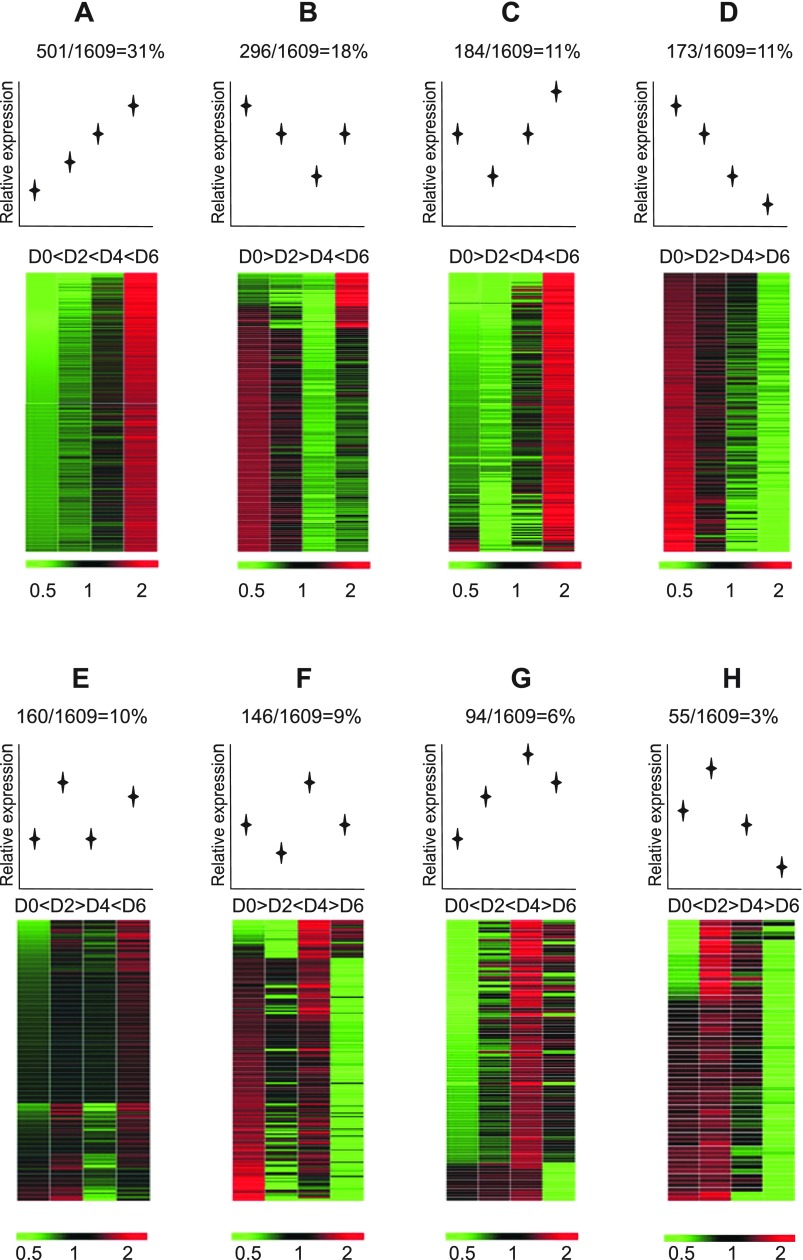
Having observed general trends in gene expression changes with heat map imaging and PCA, we wanted to more precisely identify expression profiles of individually identified genes to gain better understanding of how certain genes may be regulated. Therefore, according to gene expression patterns at the four time points, the 1,609 genes were evaluated and divided into eight separate classes of expression profiles. As shown in Fig. 4, the majority of the genes (group A, 31%) were progressively upregulated over time. However, the next highest number of genes (group B) displayed a unique progressively downward expression profile to day 4 that then rebounded at day 6. Interestingly, genes that were progressively downregulated throughout the course of the model (group D) were the third most abundant (11%; tied with group C genes showing an initial decrease in expression followed by an increase). Together, gene expression classification of the first four groups (groups A–D) showed predominant up- or downregulation of gene expression with only one time point of expression fluctuation (groups B and C) during the progression of DSS-mediated colitis. Conversely, the last four groups (groups E–H) indicate a smaller percentage of genes with dynamic up and down changes in expression. Supplemental Tables S1–S8 provide specific gene names and fold change in expression for group classifications A–H, respectively.1
Fig. 4.
Different gene expression profile classes during the progression of DSS colitis. The identified 1,609 differentially expressed genes during DSS colitis were subsequently separated into discrete expression profile classes (groups A–H). Expression profile classes are ordered from most to least abundant percentage within the total cohort of 1,609 genes.
Quantitative real-time PCR validation of DSS microarray data.
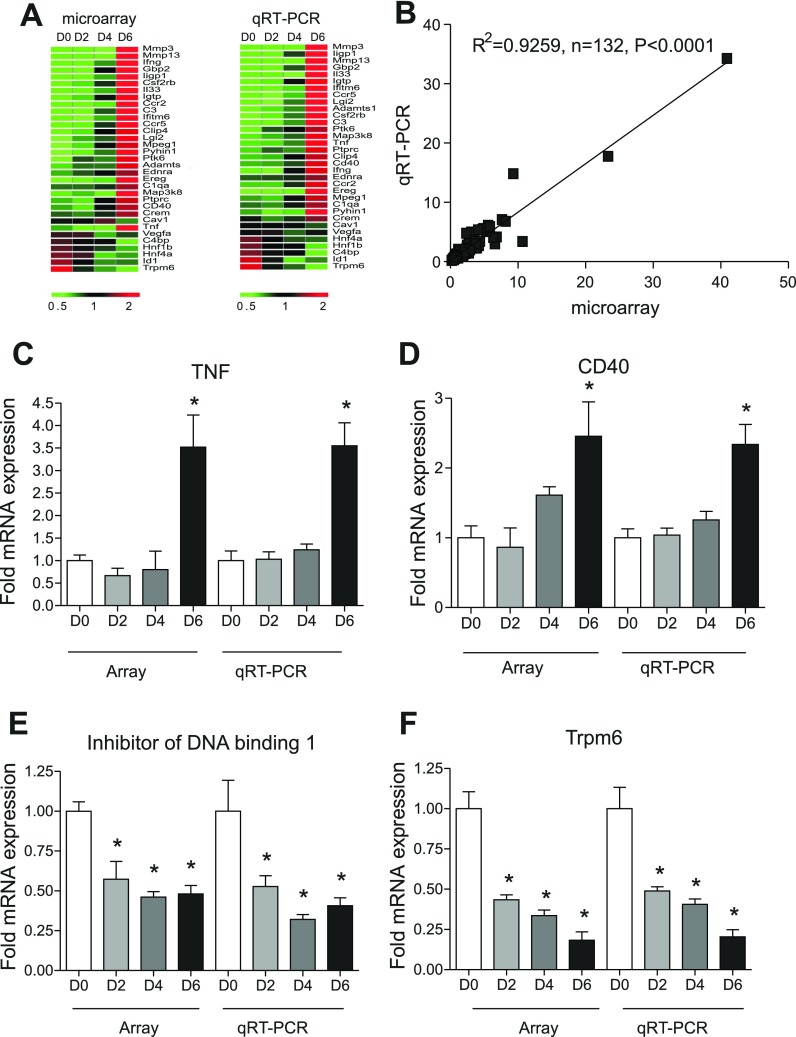
We next sought to confirm our gene array data and observations by qRT-PCR analysis of several genes representing the various classifications of gene expression profiles we identified. We used the same samples and the same number of samples in each time point as were used for microarray analysis. Thirty-three genes associated with inflammation, cell adhesion, extracellular matrix, and angiogenesis were selected for real-time PCR analysis, with 759 (23 individual samples over time × 33 genes per sample) qRT-PCR reactions performed in triplicate from total RNA samples from each specimen at each time point. The changes in gene expression obtained from qRT-PCR analysis were uploaded to Genesifter to construct heat map plots. As shown in Fig. 5A, the qRT-PCR expression profiles are highly similar to microarray data. Importantly, Fig. 5B illustrates the correlation between qRT-PCR and microarray data, with a resulting correlation coefficient of R2 = 0.9259, P < 0.0001 at 95% confidence level. Table 2 reports the actual expression changes between qRT-PCR and microarray data. Importantly, Fig. 5, C and D, illustrate that the array and qRT-PCR gene expression profiles of either TNF-α or CD40 were similarly upregulated over time. Likewise, Fig. 5, E and F, show that the array and qRT-PCR gene expression profiles for either Inhibitor of DNA binding 1 (Id1) or Trpm6 were also similarly downregulated over time. Together, these data validate our microarray findings and verify a diverse array of gene expression changes during DSS colitis.
Fig. 5.
Quantitative real-time PCR (qRT-PCR) validation of DSS microarray data. qRT-PCR validation was performed for numerous genes identified by microarray. A: microarray vs. qRT-PCR expression heat map for 33 individual genes found across all classification ranges as indicated in Fig. 4. B: correlation in gene expression between microarray (x-axis) and qRT-PCR (y-axis) changes in gene expression with r2 = 0.9259, P < 0.0001 at the 95% confidence level, n = 132. C and D: representative validation between microarray and qRT-PCR gene expression for upregulated genes TNF and CD40, respectively. E and F: representative validation between microarray and qRT-PCR gene expression for downregulated genes Id1 and Trpm6, respectively. *P < 0.01 vs. day 0 (n = 5), at days 2, 4, and 6 (n = 6).
Table 2.
Quantitative real-time PCR validation of microarray data
| Affymetrix Gene Array |
RT-PCR |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Symbol | Accession No | D0 | D2 | D4 | D6 | D0 | D2 | D4 | D6 |
| Adamts1 | D67076 | 1 ± 0.15 | 1.26 ± 0.11 | 1.25 ± 0.11 | 3.11 ± 0.37* | 1 ± 0.11 | 1.34 ± 0.16 | 1.61 ± 0.23* | 5.01 ± 0.87* |
| C1qa | NM_007572 | 1 ± 0.07 | 1 ± 0.08 | 1.14 ± 0.11 | 2.08 ± 0.28* | 1 ± 0.11 | 0.93 ± 0.07 | 1.2 ± 0.13 | 2.06 ± 0.29* |
| C3 | K02782 | 1 ± 0.03 | 1.14 ± 0.12 | 2.01 ± 0.16* | 5.81 ± 0.88* | 1 ± 0.1 | 0.98 ± 0.1 | 1.67 ± 0.15* | 5.9 ± 1.27* |
| C4bp | NM_007576 | 1 ± 0.05 | 0.85 ± 0.06 | 0.85 ± 0.03* | 0.42 ± 0.05* | 1 ± 0.13 | 0.77 ± 0.07 | 0.68 ± 0.05* | 0.31 ± 0.05* |
| Cav1 | AB029929 | 1 ± 0.04 | 1.11 ± 0.03 | 1.43 ± 0.01* | 0.77 ± 0.08 | 1 ± 0.06 | 0.94 ± 0.07 | 1.02 ± 0.07 | 0.75 ± 0.12 |
| CcR2 | BB148128 | 1 ± 0.14 | 1.16 ± 0.11 | 1.47 ± 0.24* | 6.56 ± 1* | 1 ± 0.23 | 0.99 ± 0.2 | 1.4 ± 0.2* | 3.0 ± 0.37* |
| Ccr5 | D83648 | 1 ± 0.13 | 1.28 ± 0.21 | 1.98 ± 0.09* | 4.87 ± 0.61* | 1 ± 0.16 | 1.19 ± 0.2 | 1.48 ± 0.13* | 5.61 ± 0.96* |
| CD40 | BB220422 | 1 ± 0.17 | 0.86 ± 0.28 | 1.61 ± 0.12* | 2.45 ± 0.49* | 1 ± 0.13 | 1.04 ± 0.1 | 1.26 ± 0.12 | 2.81 ± 0.55* |
| Clip4 | BM217861 | 1 ± 0.1 | 1.3 ± 0.06 | 2.3 ± 0.31 | 4.17 ± 0.42* | 1 ± 0.16 | 1.04 ± 0.06 | 1.56 ± 0.08* | 2.7 ± 0.57* |
| Crem | AI467599 | 1 ± 0.04 | 0.91 ± 0.12 | 1.12 ± 0.07 | 2.03 ± 0.15* | 1 ± 0.11 | 0.77 ± 0.07 | 0.93 ± 0.07 | 1.55 ± 0.14* |
| Csf2rb | NM_007780 | 1 ± 0.13 | 1.87 ± 0.17* | 2.71 ± 0.35* | 6.82 ± 1* | 1 ± 0.25 | 1.09 ± 0.13 | 1.44 ± 0.29* | 4.17 ± 0.43* |
| Ednra | AW558570 | 1 ± 0.14 | 1.21 ± 0.13 | 1.45 ± 0.13* | 2.33 ± 0.26* | 1 ± 0.09 | 1.04 ± 0.13 | 1.11 ± 0.09 | 1.87±0.22* |
| Ereg | NM_007950 | 1 ± 0.24 | 0.87 ± 0.13 | 1.13 ± 0.15 | 4.76 ± 1.05* | 1 ± 0.31 | 0.96 ± 0.26 | 0.82 ± 0.09 | 5.44 ± 1.48* |
| Gbp2 | BE197524 | 1 ± 0.14 | 1.68 ± 0.17* | 3.74 ± 0.49* | 8.14 ± 1.68* | 1 ± 0.23 | 1.26 ± 0.15 | 2.29 ± 0.43* | 6.8 ± 1.97* |
| Hnf1b | AB008174 | 1 ± 0.1 | 0.85 ± 0.06 | 0.63 ± 0.05* | 0.49 ± 0.04* | 1 ± 0.08 | 0.71 ± 0.08* | 0.71 ± 0.05* | 0.41 ± 0.05* |
| Hnf4a | NM_008261 | 1 ± 0.06 | 0.88 ± 0.1 | 0.42 ± 0.04* | 0.48 ± 0.04* | 1 ± 0.08 | 0.71 ± 0.07* | 0.58 ± 0.04* | 0.55 ± 0.06* |
| ID1 | U43884 | 1 ± 0.06 | 0.63 ± 0.06* | 0.46 ± 0.03* | 0.48 ± 0.05* | 1 ± 0.19 | 0.52 ± 0.07* | 0.32 ± 0.03* | 0.41 ± 0.05* |
| Ifitm6 | BB193024 | 1 ± 0.11 | 1.17 ± 0.12 | 1.56 ± 0.29 | 5.58 ± 1.1* | 1 ± 0.16 | 1.19 ± 0.2 | 1.48 ± 0.13* | 5.61 ± 0.96* |
| Ifng | K00083 | 1 ± 0.59 | 1.7 ± 0.64 | 2.87 ± 0.77 | 10.66 ± 3.01* | 1 ± 0.25 | 0.89 ± 0.29 | 2 ± 0.46 | 3.43 ± 0.84* |
| igtp | NM_018738 | 1 ± 0.14 | 1.58 ± 0.21 | 2.41 ± 0.4* | 5.29 ± 0.93* | 1 ± 0.11 | 1.4 ± 0.28 | 2.31 ± 0.54 | 5.08 ± 1.24* |
| IL33 | NM_133775 | 1 ± 0.06 | 1.17 ± 0.09 | 1.96 ± 0.26* | 7.63 ± 1.06* | 1 ± 0.18 | 1 ± 0.08 | 1.33 ± 0.14 | 7.13 ± 1.66* |
| Lgi2 | BE947711 | 1 ± 0.12 | 1.36 ± 0.2 | 1.61 ± 0.17* | 4.09 ± 0.9* | 1 ± 0.1 | 1.16 ± 0.16 | 1.71 ± 0.12* | 5.37 ± 1.12* |
| ligp1 | BM239828 | 1 ± 0.21 | 1.16 ± 0.08* | 2.34 ± 0.35* | 9.29 ± 0.77* | 1 ± 0.29 | 2.13 ± 0.59 | 4.83 ± 1.56 | 14.85 ± 4.1* |
| Map3k8 | NM_007746 | 1 ± 0.12 | 1.14 ± 0.08 | 0.96 ± 0.12 | 4.01 ± 0.58* | 1 ± 0.02 | 1.13 ± 0.07 | 1.35 ± 0.09* | 3.36 ± 0.62* |
| Mmp13 | NM_008607 | 1 ± 0.12 | 0.88 ± 0.1 | 3.9 ± 1.02* | 23.32 ± 4.63* | 1 ± 0.11 | 0.88 ± 0.11 | 2.36 ± 0.66 | 17.8 ± 3.67* |
| Mmp3 | NM_010809 | 1 ± 0.14 | 1.98 ± 0.18* | 6.35 ± 1.07* | 40.9 ± 6.59* | 1 ± 0.09 | 1.68 ± 0.04* | 4.09 ± 0.75* | 34.27 ± 6.16* |
| Mpeg1 | L20315 | 1 ± 0.07 | 1.21 ± 0.05 | 2.18 ± 0.22* | 3.6 ± 0.44* | 1 ± 0.09 | 0.92 ± 0.06 | 1.39 ± 0.16 | 2.5 ± 0.45* |
| Ptk6 | NM_009184 | 1 ± 0.1 | 1.61 ± 0.16 | 1.43 ± 0.25 | 3.74 ± 0.52* | 1 ± 0.04 | 1.49 ± 0.12 | 1.8 ± 0.29* | 3.03 ± 0.31* |
| Ptprc | NM_011210 | 1 ± 0.12 | 0.88 ± 0.06 | 1.53 ± 0.14* | 2.7 ± 0.34* | 1 ± 0.05 | 1.29 ± 0.22 | 1.15 ± 0.22 | 3.03 ± 0.55* |
| Pyhin1 | BM241008 | 1 ± 0.17 | 1.12 ± 0.13 | 1.48 ± 0.22 | 4.35 ± 0.8* | 1 ± 0.26 | 0.61 ± 0.1 | 0.87 ± 0.12 | 3.44 ± 0.87* |
| TNF | NM_013693 | 1 ± 0.12 | 0.66 ± 0.16 | 0.8 ± 0.41 | 3.52 ± 0.7* | 1 ± 0.21 | 1.03 ± 0.17 | 1.24 ± 0.13 | 3.55 ± 0.51* |
| Trpm6 | BC022929 | 1 ± 0.11 | 0.43 ± 0.03* | 0.33 ± 0.03* | 0.18 ± 0.05* | 1 ± 0.13 | 0.49 ± 0.03* | 0.41 ± 0.03* | 0.2 ± 0.04* |
| VEGF-A | U50279 | 1 ± 0.06 | 1.02 ± 0.04 | 0.53 ± 0.06* | 0.67 ± 0.05* | 1 ± 0.1 | 1.06 ± 0.1 | 0.7 ± 0.05* | 0.83 ± 0.09 |
Values are mean fold change ± SE; n = 6. D0, D2, D4, D6, days 0, 2, 4, 6, respectively.
P < 0.01 vs. day 0.
Ingenuity network analysis.
We next sought to determine which biological processes were associated with identified changes in gene expression during DSS colitis. Table 3 shows that 380 genes we identified were associated with 25 established Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Not surprisingly, the cytokine-cytokine receptor interaction pathway, complement and coagulation cascades, and other inflammation-associated pathways were highly associated with DSS-mediated disease. However, KEGG analysis is limited in that it cannot identify interactions between or influence of the change in expression of one gene on other “off-target” or “out of pathway” molecules.
Table 3.
KEGG pathway classification of gene expression during DSS colitis
| Pathway | List | Array | Z-Score |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | 55 | 202 | 7.78 |
| Complement and coagulation cascades | 15 | 47 | 4.69 |
| Jak-STAT signaling pathway | 26 | 124 | 3.72 |
| Cell adhesion molecules (CAMs) | 22 | 110 | 3.16 |
| Toll-like receptor signaling pathway | 16 | 73 | 3.09 |
| ECM-receptor interaction | 12 | 54 | 2.72 |
| Natural killer cell-mediated cytotoxicity | 15 | 83 | 2.16 |
| Hematopoietic cell lineage | 12 | 64 | 2.06 |
| Glycan structures-biosynthesis 2 | 9 | 46 | 1.92 |
| TGF-β signaling pathway | 13 | 74 | 1.89 |
| Axon guidance | 17 | 105 | 1.8 |
| Cell communication | 12 | 70 | 1.72 |
| Apoptosis | 10 | 59 | 1.53 |
| Hedgehog signaling pathway | 8 | 51 | 1.13 |
| Leukocyte transendothelial migration | 13 | 90 | 1.12 |
| Wnt signaling pathway | 15 | 110 | 0.97 |
| Adhesion junction | 9 | 64 | 0.84 |
| Focal adhesion | 17 | 133 | 0.75 |
| T-cell receptor signaling pathway | 9 | 73 | 0.42 |
| Glycan structures-biosynthesis 1 | 9 | 87 | −0.14 |
| Calcium signaling pathway | 14 | 136 | −0.2 |
| Insulin signaling pathway | 9 | 97 | −0.5 |
| MAPK signaling pathway | 16 | 189 | −1.08 |
| Regulation of actin cytoskeleton | 9 | 125 | −1.33 |
| Neuroactive ligand-receptor interaction | 18 | 223 | −1.37 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; DSS, dextran sodium sulfate.
To obtain novel insight into interrelated biological processes and signaling networks involved in the DSS response, we performed network analysis using Ingenuity software. Ingenuity network analysis allowed us to identify the functional classification and physical, transcriptional, and enzymatic interactions between genes and their molecules, which are mined from peer-reviewed scientific publications. Network analysis performed on the 1,609 genes identified revealed 55 networks with at least 9 focus molecules and a score over 5, as shown in Supplemental Table S9.
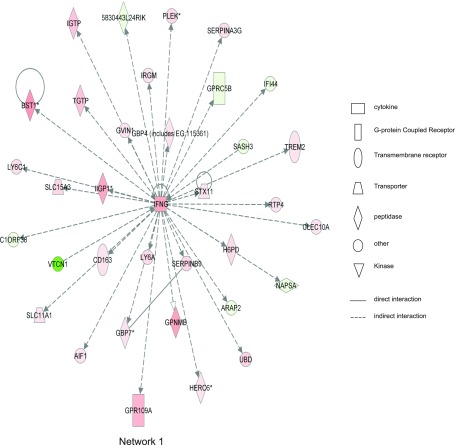
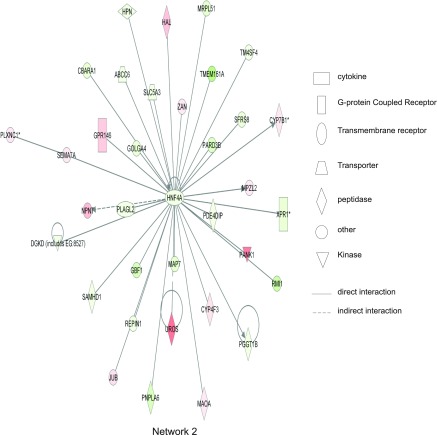
Ingenuity network analysis also revealed that inflammation-related genes changed greatly, such as IL-6, IFN-γ, chemokine (C-C motif) receptor 2, and matrix metalloproteinase 3 (Mmp3). IFN-γ, IL-6, and others were found to be located in the center of the network, demonstrating that they exert strong influence over other molecules. Figure 6 shows the first network identified, which had a score of 48 and contained all 35 focus molecules contained within that network [many of which have been implicated in colitis based on previous studies (e.g., IFN-γ, STX1, IRGM, CD163, LY6A, AIF1; Refs. 13, 18–20, 30, 35)]. The top biological functions of network 1 are associated with antigen presentation, cell morphology, and cell-cell signaling and interaction. Figure 7 illustrates network 2, with top biological functions of genetic disorders, neurological disease, and amino acid metabolism, which also had a score of 48 with all 35 focus molecules involved. However, several novel genes are found within this network with no known or poorly understood roles in colitis such as the inflammation-related gene HNF4A (23), the colon cancer-related gene Map7 (3), innate immune response-related SAMHD1 (40), mitochondrial ribosomal protein-related MRPL51 (21), and antigen presentation-related ABCC6 (2). Together, these data demonstrate that Ingenuity network analysis reveals previously unknown relationships among diverse genes identified in our global expression profile studies that would not have been identified with more traditional pathway analysis.
Fig. 6.
Ingenuity network 1 from DSS-mediated changes in gene expression. Of 1,609 differentially expressed genes, a total of 55 statistically significant networks were identified. Network 1 has a score of 48 and involved all 35 genes contained within that network. Major biological functions of network 1 are antigen presentation, cell morphology, and cell-cell signaling and interaction. Interferon γ is centrally located within this network, indicating a high degree of relationships with this molecule and all others. Red shading indicates that gene expression is greater than at day 0; green shading means that gene expression is less than at day 0. Symbol legend on right indicates the nature of the protein product and the type of relationship.
Fig. 7.
Ingenuity network 2 from DSS-mediated changes in gene expression. Ingenuity network 2 has a score of 48 with all 35 genes involved. Major biological functions of network 2 are genetic disorders, neurological disease, and amino acid metabolism. HNF4A is centrally located within this network, indicating a high degree of relationships between this molecule and others. Red shading indicates that gene expression is greater than at day 0; green shading means that gene expression is less than at day 0. Symbol legend on right indicates the nature of the protein product and the type of relationship.
Comparison of DSS colitis and UC patient microarray gene expression.
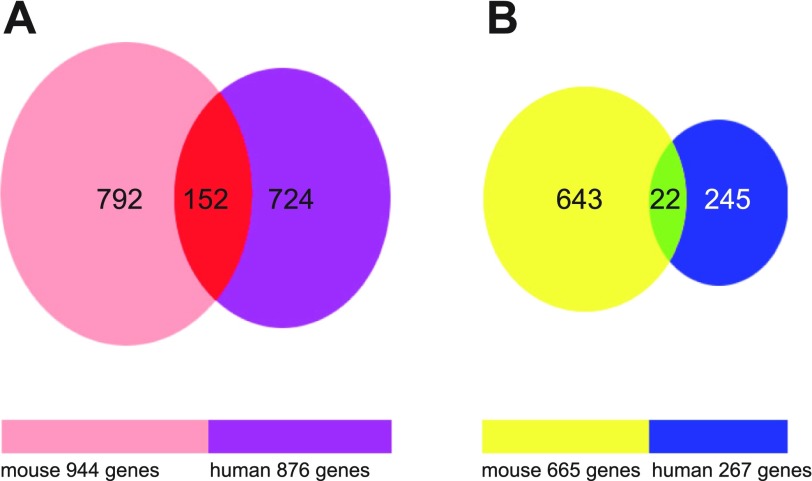
While the DSS-induced colitis model is widely used, relatively little is known of the differences and similarities between the DSS model and UC. Previous articles have attempted to address this question with smaller, more focused arrays that were also less well annotated compared with currently available arrays (7, 41). Recent refinements in annotation and array coverage make species comparison approaches much more informative than those of the past. Therefore, we next compared our identified genes in the DSS model to those of tissue specimens from UC patients in a study that used a similar highly annotated and comprehensive human Affymetrix array. Data from the Wu et al. study (47) reported that 876 genes were significantly increased in active UC patients and 267 genes were significantly decreased (for a combined total of 1,143 genes). These findings are comparable with our 1,609 genes identified from the DSS colitis model. Figure 8 shows Venn diagrams of gene expression similarity between the DSS model and UC patients. Interestingly, 152 genes were found to be upregulated in a similar fashion in either DSS colitis or UC patients (Fig. 8A). Supplemental Table S10 lists all of the 152 similarly upregulated genes along with the fold change in gene expression. Conversely, only 22 genes were similarly downregulated in DSS colitis and UC patients (Fig. 8B). Table 4 lists all of the 22 similarly downregulated genes along with the fold change in gene expression. These data reveal that the majority of differentially expressed genes during both DSS colitis and UC are upregulated, while a lesser number are downregulated. Interestingly, these data identify a greater number of similarly regulated genes than previously reported (41, 29).
Fig. 8.
Venn diagram illustration of gene expression similarity between DSS-induced colitis and ulcerative colitis (UC) patient sample microarray data. A: 944 genes were upregulated at day 6 in DSS colitis compared with 876 upregulated genes from UC patients; 152 of these genes were similarly upregulated between both data sets. B: 665 genes were downregulated at day 6 during DSS colitis compared with 267 downregulated genes from UC patients; 22 of these genes were similarly downregulated between both data sets.
Table 4.
Similarly downregulated genes between DSS colitis and UC patients
| Gene Name | Mouse Gene Accession No. | Human Homolog Gene Accession No. | DSS/Control | UC/Control |
|---|---|---|---|---|
| Activin A receptor, type IC | BB432539 | NM_145259 | −2.27 | −2.22 |
| Alcohol dehydrogenase 1 (class I) | BC013477 | NM_000669 | −2.08 | −8.33 |
| Ankyrin 3, epithelial | BC021657 | NM_020987 | −2 | −2.08 |
| Aquaporin 8 | NM_007474 | NM_001169 | −2.33 | −14.29 |
| Calcium/calmodulin-dependent protein kinase ID | BG071931 | AA835485 | −3.13 | −2.08 |
| Dopa decarboxylase | AF071068 | NM_000790 | −2.33 | −2.7 |
| Fibroblast growth factor receptor 3 | NM_008010 | NM_000142 | −2.22 | −3.57 |
| Forkhead box A2 | NM_010446 | AB028021 | −2.56 | −2.13 |
| Meprin 1α | AI098089 | NM_005588 | −2.33 | −2.27 |
| Peroxiredoxin 6 | AW319648 | NM_004905 | −3.13 | −2 |
| Peroxisome proliferative activated receptor, γ, coactivator 1α | BB745167 | NM_013261 | −5.56 | −2.78 |
| Phosphoenolpyruvate carboxykinase 1, cytosolic | AW106963 | NM_002591 | −3.33 | −6.67 |
| Prolactin receptor | M22958 | AA843963 | −1.75 | −2.44 |
| Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6A | AF288666 | AB002438 | −5 | −2.13 |
| Solute carrier family 26 (sulfate transporter), member 2 | NM_007885 | AI025519 | −2.78 | −5 |
| Solute carrier family 4 (anion exchanger), member 4 | NM_018760 | NM_003759 | −2.33 | −2.22 |
| Special AT-rich sequence binding protein 2 | AF319623 | AB028957 | −2.08 | −2.38 |
| Sulfotransferase family 1A, phenol-preferring, member 1 | AK002700 | U37025 | −2.04 | −2.7 |
| Transient receptor potential cation channel, subfamily M, member 6 | BC022929 | BF447669 | −7.14 | −4 |
| UDP-GlcNAc:βGal β-1,3-N-acetylglucosaminyltransferase 7 | BF578266 | CA503291 | −2.63 | −3.13 |
| Vasoactive intestinal peptide receptor 1 | BF224468 | NM_004624 | −2.04 | −2.44 |
| WAP four-disulfide core domain 2 | AF334269 | NM_006103 | −3.45 | −3.23 |
DSS colitis array data from day 6 against day 0 were used to generate fold downregulation differences to compare with clinical ulcerative colitis (UC) specimen data.
DISCUSSION
It is now well appreciated that IBD etiology involves several different pathological factors ranging from genetic susceptibility to microbial flora and inflammatory exacerbates. As such, it is becoming increasingly clear that future therapeutic interventions may benefit from targeting multiple mediators involved in disease pathogenesis. However, there is a paucity of information regarding genomewide changes in gene expression during either clinical IBD or experimental colitis over time. To better understand the nature of gene expression profile changes, we examined temporal changes in genomewide expression patterns in the DSS colitis model and compared these results with recent findings from whole genome array profiles of active disease in UC patients (9). Importantly, all previous gene array studies of experimental colitis models were performed with arrays containing smaller and less well-annotated probe sets, performed with different experimental colitis models, or performed on specimens harvested at the end of the disease model (7, 41). We found 1,609 genes that were significantly altered in a temporal manner by DSS treatment, and these genes could be grouped into 55 separate functional networks based on peer-reviewed publications at the present time. Interestingly, these data showed that the gene expression changes are not linear; rather, they are multidimensional, involving diverse changes in gene expression groups over time. Finally, KEGG pathway analysis revealed that 25 classical pathways were differentially affected during the colitis induction, with 380 genes showing significant changes in expression. However, Ingenuity network analysis revealed a large number of novel functional networks that have not been previously considered during DSS disease pathogenesis.
Segregation of temporal gene expression changes revealed unique results demonstrating complex patterns of cytopathological changes during DSS colitis. Data from the expression class of genes that continually increase (group A) reveal that innate immune response mediators are quickly induced, with chemokines, chitinase, and epithelial growth regulatory molecules primarily affected at day 2. Inflammatory activation is increased further by day 4, with significant upregulation of C-type lectin, integrin, and immunoglobulin cell adhesion molecule expression, which critically regulate leukocyte recruitment and activation. In addition, changes in angiogenic gene expression become evident with increased expression of extracellular matrix proteins, matrix metalloproteinases, and endothelial cell signaling mediators [e.g., endothelial cell-specific chemotaxis regulator/endothelial cell-specific molecule 2 (Ecscr/ECSM2) and ephrin A2]. At day 6 multiple biological targets are now affected, with changes in gene expression modulating inflammation, coagulation, angiogenic, and tissue repair responses. Conversely, data from the expression class of genes that continually decrease (group D) show significant loss of genes responsible for controlling water and ion transport, intracellular signal transduction regulation, antioxidant defense, mitochondrial function, cell motility, and transcriptional activation. Together, these data strongly suggest that DSS proceeds through initial activation of innate inflammatory responses transitioning to changes in vascular architecture and function with a later repair response. These changes are likely affected by loss of gene expression involved in maintaining tissue homeostasis; however, additional studies will be necessary to determine how these responses influence one another.
Our laboratory has previously reported (11, 12) that increased angiogenic activity contributes to DSS and T-cell-mediated experimental colitis that is highly linked with inflammatory activity. Moreover, work from our lab (10, 11, 22) and others (15–17) has demonstrated similar features in clinical IBD specimens. The mechanisms by which pathological angiogenesis contributes to colitis disease are complex and diverse depending on the model system studied (11). Importantly, only a few known angiogenesis-associated genes have been previously identified (11). Here we identified several novel genes that were differentially expressed and have been implicated in regulating angiogenic activity and vascular function, such as Ecscr/ECSM2, endothelin receptors A and B, ephrin A2, ephrin B1 and B2, EphA1, follistatin, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), and quaking (6, 24, 26–28 32, 37, 45, 46). Moreover, we identified (11, 12) several inflammatory molecules that have been previously associated with angiogenesis during inflammatory disease states (e.g., P-selectin, β2-integrin, VCAM-1, versican, and MMP2). However, to our knowledge, we are the first to report differential expression of Ecscr/ECSM2, Eph/Ephrins, follistatin, quaking, and LYVE-1 with experimental colitis, which provides important new insight into pathological angiogenesis during DSS colitis.
By comparing our gene array data with those from UC patients, we found that the RNA binding protein Quaking is similarly upregulated in both the DSS colitis model and UC patients. Quaking belongs to the evolutionarily conserved RNA protein binding family that contains a STAR domain along with P and Y motifs (9). Quaking is expressed in multiple cell types but is best understood for its role in regulating axon myelination, and its defective function is associated with demyelinating neurological disorders (9). Interestingly, genetic deletion of Quaking is embryonic lethal, which appears to be due to dysfunctional visceral endoderm function, which is required for normal vascular development (4, 37). These findings suggest that quaking may play an important role in governing vascular function and remodeling during chronic inflammation observed during colitis. Moreover, recent studies have shown that Quaking is normally expressed in the colon epithelium while downregulated or absent in some colon cancers, suggesting that its expression may play a role in modulating colon cancer cell proliferation (49). In the DSS model system, upregulation of Quaking expression likely leads to higher levels of Quaking protein that may simultaneously inhibit epithelial cell proliferation while driving angiogenesis and vascular remodeling. Further evaluation of Quaking protein expression by various cell types during the development of experimental colitis will be necessary to better understand the role of this molecule during colitis.
Experimental models of colitis have proven useful as tools to better understand pathological progression of disease (38, 39). While no experimental colitis model fully replicates all pathological processes, the DSS colitis model has been widely used as a reproducible model of acute colitis with ulceration (12, 14, 44). With the use of microarray technology, previous studies have been reported in various experimental colitis models documenting diverse changes in gene expression. Brudzewsky et al. (7) used the T-cell transfer SCID mouse colitis model and found that 152 genes were significantly changed. This is a relatively small number of changes compared with our results, which is likely influenced by the MOE 430A array used (only 22,626 probe sets) to test gene expression differences and only one time point studied (12 wk after transfer of activated T cells). In contrast, Rivera et al. (41) used a rat microarray containing even fewer genes (1,252 genes) with the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model. After 72 h of TNBS treatment, they found that 395 genes were differentially regulated between experimental and control groups (41). Finally, Martinez-Augustin et al. (33) used the Affymetrix Rat Expression Array 230 2.0 system to test the TNBS-induced colitis model at 2, 5, 7 and 14 days after the TNBS induction and found that the expression of 5,752 genes were significantly modified. This last study is consistent with our findings and clearly highlights the impact of array composition, model studied, and time course of study. Nonetheless, comparison of array data from the SCID T-cell transfer study, TNBS study, our DSS study, and UC patients' microarray data revealed that S100A8 and S100A9 mRNA increased greatly, suggesting that these two molecules may be useful as biomarkers of colitis. Clearly, additional cross-comparison analysis of these studies is warranted and may reveal additional novel targets or markers for the development of colitis.
In summary, we found that temporal genomewide gene expression analysis of the DSS colitis model compared with similar array studies of human UC specimens identified numerous genes that are similarly up- and downregulated. We have also identified several novel genes and functional biological networks associated with experimental colitis that were previously unknown. Together, these data suggest that multiple changes in gene expression likely underlie the progression of colitis, which substantiates the notion that combination therapeutic approaches may be useful for clinical management while also identifying target candidate genes for intervention and biomarkers.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-43875-18, project 4, and the PPG animal model and histopathology core laboratories.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Sawyer Bonsib for his editorial assistance.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Abdelbaqi M, Chidlow JH, Matthews KM, Pavlick KP, Barlow SC, Linscott AJ, Grisham MB, Fowler MR, Kevil CG. Regulation of dextran sodium sulfate induced colitis by leukocyte beta2 integrins. Lab Invest 86: 380–390, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Bergen AA, Plomp AS, Hu X, de Jong PT, Gorgels TG. ABCC6 and pseudoxanthoma elasticum. Pflügers Arch 453: 685–691, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Blum C, Graham A, Yousefzadeh M, Shrout J, Benjamin K, Krishna M, Hoda R, Hoda R, Cole DJ, Garrett-Mayer E, Reed C, Wallace M, Mitas M. The expression ratio of Map7/B2M is prognostic for survival in patients with stage II colon cancer. Int J Oncol 33: 579–584, 2008. [PMC free article] [PubMed] [Google Scholar]
- 4. Bohnsack BL, Lai L, Northrop JL, Justice MJ, Hirschi KK. Visceral endoderm function is regulated by quaking and required for vascular development. Genesis 44: 93–104, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics 3: 77–97, 2009. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis 7: 17–28, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Brudzewsky D, Pedersen AE, Claesson MH, Gad M, Kristensen NN, Lage K, Jensen T, Tommerup N, Larsen LA, Knudsen S, Tumer Z. Genome-wide gene expression profiling of SCID mice with T-cell-mediated colitis. Scand J Immunol 69: 437–446, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature 437: 1032–1037, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Chenard CA, Richard S. New implications for the QUAKING RNA binding protein in human disease. J Neurosci Res 86: 233–242, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Chidlow JHz, Jr, Greer JJ, Anthoni C, Bernatchez P, Fernandez-Hernando C, Bruce M, Abdelbaqi M, Shukla D, Granger DN, Sessa WC, Kevil CG. Endothelial caveolin-1 regulates pathologic angiogenesis in a mouse model of colitis. Gastroenterology 136: 575–584, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chidlow JH, Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG. Differential angiogenic regulation of experimental colitis. Am J Pathol 169: 2014–2030, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chidlow JH, Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol 293: G5–G18, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Cimmino L, Martins GA, Liao J, Magnusdottir E, Grunig G, Perez RK, Calame KL. Blimp-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J Immunol 181: 2338–2347, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin 28: 1450–1459, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 130: 2060–2073, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Danese S, Sans M, Spencer DM, Beck I, Donate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56: 855–862, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. Am J Pathol 172: 1457–1466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dema B, Fernandez-Arquero M, Maluenda C, Polanco I, Figueredo MA, de la Concha EG, Urcelay E, Nunez C. Lack of association of NKX2–3, IRGM, and ATG16L1 inflammatory bowel disease susceptibility variants with celiac disease. Hum Immunol 70: 946–949, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Ferens WA, Hovde CJ. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect Immun 68: 4462–4469, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flanagan K, Modrusan Z, Cornelius J, Chavali A, Kasman I, Komuves L, Mo L, Diehl L. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J Immunol 180: 3874–3881, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Gan X, Kitakawa M, Yoshino K, Oshiro N, Yonezawa K, Isono K. Tag-mediated isolation of yeast mitochondrial ribosome and mass spectrometric identification of its new components. Eur J Biochem 269: 5203–5214, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Goebel S, Huang M, Davis WC, Jennings M, Siahaan TJ, Alexander JS, Kevil CG. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 290: G648–G654, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Guo H, Gao C, Mi Z, Zhang J, Kuo PC. Characterization of the PC4 binding domain and its interactions with HNF4alpha. Biochem J 141: 635–640, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res 312: 642–650, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol 104: 2100–2109, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS 112: 526–538, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Knowles J, Loizidou M, Taylor I. Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol 3: 309–314, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Kozian DH, Ziche M, Augustin HG. The activin-binding protein follistatin regulates autocrine endothelial cell activity and induces angiogenesis. Lab Invest 76: 267–276, 1997. [PubMed] [Google Scholar]
- 29. Kristensen NN, Olsen J, Gad M, Claesson MH. Genome-wide expression profiling during protection from colitis by regulatory T cells. Inflamm Bowel Dis 14: 75–87, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Liu S, Russo PA, Baldassano RN, Sullivan KE. CD68 expression is markedly different in Crohn's disease and the colitis associated with chronic granulomatous disease. Inflamm Bowel Dis 15: 1213–1217, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126: 1504–1517, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Ma F, Zhang D, Yang H, Sun H, Wu W, Gan Y, Balducci J, Wei YQ, Zhao X, Huang Y. Endothelial cell-specific molecule 2 (ECSM2) modulates actin remodeling and epidermal growth factor receptor signaling. Genes Cells 14: 281–293, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Martinez-Augustin O, Merlos M, Zarzuelo A, Suarez MD, de Medina FS. Disturbances in metabolic, transport and structural genes in experimental colonic inflammation in the rat: a longitudinal genomic analysis. BMC Genomics 9: 490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mori M, Stokes KY, Vowinkel T, Watanabe N, Eirod JW, Harris NR, Lefer DJ, Hibi T, Granger DN. Colonic blood flow responses in experimental colitis: time course and underlying mechanisms. Am J Physiol Gastrointest Liver Physiol 289: G1024–G1029, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Morohashi T, Iwabuchi K, Watano K, Dashtsoodol N, Mishima T, Nakai Y, Shimada S, Nishida R, Fujii S, Onoe K. Allograft inflammatory factor-1 regulates trinitrobenzene sulphonic acid-induced colitis. Immunology 110: 112–119, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano S, Ohara S, Kubota T, Saigenji K, Hotta K. Compensatory response of colon tissue to dextran sulfate sodium-induced colitis. J Gastroenterol 34: 207–214, 1999. [DOI] [PubMed] [Google Scholar]
- 37. Noveroske JK, Lai L, Gaussin V, Northrop JL, Nakamura H, Hirschi KK, Justice MJ. Quaking is essential for blood vessel development. Genesis 32: 218–230, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol 296: G135–G146, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol Med 9: 218–222, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, Ali M, Gornall H, Couthard LR, Aeby A, Attard-Montalto SP, Bertini E, Bodemer C, Brockmann K, Brueton LA, Corry PC, Desguerre I, Fazzi E, Cazorla AG, Gener B, Hamel BC, Heiberg A, Hunter M, van der Knaap MS, Kumar R, Lagae L, Landrieu PG, Lourenco CM, Marom D, McDermott MF, van der Merwe W, Orcesi S, Prendiville JS, Rasmussen M, Shalev SA, Soler DM, Shinawi M, Spiegel R, Tan TY, Vanderver A, Wakeling EL, Wassmer E, Whittaker E, Lebon P, Stetson DB, Bonthron DT, Crow YJ. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet 41: 829–832, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rivera E, Flores I, Rivera E, Appleyard CB. Molecular profiling of a rat model of colitis: validation of known inflammatory genes and identification of novel disease-associated targets. Inflamm Bowel Dis 12: 950–966, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Stevens C, Walz G, Singaram C, Lipman ML, Zanker B, Muggia A, Antonioli D, Peppercorn MA, Strom TB. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci 37: 818–826, 1992. [DOI] [PubMed] [Google Scholar]
- 43. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 20: 495–549, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Tlaskalova-Hogenova H, Tuckova L, Stepankova R, Hudcovic T, Palova-Jelinkova L, Kozakova H, Rossmann P, Sanchez D, Cinova J, Hrncir T, Kverka M, Frolova L, Uhliq H, Powrie F, Bland P. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann NY Acad Sci 1051: 787–798, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Verissimo AR, Herbert JM, Heath VL, Legg JA, Sheldon H, Andre M, Swain RK, Bicknell R. Functionally defining the endothelial transcriptome, from Robo4 to ECSCR. Biochem Soc Trans 37: 1214–1217, 2009. [DOI] [PubMed] [Google Scholar]
- 46. Verma A, Bhattacharya R, Remadevi I, Li K, Pramanik K, Samant GV, Horswill M, Chun CZ, Zhao B, Wang E, Miao RQ, Mukhopadhyay D, Ramchandran R, Wilkinson GA. Endothelial cell-specific chemotaxis receptor (ecscr) promotes angioblast migration during vasculogenesis and enhances VEGF receptor sensitivity. Blood 115: 4614–4622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2alpha. Gastroenterology 135: 1624–1635, 2008. [DOI] [PubMed] [Google Scholar]
- 48. Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4: e6073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang G, Fu H, Zhang J, Lu X, Yu F, Jin L, Bai L, Huang B, Shen L, Feng Y, Yao L, Lu Z. RNA-binding protein quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology 138: 231–240, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.