Abstract
Vitamin A (retinol) is an essential precursor for the production of retinoic acid (RA), which in turn is a major regulator of gene expression, affecting cell differentiation throughout the body. Understanding how vitamin A nutritional status, as well as therapeutic retinoid treatment, regulates the expression of retinoid homeostatic genes is important for improvement of dietary recommendations and therapeutic strategies using retinoids. This study investigated genes central to processes of retinoid uptake and storage, release to plasma, and oxidation in the liver of rats under steady-state conditions after different exposures to dietary vitamin A (deficient, marginal, adequate, and supplemented) and acutely after administration of a therapeutic dose of all-trans-RA. Over a very wide range of dietary vitamin A, lecithin:retinol acyltransferase (LRAT) as well as multiple cytochrome P-450s (CYP26A1, CYP26B1, and CYP2C22) differed by diet and were highly correlated with one another and with vitamin A status assessed by liver retinol concentration (all correlations, P < 0.05). After acute treatment with RA, the same genes were rapidly and concomitantly induced, preceding retinoic acid receptor (RAR)β, a classical direct target of RA. CYP26A1 mRNA exhibited the greatest dynamic range (change of log 26 in 3 h). Moreover, CYP26A1 increased more rapidly in the liver of RA-primed rats than naive rats, evidenced by increased CYP26A1 gene expression and increased conversion of [3H]RA to polar metabolites. By in situ hybridization, CYP26A1 mRNA was strongly regulated within hepatocytes, closely resembling retinol-binding protein (RBP)4 in location. Overall, whether RA is produced endogenously from retinol or administered exogenously, changes in retinoid homeostatic gene expression simultaneously favor both retinol esterification and RA oxidation, with CYP26A1 exhibiting the greatest dynamic change.
Keywords: diet, gene expression, homeostasis, localization
all-trans-retinoic acid (RA) is generated in vivo from the oxidation of vitamin A (retinol). RA functions as the physiological ligand for nuclear hormone receptors of the retinoic acid receptor (RAR) family, RARα, β, and γ (5, 61). RA is well known as a potent inducer of cell differentiation (1, 46). Clinically, it has important applications in the treatment of certain leukemias, other cancers, and hyperproliferative disorders (1, 13, 43, 57, 60). A large number of genes are either proven or putative direct targets of RA and related retinoids, and numerous other genes are regulated by RA in a physiological but probably indirect manner (1, 3). Overall, RA is important in many biological processes including cell fate determination, renewal, maturation, cell-cell interactions, embryogenesis, and metabolic regulation (1, 3).
It is well understood that vitamin A status in humans ranges widely as a result of differences in dietary intake (47). However, there is relatively little information on the patterns of retinoid homeostatic genes that are associated with these nutritional-physiological states. Currently, there is interest in using biochemical and molecular data from dietary studies in risk assessment models to examine dose response and thresholds (19), and vitamin A has been considered an exemplary nutrient for such an analysis (44). Retinoid homeostasis is understood to be complex, consisting of processes that include the reversible removal of excess dietary retinol into storage as retinyl esters, catalyzed by lecithin:retinol acyltransferase (LRAT); the transport of retinol in plasma by retinol-binding protein (RBP)4; the dynamic recycling of retinol between plasma, liver, and extrahepatic organs (7); the production of bioactive retinoids (30); and the irreversible degradation of retinol and RA when they are present in excess (8, 24). The liver is a central player in vitamin A homeostasis, including retinol esterification, uptake of plasma retinol bound to RBP4 and of RA bound to albumin, and oxidation of RA to polar compounds that mostly are excreted in bile (45). However, it is still not well understood how hepatic RA metabolism is modulated in response to variations in dietary vitamin A intake and after treatment with RA. We previously reported (8, 9) that the conversion of RA to polar metabolites is increased in the liver of rats fed a high-vitamin A diet and after treatment with RA. Retinoid catabolism is also induced when RA is administered at pharmacological levels (41, 55, 64). Currently, evidence suggests that multiple cytochrome P-450 (CYP) genes may play a role in RA oxidation, including CYP26A1 and CYP26B1 encoding RA-4-hydroxylase activity (6, 24, 52). Recently we reported (36) that genes of the CYP2C family (CYP2C22 in the rat and CYP2C8 and CYP2C9 in human hepatocytes) respond directly to RA. Although the CYP2C family is better known for metabolizing polyunsaturated fatty acids, drugs, and xenobiotics (4, 17, 54), the CYP2C family may also facilitate the oxidation of RA (2, 14, 25, 26, 28, 34, 62). It is unknown whether the response of multiple retinoid homeostatic genes in the liver occurs coordinately and in a well-correlated manner. The aim of the present study was to determine the responses at the gene transcript level of several retinoid homeostatic genes over a wide range of steady-state dietary conditions and after acute treatment with RA. These genes include LRAT, CYP26A1 as the putative major regulator of RA oxidation (32, 53, 59, 63), CYP26B1, and CYP2C22, as well as the transport protein RBP4. Our results provide evidence for the concomitant and significantly correlated regulation of multiple retinoid homeostatic genes in the liver, while showing that CYP26A1 is the most dynamically regulated of the retinoid-metabolizing CYP genes and that the induction of its expression can be “primed” by prior exposure to RA. We also provide evidence for the cellular location of the CYP26A1 transcript in the liver because, first, it plays a central role in RA catabolism during normal homeostasis (52, 53) and, second, CYP26A1 is believed to be the major contributor to retinoid tolerance when RA is used therapeutically (35). Thus understanding its localization may facilitate studies to target CYP26A1 for inhibition.
MATERIALS AND METHODS
Rats, diets, and experimental treatments.
All procedures for animal care and use were approved by the Institutional Animal Care and Use Committee of Pennsylvania State University. Rats were housed in a room maintained at 22°C with a 12:12-h dark-light cycle, and food and water were freely available. For the study of steady-state vitamin A status (experiment 1) and RA administration (experiment 2), lactating female Sprague-Dawley rats with 12 female pups (purchased from Charles River Laboratories, Wilmington, MA) were fed a vitamin A-deficient purified diet [AIN-93G diet (37), prepared by Research Diets, New Brunswick, NJ] to reduce the transfer of vitamin A in milk from mother to pups before the start of the study. In experiment 1, the offspring were fed from weaning the same diet modified to contain vitamin A at one of four levels: 0 (vitamin A deficient; n = 16), 0.4 mg retinol/kg diet (vitamin A marginal; n = 4), 4 mg retinol/kg (vitamin A adequate control; n = 4), or 100 mg retinol/kg (vitamin A supplemented; n = 4). All rats were studied at 8 wk of age. Rats were euthanized by carbon dioxide asphyxiation, and blood and liver were collected rapidly and frozen in liquid nitrogen for storage at −80°C before analysis (8, 66). Plasma total retinol was analyzed by HPLC after saponification (9). In experiment 2 (16-h RA kinetic study), 16 female vitamin A-deficient rats were treated with ∼100 μg of all-trans-RA (59) for times of 0 (vehicle only), 3, 6, 10, or 16 h (n = 3 or 4/group). Tissues were collected and RNA was prepared in the same manner as in experiment 1. For experiment 3 (90-min “first-pass” kinetic study), 23 female rats were purchased at 6 wk of age and fed a stock rodent diet. When rats were 8 wk old they were randomly assigned to either a control (naive) group (n = 11, experiments 3 and 4) or an RA-primed group (n = 12, experiment 4). Rats in the RA-primed group received an oral dose of 500 μg of all-trans-RA in ∼30 μl of vehicle (vegetable oil-5% ethanol), whereas rats in the naive group received an equal amount of vehicle only. Food was removed immediately. Sixteen hours after priming, each rat was lightly anesthetized by isoflurane-oxygen inhalation and treated with ∼20 μg all-trans-RA bound to albumin (10 μg RA per 100 g body wt, similar to Refs. 8, 9) injected into the exposed left common iliac vein (8, 9). The incision was closed with a surgical staple. The rats were allowed to recover from the anesthesia. Rats were killed at 0 min (vehicle injection) and 30, 60, and 90 min (n = 3/group) after injection of the RA test dose. Blood and liver tissue were collected as in experiments 1 and 2.
RNA preparation and analysis.
In the present work, we used an array procedure as a multiplex analytical tool to compare the expression of the genes of main interest: CYP26A1 (154985, 1387583_at); CYP26B1 (312495, 1384392_at); CYP2C22 [also referred to as CYP2C70 (171518, 1387949_at)]; LRAT (64047, 1368570_at); RARβ (24706, 1376755_at); POR (NADPH oxidoreductase) (29441, NM_031576); RBP4 (plasma retinol-binding protein) (25703, 1371762_at) (50), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (24383, 1367557_s_at) as a housekeeping gene. Array data have been deposited with NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) as accession number GSE24104.
RNA was prepared from each individual rat liver by TRIzol extraction followed by repurification on an RNeasy column (Qiagen, Valencia, CA), checked for quality by an A260-to-A280 ratio > 1.64, quantified, and diluted to 0.2 μg/ml (66). Ten microliters each was shipped on dry ice to the National Cancer Institute (NCI) Microarray Facility (Frederick, MD). The RNA samples from the various treatment groups were coded before shipment so that samples analyzed together would represent different treatment groups. cDNA targets for the Affymetrix RAE230_2 arrays were synthesized, labeled, purified, and prepared for analysis as we have described previously (33). The arrays were hybridized overnight at 45°C, washed and stained with streptavidin-phycoerythrin, and scanned at 488 nm and 11-μm resolution. Data were acquired with Genechip Operating Software (GCOS, Affymetrix) and uploaded onto the NCI website mAdb (http://nciarray.nci.nih.gov/). Data from experiments 1–3 above were analyzed in our laboratory by setting the signal floor value to 1 and centering each array to the median signal intensity. The results were expressed as log2-transformed signal intensities, and the log2 of the median intensity for each array was set to 0. For experiment 4, RNA samples were analyzed by quantitative real-time RT-PCR (qRT-PCR) using a final volume of 25 μl of PCR product (Real Time PCR iQ SYBR Green Supermix system, Bio-Rad) and a DNA Engine Opticon 2 System (MJ Research), and 18S RNA or GAPDH was analyzed as an internal control (33).
Plasmid DNA for preparation of RNA probes.
Rat CYP26A1 cDNA covering from nucleotide number 300 to the end of the 3′ end (insert size was ∼1,500 bp based on accession no. AF439720) in pGEM-T Easy vector (Promega) was cut with EcoRI, blunt ended, and subcloned into the SmaI site of pGEM-4 vector. A pGEM4-rCYP26A1 clone was then linearized with EcoRI or HindIII and used as a template to synthesize antisense or sense RNA probes with T7 or SP6 RNA polymerase, respectively. The rat RBP4 cDNA (548 bp) in pGEM4 vector (51) was linearized with either NarI or HindIII and used as the template to synthesize antisense or sense RNA probe by T7 or SP6 RNA polymerase, respectively. The BglI fragment (1,200 bp) of pRbA-1 containing the full rat β-actin cDNA (15, 31) was subcloned into pGEM3 vector, which was then linearized with either HindIII or EcoRI and used as template to synthesize antisense or sense RNA probe with either SP6 or T7 RNA polymerase, respectively. For α-smooth muscle actin (α-SMA) probe, the first-strand cDNA was first synthesized from total RNA of HSC-T6 rat stellate cells (58) and then used as the template to amplify rat α-SMA (NCBI accession no. NM_031004) by PCR in a reaction buffer containing GCTCTGGTGTGTGACAATGG as the sense primer and GACAGGCCAGGGCTAGAAG as the antisense primer with High Fidelity AccuePrime Taq DNA polymerase (Invitrogen) according to the protocol recommended by the manufacturer. The amplified DNA was purified by agarose gel electrophoresis and then cloned in pGEM-T Easy vector (Promega). The α-SMA cDNA was cut with EcoRI and then subcloned into pGEM-4 as described above. pGEM-4-rα-SMA was cut with either EcoRI or XbaI to linearize and then use T7 or SP6 to synthesize sense or antisense riboprobe, respectively. For each probe, a digoxigenin (DIG)-labeled sense or antisense RNA riboprobe was prepared with the DIG RNA Labeling Kit in a reaction with ribonucleotides including DIG-UTP (Roche Biotechnology). The labeled RNA was isolated, checked for size by ethidium bromide-stained agarose gel electrophoresis, and then treated in alkaline solution to prepare 100- to 150-base polynucleotide lengths as probe (18).
In situ hybridization.
Liver sections, 5–10 μm in thickness, were cut from frozen liver tissue blocks at −20°C, mounted on electrostatically charged slides (Fisher Scientific, Pittsburgh, PA), and then dried at 40°C for 5 min before storage at −80°C prior to use. For hybridization, the slides were first dipped into acetone for 5 min, dried, and then fixed with 4% paraformaldehyde in PBS as described previously (11). After prehybridization in 50% formamide at 60°C for 10 min, each slide was covered with 150 μl of hybridization solution containing 50% formamide, 10% dextran sulfate, and 1× Denhardt's solution with 50 ng of DIG-labeled riboprobe and hybridized at 42°C overnight (18). As previously described (36), the slides were washed, blocked, incubated with alkaline phosphatase-conjugated anti-DIG antibody in blocking buffer, and then washed and incubated with 5-bromo-4-chloro-3-indolyl phosphate (BCIP)-nitro blue tetrazolium (NBT) as synthetic substrate for alkaline phosphatase (Roche Biotechnology), with levamisole overnight. The slides were rinsed in Tris-EDTA (TE) buffer for at least 5 min, dried, and then evaluated under a digital color microscope in the Center for Quantitative Cell Analysis at Pennsylvania State University.
Enzyme assay.
Retinoic acid metabolizing activity was measured as described by Yamamoto et al. (64), with 200 μg of microsomal protein incubated with 45 nM 11,12-[3H(N)]retinoic acid (specific activity 1.96 TBq/nmol; PerkinElmer, Boston, MA) in 0.25 ml of buffer (pH 7.4) and an NADPH regenerating system (2.5 U glucose-6-phosphate dehydrogenase, 500 nmol NADP, and 0.5 μmol glucose-6-phosphate per incubation). The reaction was initiated by adding the [3H]RA, incubated at 37°C for 50 min, and terminated by the addition of 5 ml of chloroform-methanol (2:1, vol/vol). Solid-phase extraction on Supelclean LC-18 SPE columns (Supelco, Bellefonte, PA) was used to separate the parent compound, [3H]RA, from its oxidation products (8), with all-trans and 4-oxo-RA as standards to monitor separation, and fractions were dried and analyzed by liquid scintillation spectrometry.
Statistics.
Effects on gene expression of diet and time after treatment (experiments 1–3) were compared by one-way ANOVA on log2-transformed data. Experiment 4 (naive vs. RA-primed rats) was analyzed by two-way ANOVA. Differences between treatment groups were determined by least significant difference test (SuperAnova, Abacus Concepts). Linear regression analysis was performed with log2-transformed gene expression values or log10-transformed liver total retinol concentrations, except for enzyme assay results. For post hoc tests, P < 0.05 was considered significant.
RESULTS
Steady-state dietary vitamin A regulates multiple hepatic CYPs, LRAT, and RARβ.
Conditions were established to produce a wide range of vitamin A status in growing, healthy animals. To validate our dietary study, we first assessed liver vitamin A as the major indicator of vitamin A status (47). Liver total retinol differed among all of the groups (Fig. 1A), with vitamin A deficient << marginal < adequate (defined as the reference group) << supplemented (P < 0.01 between all groups). The liver of vitamin A-deficient rats had only 0.0035 times the concentration present in the vitamin A-adequate rats, while vitamin A-supplemented rats had 40.8 times more. Body weights did not differ (data not shown). Plasma total retinol exhibited more modest differences, as expected, with low retinol in the vitamin A-deficient rats (<0.2 μM, P < 0.05 vs. vitamin A-adequate rats). However, retinol was normal (>1 μM) in the vitamin A-marginal rats (Fig. 1B), indicating that their remaining liver vitamin A was still being mobilized to maintain plasma retinol. Plasma retinol was ∼30% higher in vitamin A-supplemented rats compared with the vitamin A-adequate control group but still within the normal range. Thus the dietary conditions employed in this study resulted in a wide range of vitamin A status that models that found in human populations (47).
Fig. 1.
Liver total retinol (A), plasma total retinol (B), and relative gene expression levels in liver (C–H) of rats fed vitamin A-deficient, -marginal, -adequate, and -supplemented diets from weaning to 8 wk of age. For C–H, the values shown are log2 of signal intensity, with median intensity for each array centered on 0. Data are means ± SE for each group; n = 5, 7, 6, and 2 rats, respectively. Differences between groups, P < 0.05, were determined by least significance difference test when the ANOVA value was P < 0.001 or lower. CYP, cytochrome P-450; LRAT, lecithin:retinol acyltransferase; RAR, retinoic acid receptor.
To compare hepatic gene expression, signal intensities for the genes of interest were transformed (log2 values), where the average intensity for each gene array is represented by a value of 0. The mean value for vitamin A-adequate rats was normalized to 1.00, and the relative values for the other diet groups were calculated compared to this reference group. Vitamin A status regulated the levels of several genes considered central to retinoid homeostasis. CYP26A1 (Fig. 1C) exhibited graded expression over a wide range of nearly 100-fold, as the relative expression was reduced to 0.23 in vitamin A-deficient rats and elevated 20.8 times in vitamin A-supplemented rats, both compared with the reference group. CYP26B1 (Fig. 1D) also exhibited graded expression, with lower expression in vitamin A-deficient and vitamin A-marginal rats compared with both vitamin A-adequate and -supplemented rats. However, the range of expression for CYP26B1 was less than for CYP26A1. CYP2C22 (Fig. 1E) was much higher than for either CYP26A1 or CYP26B1 in terms of log2 signal intensity, but nevertheless the relative expression of CYP2C22 was significantly reduced in the vitamin A-deficient group (0.034 times the reference level) and differed between vitamin-A marginal and -supplemented rats. Thus the range for this gene was also large, ∼70-fold between deficient and supplemented groups. LRAT (Fig. 1F) also exhibited graded expression with vitamin A intake, with a significant reduction in the vitamin A-deficient group and a modest elevation in the vitamin A-supplemented group. For RARβ (Fig. 1G), only the vitamin A-deficient group differed from other groups. GAPDH (Fig. 1H), monitored as a housekeeping gene, remained steady across all dietary treatments.
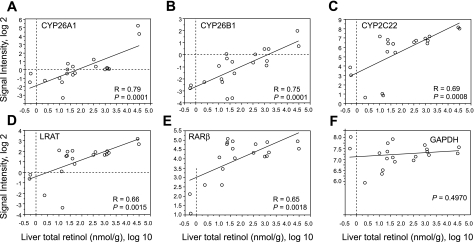
To further assess the relationship between vitamin A status and the expression of CYP26A1, CYP26B1, CYP2C22, LRAT, and RARβ in liver, linear regression analysis was performed with liver total retinol (log10) as the independent variable. The correlations were highest for CYP26A1 (Fig. 2A) and CYP26B1 (Fig. 2B) (both R = 0.76, P < 0.0001); however, liver total retinol was also significantly correlated with the level of CYP2C22, LRAT, and RARβ mRNAs (Fig. 2, C–E, all P < 0.005). Liver total retinol was not correlated with the housekeeping gene, GAPDH. Intergene correlation analysis between individual pairs of genes was also performed (Table 1). The transcript levels of all of these genes were strongly correlated, with the highest correlation coefficient between LRAT and CYP2C22 (R = 0.92, P < 0.0001).
Fig. 2.
Regression analysis for liver total retinol (log10) vs. gene expression signal intensity (all log2) in the liver of n = 20 rats fed vitamin A-deficient, -marginal, -adequate, or -supplemented diets from weaning to 8 wk of age.
Table 1.
Correlation matrix of CYP26A1, CYP26B1, CYP2C22, LRAT, and RARβ genes in steady state in liver of vitamin A-deficient, -marginal, -adequate, and -supplemented rats
| Gene | CYP26B1 | CYP2c22 | LRAT | RARβ |
|---|---|---|---|---|
| CYP26A1 | R = 0.60 | R = 0.67 | R = 0.70 | R = 0.52 |
| R2 = 0.36 | R2 = 0.45 | R2 = 0.49 | R2 = 0.27 | |
| P = 0.0054 | P = 0.0011 | P = 0.0006 | P = 0.0195 | |
| CYP26B1 | R = 0.72 | R = 0.71 | R = 0.62 | |
| R2 = 0.52 | R2 = 0.51 | R2 = 0.39 | ||
| P = 0.0187 | P = 0.0230 | P = 0.0851 | ||
| CYP2C22 | R = 0.922 | R = 0.81 | ||
| R2 = 0.85 | R2 = 0.66 | |||
| P = 0.0001 | P = 0.0001 | |||
| LRAT | R = 0.71 | |||
| R2 = 0.50 | ||||
| P = 0.0005 |
Data shown are R, R2, and P values for regression using log2 values, from n = 20 rats in experiment 1.
CYP, cytochrome P-450; LRAT, lecithin:retinol acyltransferase; RARβ, retinoic acid receptor β.
RA transiently induces multiple retinoid metabolic genes in vitamin A-deficient rats.
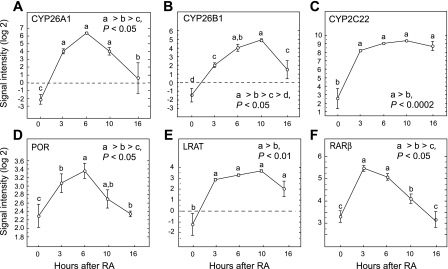
We assessed the kinetics of the response to exogenous RA in vitamin A-deficient rats chosen because they could produce very little RA endogenously. Each rat was treated with a single oral dose of RA, and gene expression was determined in animals killed over the next 16 h. Because the half-life for all-trans-RA had been shown to be ∼1.5 h in rats administered RA orally (12), essentially all of the RA that was administered can be expected to have been metabolized in the earlier part of the 16-h time course. CYP26A1 expression was increased significantly by 3 h after RA (Δlog2 signal intensity > 26), peaked at 6 h, and then declined by 16 h (Fig. 3A). CYP26B1 (Fig. 3B) increased with similar kinetics, but the fold increase was not as great as for CYP26A1. CYP2C22 expression started at a much higher value, similar to Fig. 1, yet it also increased very rapidly (Δlog2 > 25 in 3 h) (Fig. 3C), while in contrast to CYP26A1 and CYP26B1, the elevation in CYP2C22 was sustained to the end of the 16-h study, suggesting that this mRNA, once induced by RA, is relatively stable. We also noted that POR (NADPH oxidoreductase) was moderately induced by RA (Fig. 3D). The increase in POR, an electron donor to all CYP enzymes, concomitantly with CYP26A1, CYP26B1, and CYP2C22 suggests the possibility that increased POR activity could cooperate in increasing CYP-mediated enzymatic activity in the liver after treatment with RA, as a previous study reported that mouse embryos lacking POR exhibited impaired RA signaling (39).
Fig. 3.
Kinetics of gene expression in liver of vitamin A-deficient rats after acute administration of a single dose of 100 μg of all-trans-retinoic acid (RA) (ip; experiment 2) for CYP26A1 (A), CYP26B1 (B), CYP2C22 (C), POR (D), LRAT (E), and RARβ (F). Data are means ± SE for n = 6, 3, 3, 4, and 3 rats at 0, 3, 6, 10 and 16 h, respectively. Differences between groups, P < 0.05, were determined by least significance difference test when the ANOVA value was P < 0.001 or lower.
Concomitantly with increases in genes capable of RA oxidation, LRAT mRNA increased by 3 h and remained somewhat elevated up to 16 h (Fig. 3E). RARβ is considered a model of autoregulation through ligand-dependent nuclear receptor-mediated activation of the RARE in its promoter (10, 16). RARβ exhibited a rapid increase in 3 h that, however, was transient, with a return to initial intensity at 16 h (Fig. 3F). Overall, these data indicate that the capacity for RA oxidation can be upregulated very quickly, even in animals lacking stores of vitamin A, consistent with previous findings for [3H]RA metabolism in vitamin A-marginal rats (8). The increase in LRAT expression in animals depleted of retinol further supports the regulation of the LRAT gene by RA itself, which, in circumstances where vitamin A is available from the diet, would be produced endogenously by the oxidation of retinol.
Retinoid metabolic genes are induced under conditions of first-pass RA metabolism.
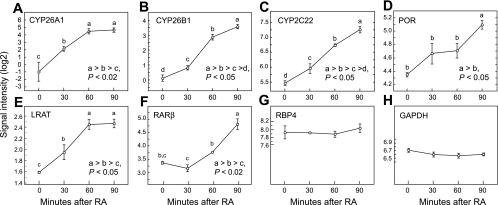
To further test the regulation of these genes by RA, we conducted experiment 3, in which gene expression was determined over a 90-min time study designed to resemble hepatic first-pass metabolism of RA. Previous research had shown that when RA was injected intravenously >90% was removed from the circulation within 3–5 min (8, 21), indicative of first-pass clearance. Under these conditions over one-fourth of the intravenously injected [3H]RA was taken up by the liver (8, 9, 21). Therefore, we asked whether RA induces very early changes in the expression of genes involved in retinoid metabolism, covering the period of time immediately after the hepatic uptake of RA. Vitamin A-adequate rats were treated with a single small test dose of RA, 20 μg/200 g rat, delivered intravenously bound to albumin, its physiological carrier, and liver was rapidly collected at t = 0 (mock dose) and at 30, 60, and 90 min after RA administration. For CYP26A1, CYP26B1, and CYP2C22 (Fig. 4, A–C) each transcript level increased significantly (P < 0.05), even at 30 min, and expression continued to increase up to the end of the 90-min study. The expression of POR also increased, but this was significant only at 90 min (P < 0.05, Fig. 4D). At the same time that the expression of genes implicated in RA oxidation increased, LRAT also increased significantly and with similar kinetics (Fig. 4E). By comparison, the expression of RARβ did not increase significantly until 90 min (Fig. 4F). RBP4 expression remained constant (Fig. 4G), as did GAPDH measured as a housekeeping gene (Fig. 4H). Thus in the liver of vitamin A-adequate rats the genes for CYP26A1, CYP26B1, CYP2C22, as well as LRAT, were all rapidly regulated by RA under conditions of first-pass hepatic metabolism, even more quickly than RARβ.
Fig. 4.
Kinetics of rapid changes in gene expression under conditions of first-pass hepatic metabolism of RA in vitamin A-adequate rats (experiment 3). Rats were treated with 20 μg of intravenous all-trans-RA. Data are for CYP26A1 (A), CYP26B1 (B), CYP2C22 (C), POR (D), LRAT (E), RARβ (F), retinol-binding protein (RBP)4 (G), and, as a control gene, GAPDH (H). Data are means ± SE for n = 3, 3, 3, and 2 for 0, 30, and 90 min, respectively. Differences between groups, P < 0.05, were determined by least significance difference test when the ANOVA value was P < 0.001 or lower.
Oral priming with RA accelerates hepatic CYP26A1 response to RA but does not alter its magnitude.
Previous reports indicate that patients treated with all-trans-RA often become refractory to further treatment with RA; however, the basis for this lack of therapeutic response is only beginning to become understood (27, 35). To explore whether prior treatment with RA modifies the response of CYP26A1 to RA, we tested whether priming with a large (500 μg) oral dose of RA would accelerate the response of CYP26A1 to a subsequent 20-μg test dose. CYP26A1 mRNA levels were compared between naive rats and RA-primed rats by qRT-PCR. At t = 0 (16 h after priming dose and before iv injection of 20 μg RA), CYP26A1 mRNA was 60-fold higher in the liver of RA-primed rats compared with naive rats pretreated with oil as a vehicle (Fig. 5A). After administration of the 20-μg RA test dose, CYP26A1 mRNA increased significantly in both the naive rats and the RA-primed rats. Notably, the rate of increase in CYP26A1 expression, as determined by the slope of the response line from 30 to 60 min in naive rats, was nearly identical to the slope from 0 to 30 min in RA-primed rats. Similarly, the slope of the line from 60 to 90 min in naive rats was nearly identical to the slope from 30 to 60 min in RA-primed rats. Therefore, the initial 0–30 min response of CYP26A1 mRNA in the RA-primed rats occurred more rapidly and without a lag time, compared with the initial response in naive rats. By left-shifting the curve for the naive rats by ∼40 min, the two curves were nearly superimposable. Thus, even though the first-pass response of the naive rats to the 20-μg RA test dose was significant after 30 min, the response lagged ∼40 min behind that of RA-primed naive rats given the same dose of RA.
Fig. 5.
Kinetics of CYP26A1 mRNA expression and protein in the liver of vitamin A-adequate RA-primed rats compared with naive rats after a test dose of 20 μg of RA. RA-primed rats were pretreated with 500 μg of RA given orally, 16 h before administration of the test dose. Naive rats were pretreated with vehicle only, 16 h before the test dose. A: total RNA from the liver of 2 or 3 rats per time point was analyzed for CYP26A1 transcript level by quantitative real-time PCR, which was normalized to 18S ribosomal RNA measured simultaneously on the same samples. Results of 2-way ANOVA (RA pretreatment, time after test dose as factors) are shown in inset. Different letters (a, b, c) for time and different letters with primes (a′, b′) for naive vs. RA-primed rats indicate differences by 1-way ANOVA, P < 0.05. B: conversion of [3H]RA (45 nM total RA) to [3H]-polar metabolites in a 50-min in vitro assay by rat liver microsomes (200 μg protein) prepared from naive and RA-primed rats. Values are means ± SE for n = 3 rats/group at each time. By paired t-test, means for RA-primed rats differed significantly from means for naive rats at the same times, P < 0.05. Activity vs. time was significant, P < 0.01, for naive (R2 = 0.96) and RA-primed (R2 = 0.77) rats.
RA-metabolizing activity was also increased in response to intravenous RA in both naive and RA-primed rats. At t = 0, microsomal [3H]RA-metabolizing activity was approximately three times greater in the RA-primed rats than in the naive rats (Fig. 5B), and the amount of [3H]metabolites increased over the time of treatment in vivo in both the naive and RA-primed rats. Although the differences at each time were not statistically significant, the average formation of [3H]metabolites from [3H]RA was greater in the RA-primed rats than in the naive rats at each of the time points after injection. The increase in [3H]metabolite production was linear in both the naive (R2 = 0.96) and RA-primed (R2 = 0.77) rats. Furthermore, the rate of [3H]metabolite formation, as determined by the slope of the line, was similar in both groups.
CYP26A1 mRNA transcript is expressed in hepatocytes in adult rats.
Liver consists of several cell types, of which hepatocytes constitute ∼80% of parenchyma (48). Whereas vitamin A is metabolized and secreted as retinol from hepatocytes, it is stored in lipid droplets as retinyl esters, mainly in hepatic stellate cells (42), which constitute not more than 2% cell mass in the liver (48). Previously, we showed (36) that CYP2C22 is synthesized in the hepatocytes and that its mRNA transcript is detectable in the hepatocytes of vitamin A-adequate adult rats and increased in those cells when the rats are treated with RA. LRAT, on the other hand, is expressed in stellate cells as well as endothelial cells of the adult rat liver (29). As CYP26A1 may be the major RA hydroxylase activity enzyme in the liver (53), we examined its localization by in situ hybridization in the liver of vitamin A-deficient and vitamin A-adequate rats treated with exogenous RA. For comparison, the frozen sections of the liver of the vitamin A-adequate control rats were also evaluated for the expression of RBP4 mRNA transcript, an abundant transcript and marker for hepatocytes (50), and β-actin mRNA and α-SMA mRNA for nonparenchymal cells in the whole sections of the liver. Similar to the pattern of the qRT-PCR results, a faint signal for CYP26A1 was observed in the liver sections of vitamin A-adequate rats but no signal was apparent in those of vitamin A-deficient rats (Fig. 6A). Upon treatment of rats with RA, signal intensity was markedly increased, limited to the hepatocytes, with more intensity in the liver of vitamin A-adequate than vitamin A-deficient rats (Fig. 6A). CYP26A1 mRNA was more highly expressed in hepatocytes around rather than distal from the portal veins (Fig. 6A, +RA) and more uniformly in hepatocytes in all area of the liver in vitamin A-adequate, RA-treated rats. Signal was not observed in fibroblast-like cells around the portal vein, in endothelial cells around the space of Disse, or in the perisinusoidal region (Fig. 6B) or, as a control, when DIG-labeled sense RNA was used as the probe (Fig. 6A).
Fig. 6.
In situ hybridization analysis of CYP26A1, RBP4, β-actin, and α-smooth muscle actin (α-SMA) mRNA transcripts in the liver of adult rats. Glass slides containing liver sections of vitamin A-deficient and vitamin A-adequate rats orally treated with either oil as vehicle or all-trans-RA for 6 h (A) or sections of VA-adequate rat liver (B, C) were first prehybridized and then hybridized to either digoxigenin-labeled sense or antisense RNA riboprobe of CYP26A1 (A and B) or RBP4, β-actin, or α-SMA (C). After washing, the slides were incubated first with alkaline phosphatase conjugated anti-digoxigenin antibody and then with phosphatase synthetic substrate as described in materials and methods. The slides were washed and imaged under a digital microscope. In A, dashed circle surrounds a portal area where signal was more intense. In C, for β-actin, white arrow denotes signal around portal area and black arrow denotes signal scattered in what appear as stellate cells; for α-SMA, white arrows denote signal around portal areas and bile ducts. Scale bars: A, 100 μm; B and C, 50 μm.
It has been known for some time that plasma retinol-binding protein (RBP4) is synthesized in and secreted to plasma from hepatocytes (50). Since RBP4 mRNA is highly expressed in the liver of adult control rats and its expression is not highly regulated by vitamin A (Ref. 50 and data not shown), we examined RBP4 in the liver sections of vitamin A-adequate rats (Fig. 6C) as a control marker for hepatocytes. RBP4 mRNA was highly expressed in hepatocytes throughout the liver, in which it exhibited a perinuclear and cytoplasmic distribution, but not in other cells including fibroblasts around the portal vein and bile ducts, endothelial cells, or stellate cells (Fig. 6C). Again no signal was observed when sense RNA was used as the control probe (Fig. 6C). Interestingly, when DIG-labeled β-actin antisense RNA probe was hybridized to liver sections of vitamin A-adequate rats it produced strongly intense signals around and away from the portal tracts (Fig. 6C, white arrow) and scattered throughout the liver in nonparenchymal cells, which may be stellate cells (e.g., Fig. 6C, black arrow). In addition, hepatocytes exhibited a fainter, more uniform signal barely above the level of the sense probe control (Fig. 6C). In contrast, no signal was detected when DIG-labeled sense RNA was used as the probe. As a positive marker for nonparenchymal cells, an α-SMA riboprobe was used; this probe hybridized in the smooth muscle cells around portal vein areas (Fig. 6C, white arrows), consistent with a previous report (29).
DISCUSSION
The presence of several RA-responsive CYP genes in liver suggests that RA could be metabolized through multiple pathways. CYP26A1, the best-studied of the RA-metabolizing CYPs, and CYP26B1 have been shown to possess RA-4-hydroxylase activity against the all-trans isomer of RA (24), while we have recently shown (36) that CYP2C22, expressed nearly exclusively in liver, can convert all-trans-RA to polar metabolites when expressed in HEK293T cells. On the other hand, LRAT, which diverts retinol into retinyl esters, may indirectly regulate the concentration of RA by controlling the availability of retinol. The similar response patterns of CYP26A1, CYP26B1, CYP2C22, and LRAT to graded levels of dietary vitamin A under steady-state conditions along with the similar temporal patterns in response to acute administration of RA suggest that when RA builds up in the liver, whether as a result of vitamin A supplementation or because of the rapid uptake of exogenous RA (8, 21), the mRNAs of all of these genes are substantially increased. It is presently not known whether the concomitant regulation of CYP26A1, CYP26B1, and CYP2C22 represents redundant mechanisms. From the present studies, CYP2C22 was the most highly expressed transcript in the basal state (CYP2C22 > CYP26B1 > CYP26A1 for vitamin A-adequate liver; Fig. 1), and its increase by RA was the longest lasting. The higher expression of CYP2C22 in vitamin A-marginal rats, in which the liver still contained some retinol, may suggest that retinol as well as RA helps to maintain the steady-state expression level of this enzyme. However, CYP26A1 showed the largest dose-dependent range to dietary vitamin A (vitamin A-deficient compared with control compared with supplemented groups; Fig. 1) as well as the greatest fold increase after oral RA (Fig. 3). This suggests that CYP26A1 could function as an “emergency CYP,” sensing and responding rapidly to elevations in RA. In the developing embryo, CYP26A1 has been shown to tightly regulate the concentration of RA as is necessary for normal embryonic development (38). On the basis of in vitro kinetic studies of several CYPs in human liver specimens, it was concluded that CYP26A1 is the major RA-metabolizing CYP in human liver (53). RA clearance values by CYP26A1 have been estimated at 0.19 l/min (32) to 0.01 and 0.07 l/min (53), depending on the methods and assumptions used, with the overall conclusion that CYP26A1 represents the major RA-clearing CYP in the human liver, especially at therapeutically achievable levels of RA. The CYP26A1 gene promoter region contains proximal and distal retinoic acid response elements (RAREs) that cooperatively increase CYP26A1 expression (22, 65), and each of these elements has been shown to be functionally active in the liver (65). The presence of these response elements provides a likely explanatory mechanism for the rapid, high-level response of CYP26A1 to RA that we have observed in vivo (Figs. 3–5). Interestingly, the elevation of CYP26A1 mRNA in RA-primed rats was even faster than in naive rats (Fig. 5A), although the total area under the response curves did not differ. The more rapid response of CYP26A1 in RA-primed rats suggests that prior retinoid exposure may modify the chromatin in a way that allows for a more rapid response to RA, and the Cyp26A1 protein expression and enzymatic activity analyses indicated a more rapid response to the 20-μg test dose of RA in the RA-pretreated compared with naive rats (Fig. 5, B and C). Although we do not know the mechanism at this time, a relaxation of chromatin leading to a more open, accessible gene after the first exposure to RA would be a plausible possibility, and an effect of this type has been observed for the CYP24 gene that is responsible for the 24-hydroxylation of vitamin D compounds (56). The expression of nuclear retinoid receptors is probably not a limiting factor because among RARα, RARβ, RARγ, and retinoid X receptor (RXR)α, only the expression of RARβ mRNA has been found to be lower in vitamin A-deficient than vitamin A-adequate rat liver (20, 68) and RARα, RARβ, and RARγ were equally effective in inducing the CYP26A1 promoter in liver cells (65).
Like plasma RBP4, which is majorly expressed in hepatocytes (50), CYP26A1 mRNA transcript is expressed essentially exclusively in hepatocytes in adult rats. However, unlike the RBP4 transcript, which is highly expressed even in the vitamin A-deficient state (50), the CYP26A1 transcript was barely detected in the hepatocytes of vitamin A-deficient as well as vitamin A-adequate rats. However, CYP26A1 mRNA was observed in the hepatocytes when rats were treated with RA. This transcript seems to be more highly expressed in periportal hepatocytes than in those nearer the central vein, probably because of the accessibility of RA from circulation. Although hepatocytes may have some capacity to synthesize RA, it has been reported that >90% of the RA content of the liver is contributed from plasma (21). Plasma RA may provide a signal to the liver from peripheral tissue to control the metabolism of retinol for either storage as retinyl ester or secretion from hepatocytes bound to RBP4.
CYP2C22 appears to have a regulation pattern and cellular distribution similar to those of CYP26A1. Recently we showed (36) that CYP2C22 is exclusively expressed in the hepatocytes of rats and that the transcript is easily detectable in cells of the liver from vitamin A-adequate rats as well as being highly regulated by vitamin A and exogenous RA. This apparent colocalization raises unanswered questions about the functions of this CYP gene. For CYP2C22, the basal expression in vitamin A-adequate rats was much higher than for CYP26A1 or CYP26B1. Nonetheless, its expression was significantly reduced in vitamin A-deficient rats and increased in vitamin A-supplemented rats (Fig. 1) as well as by RA (Figs. 3 and 4). CYP2C22 contains a functional RARE in its 5′ upstream sequence, and the expressed protein was shown to be capable of metabolizing RA to polar metabolites (36). The CYP2C family is best known for the metabolism of long-chain fatty acids, such as linoleic and arachidonic acid (4, 23, 40, 54), and various xenobiotics including several clinically important drugs (14, 54). Given the high level of CYP2C22 in rat liver, it might be a significant player in RA homeostasis. However, conversely, if CYP2C22 primarily metabolizes other substrates such as fatty acids or xenobiotics, then our results suggest that vitamin A nutritional status and RA treatment could have a significant influence on the metabolism of other substrates. Further studies are needed to clarify the relative affinity of RA for these various CYPs. Additionally, our results for POR, an electron donor to all CYPs (39), suggests yet another possible means through which RA could augment CYP-mediated oxidative metabolism.
RA has been shown previously to act as a “feedback” inducer of LRAT mRNA and enzyme activity (45, 49, 67). The temporal similarity in the regulation of LRAT and several CYPs suggests that a rise in the level of RA in the liver provides a signal not only for increased oxidation of RA but also for the diversion of retinol into retinyl ester. Overall, our data suggest a dual rheostatic mechanism in which RA concomitantly increases both LRAT, which diverts retinol away from oxidation, and several CYP enzymes with the capacity to metabolize the excess RA. The diversion of retinol by LRAT is also a mechanism for conserving retinol for later use, as retinyl esters are considered a major precursor that can be mobilized for RA biogenesis. Both the diversion of retinol and the oxidation of excess RA appear crucial for retinoid homeostasis. These mechanisms may protect the liver directly, and also protect extrahepatic tissues indirectly, from fluctuations in RA concentration that could perturb the expression patterns of many other genes that can also respond to RA.
GRANTS
This work was supported by National Institutes of Health Grants CA-90214, BANGEO supplement through the Nutritional Science Research Group, Division of Cancer Prevention, CA-90214S, and DK-41479, funds from the Howard Heinz Endowment, and the Graduate Program in Nutrition (C. J. Cifelli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the Bioactive Nutrient Gene Expression Omnibus (BANGEO) program, the Microarray Facility, NCI (Frederick, MD), and mAdb support staff for excellent support.
REFERENCES
- 1. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6: 793–810, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Andreola F, Hayhurst GP, Luo G, Ferguson SS, Gonzalez FJ, Goldstein JA, De Luca LM. Mouse liver CYP2C39 is a novel retinoic acid 4-hydroxylase—its down-regulation offers a molecular basis for liver retinoid accumulation and fibrosis in aryl hydrocarbon receptor-null mice. J Biol Chem 279: 3434–3438, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 43: 1773–1808, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Barbarosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH. Eicospentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C family. Biochem Biophys Res Commun 329: 1275–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328: 1–16, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chithalen JV, Luu L, Petkovich M, Jones G. HPLC-MS/MS analysis of the products generated from all-trans-retinoic acid using recombinant human CYP26A. J Lipid Res 43: 1133–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. In: Vitamins and Hormones, edited by Litwack G. London: Academic, 2007, p. 161–195 [DOI] [PubMed] [Google Scholar]
- 8. Cifelli CJ, Ross AC. All-trans-retinoic acid distribution and metabolism in vitamin A-marginal rats. Am J Physiol Gastrointest Liver Physiol 291: G195–G202, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cifelli CJ, Ross AC. Chronic vitamin A status and acute repletion with retinyl palmitate are determinants of the distribution and catabolism of all-trans-retinoic acid in rats. J Nutr 137: 63–70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Thé H, Marchio A, Tollais P, Dejean A. Differential expression and ligand regulation of the retinoic acid receptor a and b genes. EMBO J 8: 429–433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Décimo D, Georges-Labouesse E, Dollé P. In situ hybridization of nucleic acid probes to cellular RNA. In: Gene Probes, a Practical Approach, edited by Hames BD, Higgins SJ. Oxford, UK: Oxford Univ. Press, 1996, p. 183–210 [Google Scholar]
- 12. El Mansouri S, Tod M, Leclerq M, Petitjean O, Perret G, Porthault M. Time- and dose-dependent kinetics of all-trans-retinoic acid in rats after oral or intravenous administration(s). Drug Metab Dispos 23: 227–231, 1995 [PubMed] [Google Scholar]
- 13. Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem 102: 886–898, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Goldstein JA, de Morais SM. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4: 285–299, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Gunning P, Mohun T, Ng S, Ponte P, Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3′ untranslated regions of the mRNAs. J Mol Evol 20: 202–214, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann B, Lehmann JM, Zhang XK, Hermann T, Husmann M, Graupner T, Pfahl M. A retinoic acid receptor-specific element controls the retinoic acid receptor-beta promoter. Mol Endocrinol 4: 1727–1736, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Ioannides C, Lewis DF. Cytochromes P450 in the bioactivation of chemicals. Curr Top Med Chem 4: 1767–1788, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Jackson DP. In situ hybridization in plants. In: Molecular Plant Pathology: A Practical Approach, edited by Bowles DJ, Gurr SJ, McPherson M. Oxford, UK: Oxford Univ. Press, 1992, p. 163–174 [Google Scholar]
- 19. Julien E, Boobis AR, Olin SS, ILSI Research Foundation Threshold Working Group The key events dose-response framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit Rev Food Sci Nutr 49: 682–689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kato S, Mano H, Kumazawa T, Yoshizawa Y, Kojima R, Masushige S. Effect of retinoid status on α, β and γ retinoic acid receptor mRNA levels in various rat tissues. Biochem J 286: 755–760, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem 270: 17850–17857, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem J 392: 241–248, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA. Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch Biochem Biophys 357: 45–57, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Luu L, Ramshaw H, Tahayato A, Stuart A, Jones G, White J, Petkovich M. Regulation of retinoic acid metabolism. Adv Enzyme Regul 41: 159–175, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Marill J, Cresteil T, Lanotte M, Chabot GG. Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol Pharmacol 58: 1341–1348, 2000 [DOI] [PubMed] [Google Scholar]
- 26. McSorley LC, Daly AK. Identification of human cytochrome P450 isoforms that contribute to all-trans-retinoic acid 4-hydroxylation. Biochem Pharmacol 60: 517–526, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Muindi JRF, Young CW, Warrell RP., Jr Clinical pharmacology of all-trans retinoic acid. Leukemia 8: 1807–1812, 1994 [PubMed] [Google Scholar]
- 28. Nadin L, Murray M. Participation of CYP2C8 in retinoic acid 4-hydroxylation in human hepatic microsomes. Biochem Pharmacol 58: 1201–1208, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Nagatsuma K, Hayashi Y, Hano H, Sagara H, Murakami K, Saito M, Masaki T, Lu T, Tanaka M, Enzan H, Aizawa Y, Tajiri H, Matsuura T. Lecithin: retinol acyltransferase protein is distributed in both hepatic stellate cells and endothelial cells of normal rodent and human liver. Liver Int 29: 47–54, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Napoli JL. Enzymology and biogenesis of retinoic acid. In: Vitamin A and Retinoids: An Update of Biological Aspects and Clinical Applications, edited by Livrea MA. Basel: Birkhèuser, 2000, p. 17–27 [Google Scholar]
- 31. Nudel U, Zakut R, Shani M, Neuman S, Levy Z, Yaffe D. The nucleotide sequence of the rat cytoplasmic β-actin gene. Nucleic Acids Res 11: 1759–1771, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozpolat B, Lopez-Berestein G, Adamson P, Fu CJ, Williams AH. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci 6: 292–301, 2003 [PubMed] [Google Scholar]
- 33. Pai T, Zolfaghari R, Chen Q, Zhang Y, Ross AC. Galactomutarotase and other galactose-related genes are rapidly induced by retinoic acid in human myeloid cells. Biochemistry 46: 15198–15207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem W. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys 1619: 243–253, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Pavez Loriè E, Chamcheu JC, Vahlquist A, Törmä H. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch Dermatol Res 301: 475–485, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Qian L, Zolfaghari R, Ross AC. Liver-specific cytochrome P450 CYP2C22 is a direct target of retinoic acid and a retinoic acid-metabolizing enzyme in rat liver. J Lipid Res 51: 1781–1792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Ribes V, Fraulob V, Petkovich M, Dollé P. The oxidizing enzyme CYP26a1 tightly regulates the availability of retinoic acid in the gastrulating mouse embryo to ensure proper head development and vasculogenesis. Dev Dyn 236: 664–653, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Ribes V, Otto DME, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dollé P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol 303: 66–81, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Rifkind AB, Lee C, Chang TK, Waxman DJ. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys 320: 380–389, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Rigas JR, Francis PA, Muindi JRF, Kris MG, Huselton C, DeGrazia F, Orazem JP, Young CW, Warrell RP., Jr Constitutive variability in the pharmacokinetics of the natural retinoid, all-trans-retinoic acid, and its modulation by ketoconazole. J Natl Cancer Inst 85: 1921–1926, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Ross AC. Hepatic metabolism of vitamin A. In: Hepatology: A Textbook of Liver Disease (4th ed.), edited by Zakim DB, Boyer TD. Philadelphia, PA: Saunders, 2003, p. 149–168 [Google Scholar]
- 43. Ross AC. Vitamin A. In: Bioactive Compounds and Cancer, edited by Milner JA, Romagnolo DF. New York: Humana, 2010, p. 335–356 [Google Scholar]
- 44. Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: perspectives from studies on vitamin A status. J Nutr 134: 269S–275S, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Ross AC, Russell RM, Miller SA, Munro IC, Rodricks JV, Yetley EA, Julien E. Application of a key events dose-response analysis to nutrients: a case study with vitamin A (retinol). Crit Rev Food Sci Nutr 49: 708–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev 80: 1021–1054, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr 71: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Saxena R, Zucker SD, Crawford JM. Anatomy and physiology of the liver. In: Hepatology: A Textbook of Liver Disease (4th ed.), edited by Zakim DB, Boyer TD. Philadelphia, PA: Saunders, 2003, p. 3–30 [Google Scholar]
- 49. Shimada T, Ross AC, Muccio DD, Brouillette WJ, Shealy YF. Regulation of hepatic lecithin:retinol acyltransferase activity by retinoic acid receptor-selective retinoids. Arch Biochem Biophys 344: 220–227, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Soprano DR, Blaner WS. Plasma retinol-binding protein. In: The Retinoids: Biology, Chemistry and Medicine, edited by Sporn MB, Roberts AB, Goodman DS. New York: Raven, 1994, p. 257–281 [Google Scholar]
- 51. Soprano DR, Soprano KJ, Wyatt ML, Goodman DS. Induction of the expression of retinol-binding protein and transthyretin in F9 embryonal carcinoma cells differentiated to embryoid bodies. J Biol Chem 263: 17897–17900, 1988 [PubMed] [Google Scholar]
- 52. Thatcher JE, Isoherranen N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol 5: 875–886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thatcher JE, Zelter A, Isoherranen N. The relative importance of CYP26A1 in hepatic clearance of all-trans retinoic acid. Biochem Pharmacol 80: 903–912, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Totah RA, Rettie AE. Cytochrome P450 2C8: substrates, inhibitors, pharmacogenetics, and clinical relevance. Clin Pharmacol Ther 77: 341–352, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Tzimas G, Nau H, Hendrickx AG, Peterson PE, Hummler H. Retinoid metabolism and transplacental pharmacokinetics in the cynomolgus monkey following a nonteratogenic dosing regimen with all-trans-retinoic acid. Teratology 54: 255–265, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D-3. J Mol Biol 350: 65–77, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Vitoux D, Nasr R, de Thé H. Acute promyelocytic leukemia: new issues on pathogenesis and treatment response. Int J Biochem Cell Biol 39: 1063–1070, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res 41: 882–893, 2000 [PubMed] [Google Scholar]
- 59. Wang Y, Zolfaghari R, Ross AC. Cloning of rat cytochrome P450RAI (CYP26) cDNA and regulation of its gene expression by all-trans-retinoic acid in vivo. Arch Biochem Biophys 401: 235–243, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111: 2505–2515, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Wei LN. Retinoid receptors and their coregulators. Annu Rev Pharmacol Toxicol 43: 47–72, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Westin S, Sonneveld E, Van der Leede BM, van der Saag PT, Gustafsson JÅ, Mode A. CYP2C7 expression in rat liver and hepatocytes: regulation by retinoids. Mol Cell Endocrinol 129: 169–179, 1997 [DOI] [PubMed] [Google Scholar]
- 63. Xi J, Yang Z. Expression of RALDHs (ALDH1As) and CYP26s in human tissues and during the neural differentiation of P19 embryonal carcinoma stem cell. Gene Expr Patterns 8: 438–442, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Yamamoto Y, Zolfaghari R, Ross AC. Regulation of CYP26 (cytochrome P450RAI) mRNA expression and retinoic acid metabolism by retinoids and dietary vitamin A in liver of mice and rats. FASEB J 14: 2119–2127, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y, Zolfaghari R, Ross AC. Multiple retinoic acid response elements cooperate to enhance the inducibility of CYP26A1 gene expression in liver. Gene 464: 32–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zolfaghari R, Cifelli CJ, Lieu SO, Chen Q, Li NQ, Ross AC. Lipopolysaccharide opposes the induction of CYP26A1 and CYP26B1 gene expression by retinoic acid in the rat liver in vivo. Am J Physiol Gastrointest Liver Physiol 292: G1029–G1036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zolfaghari R, Ross AC. An essential set of basic DNA response elements is required for receptor-dependent transcription of the lecithin:retinol acyltransferase (Lrat) gene. Arch Biochem Biophys 489: 1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zolfaghari R, Ross AC. Chronic vitamin A intake affects the expression of mRNA for apolipoprotein A-I, but not for nuclear retinoid receptors, in liver of young and aging Lewis rats. Arch Biochem Biophys 323: 258–264, 1995 [DOI] [PubMed] [Google Scholar]