Abstract
Background
In 2008 and 2009, the authors saw in their institution three women who had undergone oocyte donation and went on to develop severe de novo hypertension before the 26th week of gestation, with values above 180/110 mm Hg. Pregnancy was prematurely terminated in these cases because of the acute threat to the mother’s life, and none of the three neonates survived. Five further cases with better outcomes were found to have occurred from 2006 to 2010. On the basis of this experience, the authors performed a meta-analysis to determine whether oocyte donation elevates the risk of pregnancy-induced hypertension (PIH). The cases are discussed in detail.
Methods
Systematic review of the literature on PIH after oocyte donation, with meta-analysis and calculation of an odds ratio. We also provide a retrospective chart review of our own case series.
Results
28 publications were evaluated. The overall rate of PIH in a total of 2308 deliveries after oocyte donation was 22.6%. With the aid of data from 11 studies, the course of pregnancy in a total of 644 oocyte recipients was compared to that in a control group of 2320 women who were not oocyte recipients. The calculated odds ratio for PIH after oocyte donation, compared to conventional reproductive therapy, was 2.57 (95% CI, 1.91–3.47), while the calculated odds ratio for PIH after oocyte donation, compared to other women in the control group, was 6.60 (95% CI, 4.55–9.57).
Conclusion
The data reveal that oocyte donation confers a considerable risk that the recipient will develop PIH. The very early and severe cases of preeclampsia that we report here are rather atypical; similar cases may have occurred elsewhere without finding their way into the relevant literature. The authors recommend close surveillance of pregnancies following allogenic oocyte transplantation by physicians with special expertise in prenatal medicine.
Oocyte donation has been used for more than 25 years to treat unwanted childlessness (1). As the procedure has been prohibited in Germany since the law on the protection of embryos was introduced in December 1990, couples have been using medical services in neighboring countries, such as the Czech Republic or Spain (2). After a successful embryo transfer, the pregnant woman is cared for in Germany in accordance with statutory maternity provision.
As early as in the late 1980s, an increase in the incidence of hypertensive disorders of pregnancy (pregnancy-induced hypertension or pre-eclampsia) after implantation of donated eggs was reported (3). These disorders are defined as new onset hypertension ≥ 140/90 mm Hg after the 20th week of gestation. However, the risk for hypertensive complications as a result of oocyte donation has been the subject of controversy ever since the procedure was first introduced. An increased incidence in hypertensive disorders of pregnancy has also been ascribed to the causative factors of infertility that are responsible for the decision to undergo assisted reproduction. These include older maternal age or primary infertility in the woman (4). Multiple pregnancy, which is common after the implantation of several embryos (in Germany up to 3 are allowed), are an additional risk factor (5).
Recently, a death subsequent to postpartum eclampsia with HELLP syndrome following egg donation was reported in the Netherlands (6).
In the past 4 years we have treated 8 women with pre-eclampsia following oocyte donation. The following report describes in detail 3 particularly severe cases in 2008/09. Another 5 cases with a milder course are shown in a table. In order to assess the risk of hypertensive disorders of pregnancy after egg donation—while bearing in mind maternal age and multiple pregnancies—we conducted a meta-analysis of the topic.
Method
Some case reports relate to patients seen in 2006–2010, who, as the clinicians concerned recall, developed pre-eclampsia following egg donation. Pre-eclampsia was defined using standard criteria (7). A case note review was conducted. Basic clinical information including risk factors for hypertensive disorders of pregnancy were recorded, along with pregnancy outcome, gestation at birth, mode of delivery, and data on the neonatal outcome were recorded.
We searched PubMed and the search engines MedPilot and Google (only the first 250 hits) for combinations of the search terms “pre-eclampsia or hypertension” and “maternal, perinatal, or obstetric outcome” and “oocyte or ovum donation”. In addition, we reviewed the reference lists of relevant articles. The included studies were not subject to any language restriction. Case reports, studies including fewer than 6 egg donations, and reviews were not included. Where the same working groups had posted several publications that reported on patients from the same recruitment center and over the same time period, the most recent study was included or patient groups with identical characteristics excluded. We counted pregnancies with deliveries after the 24th week of gestation (a patient may be counted several times if she gave birth to more than one baby during the study period). The number of women with hypertensive disorders of pregnancy from each group was recorded. Control groups, where they existed, were recorded as ART (other assisted reproductive technologies, such as IVF with autologous oocytes or insemination) or NC (natural conception or mixed groups with natural conception and ART).
The software package SAS was used to evaluate the data. The graphical representations were done using MedCalc. We calculated odds rations (OR) with 95% confidence intervals for the development of hypertensive disorders of pregnancy according to the random effects model (DerSimonian–Laird) while considering heterogeneity between groups.
Case series
Case 1
A 36 year old primigravida with no pre-existing pathology decided to undergo oocyte donation after several unsuccessful attempts at intracytoplasmic sperm injection (ICSI) with autologous oocytes because of androgen related infertility. After successful fertilization with her partner’s sperm and uncomplicated implantation, the early pregnancy took a normal course up to week 16. At that time, a fetal developmental delay of around 10 days was seen on ultrasonography. Karyotyping by chorionic villus biopsy yielded a normal result. In the 19th week of gestation the patient was admitted to the authors’ hospital with new onset arterial hypertension (peak values 220/120 mm Hg), proteinuria (3 g/dl), and increasing edema, as well as pleural effusions and increasing sickness. Nephrotic syndrome and an antibody-associated systemic disorder were ruled out, as were infectious causes (via TORCH serology). During the course of the patient’s illness she developed hemodynamic insufficiency with pericardial effusion and pulmonary hypertension with new onset mitral and tricuspid regurgitation. In view of the risk to the life of the mother and poor prognosis for the growth restricted fetus, the pregnancy was terminated in the 20th week. The fetus weighed 120 g. Placental histology was inconclusive. Within 3 months the woman had recovered, except for persistent mild mitral insufficiency.
Case 2
A 46 year old normotensive woman with normal renal function decided to undergo oocyte donation because of primary infertility, and her age. The egg was fertilized using her husband’s sperm. During the preparation for the implantation of 2 zygotes the endometrium was treated with steroids. A singleton pregnancy resulted. When the patient was referred to the perinatal center at the authors’ hospital in the 22nd week of gestation, she had developed the complete clinical picture of pre-eclampsia, with a mean blood pressure of 180/115 mm Hg on 24 hour monitoring, 3+ of proteinuria, and marked pulmonary edema. Because she rapidly developed HELLP syndrome, the decision was made at 21+6 weeks of gestation to terminate the pregnancy by caesarean section. The female neonate weighed 260 g and died immediately after delivery while receiving pediatric palliative treatment. The patient still had mild hypertension one year later.
Case 3
A 45 year old primigravida underwent several inseminations and one attempt at ICSI for primary ovarian failure. Owing to her life partner’s oligo-astheno-teratozoospermia (OAT III) syndrome, the donor oocytes were fertilized using donor sperm. The early pregnancy was accompanied by severe hyperemesis gravidarum. At 25+3 weeks of gestation the patient was referred to the authors’ hospital by her gynecologist with discomfort, abdominal pain, headache, and diarrhea of unknown origin. Her blood pressure measurement was 185/105 mm Hg and urinalysis showed 3+ of proteinuria. Her blood pressure was only moderately controlled with maximal treatment. About a week later, the pregnancy was terminated when she excreted 7 g/d of protein and had oliguria and raised transaminases. The female fetus weighed 760 g. In the postpartum period, the patient developed dyspnea with bilateral pleural effusion and was temporarily admitted to intensive care. After her clinical condition had improved she was discharged on the 16th day after the birth while remaining on antihypertensive medication. The premature baby died after 8 weeks.
Two years later the patient decided to undergo another attempt at blastocyst transfer in the same fertilization center. According to the patient, an increased risk for pre-eclampsia after oocyte donation was denied in spite of her asking the question explicitly. She received a cryopreserved blastocyst from the same donors (oocyte/sperm). This time the pregnancy was monitored closely at the patient’s own request and after expert opinion had been sought in Germany, and secondary prophylaxis with acetylsalicylic acid was given. After her pregnancy progressed normally initially, in the 35th week her weight increased drastically, at 2 kg per week, with pronounced edema and bilateral carpal tunnel syndrome. Because of rising blood pressure up to 145/80 mm Hg and in view of her medical history the patient was delivered of a normal-weight infant by elective caesarean section at 36+0 weeks’ gestation. Three months after giving birth, she still had bilateral carpal tunnel syndrome; otherwise her postpartum history was normal.
Cases 4–8 are shown in Table 1.
Table 1. Clinical characteristics and obstetric development in 5 patients with hypertensive disorder of pregnancy in 2005-2009, who had undergone oocyte transfer.
| Case | Age (years) | Week of gestation on admission | Occasional blood pressure on admission (mg Hg) | Risk factors (RF) and Complications (C) | Week of gestation at birth | Mode of birth | Outcome for the fetus |
| 4 | 40 | 36 + 0 | 170/115 | RF: Obesity, gestational diabetes requiring insulin | 37 + 3 | Elective caesarean section | Liveborn hypertrophic neonate >90th percentile |
| 5 | 36 | 36 + 5 | 170/110 | RF: Pre-existing hypertension,C: fetal growth restriction | 37 + 0 | Elective caesarean section | Liveborn, hypotrophic neonate <10th percentile |
| 6 | 50 | 29 + 4 | 165/110 | RF: Pre-existing hypertension, C: proteinuria 8 g/d, signs of fetal hypoxia | 30 + 2 | Emergency caesarean section | Liveborn premature singleton, normal weight |
| 7 | 39 | 35 + 4 | 155/90 | RF: Twin pregnancy | 36 + 2 | Elective caesarean section | Liveborn premature twins, normal weight |
| 8 | 44 | 31 + 6 | 140/90 | C: Fetal growth restriction | 33 + 1 | Elective caesarean section | Liveborn premature hypotrophic singleton <10th percentile |
Result of the literature review
Altogether 863 hits from the search engines were reviewed for relevance. Of these, 33 reports contained data on progressions of pregnancies and complications after 24+0 weeks’ gestation after egg donation. Five publications were excluded because they were publications of cases described previously. 28 reports (2308 deliveries after oocyte donation) were included in our evaluation (table 2). The rate of hypertensive disorder of pregnancy in these women was 22.6% (522/2308). 26 studies reported a proportion of multiple pregnancies (24.3%; 541/2223). In 15 studies from which the mean age of all patients could be concluded, this was 42.1 years. In these women the rate of hypertensive disorders of pregnancy after oocyte donation was 28.3% (250/882).
Table 2. Meta-analysis of 28 studies of the risk of hypertensive disorders of pregnancy after occyte donation.
| Authors | Year | Aim of the study | Study design | Study groups | Mean maternal age (years) | (n) | % hypertensive disorders of pregnancy | |||||
| OD | ART | NC | OD | ART | NC | |||||||
| (e1) Serhal et al. | 1989 | Report of experiences with oocyte donation from 75 treatment cycles | Retrospective | Oocyte donation | *38.6 | 21 | – | – | 38.1 | – | – | |
| (e2) Pados et al. | 1994 | Pregnancy rate and outcome after 336 cycles of oocyte donation in 199 patients with regard to whether the ovaries are functional (premature ovarian dysfunction vs functional ovaries) | Retrospective | Oocyte donation | *32.8 | 52 | – | – | 32.7 | – | – | |
| (e3) Michalas et al. | 1996 | Pregnancy risks after oocute donation in women <40 years vs >40 years, controlled for multiple fetuses | Retrospective | Oocyte donation | N/A | 57 | – | – | 14.0 | – | – | |
| (e4) Sauer et al. | 1996 | Report of experiences with pregnancy rates and outcomes after 218 oocyte donations in 162 women ≥45 years of age | Prospective observational | Oocyte donation | 47.3 | 74 | – | – | 16.2 | – | – | |
| (e5) Koopersmith et al. | 1997 | Obstetric result and risk factors after oocyte donation in women >40 years vs women <40 years | Retrospective | Oocyte donation | N/A | 30 | – | – | 16.7 | – | – | |
| (e6) Remohi et al. | 1997 | Evaluation of cumulative pregnancy rate and proportion of live births after oocyte donation | Retrospective | Oocyte donation | N/A | 254 | – | – | 11.4 | – | – | |
| (e7) Wolff et al. | 1997 | Comparison of perinatal results after oocyte donation vs natural conception paired by several demographic and medical variables and controlled for age and multiple fetuses | Retrospective case control study | Oocyte donation | N/A | 46 | – | – | 19.6 | – | – | |
| Natural conception | N/A | – | – | 49 | – | – | 2.0 | |||||
| (e8) Abdalla et al. | 1998 | Obstetric result after oocyte donation in women <40 years vs ≥40 years | Retrospective | Oocyte donation | N/A | 140 | – | – | 22.9 | – | – | |
| (e9) Söderström-Anttila et al. | 1998 | Pregnancy outcome after oocyte donation in women <35 years vs ≥35 years and controlled for multiple fetuses compared with art | Prospective observational cohort study | Oocyte donation | 33.5 | 51 | – | – | 27.5 | – | – | |
| ART | 33.4 | – | 97 | – | – | 13.4 | – | |||||
| (e10) Yaron et al. | 1998 | Factors influencing the pregnancy rate after oocyte donation in 1011 oocyte donation cycles; calculation of the incidence of pregnancy-related complications and the obstetric outcome | Retrospective | Oocyte donation | N/A | 155 | – | – | 15.5 | – | – | |
| (e11) Foudila et al. | 1999 | Report of experiences with oocyte donation in 18 women with Turner syndrome | Retrospective | Oocyte donation | *30.0 | 11 | – | – | 36.3 | – | – | |
| (e12) Salha et al. | 1999 | Influence of donor gametes (oocyte donation/sperm donation) on the incidence of hypertensive disorders of pregnancy in cases paired by age, parity, and demographic background after excluding high risk chronic disorders | Retrospective case control study | Gamete donation | ||||||||
| – | donor insemination | 30.9 | 33 | – | – | 27.3 | – | |||||
| – | oocyte donation | 38.1 | 27 | – | – | 33.3 | – | – | ||||
| – | embryo donation NC | 36.7 | 12 | – | – | 33.3 | – | – | ||||
| – | partner insemination | 37.2 | – | 33 | – | – | 3.0 | – | ||||
| – | natural conception | 30.9 | – | – | 27 | – | – | 7.4 | ||||
| – | natural conception | 36.2 | – | – | 12 | – | – | 0.0 | ||||
| (e13) Anselmo et al. | 2001 | Oocyte donation after chemotherapy and/or radiotherapy for Hodgkin’s disease | Retrospective | Oocyte donation | 31.2 | 6 | – | – | 50.0 | – | – | |
| (e14) Antinori et al. | 2002 | Obstetric outcome and risk factors after oocyte donation in women aged 45–50 years vs >50 years in a carefully selected cohort after exclusion of chronic disorders—a 12-year report | Retrospective | Oocyte donation ≥45 | N/A | 363 | – | – | 13.0 | – | – | |
| (e15) Gielchinsky et al. | 2002 | Obstetric result after oocyte donation and natural conception in women ≥45 years | Retrospective case control study | Oocyte donation | 47.6 | N/A | 27 | – | 11.0 | – | ||
| Natural conception | – | – | 231 | – | – | |||||||
| (e16) Paulson et al. | 2002 | Pregnancy outcome after 121 oocyte donations in 77 postmenopausal women ≥50 years in a selected cohort, excluding chronic diseases; comparison <55 years vs ≥55 years | Retrospective | Oocyte donation | *52.8 | 40 | – | – | 35.0 | – | – | |
| (e17) Sheffer-Mimouni et al. | 2002 | Perinatal outcome in singleton pregnancies after oocyte donation in mothers <45 years vs ≥45 years | Retrospective | Oocyte donation | 41.3 | 134 | – | – | 27.6 | – | – | |
| (e18) Porreco et al. | 2005 | Obstetric outcome in women ≥45 years vs control group (NC<36 years), randomized and paired by parity and multiple fetuses | Retrospective case control study | Oocyte donation ≥45 years | *47.0 | 39 | – | – | 51.3 | – | – | |
| NC ≥45 years | *47.0 | – | – | 11 | – | – | 22.0 | |||||
| NC <36 years | 29.0 | – | – | 50 | – | – | 9.1 | |||||
| (e19) Soares et al. | 2005 | Results of 3089 embryo transfers after oocyte donation for successful nidation and pregnancy outcome comparing women <45 years of age vs ≥45 years, and controlled for multiple fetuses | Retrospective | Oocyte donation | *38.9 | 106 | – | – | 13.2 | – | – | |
| (e20) Wiggins et al. | 2005 | Pregnancy outcomes after oocyte donation compared with ART in patients paired by socioeconomic status | Retrospective case control study | Oocyte donation | 41.9 | 50 | – | – | 38 | – | – | |
| ART | 37.7 | – | 50 | – | – | 12 | – | |||||
| (e21) Bodri et al. | 2006 | Report of experiences with oocyte donations in 21 women with Turner syndrome | Retrospective | Oocyte donation | *33.1 | 8 | – | – | 62.5 | – | – | |
| (e22) Henne et al. | 2007 | Pregnancy-related complications after oocyte donation compared with women of advanced age and autologous oocytes | Retrospective cohort study | Oocyte donation | 45.3 | 69 | – | 37.7 | – | – | ||
| Autologous oocyte | 41.6 | – | – | 681 | – | – | 9.3 | |||||
| (e23) Keegan et al. | 2007 | Pregnancy outcomes after oocyte donation in women <35 years and ≥40 years compared with art (ivf) patients of the same age, controlled for multiple fetuses | Retrospective case control study Questionnaire | Oocyte donation | N/A | 190 | – | – | 27.9 | – | – | |
| ART | N/A | – | 488 | – | – | 12.5 | – | |||||
| (e24) Poranen et al. | 2007 | Obstetric result after oocyte donation in multiple fetuses vs singletons | Retrospective | Oocyte donation | 35.8 | 92 | – | – | 22.1 | – | – | |
| (e25) Krieg et al. | 2008 | Pregnancy related complications after oocyte donation compared with ART with autologous oocytes controlled for age and multiple fetuses | Retrospective case control study | Oocyte donation | 42.7 | 71 | – | – | 18 | – | – | |
| ART | 41.3 | – | 108 | – | – | 15 | – | |||||
| (e26) Ameratunga et al. | 2009 | Pregnancy rates and obstetric result after oocyte donation in women with premature ovarian dysfunction (<40 years) and postmenopausal women (≥40 years) | Retrospective case control study | Oocyte donation | N/A | 58 | – | – | 13.8 | – | – | |
| (e27) Simchen et al. | 2009 | Investigation of the pregnancy risk in women ≥40 years with multiple fetuses | Retrospective case control study | Oocyte donation | N/A | 125 | – | – | 44.8 | – | – | |
| NC | 31.6 | – | – | 417 | – | – | 9.1 | |||||
| (e28) Gundogan et al. | 2010 | Placental morphology and immunohistochemistry with regard to immune-mediated pathology in 20 oocyte donations vs 33 IVF procedures, while capturing the clinical/obstetric outcomes | Retrospective case control study | Oocyte donation | 43.0 | 20 | – | – | 30.0 | – | – | |
| ART | 37.3 | – | 33 | – | – | 9.1 | – | |||||
| – | – | – | – | – | – | |||||||
Inclusion criteria: studies in any language were included that reported pregnancy outcomes and obstetric complications for deliveries from 24+0 weeks’ gestation after embryo transfer of a heterologous oocyte (oocyte donation [OD]). Control groups, where they existed, were noted as ART=other assisted reproductive technologies. such as in vitro fertilization (IVF) with autologous oocytes or insemination) or as NC (natural conception or mix of natural conception and ART). Case reports and studies with fewer than 6 oocyte donations and reveiws were not included. Where the same working group had published several study reports on patients from the same recruitment center and period, the most recent study was included or patients with the same characteristics excluded.
N/A: data were not published or only for subgroups; *mean value relates to all patients who were initially included in the study and does not reflect the mean for those women whose data were included in the final evaluation of pregnancy outcomes
Nine articles calculated the proportion of hypertensive disorders of pregnancy at 24% (164/683). Multiple pregnancies after oocyte donation are affected by hypertensive disorders of pregnancy almost twice as often as others (7 studies, odds ratio [OR] 1.95, 95% confidence interval [CI] 1.368 to 2.796). Maternal age has a significant effect (7 studies comparing 2 age groups, OR 1.65, CI 1.206 to 2.246). In 11 studies the progression of the pregnancies after oocyte donation was compared with a control group (644 oocyte donations, 2320 NC+ART). The results are shown in Figures 1 and 2 and in Table 2.
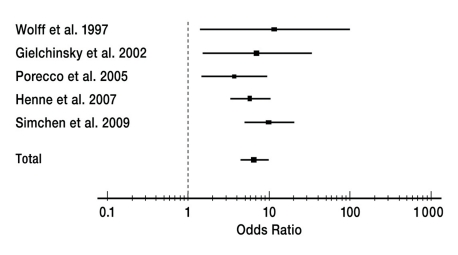
Figure 1.
The forest plot shows the risk calculation for hypertensive disorders of pregnancy as odds ratios with 95% confidence intervals according to the random effects model after oocyte donation compared with women with deliveries of autologous oocytes. It was not clear from the 5 studies whether or to what extent assisted reproductive interventions preceded the pregnancies in the control group. (e7, e15, e18, e22, e27). df=4, I2=21.2%. Test for heterogeneity: p = 0.509.
Studies in any language were included that compared the outcome of pregnancy and obstetric complications during delivery after 24+0 weeks’ gestation after embryo transfer of a heterologous oocyte (egg donation) with a control group. Studies with fewer than 6 participants were not included
Figure 2.
Forest plot showing the risk for hypertensive disorders of pregnancy after oocyte donation as odds ratios with 95% confidence intervals according to the random effects model compared with women with pregnancy after assisted reproductive interventions, but of autologous oocytes, such as for example IVF or insemination. The association was investigated in 6 studies. (e9, e12, e20, e23, e25, e28). df=5, I2=10.6%. Test for heterogeneity: p = 0.477.
Studies in any language were included that compared the outcome of pregnancy and obstetric complications during delivery after 24+0 weeks’ gestation after embryo transfer of a heterologous oocyte (egg donation) with a control group. Studies with fewer than 6 participants in each group were not included.
The OR for developing a hypertensive disorder of pregnancy after oocyte donation compared with ART was 2.57 (CI 1.91 to 3.47) and compared with NC 6.60 (CI 4.55 to 9.57). Only one controlled study investigated the rate of hypertensive disorders of pregnancy specifically in relation to maternal age (<35 years vs ≥40 years) and adjusted for multiple pregnancies after oocyte donation compared with ART. The authors of that particular study concluded that young recipients of oocyte donations have the highest relative risk for hypertensive disorders of pregnancy (singletons: OR 8.73, CI 1.896 to 40.224) compared with the ART control group, and independently of the number of fetuses (e23).
However, in terms of their methodology, most of the studies are retrospective clinical reports. Only 2 studies reported a randomization protocol for the allocation of the controls (e9, e18). Two studies had a prospective design (e4, e8).
Discussion
About 2% of all deliveries in Germany occurred following reproductive technologies (8). In some instances, however, unwanted childlessness cannot be treated using the procedures that are licensed under German law. Couples therefore take opportunities to receive oocyte donations in the fertility centers of neighboring countries. Internet advertisements from companies abroad are highly targeted at the German speaking clientele, and the term used in this context is that of reproductive medical tourism (2).
Following embryo transfer, further care is provided in Germany according to the maternity guidelines of the Federal Joint Committee (G-BA) of physicians and health insurers. The primary aim of medical care during pregnancy is the early detection of risk pregnancies and births. If existing risks are not identified then adequate monitoring of the pregnancy is not possible. The low acceptance of egg donation in the German population (2) may cause women to fear stigmatization. Legal aspects can cause further uncertainties for couples and result in patients’ withholding from their doctor in Germany the origin of the pregnancy. In addition to the prohibition of egg donation in Germany, the fact that the genetic and birth mothers are not identical can lead to situations of legal conflict. In Germany the woman giving birth is always the natural mother (9), but this is not the case in other countries.
The scant experience of Germany’s gynecologists with oocyte donation may result in reduced attention. Furthermore, a lack of patient information given in fertility centers abroad may result in the patients feeling safe when this is not warranted.
The incidence of hypertensive disorders of pregnancy is 5% to 7%. With more than 50 000 deaths per year, these disorders account for 12% to 18% of maternal mortality worldwide (7). An early diagnosis can favorably influence the outcome. However, the only causative treatment in potential life threatening multiorgan disease that is currently available is termination of the pregnancy (7, 10). Women who are desperate to have children may find this particularly difficult to accept, even in situations of acute danger, especially when the fetus is close to being viable (usually from 24+0 weeks’ gestation).
On the basis of the available data, this meta-analysis found an increased relative risk for hypertensive disorders of pregnancy subsequent to oocyte donation. This effect is independent of maternal age, multiple pregnancies, and reproductive interventions. In 28 studies, the respective authors found multiple associations between hypertensive disorders of pregnancy and egg donation. Only the study reported by Krieg et al. did not find a significant difference between women after oocyte donation (n = 71) compared with ART using autologous oocytes (n = 108). Maternal characteristics including age (mean 42.7 years in oocyte donation and 41.3 years in ART) were similar in this retrospective study. The absolute numbers still show an increased incidence of hypertensive disorders of pregnancy in both study groups (oocyte donation 19%, ART 15%) compared with their incidence in the normal population (e25). Krieg et al. advised caution when interpreting studies with non-comparable control groups. It is difficult to set up an adequate control group because the rate of spontaneous conception in the over 40s drops notably, and it is these women who constitute the main clientele for oocyte donation programs.
Keegan et al. did justice to this problem and in 2007 published a controlled retrospective study including 190 deliveries after oocyte donation compared with 488 live births after ART. They studied the incidence of hypertensive disorders of pregnancy dependent on maternal age (<35 years vs >40 years) and multiple pregnancies. Surprisingly, they found an increased incidence in hypertensive disorders of pregnancy especially in the group of women younger than 35 who had undergone oocyte donation compared with the relevant control group. Keegan et al. explain the increased rates of hypertensive disorders of pregnancy in this group with ovarian dysfunction in these patients (e23).
Other authors explain the development of hypertensive disorders of pregnancy after oocyte donation with immunological processes (e1, e12, e17, e20, e23). Controlling the immune response to the developing fetoplacental unit as an allograft is one of the biggest challenges for the pregnancy. Disrupted immune adaptation is assumed to be the central cause in the development of pre-eclampsia (11). The focus is on the interaction of fetal HLA-C antigen with maternal natural killer cells (NK cells) (12, 13). Through the NK cells, produced cytokines influence the trophoblast-mediated modulation of the uterine spiral arteries (4, 14, 15). Disturbed trophoblast invasion and therefore reduced endovascular transformation of the spiral arteries will result in pre-eclampsia via placental ischemia (16). This means that according to what is currently known the onset of pre-eclampsia occurs in the early stages of pregnancy (17). Figure 3 shows the hypothetical pathomechanism for the development of pre-eclampsia.
Figure 3.
Hypothetical causes and pathomechanisms of pre-eclampsia (according to Sibai et al, 2005)
Furthermore, the circumstances that lead to choosing oocyte donation may favor the development of hypertensive disorders of pregnancy. Early ovarian dysfunction, for example, is associated with maternal antibodies against the zona pellucida and against granulosa cells, which leads to interference with invasive trophoblast cells on the endometrial border and can cause disrupted trophoblast invasion, such as occurs in pre-eclampsia (18).
In most studies, terminations of pregnancies and miscarriages/births before 25 weeks’ gestation were not captured. The rate of terminations after oocyte donation for maternal indications could therefore not be found out. The 3 cases presented by the authors in this article are extreme cases but may not be exceptions.
Conclusion
The results reported in the recent literature show an increased relative risk for hypertensive disorders in pregnancy after fertilization by means of a heterologous oocyte (egg/oocyte donation). Patients who have undergone oocyte donation in fertilization centers abroad should be explicitly asked whether they have undergone egg donation. In addition to close monitoring of the pregnancy, the women should if possible be under the care of obstetricians specializing in maternofetal medicine.
Table 3. Risk calculation for hypertensive disorders of pregnancy after oocyte donation compared with natural conception (NC) and/or other assisted reproductive interventions (ART).
| Control group comparison | OD | NC/ART | OR | 95% CI |
| 11 studies comparing oocyte donation vs NC + ART | 3.87 | 2.61–5.74 | ||
| No of patients who had undergone oocyte donation (n) | 644 | 2270 | ||
| No of patients who developed pregnancy-induced hypertensive disorders (n) | 197 | 226 | ||
| % hypertensive disorders of pregnancy | 30.6 | 10 | ||
| 6 studies comparing oocyte donation vs ART | 2.57 | 1.91–3.47 | ||
| No of patients who had undergone oocyte donation (n) | 421 | 842 | ||
| No of patients who developed pregnancy-induced hypertensive disorders (n) | 120 | 115 | ||
| % hypertensive disorders of pregnancy | 28.5 | 13.7 | ||
| 1 study comparing oocyte donation vs ART controlled for maternal age (age <35years) | 5.42 | 2.04–14.40 | ||
| No of patients who had undergone oocyte donation (n) | 19 | 296 | ||
| No of patients who developed pregnancy-induced hypertensive disorders (n) | 8 | 35 | ||
| % hypertensive disorders of pregnancy | 42.11 | 11.82 | ||
| Mean age (years) | 31.7 | 31 | ||
| 1 study comparing oocyte donation vs ART controlled for maternal age (age >40 years) | 2.21 | 1.29–3.78 | ||
| No of patients who had undergone oocyte donation (n) | 171 | 192 | ||
| No of patients who developed pregnancy-induced hypertensive disorders (n) | 44 | 26 | ||
| % hypertensive disorders of pregnancy | 25.73 | 13.54 | ||
| Mean age (years) | 43.9 | 41.4 | ||
| 2 studies comparing oocyte donation vs ART, only singleton pregnancies | 3.25 | 1.94–5.47 | ||
| No of patients who had undergone oocyte donation (n) | 153 | 395 | ||
| No of patients who developed pregnancy-induced hypertensive disorders (n) | 35 | 33 | ||
| % hypertensive disorders of pregnancy | 22.9 | 8.4 |
The risk is shown as odds ratios (OR) with 95% confidence intervals (95% CI). In the 6 studies that compared oocyte donation (OD) with ART, subgroup analyses were conducted of the influence of maternal age and the risk for singleton pregnancies (e7, e9, e12, e15, e18, e20, e22, e23, e25, e27, e28). Studies in any language were included that compared the outcome of pregnancy and obstetric complications in deliveries from 24+0 weeks’ gestation after embryo transfer of a heterologous oocyte (oocyte donation) with a control group. Reports of less than 6 patients in each group were not included. Where a working group had published several reports on patients from the same recruitment center and recruitment period, the most recent report was included or patient groups with the same characteristics excluded
Key Messages.
In Germany, egg (oocyte) donation is not permitted as a reproductive technology. “Reproductive tourism” to neighboring European countries has been observed.
The fear of stigmatization and uncertainties about the legal situation may lead patients to conceal the origin of the pregnancy to the doctor treating them in Germany.
Pregnant woman who have conceived as a result of oocyte donation are to be categorized as high risk patients, with a substantial risk especially for hypertensive disorders of pregnancy.
The increased relative risk for hypertensive disorders of pregnancy after oocyte donation exists independently of the rate of multiple pregnancies, maternal age, and the type of reproductive treatment.
In addition to close antenatal monitoring women pregnant from oocyte donation should ideally be under the care of obstetricians specializing in maternofetal medicine.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Trounson A, Leeton J, Besanko M, Wood C, Conti A. Pregnancy established in an infertile patient after transfer of a donated embryo fertilised in vitro. Br Med J (Clin Res Ed) 1983;286:835–838. doi: 10.1136/bmj.286.6368.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stobel-Richter Y, Goldschmidt S, Brahler E, Weidner K, Beutel M. Egg donation, surrogate mothering, and cloning: attitudes of men and women in Germany based on a representative survey. Fertil Steril. 2009;92:124–130. doi: 10.1016/j.fertnstert.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Serhal PF, Craft IL. Oocyte donation in 61 patients. Lancet. 1989;1:1185–1187. doi: 10.1016/s0140-6736(89)92762-1. [DOI] [PubMed] [Google Scholar]
- 4.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 5.Sibai BM, Hauth J, Caritis S, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938–942. doi: 10.1016/s0002-9378(00)70350-4. [DOI] [PubMed] [Google Scholar]
- 6.Schutte JM, Schuitemaker NW, Steegers EA, van Roosmalen J. Maternal death after oocyte donation at high maternal age: case report. Reprod Health. 2008;5 doi: 10.1186/1742-4755-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rath W, Fischer T. The diagnosis and treatment of hypertensive disorders of pregnancy: new findings for antenatal and inpatient care. Dtsch Arztebl Int. 2009;106(45):733–738. doi: 10.3238/artebl.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Mouzon J, Goossens V, Bhattacharya S, et al. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod. 2010;25:1851–1862. doi: 10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- 9.Frommel M, Taupitz J, Ochsner A, Geisthövel F. Rechtslage der Reproduktionsmedizin in Deutschland. J Reproduktionsmed Endokrinol. 2010;7:96–105. [Google Scholar]
- 10.Rath W, Bartz C. [Treatment of severe preeclampsia and HELLP syndrome] Zentralbl Gynakol. 2004;126:293–298. doi: 10.1055/s-2004-820420. [DOI] [PubMed] [Google Scholar]
- 11.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24(Suppl A):S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 12.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 13.Hiby SE, Walker JJ, O’Shaughnessy KM, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croy BA, He H, Esadeg S, et al. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003;126:149–160. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 2010;221:363–378. doi: 10.1002/path.2719. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 17.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 18.Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Reprod Immunol. 2005;66:53–67. doi: 10.1016/j.jri.2005.02.003. [DOI] [PubMed] [Google Scholar]
- e1.Serhal PF, Craft IL. Oocyte donation in 61 patients. Lancet. 1989;1:1185–1187. doi: 10.1016/s0140-6736(89)92762-1. [DOI] [PubMed] [Google Scholar]
- e2.Pados G, Camus M, Van Steirteghem A, Bonduelle M, Devroey P. The evolution and outcome of pregnancies from oocyte donation. Hum Reprod. 1994;9:538–542. doi: 10.1093/oxfordjournals.humrep.a138541. [DOI] [PubMed] [Google Scholar]
- e3.Michalas S, Loutradis D, Drakakis P, et al. Oocyte donation to women over 40 years of age: pregnancy complications. Eur J Obstet Gynecol Reprod Biol. 1996;64:175–178. doi: 10.1016/0301-2115(95)02335-6. [DOI] [PubMed] [Google Scholar]
- e4.Sauer MV, Paulson RJ, Lobo RA. Oocyte donation to women of advanced reproductive age: pregnancy results and obstetrical outcomes in patients 45 years and older. Hum Reprod. 1996;11:2540–2543. doi: 10.1093/oxfordjournals.humrep.a019155. [DOI] [PubMed] [Google Scholar]
- e5.Koopersmith TB, Lindheim SR, Lobo RA, Paulson RJ, Sauer MV. Outcomes of high-order multiple implantations in women undergoing ovum donation. J Matern Fetal Med. 1997;6:268–272. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<268::AID-MFM5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- e6.Remohi J, Gartner B, Gallardo E, Yalil S, Simon C, Pellicer A. Pregnancy and birth rates after oocyte donation. Fertil Steril. 1997;67:717–723. doi: 10.1016/s0015-0282(97)81372-6. [DOI] [PubMed] [Google Scholar]
- e7.Wolff KM, McMahon MJ, Kuller JA, Walmer DK, Meyer WR. Advanced maternal age and perinatal outcome: oocyte recipiency versus natural conception. Obstet Gynecol. 1997;89:519–523. doi: 10.1016/S0029-7844(97)00051-3. [DOI] [PubMed] [Google Scholar]
- e8.Abdalla HI, Billett A, Kan AK, et al. Obstetric outcome in 232 ovum donation pregnancies. Br J Obstet Gynaecol. 1998;105:332–337. doi: 10.1111/j.1471-0528.1998.tb10096.x. [DOI] [PubMed] [Google Scholar]
- e9.SÖderström-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod. 1998;13:483–490. doi: 10.1093/humrep/13.2.483. [DOI] [PubMed] [Google Scholar]
- e10.Yaron Y, Ochshorn Y, Amit A, Kogosowski A, Yovel I, Lessing JB. Oocyte donation in Israel: a study of 1001 initiated treatment cycles. Hum Reprod. 1998;13:1819–1824. doi: 10.1093/humrep/13.7.1819. [DOI] [PubMed] [Google Scholar]
- e11.Foudila T, Soderstrom-Anttila V, Hovatta O. Turner’s syndrome and pregnancies after oocyte donation. Hum Reprod. 1999;14:532–535. doi: 10.1093/humrep/14.2.532. [DOI] [PubMed] [Google Scholar]
- e12.Salha O, Sharma V, Dada T, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14:2268–2273. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- e13.Anselmo AP, Cavalieri E, Aragona C, Sbracia M, Funaro D, Maurizi Enrici R. Successful pregnancies following an egg donation program in women with previously treated Hodgkin’s disease. Haematologica. 2001;86:624–628. [PubMed] [Google Scholar]
- e14.Antinori S, Gholami GH, Versaci C, et al. Obstetric and prenatal outcome in menopausal women: a 12-year clinical study. Reprod Biomed Online. 2003;6:257–261. doi: 10.1016/s1472-6483(10)61718-x. [DOI] [PubMed] [Google Scholar]
- e15.Gielchinsky Y, Mankuta D, Samueloff A, et al. Pregnancies from oocyte donation: increased obstetric complications in women over 45 years of age. Am J Obstet Gynecol. 2002;187 Abstract 91. [Google Scholar]
- e16.Paulson RJ, Boostanfar R, Saadat P, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. Jama. 2002;288:2320–2323. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- e17.Sheffer-Mimouni G, Mashiach S, Dor J, Levran D, Seidman DS. Factors influencing the obstetric and perinatal outcome after oocyte donation. Hum Reprod. 2002;17:2636–2640. doi: 10.1093/humrep/17.10.2636. [DOI] [PubMed] [Google Scholar]
- e18.Porreco RP, Harden L, Gambotto M, Shapiro H. Expectation of pregnancy outcome among mature women. Am J Obstet Gynecol. 2005;192:38–41. doi: 10.1016/j.ajog.2004.07.035. [DOI] [PubMed] [Google Scholar]
- e19.Soares SR, Troncoso C, Bosch E, et al. Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J Clin Endocrinol Metab. 2005;90:4399–4404. doi: 10.1210/jc.2004-2252. [DOI] [PubMed] [Google Scholar]
- e20.Wiggins DA, Main E. Outcomes of pregnancies achieved by donor egg in vitro fertilization—a comparison with standard in vitro fertilization pregnancies. Am J Obstet Gynecol. 2005;192:2002–2006. doi: 10.1016/j.ajog.2005.02.059. discussion 6-8. [DOI] [PubMed] [Google Scholar]
- e21.Bodri D, Vernaeve V, Figueras F, Vidal R, Guillen JJ, Coll O. Oocyte donation in patients with Turner’s syndrome: a successful technique but with an accompanying high risk of hypertensive disorders during pregnancy. Hum Reprod. 2006;21:829–832. doi: 10.1093/humrep/dei396. [DOI] [PubMed] [Google Scholar]
- e22.Henne MB, Zhang M, Paroski S, Kelshikar B, Westphal LM. Comparison of obstetric outcomes in recipients of donor oocytes vs. women of advanced maternal age with autologous oocytes. J Reprod Med. 2007;52:585–590. [PubMed] [Google Scholar]
- e23.Keegan DA, Krey LC, Chang HC, Noyes N. Increased risk of pregnancy-induced hypertension in young recipients of donated oocytes. Fertil Steril. 2007;87:776–781. doi: 10.1016/j.fertnstert.2006.08.105. [DOI] [PubMed] [Google Scholar]
- e24.Poranen AK. Single embryo transfer minimizes obstetric complications after ovum and embryo donations. Acta Obstet Gynecol Scand. 2007;86 Abstract 13. [Google Scholar]
- e25.Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil Steril. 2008;90:65–70. doi: 10.1016/j.fertnstert.2007.06.014. [DOI] [PubMed] [Google Scholar]
- e26.Ameratunga D, Weston G, Osianlis T, Catt J, Vollenhoven B. In vitro fertilisation (IVF) with donor eggs in post-menopausal women: are there differences in pregnancy outcomes in women with premature ovarian failure (POF) compared with women with physiological age-related menopause? J Assist Reprod Genet. 2009;26:511–514. doi: 10.1007/s10815-009-9351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e27.Simchen MJ, Shulman A, Wiser A, Zilberberg E, Schiff E. The aged uterus: multifetal pregnancy outcome after ovum donation in older women. Hum Reprod. 2009;24:2500–2503. doi: 10.1093/humrep/dep238. [DOI] [PubMed] [Google Scholar]
- e28.Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010;93:397–404. doi: 10.1016/j.fertnstert.2008.12.144. [DOI] [PubMed] [Google Scholar]