Abstract
Background
The diagnosis of soft-tissue sarcomas of the limbs is often delayed, sometimes markedly so, even though prompt and appropriate treatment improves survival and lowers the amputation rate.
Methods
On the basis of a selective literature review and consideration of the relevant guidelines, we developed an algorithm that can serve as a guide to the diagnosis of soft-tissue tumors in general and to the treatment of soft-tissue sarcomas of the limbs.
Results
Surgical resection accompanied by multimodal therapy is the only treatment strategy for soft-tissue sarcoma that provides a chance of cure. Particularly when the tumor is located in the distal part of a limb, plastic-reconstructive surgical techniques often enable adequate local control, along with limb salvage and preservation of function. The role of adjuvant or neo-adjuvant radiotherapy and/or chemotherapy is currently debated. The overall survival rate at 5 years is 87% for low-grade sarcomas and 62% for high-grade sarcomas.
Conclusion
Any solid mass of the limbs that has been present for more than four weeks requires diagnostic evaluation. Excisional biopsy is suitable only for epifascial lesions measuring less than 5 cm in diameter. All other lesions should be imaged with MRI and then diagnosed with an incisional biopsy. Patients with soft tissue sarcomas must be treated in an interdisciplinary collaboration so that they can undergo multimodal treatment. The proposed algorithm should help avoid delays in diagnosis and optimize treatment strategies.
The aim of this review is to set out the correct procedures for the diagnosis and treatment of soft tissue sarcomas. This seems to be necessary because these rare tumors are still often recognized late and therefore not treated promptly (1, 2), although no specialized knowledge is required for correct implementation of the initial diagnostic measures.
An essential role is played by timely referral to a specialized center (3, e1, e2). It has clearly been shown that prompt treatment at such an institution improves survival and lowers the amputation rate (1). Unfortunately, a large proportion of patients are still initially managed in institutions with low numbers of cases, mostly in hospitals that see less than three such patients per year (2). Seventy-two percent of the patients in our own collective had already undergone surgery elsewhere (4).
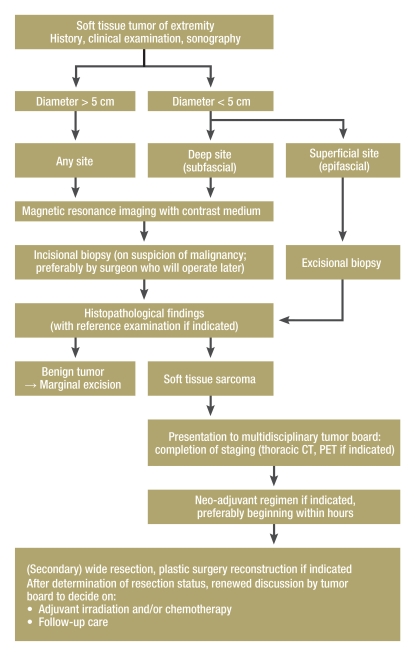
Sarcomas can occur throughout the body; therefore, surgeons from any specialty can be confronted with them. Sixty percent of soft tissue sarcomas in adults occur in the limbs (15% in the arms, 45% in the legs), and this article focuses on these localizations (5). Modern multimodal treatment strategies including improved options for reconstruction achieve good local control and avoid amputation in more than 95% of cases (2, e3). On the basis of a selective literature review, we present a suggested algorithm for the correct diagnosis of solid tumors of the extremities and timely initiation of interdisciplinary treatment (figure 1). This algorithm was conceived particularly as an aid for physicians working outside the hospital environment.
Figure 1.
A suggested algorithm for diagnosis of solid tumors of the extremities and overview of multimodal treatment
Epidemiology and clinical characteristics
The incidence of soft tissue sarcomas is relatively low at 2 to 3 per 100 000 per year. Around 50 sarcoma patients present each year at our own center in Germany. The term “sarcoma” does not denote a group of tumors with uniform presentation; rather, there are numerous histologically distinct subgroups (6, 7). The most frequent sarcomas in adults are liposarcoma, fibrosarcoma, and pleomorphic sarcoma (previously known as malignant fibrous histiocytoma). Soft tissue sarcomas comprise only 1% of all malignancies in adults (8, e4). The 5-year survival rate is 87% for low-grade sarcomas and 62% for high-grade sarcomas (6).
Problems arise from the fact that slow growth of a tumor by no means proves it is benign, even though only one in every 200 soft tissue tumors is malignant (e5). Subcutaneous tumors are usually discovered by self-palpation, although accounts of duration and progression are often unreliable. Occasionally the patient reports a (putative) association with trivial trauma (figure 2). Tumors that lie deeper, e.g., at the typical localization in the adductor compartment of the proximal upper thigh, rarely cause symptoms at an early stage. Refractory swellings that persist for more than 4 weeks should not perforce be interpreted as a pulled muscle or suchlike, but should prompt further diagnostic investigation (9).
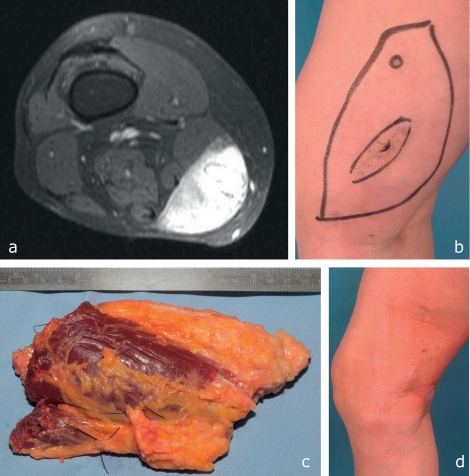
Figure 2.
A 53-year-old woman with a myxoid liposarcoma (T2b, N0, M0, G1) in the medial compartment of the right distal thigh.
MRI elsewhere showed a large, contrast-enhancing solid tumor, but because of temporal association with trauma this was misinterpreted as an organizing hematoma.
Inadequate enucleation with an incorrect drainage pathway was carried out at the same institution.
After histopathological diagnosis an R0 situation could be achieved only by wide resection including the distal portions of the sartorius and gracilis muscles, because the previous enucleation prevented palpatory assessment of the tumor bed.
Result after surgery and adjuvant irradiation
Biopsy and diagnosis
Even imaging procedures do not permit confident classification of a tumor as benign or malignant independent of histopathological investigation, but some characteristics may correlate positively with the later diagnosis of soft tissue sarcoma. These include diameter exceeding 5 cm, increase in size, painfulness of the swelling, and deep tumor site (9, e6). Every swelling that displays one of these features should be regarded and treated as malignant until it is histologically demonstrated to be benign. Eighty-six percent of tumors that meet all these criteria are malignant (9).
Smaller tumors that have been present for a long time and are definitely subcutaneous (epifascial) in location on the basis of clinical examination and ultrasonography can initially be managed by excisional biopsy. This term denotes complete excision of a tumor with a narrow margin of safety. No further imaging procedures are necessary before operation. Some 25% of all soft tissue sarcomas are epifascial in location and less than 3 cm across. If histopathological examination reveals sarcoma, wider resection is usually possible with no negative impact on the prognosis. In 60% of such cases residual tumor cells are found in the secondarily resected material (e7, e8, e9). Note that this course of action is not appropriate in subcutaneous tumors over 5 cm in diameter or in tumors of any size at subfascial sites (e10).
All solid subfascial soft tissue tumors and all larger soft tissue masses at any site should be investigated by means of contrast-enhanced MRI, the imaging procedure that provides the most information about such tumors (10). Every tumor that takes up contrast medium must be regarded as malignant until proved benign. Occasional tumors are misinterpreted as benign on MRI (smooth-bordered, benign-appearing tumor). In contrast to bone tumors, no diagnostic imaging procedure provides certain characterization of soft-tissue tumors (e11). Therefore, some surgeons will occasionally wait, or make the mistake of enucleating a subfascial tumor with no margin of safety (figure 2).
However, MRI does permit precise three-dimensional anatomic analysis and good biopsy planning. A subfascially located soft tissue tumor that takes up contrast medium should be subjected to diagnostic incisional biopsy. This intervention is not as trivial as is often thought. It should be carried out as an open procedure, ideally by the surgeon who is going to perform the subsequent operation. Errors that may occur, hampering or completely preventing later ideal oncosurgical resection and reconstruction, include incorrect or excessively wide routes of access and incorrectly chosen drainage pathways (2, 8).
Ideally the pathologist receives a sufficient amount of material from the margins of the tumor (pseudocapsule). Punch biopsies are possible in principle, provided the pathologist can work with the lower volume of relevant material. Aspiration biopsy is of limited utility (e12).
Following confirmation of soft tissue sarcoma, the treatment depends on the histopathological grade and on the staging. The grading system most widely used in Europe, the classification of the Fédération Nationale des Centres de Lutte Contre le Cancer, distinguishes grades 1 to 3, where grade 3 is highly malignant. Correct histopathological classification is of central importance for the choice of treatment, because some subtypes of sarcoma, such as primitive neuroectodermal tumor and extraosseous Ewing sarcoma, profit greatly from neo-adjuvant procedures (e13). Because of the frequent discrepancies, one should not hesitate to order reference examinations (3, 11).
Staging
First and foremost, spiral CT of the thorax is indicated, because soft tissue sarcomas primarily metastasize hematogenously to the lungs. At the time of diagnosis, however, only 10% of patients have localizable lung metastases that are amenable to thoracosurgical resection if indicated (e14). Positron emission tomography has not yet been assigned a precise role in guidelines for diagnosis of soft tissue sarcomas (e15).
The staging of soft tissue sarcomas is accomplished as usual in the TNM system, with the exception that not only the size of the mass but also its site—epi- or subfascial—is classified owing to the influence of tumor location on the prognosis (6).
Tumor board review: the planning of interdisciplinary multimodal treatment
Following diagnosis of a soft tissue sarcoma the patient must be referred to a center offering interdisciplinary multimodal care. On arrival there, staging should be completed and the continuing multimodal treatment plan discussed (7, 12). This section sets out the basic features of the multimodal approach to the treatment of soft tissue sarcomas in adult patients with particular attention to the oncosurgical and plastic–reconstructive aspects (2, 10).
Resection
Decisive for the cure of patients with soft tissue sarcomas of the extremities is radical extirpation of the tumor. Previously this often meant loss of the limb, but nowadays amputation is seldom necessary (13).
Central to the treatment and indispensable for cure is oncologically sufficient surgical resection of the tumor. No other adjuvant or neo-adjuvant treatment option provides adequate protection against local recurrence (e16). If surgery has already been carried out but the result is judged inadequate, usually on grounds of insufficient margins of safety, one must first determine whether greater margins can be achieved by reoperation. Reoperation has clearly been shown to be superior to radiotherapy alone in cases of R1 resection (1).
The technique most frequently employed for tumor removal is the so-called wide resection. This term implies resection of a large amount of surrounding healthy tissue, with safety margins of 4 to 5 cm to the sides and 1 to 2 cm deep to the tumor (14).
If certain anatomical structures around the sarcoma (muscle fascia, perineurium, adventitia of large vessels) are free of tumor, reduction of the safety margins is possible provided these sheathing tissues are included in the material excised. Combined with adjuvant radiotherapy this procedure results in local tumor control in 95% of cases, with survival rates comparable to those achieved by amputation (12, 13). The so-called compartment resection involves the removal of an entire muscle group from origin to attachment. In bone tumors this degree of surgical mutilation is justified by the frequent occurrence of satellite metastases, but in the case of soft tissue sarcomas compartment resection is applicable only to very extensive tumors (2).
Amputation may occasionally still be necessary if large vessels or nerves are infiltrated close to the trunk. The decision to amputate should be taken only after due consideration of the potential reconstructive options with regard to vessel and nerve interposition. Plastic surgery techniques such as the so-called fillet flap may help to maximize residual stump length and function (2, 4, 10).
Reconstruction
The term reconstruction describes all measures that go beyond simple suturing of the wound. The goal is always unimpaired wound healing in the interests of general rehabilitation and early commencement of postoperative radiation treatment, which is frequently required. All the techniques of plastic surgery are used to avoid complications of wound healing by primary closure under tension (2, 4, 10). In particular, routine use of microsurgical flap repairs with negligible donor site morbidity has become an indispensable component of the modern reconstructive instrumentarium (e17, e18). Functional reconstructions such as nerve interpositions or classical muscle replacements, e.g., posterior tibial muscle transfer to compensate foot drop after resection of the muscles responsible for dorsiflexion, are carried out at the same time (2).
Reconstruction can usually be carried out immediately after oncosurgical resection in the same session of surgery. In most cases of MRI-aided planning a primary R0 situation can be achieved, as long as resection is adequate without any constraints due, for instance, to difficulty in defect closure (2, 3, 10). Such constraints operate in the lack of adequate knowledge of the potential for reconstruction; even at the planning stage they may have a negative impact on the overall concept, leading to insufficiently radical surgery. Particularly in distal limb segments, adequate resection with maximal preservation of function is impossible without detailed preoperative planning of reconstruction (Figure 3). From the viewpoint of plastic surgery, the preoperative evaluation of tumor respectability should always be carried out by a surgeon well versed in all options for reconstruction (2, 3, 10). Furthermore, in order to participate meaningfully in the tumor board’s interdisciplinary decisions, e.g., on the potential benefits of preoperative downstaging, the surgeon must be familiar with the principles of (neo-)adjuvant measures (10).
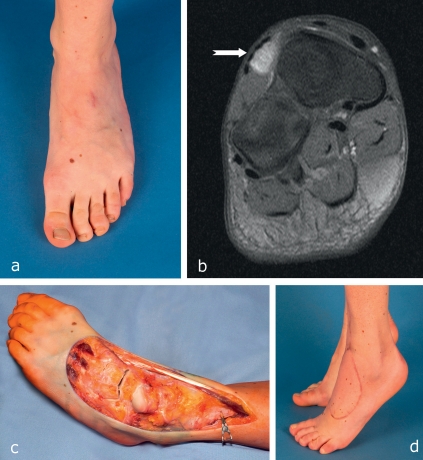
Figure 2.
A 27-year-old woman with fibrosarcoma (T1b, N0, M0, G3) on the dorsum of the left foot.
R1 resection elsewhere
MRI showed a residual contrast-enhancing mass; below-knee amputation was recommended elsewhere.
Oncologically adequate secondary wide resection including the extensor muscles of the toes and the dorsal cortex of the tarsal bones. The tendon of the anterior tibial muscle could be preserved owing to its isolated synovial position, and the divided toe extensors were tenodesed to prevent the development of claw toes. The defect was covered by the microsurgical transfer of a fasciocutaneous flap from the upper arm. Adjuvant radiotherapy was carried out postoperatively.
Functional outcome 3 years after operation
(Neo-)adjuvant measures
Radiotherapy
Since the publication of the work of Rosenberg et al. at the beginning of the 1980s, radiotherapy has been firmly established as an adjuvant to wide excision in all subfascial and G2/G3 sarcomas (13). This multimodal regimen achieves local tumor control in 95% of cases (15). It is uncertain whether adjuvant radiotherapy improves overall survival (16, 17). At this juncture it should again be stressed that radiotherapy alone following excision with narrow margins is inferior to (primary or secondary) wide resection (10, 18). The timing of radiotherapy (pre-, peri-, or postoperative) remains controversial; the arguments for and against each modality cannot be given in full here (19). The most frequently used modality is postoperative irradiation in doses of 50 to 60 Gy, sometimes boosted to 66 Gy (12). Arguments in favor of preoperative radiotherapy are application of a lower dose over a smaller field, potential prevention of tumor seeding during surgery, and potential simplification of surgery by reduction in tumor size (19). A neo-adjuvant combination of radiotherapy and chemotherapy (MAID protocol) followed by surgery and postoperative chemotherapy with or without irradiation achieved a distinct improvement in overall survival compared with a historical control group (20); however, other authors were critical of this protocol’s toxicity (15). In a large series, O’Sullivan et al. showed that patients with preoperative irradiation had a much higher rate of impaired wound healing than those with postoperative radiotherapy (18). On closer inspection, this was not true for the upper limb and late adverse effects were significantly less common in the neo-adjuvantly treated group than in the group with adjuvant radiotherapy (e19). The reconstructive options with flap repair ensuring tension-free wound closure also play a major part (10). This brief account of the controversy demonstrates once more the necessity of interdisciplinary discussions among the members of the tumor board.
Chemotherapy
The benefit of neo-adjuvant and/or adjuvant chemotherapy in the treatment of soft tissue sarcomas remains controversial. Exceptions to this are Ewing sarcoma and rhabdomyosarcoma, in which the addition of neo-adjuvant and/or adjuvant chemotherapy to local therapy can prolong progression-free survival and lower the risk of local recurrence. Patients with Ewing sarcoma should therefore be treated in the framework of the ongoing studies by the EURO-EWING study group. In the case of rhabdomyosarcoma, particularly embryonal rhabdomyosarcoma, treatment according to pediatric protocols should also be considered for young adults. For the remaining soft tissue sarcomas the role of neo-adjuvant or adjuvant chemotherapy is still not clearly defined despite numerous studies and meta-analyses. In a meta-analysis published in 2008, Pervaiz et al. found an extension of overall survival after adjuvant chemotherapy with a combination of doxorubicin and ifosfamide (21). In contrast, two EORTC studies showed no advantage of adjuvant chemotherapy with doxorubicin and ifosfamide for overall survival, with the exception of R1 resection (Woll et al.: Adjuvant chemotherapy with doxorubicin and ifosfamide in resected soft tissue sarcoma [STS]: interim analysis of a randomised phase III trial [abstract]. J Clin Oncol 2007; 25: 547s; Le Cesne et al.: The end of adjuvant chemotherapy era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma: Pooled analysis of the two STBSG-EORTC phase III clinical trials [abstract]. J Clin Oncol 2008; 26: 559s). Given these conflicting data it is not possible to make a general recommendation in favor of adjuvant chemotherapy. If possible, therefore, patients should be included in clinical studies. For patients treated outside the framework of studies, adjuvant chemotherapy must be decided on a case-by-case basis, taking account of the individual characteristics of the patient and the tumor.
The role of chemotherapy in the neo-adjuvant situation has also not been clarified. An EORTC study showed no advantage of neo-adjuvant chemotherapy with doxorubicin and ifosfamide as sole treatment over surgery alone, although it must be pointed out that the ifosfamide dose was low (22). To what extent additional chemotherapy—whether in combination or sequentially—improves the results of neo-adjuvant radiotherapy is also not yet clear. The guidelines of the National Comprehensive Cancer Network present neo-adjuvant chemotherapy with postoperative radiotherapy, neo-adjuvant radiotherapy alone, and combined radiochemotherapy as alternatives of equal value. In general, locally advanced high-grade sarcoma with tumor diameter exceeding 5 cm is seen as an indication for neo-adjuvant treatment, particularly if surgery alone would involve amputation or severe functional limitation. One interesting treatment option in this situation is regional hyperthermia in combination with chemotherapy, followed by local treatment measures and adjuvant chemotherapy. A phase III study of this regimen showed improvements in local progression-free survival and in disease-free survival (Issels et al.: Regional hyperthermia improves response and survival when combined with systemic chemotherapy in the management of locally advanced, high-grade soft tissue sarcomas of the extremities, the body wall, and the abdomen: a phase III randomised prospective trial [abstract]. J Clin Oncol 2007; 25: 547se). A treatment option that could be considered in primary inoperable sarcoma of the extremities is isolated perfusion of the affected limb with TNF-alpha, melphalan, and/or interferon or isolated infusion of the limb with, for example, melphalan or dactinomycin.
Conclusion
On the basis of a selective literature review, we present an algorithm intended to reduce delays in the diagnosis of soft tissue sarcomas and ensure that all potentially beneficial treatments are exploited. While the role of surgery can be defined relatively clearly, the options for adjuvant and neo-adjuvant strategies are highly complex and in flux. Treatment can therefore take place only at a center that has a multimodal interdisciplinary tumor board for this relatively rare disease.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high–volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinau HU, Homann HH, Drücke D, Torres A, Soimaru D, Vogt P. Resektionsmethodik und funktionelle Wiederherstellung bei Weichgewebssarkomen der Extremität. Chirurg. 2001;72:501–513. doi: 10.1007/s001040051339. [DOI] [PubMed] [Google Scholar]
- 3.Lehnhardt M, Daigeler A, Homann HH, et al. Importance of specialized centers in diagnosis and treatment of extremitiy-soft tissue sarcomas: Review of 603 cases. Chirurg. 2009;80:341–347. doi: 10.1007/s00104-008-1562-2. [DOI] [PubMed] [Google Scholar]
- 4.Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg. 2009;129:43–49. doi: 10.1007/s00402-008-0576-z. [DOI] [PubMed] [Google Scholar]
- 5.Lahat G, Lazar A, Lev D. Sarcoma epidemiology and etiology: potential environmental and genetic factors. Surg Clin N Am. 2008;88:451–481. doi: 10.1016/j.suc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Lahat G, Tuvin D, Wei C, et al. New perspectives for staging and prognosis in soft tissue sarcomas. Ann Surg Oncol. 2008;15:2739–2748. doi: 10.1245/s10434-008-9970-6. [DOI] [PubMed] [Google Scholar]
- 7.Deutsche Gesellschaft für Hämatologie und Onkologie. Leitlinien für Weichteilsarkome des Erwachsenen. www.dgho.de/informationen/leitlinien/solide-tumore [Google Scholar]
- 8.Bruns J, Delling G, Henne-Bruns D, Hossfeld DK. Die Biopsie bei Tumoren des muskuloskeletalen Systems. Dtsch Arztebl. 2008;105:492–497. doi: 10.3238/arztebl.2008.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson CJ, Pynsent PB, Grimer RJ. Clinical features of soft tissue sarcomas. Ann R C Surg E. 2001;83:203–205. [PMC free article] [PubMed] [Google Scholar]
- 10.Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. JPRAS. 2009;62:161–174. doi: 10.1016/j.bjps.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Lehnhardt M, Daigeler A, Hauser J, et al. The value of expert second opinion in diagnosis of soft tissue sarcomas. J Surg Oncol. 2008;97:40–43. doi: 10.1002/jso.20897. [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Jost L, Sleijfer S, et al. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(s):ii89–ii93. doi: 10.1093/annonc/mdn101. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of softtissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculosceletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 15.Kaushal A, Citrin D. The role of radiation therapy in the managment of sarcomas. Surg Clin N Am. 2008;88:629–646. doi: 10.1016/j.suc.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichter AS, Lawrence Recent advances in radiation oncology. N Eng J Med. 1995;332:371–379. doi: 10.1056/NEJM199502093320607. [DOI] [PubMed] [Google Scholar]
- 17.Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncologica. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 19.Hohenberger P, Wysocki WM. Neoadjuvant treatment of locally advanced soft tissue sarcoma of the limbs: which treatment to choose? The Oncologist. 2008;13:175–186. doi: 10.1634/theoncologist.2007-0165. [DOI] [PubMed] [Google Scholar]
- 20.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 21.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 22.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‚high-risk‘ adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- e1.Gustafson P, Dreinhofer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand. 1994;65:47–50. doi: 10.3109/17453679408993717. [DOI] [PubMed] [Google Scholar]
- e2.Clasby R, Tilling K, Smith MA, et al. Variable management of soft tissue sarcoma: regional audit with implications for specialist care. Br J Surg. 1997;84:1692–1696. [PubMed] [Google Scholar]
- e3.Ray-Coquard I, Thiesse P, Ranchere-Vince D, et al. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann Oncol. 2004;15:307–315. doi: 10.1093/annonc/mdh058. [DOI] [PubMed] [Google Scholar]
- e4.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- e5.Rydholm A. Improving the management of soft tissue sarcoma. Diagnosis and treatment should be given in specialist centres. BMJ. 1998;317:93–94. doi: 10.1136/bmj.317.7151.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand. 1994;259:1–31. [PubMed] [Google Scholar]
- e7.Noria S, Davis AM, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. JBJS. 1996;78:650–655. doi: 10.2106/00004623-199605000-00003. [DOI] [PubMed] [Google Scholar]
- e8.Davis AM, Kandel R, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66:81–87. doi: 10.1002/(sici)1096-9098(199710)66:2<81::aid-jso2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- e9.Goodlad JR, Fletcher CD, Smith MA. Surgical resection of primary soft-tissue sarcoma. Incidence of residual tumour in 95 patients needing re-excision after local resection. JBJS (Br) 1996;78:658–661. [PubMed] [Google Scholar]
- e10.Grimer RJ. Size matters for sarcomas! Ann R Coll Surg Engl. 2006;88:519–524. doi: 10.1308/003588406X130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Berger F, Winkler EC, Ruderer C, et al. Moderne bildgebende Diagnostik bei Weichteilsarkomen. Chirurg. 2009;80:175–185. doi: 10.1007/s00104-008-1594-7. [DOI] [PubMed] [Google Scholar]
- e12.Domanski HA. Fine-needle aspiration cytology of soft tissue lesions: diagnostic challenges. Diagn Cytopathol. 2007;35:768–773. doi: 10.1002/dc.20765. [DOI] [PubMed] [Google Scholar]
- e13.Singer S, Demetri GD, Baldini EH, et al. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75–85. doi: 10.1016/s1470-2045(00)00016-4. [DOI] [PubMed] [Google Scholar]
- e14.Dadia S, Grimer R. Characteristics, diagnosis and treatment of bone and soft tissue sarcomas. Br J Hosp Med. 2007;68:589–593. doi: 10.12968/hmed.2007.68.11.27680. [DOI] [PubMed] [Google Scholar]
- e15.Van de Luijtgaarden AC, De Rooy JW, De Geus-Oei LF, et al. Promises and challenges of positron emission tomography for assesment of sarcoma in daily clinical practice. Cancer Imaging. 2008;8(s):61–68. doi: 10.1102/1470-7330.2008.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Eng J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- e17.Doi K, Kuwata N, Kawakami F, et al. Limb sparing surgery with reinnervated free-muscle transfer following radical excision of soft-tissue sarcoma. Plast Recon Surg. 2007;120:960–969. doi: 10.1097/00006534-199911000-00011. [DOI] [PubMed] [Google Scholar]
- e18.Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg. 2003;50:90–99. doi: 10.1097/00000637-200301000-00016. [DOI] [PubMed] [Google Scholar]
- e19.O’Sullivan B, Davis AM, Turcotte R, et al. Five-year results of a randomized phase III trial of pre-operative vs. post-operative radiotherapy in extremity soft tissue sarcoma. Proc Am Soc Clin Oncol. 2004;23 [Google Scholar]