Abstract
We have recently described an efficient transient expression system mediated by Agrobacterium tumefaciens for the production of HIV-1 Nef protein in Nicotiana benthamiana plants. In order to enhance the yield of recombinant protein we assayed the effect of three gene-silencing viral suppressor proteins (P25 of Potato Virus X, P19 of Artichoke Mottled Crinckle virus and Tomato Bushy Stunt virus) on Nef expression levels. Results demonstrated that AMCV-P19 gave the highest Nef yield (1.3% of total soluble protein) and that this effect was correlated to a remarkable decrease of Nef-specific small interfering RNAs (siRNAs) indicating an effective modulation of RNA silencing mechanisms. Here we report additional data on the production of different heterologous proteins including human immunoglobulin heavy and light chains and a virus coat protein that demonstrate the robustness of this co-agroinfiltration expression system boosted by the AMCV-P19 gene-silencing suppressor.
Key words: Agrobacterium tumefaciens, molecular farming, agroinfiltration, gene-silencing suppressor, HIV, immunoglobulin
Plants represent an ideal host for the production of complex heterologous proteins and in the last decade many examples of the expression of vaccine components, antibody fragments and full-size immunoglobulins have been reported in literature.1 The major advantages of plants over traditional expression systems based on bacterial and mammalian cells are generally represented by the limited risks of contamination by human pathogens and the low production costs, although the latter is only true if high expression yields of the heterologous protein are obtained.2 A major disadvantage of using plant cells for the production of biopharmaceuticals can be also represented by the unwanted post-translational glycan modifications affecting the quality of the final product.3
Two different expression strategies are generally employed for the expression of biopharmaceuticals in plants: stable transformation of the nuclear/chloroplast genome or transient transformation mediated by recombinant viral or Agrobacterium vectors. Transient expression systems proved to offer several such as yields. The use of Agrobacterium tumefaciens (A. tumefaciens) for epichromosomal expression of heterologous proteins is generally obtained by the infiltration of bacterial suspensions in fresh plant tissues. This technology, known as agroinfiltration, allowed the production of different biopharmaceutical proteins such as viral antigens for vaccine formulations and immunoglobulins for both therapy and diagnosis.4–10 The expression yield of heterologous proteins in agroinfiltrated tissues can be dramatically diminished by the activation of post-transcriptional gene silencing (PTGS) in the plant host. The use of plant virus suppressors of gene silencing was demonstrated to greatly reduce this response, increasing by several folds the expression levels of heterologous proteins in agroinfiltrated leaves.11,12
In a recent work we assayed the effect of three gene-silencing viral suppressor proteins [P25 of Potato Virus X (PVX), P19 of Artichoke Mottled Crinckle virus (AMCV) and Tomato Bushy Stunt virus (TBSV)] in vacuum co-agroinfiltration experiments on the expression levels of the HIV-1 Nef antigen. A positive influence on Nef production yield was observed for all three suppressor proteins, but best results were obtained using AMCV-P19, reaching an accumulation level of 1.3% of total soluble protein (TSP). This represented a 4.4 fold increase of Nef yield compared to agroinfiltrated plant tissues with Nef alone. Moreover, a fine analysis of agroinfiltrated plants showed that young top leaves exhibited a significant reduction in expression yield.13
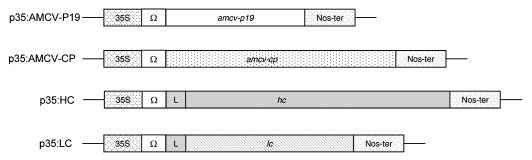
To demonstrate that this transient expression system, based on the use of AMCV-P19 gene-silencing suppressor, can be successfully employed for the high-yield production of different proteins, three constructs encoding a human immunoglobulin heavy (HC) or light chain (LC) and the AMCV coat protein (AMCV-CP) were tested (Fig. 1). Six weeks old N. benthamiana plants were infiltrated with A. tumefaciens strain LBA 4404 containing the binary vectors carrying p35:HC or p35:LC expression cassettes (Fig. 1), harboring the sequence encoding the HC or LC under the trascriptional control of Cauliflower Mosaic Virus (CaMV) 35S promoter and the translational enhancer omega (Ω) of the Tobacco Mosaic Virus.14 Plants were also co-infiltrated with either two A. tumefaciens clones harboring HC and the AMCV-P19 gene silencing suppressor or LC and AMCV-P19 as previously described.7 Leaves from the fifth node (bottom leaf) were collected at 3, 5, 7, 9 days post infiltration (d.p.i.) and expression was assayed by Double-Antibody-Sandwich (DAS)-ELISA.
Figure 1.
Schematic representation of the constructs used in this study. All genes are under the control of the CaMV 35S promoter (35S) and the Tobacco Mosaic Virus (TMV) 5′ leader sequence omega (Ω). L: murine secretory leader peptide. amcv-p19: cDNA encoding AMCV p19 silencing suppressor; amcv-cp: cDNA encoding AMCV coat protein; hc and lc: cdnas encoding a human immunoglobulin heavy chain and light chain respectively.
Quantitative ELISA was performed to evaluate expression levels of HC, LC and AMCV-CP using agroinfiltrated leaf tissue (100 mg) ground in liquid nitrogen and homogenized in PBS with the addition of 0.2% Tween 20 (Sigma-Aldrich) and protease inhibitors (Complete™ Roche). The supernatants were recovered and quantified for TSP using the Bradford colorimetric assay (Bio-Rad, Hercules, CA, USA) and the leaf extracts were normalized for TSP concentration. Expression levels reported as % TSP (Table 1) were calculated by ELISA performed on three biological replicas (bottom leaves collected from three agroinfiltrated plants). The anti-human γ chain (I6010, Sigma-Aldrich) or anti-human λ chain (L6522, Sigma-Aldrich) were used as capture antibodies while, the anti-human γ chain HRP-conjugated (A8419, Sigma-Aldrich) or anti-human λ chain HRP-conjugated (A5175, Sigma-Aldrich) as secondary antibodies. As internal standard, a human IgG1-λ (I5029, Sigma-Aldrich) at known concentrations (ranging from 1 to 100 ng) spiked in wild type N. benthamiana extract was used.
Table 1.
Expression yield of recombinant proteins by transient agroinfiltration using the AMCV-P19 gene silencing suppressor
| Recombinant protein | Yield (% TSP) (− AMCV-P19) | Yield (% TSP)a (+ AMCV-P19) |
| Nef | 0.3* ± 0.03 | 1.3* ± 0.23 |
| IgG Heavy Chain (HC) | 1.25 ± 0.05 | 10.0 ± 0.5 |
| IgG Light Chain (LC) | 5.0 ± 0.15 | 15.0 ± 0.25 |
| AMCV coat protein (AMCV-CP) | 0.5 ± 0.08 | 1.5 ± 0.12 |
Expression yield of recombinant proteins calculated by quantitative ELISA is reported as percentage of total soluble proteins (% TSP). Values are the mean ± SD of three biological replicas (protein extraction performed on leaves from three agroinfiltrated plants). In the case of Nef and AMCV-CP, maximum expression levels were obtained at day 9 post infiltration. HC and LC highest levels were obtained 7 days post infiltration.
Data reported by Lombardi et al.13
Maximum expression levels in plants infiltrated with just the LC construct were obtained at 5 d.p.i. with a decrease from day 5 to day 9 post-infiltration as showed by quantitative ELISA results (Fig. 2A). In the case of plants co-infiltrated with both LC and AMCV-P19 the expression peak was observed at 7 d.p.i. with a decrease at 9 d.p.i. reaching maximum levels of 15% TSP (Table 1). Plant extracts expressing HC and LC, normalized for TSP content, were also analysed by western blot analysis. The results showed the presence of a band of the expected size (∼25 kDa) in plants agroinfiltrated with and without the P19 silencing suppressor and confirmed a threefold increase of LC yield in the plants with P19 (Fig. 2B). Plants infiltrated with the HC construct, revealed a similar expression behaviour to those infiltrated with LC. In fact, also in this case maximum expression levels were observed at 5 d.p.i. with HC alone and at 7 d.p.i in the plants co-infiltrated with P19, reaching levels of 10% TSP (Fig. 2C, Table 1). Western blot analysis of plants agroinfiltrated with or without P19 silencing suppressor showed the presence of a band of the expected size (∼50 kDa) and about a eight fold increase of HC expression levels was detected in the plants co-infiltrated with the P19 silencing suppressor (Fig. 2D).
Figure 2.
Transient expression analysis of human immunoglobulin heavy (HC) and light (LC) chains and AMCV coat protein. Plant extracts collected at different time points [3, 5, 7, 9 days post infiltration (d.p.i.)] expressing the LC, were analysed either by DAS-ELISA (A) or western blot on reducing SDS-10% (w/v) PAGE using an anti-λ antibody (B). (A) LC: plants agroinfiltrated with LC only; LC/AMCV-P19: plants co-agroinfiltrated with LC and AMCV-P19; C+: 20 ng of purified IgG1 human antibody used as a positive control; C−: mock infiltrated plants. Plant extracts were normalized for total soluble proteins (TSP) (200 ng of TSP were loaded in each well). (B) C+: 50 ng of purified IgG1 human antibody used as a control; C−: mock infiltrated plants; MWM: Protein molecular weight marker used as reference. Plant extracts were normalized for TSP (20 µg TSP were loaded). Extracts expressing the HC, were also analysed by DAS-ELISA (C) or western blot using an anti-γ antibody (D) at exactly the same conditions used for LC. (E) ELISA of plant extracts expressing the AMCV-CP. CP: plants agroinfiltrated with AMCV-CP only; CP/AMCVP19: plants co-agroinfiltrated with AMCV-CP and P19; C−: mock infiltrated plants used as a control; C+: 100 ng of purified AMCV virus particles. Plant extracts were normalized for TSP content (50 µg TSP were loaded). Here we report representative ELISA results for each construct (HC, LC and AMCV-CP) obtained analysing leaves from a single agroinfiltrated plant and values are the mean ± standard error of the mean (SEM) of three technical replicates.
Similar results were obtained in N. benthamiana leaves co-agroinfiltrated with mixed Agrobacterium cultures carrying P35:AMCV-P19 and P35:AMCV-CP expression cassettes (Fig. 1). AMCV-CP expression levels were evaluated by ELISA, using plant extracts derived from agroinfiltrated tissues. The amount of TSP was estimated by Bradford assay and leaf extracts were normalized for TSP concentration. As primary antibody the mouse monoclonal antibody mAb F8,15 was used and an anti-mouse HRP-conjugated (GE Healthcare NXA931) as secondary antibody. Plant recombinant CP levels were estimated using a standard curve obtained from serial dilutions of purified AMCV virus particles. In this case the expression peak of the virus coat protein was observed at 9 d.p.i. and expression levels were increased three times in the presence of AMCV-P19 reaching 1.5% TSP (Fig. 2E, Table 1).
In conclusion, we have demonstrated that the Agrobacterium-based transient expression system boosted by AMCV-P19 strongly enhances the production of different types of proteins including HIV-1 Nef antigen, human immunoglobulin heavy and light chains as well as a plant virus coat protein, offering several advantages over the generation of transgenic plants represented by higher protein yields, rapidity of production and cost-effectiveness.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/11723
References
- 1.Spök A, Twyman RM, Fischer R, Ma JK, Sparrow PA. Evolution of a regulatory framework for pharmaceuticals derived from genetically modified plants. Trends Biotechnol. 2008;26:506–517. doi: 10.1016/j.tibtech.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Daniell H, Sheatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001;6:219–226. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orzáez D, Granell A, Blázquez MA. Manufacturing antibodies in the plant cell. Biotechnol J. 2009;4:1712–1724. doi: 10.1002/biot.200900223. [DOI] [PubMed] [Google Scholar]
- 4.Marusic C, Vitale A, Pedrazzini E, Donini M, Frigerio L, Bock R, et al. Plant-based strategies aimed at expressing HIV antigens and neutralizing antibodies at high levels. Nef as a case study. Transgenic Res. 2009;18:499–512. doi: 10.1007/s11248-009-9244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons CW, VanderGheynst JS. Transient coexpression of post-transcriptional gene silencing suppressors and beta-glucuronidase in harvested lettuce leaf tissue does not improve recombinant protein accumulation in planta. Biotechnol Lett. 2007;29:641–645. doi: 10.1007/s10529-006-9279-0. [DOI] [PubMed] [Google Scholar]
- 6.Sainsbury F, Lomonossoff GP. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008;148:1212–1218. doi: 10.1104/pp.108.126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villani ME, Morgun B, Brunetti P, Marusic C, Lombardi R, Pisoni I, et al. Plant pharming of a full-sized, tumour-targeting antibody using different expression strategies. Plant Biotech J. 2009;7:59–72. doi: 10.1111/j.1467-7652.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 8.Kathuria S, Sriraman R, Nath R, Sack M, Pal R, Artsaenko O, et al. Efficacy of plant-produced recombinant antibodies against HCG. Hum Reprod. 2002;17:2054–2061. doi: 10.1093/humrep/17.8.2054. [DOI] [PubMed] [Google Scholar]
- 9.Hull AK, Criscuolo CJ, Mett V, Groen H, Steeman W, Westra H, et al. Human-derived, plant-produced monoclonal antibody for the treatment of anthrax. Vaccine. 2005;18:2082–2086. doi: 10.1016/j.vaccine.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotech J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 11.Johansen LK, Carrington JC. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 2001;126:930–938. doi: 10.1104/pp.126.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi R, Circelli P, Villani ME, Buriani G, Nardi L, Coppola V, et al. High-level HIV-1 Nef transient expression in Nicotiana benthamiana using the P19 gene silencing suppressor protein of Artichoke Mottled Crinckle Virus. BMC Biotechnology. 2009;9:96. doi: 10.1186/1472-6750-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marusic C, Nuttall J, Buriani G, Lico C, Lombardi R, Baschieri S, et al. Expression, intracellular targeting and purification of HIV Nef variants in tobacco cells. BMC Biotechnol. 2007;26:12. doi: 10.1186/1472-6750-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavladoraki P, Benvenuto E, Trinca S, De Martinis D, Cattaneo A, Galeffi P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature. 1993;366:469–472. doi: 10.1038/366469a0. [DOI] [PubMed] [Google Scholar]