Summary
Synaptogenesis is required for wiring neuronal circuits in the developing brain and continues to remodel adult networks. However, the molecules organizing synapse development and maintenance in vivo remain incompletely understood. We now demonstrate that the immunoglobulin adhesion molecule SynCAM 1 dynamically alters synapse number and plasticity. Overexpression of SynCAM 1 in transgenic mice promotes excitatory synapse number, while loss of SynCAM 1 results in fewer excitatory synapses. By turning off SynCAM 1 overexpression in transgenic brains, we show that it maintains the newly induced synapses. SynCAM 1 also functions at mature synapses to alter their plasticity by regulating long-term depression. Consistent with these effects on neuronal connectivity, SynCAM 1 expression affects spatial learning, with knock-out mice learning better. The reciprocal effects of increased SynCAM 1 expression and loss reveal that this adhesion molecule contributes to the regulation of synapse number and plasticity, and impacts how neuronal networks undergo activity-dependent changes.
Keywords: synapse formation, synapse maintenance, synaptic plasticity, LTD, learning, synaptic adhesion, SynCAM, CADM, nectin-like molecule
Introduction
Synapse formation is required for the development of the nervous system and dynamic changes of synapses in the mature brain are associated with cognitive functions such as learning and memory. Notably, aberrant synapse structures are present in mental retardation and neurological disorders (Fiala et al., 2002; Irwin et al., 2001). Elucidating the molecular machinery that organizes synapses is therefore relevant to our understanding both of physiological functions as well as debilitating brain disorders.
Protein interactions across the synaptic cleft are now known to organize developing synapses (Biederer and Stagi, 2008; Giagtzoglou et al., 2009; Jin and Garner, 2008). Postsynaptic adhesion molecules of the neuroligin and SynCAM families and EphB receptors drive the differentiation of synapses (Biederer et al., 2002; Chih et al., 2005; Chubykin et al., 2007; Graf et al., 2004; Kayser et al., 2006; Nam and Chen, 2005; Scheiffele et al., 2000). In addition, neuroligins control synapse specification and maturation (Chubykin et al., 2007; Varoqueaux et al., 2006), while cadherins contribute to the structural development and plasticity of synapses (Okamura et al., 2004; Togashi et al., 2002). Mutations in neuroligin and neurexin genes have been linked to familial forms of autism-spectrum disorders, supporting the hypothesis that synapse disorganization and imbalanced neuronal excitation can result in neurodevelopmental disorders (Bourgeron, 2009; Südhof, 2008; Zoghbi, 2003). Consistent with the physiological relevance of synapse-organizing molecules, links of SynCAM 1 and cadherins to autism-spectrum disorders have also been reported (Wang et al., 2009; Zhiling et al., 2008).
Among the select proteins that drive synapse formation, SynCAM 1 (also known as Cadm1 and nectin-like molecule 2) is the founding member of a family of four immunoglobulin (Ig) proteins that are expressed throughout the developing and mature brain (Biederer, 2006; Thomas et al., 2008). SynCAM 1 participates in axo-dendritic interactions, indicating early roles in the contact-mediated differentiation of synapses (Stagi et al., 2010). At later developmental stages, SynCAM proteins are enriched in pre- and postsynaptic plasma membranes and engage in specific homo- and heterophilic adhesive interactions (Biederer et al., 2002; Fogel et al., 2007). Functionally, the heterophilic partners SynCAM 1 and SynCAM 2 drive presynaptic terminal formation in cultured neurons and increase the number of excitatory, but not inhibitory synapses (Biederer et al., 2002; Fogel et al., 2007; Sara et al., 2005).
Despite the significant molecular insights into the synapse-organizing roles of trans-synaptic interactions, three decisive aspects remain insufficiently understood. First, to which extent are synapse numbers regulated by synaptic adhesion in the brain? Second, at which steps during the lifetime of synapses are individual synaptic adhesion molecules engaged? Third, do these synapse-organizing proteins alter synaptic physiology and affect neuronal network functions?
We now demonstrate that SynCAM 1 significantly impacts these three aspects. Combining electron microscopy, Golgi staining, and electrophysiology, we demonstrate that elevated expression of SynCAM 1 in a transgenic mouse model increases functional excitatory synapse number. This activity corresponds to its endogenous role, as SynCAM 1 knock-out (KO) mice exhibit fewer excitatory synapses. Moreover, SynCAM 1 functions dynamically at developing synapses: Using the temporal control afforded by the design of our transgenic model, we show that continued SynCAM 1 elevation is required to maintain the increase in synapse number it drives in the first place. Unexpectedly, SynCAM 1 additionally alters the plasticity of synapses once they are formed, with its overexpression abrogating long-term depression (LTD) and its loss increasing LTD. These complementary changes in synapse number and plasticity correlate with altered cognitive functions and SynCAM 1 KO mice exhibit improved spatial learning. Our results reveal important contributions of SynCAM 1 to excitatory synapse number and function in the developing and adult brain. This supports a model that synaptic adhesion by SynCAM 1 can promote synapse formation and restrict synaptic plasticity to regulate the formation and remodeling of neuronal circuits.
Results
SynCAM Proteins are Prominent in Synaptic Plasma Membranes
To gain better molecular insight into SynCAM properties in vivo, we determined the abundance of the four SynCAM family members. Quantitative immunoblotting of synaptic plasma membranes purified at postnatal day 9 (P9), when excitatory synapse formation begins to peak in the forebrain, showed that the heterophilic adhesion partners SynCAM 1 and 2 accounted for 0.41 ng/μg and 4.0 ng/μg of total synaptic membrane proteins, respectively. SynCAM 3 and 4 were present at 0.46 ng/μg and 0.20 ng/μg in this fraction. Thus, 0.5% of synaptic membrane proteins were comprised of SynCAM proteins at this developmental stage, a high fraction even when compared to the most abundant synaptic protein CaMKII that constitutes 7% of the postsynaptic density (Cheng et al., 2006). Correspondingly, SynCAM proteins were prominent in brain homogenates (see Table S1) where they are considerably more abundant than neuroligins (Varoqueaux et al., 2006). This prominent expression indicated that SynCAM proteins could play important roles at synapses.
Overexpression of SynCAM 1 with Temporal Control
Redundancy and compensation between synapse-organizing proteins may preclude the identification of phenotypes in mice lacking single synaptic adhesion molecules as previously reported (Varoqueaux et al., 2006). Specifically, the single loss of SynCAM 1 in KO mice may be compensated by other SynCAM family members or functionally related proteins. We therefore reasoned that elevating SynCAM 1 in neurons may expose its in vivo activities more readily than a loss-of-function approach. To pursue the overexpression of SynCAM 1 in vivo, we generated a mouse line carrying a transgene encoding flag-epitope tagged SynCAM 1 under the control of a Tet-responsive element (TRE) (Mansuy and Bujard, 2000) (Figure 1A). This line was crossed to mice transgenically expressing the transcriptional transactivator tTA from the CaMKII promoter, which is active in excitatory forebrain neurons (Mayford et al., 1996). In the resulting TRE-SynCAM 1flag × CaMKII-tTA mice, tTA mediated the expression of the transgenic SynCAM 1flag protein throughout neurons in the forebrain, similar to its endogenous distribution (Figure S1A and S1B) (Thomas et al., 2008). Mice carrying only the CaMKII-tTA transgene did not exhibit altered SynCAM 1 protein levels (data not shown) and served as controls for transgenic SynCAM 1flag overexpressors (OE) in this study.
Figure 1. Development of a Transgenic SynCAM 1 Mouse Model.
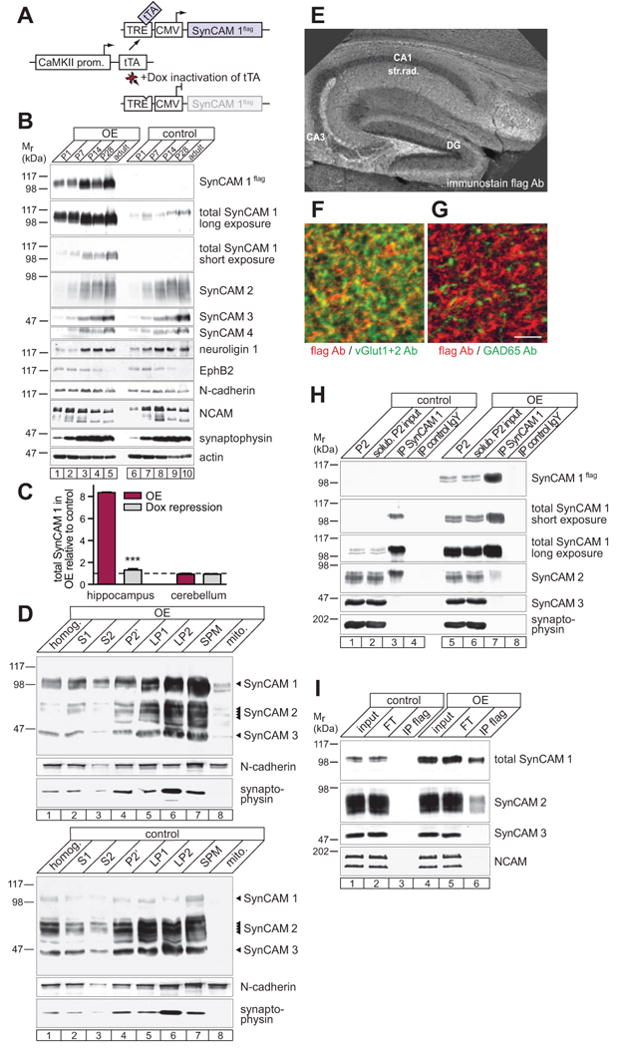
(A) SynCAM 1 Tet-Off transgenic design. The CaMKII promoter restricts expression of the transcriptional transactivator tTA to excitatory forebrain neurons. tTA binds a Tet-responsive element (TRE) to drive flag-tagged SynCAM 1. Doxycycline (Dox) inhibits tTA.
(B) Transgenic SynCAM 1flag expression in forebrain (lanes 1-5) follows the endogenous profile (lanes 6-10) as shown by immunoblotting. Other synaptic proteins are unchanged. P, postnatal day.
(C) Transgenic expression elevates SynCAM 1 in hippocampus as analyzed by quantitative immunoblotting at P28. Overexpression (OE) occurred until P28 or was repressed with Dox from P14 until 28. Signals were normalized to littermate controls.
(D) SynCAM 1flag (OE, top) fractionates with synaptic plasma membranes (SPM) similar to endogenous SynCAM 1 (tTA control, bottom). SynCAM 1-3 were detected at their distinct molecular weights with a pleio-antibody. N-cadherin marks SPM and synaptophysin synaptic vesicles (LP2). SynCAM 1 and 2 are present in LP2 is due to non-vesicular fractions (Fogel et al., 2007). S, supernatant; P, pellet; LP, lysis pellet; mito, mitochondria.
(E-G) SynCAM 1flag is sorted to excitatory synapses as analyzed at P21. (E) Anti-flag immunostaining of CA1 stratum radiatum and CA3 mossy fiber terminals of hippocampus. DG, dentate gyrus. (F and G) SynCAM 1flag localizes to excitatory but not inhibitory synapses. Both panels show the same triple-labeled hippocampal section at P21. Red marks flag staining in both panels and green represents either vGlut-positive excitatory synapses (F) or GAD65-positive inhibitory synapses (G). Scale bar, 5 μm.
(H) Co-immunoprecipitation (IP) of SynCAM 2 with SynCAM 1 from synaptosomes at P55 is reduced in SynCAM 1 OE compared to controls. SynCAM 3 and synaptophysin were negative controls. Input lanes contain 5% of the extract used for the IP. Same results were obtained at 12 months. P2, synaptosomes.
(I) SynCAM 2 is co-immunoprecipitated with overexpressed SynCAM 1flag using flag antibodies. Input from tTA animals served as negative control for the IP.
The developmental profile of SynCAM 1flag protein expression in OE mice followed the increase of endogenous SynCAM 1 in the postnatal forebrain, without altering the expression of SynCAM 2-4 (Figure 1B). No change in the expression profile of other synaptic proteins was detected (Figure 1B) and quantitative immunoblotting at P28 confirmed that the amounts of neuroligin 1, NCAM, and N-cadherin were unaltered in these mice (data not shown). Our transgenic design resulted in 8.4 ± 0.03 fold higher amounts of total SynCAM 1 in the hippocampus at P28, without affecting its expression in the cerebellum where the CaMKII promoter is inactive and tTA is not expressed (Figure 1C). This double-transgenic mouse model constitutes a Tet-Off system (Mansuy and Bujard, 2000) and administration of the tTA inhibitor doxycycline tightly repressed SynCAM 1flag approximately to control levels (Figure 1C).
Synaptic Expression of Transgenic SynCAM 1
Subcellular fractionation confirmed the enrichment of overexpressed SynCAM 1 in synaptic plasma membranes purified from forebrain of adult OE mice, similar to endogenous SynCAM 1 in controls (Figure 1D). Consequently, total SynCAM 1 amounts were increased 8-fold in synaptic plasma membranes purified from the forebrain of adult OE mice (Figure S1C). Immunohistochemical analysis confirmed the correct subcellular sorting of SynCAM 1flag to synaptic regions, while cell body layers lacked staining (Figure 1E). Analysis at higher magnification revealed that the majority of vGlut1/2-positive excitatory synapses expressed SynCAM 1flag (Figure 1F), whereas it was not detected at inhibitory synapses marked by GAD65 (Figure 1G). This agreed with previous immuno-electron microscopic results that SynCAM proteins are endogenously present at excitatory synapses (Biederer et al., 2002). We additionally immunoprecipitated SynCAM 1 to test whether overexpression alters its extracellular interactions (Figure 1H). Control mice showed strong heterophilic interaction of SynCAM 1 with SynCAM 2 (lanes 1-4) as reported previously (Fogel et al., 2007). Overexpression of SynCAM 1 reduced the amount of bound SynCAM 2 (lanes 5-8), presumably because elevation of SynCAM 1 increased the formation of homophilic SynCAM 1 adhesion complexes and competed SynCAM 2 out of the heterophilic interaction. Heterophilic SynCAM 1/2 complexes were however still readily detected in the overexpressors, consistent with the co-immunoprecipitation of endogenous SynCAM 2 with overexpressed SynCAM 1flag using anti-flag antibodies (Figure 1I). Together, this transgenic SynCAM 1 mouse model replicated the properties of endogenous SynCAM 1 and was suited to identify roles of SynCAM 1 in the postnatal brain.
SynCAM 1 Promotes the Number of Excitatory Synapses
Using electron microscopy, we determined the effects of altered SynCAM 1 expression on the density and ultrastructure of excitatory (Gray type I, asymmetric) and inhibitory (Gray type II, symmetric) synapses in the CA1 stratum radiatum of the hippocampus (Figures 2A and 2H). This area was selected as the morphological and physiological properties of its synapses are well characterized. Importantly, the density of excitatory synapses in SynCAM 1 overexpressing mice was increased by 26 ± 3%, while the number of the less abundant inhibitory synapses was not affected (Figure 2B). In addition, we used electron microscopy to count inhibitory synapses at perisomal regions of CA1 pyramidal neurons, where inhibitory synapses are prominent (Megias et al., 2001). As in CA1 stratum radiatum, no difference in perisomal inhibitory synapse density was observed between SynCAM 1 OE and control mice (Figure S2A and S2B). These results demonstrated that SynCAM 1 specifically increases excitatory synapse number. They also show that the elevation of SynCAM 1 in the complex environment of the brain is not compensated by mechanisms negatively regulating excitatory synapse number. Further, these findings agree with the synaptogenic activities of SynCAM 1 previously demonstrated in cultured hippocampal neurons (Biederer et al., 2002; Fogel et al., 2007; Sara et al., 2005). The absence of an effect on inhibitory synapse number in vivo was consistent with our transgenic design that overexpressed SynCAM 1 in excitatory forebrain neurons, similar to its endogenous expression pattern (Thomas et al., 2008). The average number of synaptic vesicles per excitatory terminal was not altered by SynCAM 1 overexpression (Figure 2C), and the thickness and length of the postsynaptic density (PSD) were also unchanged (Figures 2D and 2E). These results demonstrated that SynCAM 1 overexpression increases excitatory synapse number in vivo without altering their ultrastructure.
Figure 2. SynCAM 1 Regulates Excitatory Synapse Number.
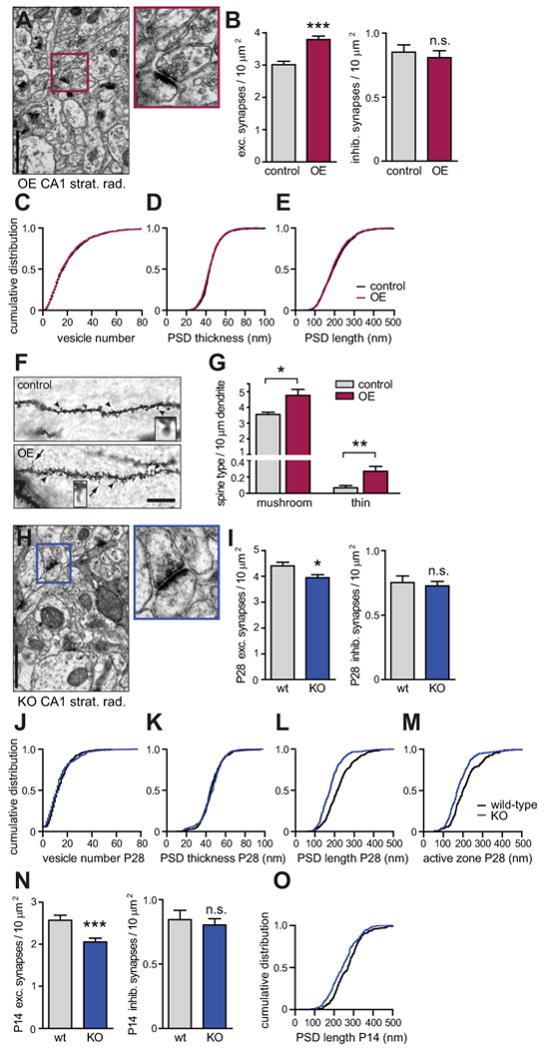
(A-E) Electron microscopy of synapses in CA1 stratum radiatum of SynCAM 1 overexpressors (OE) at 12-15 months. (A) Left, micrograph of overexpressors (magnification 26,500×). Right, excitatory synapse boxed on the left. Scale bar in (A) and (H), 1 μm. (B) SynCAM 1 overexpression increases excitatory synapse density. (tTA controls, n=180 images, 848 synapses, 3 male littermates; OE, n=180 images, 1087 synapses, 3 males) Inhibitory synapse density is unaffected by overexpression. (tTA controls, n=122 images, 160 synapses, 3 males; OE, n=95 images, 119 synapses, 3 males) (C-E) Elevated SynCAM 1 at excitatory synapses alters neither (C) average synaptic vesicle number per terminal (tTA controls 21 ± 0.6, n=793 synapses; OE 21 ± 0.6, n=1018), (D) PSD thickness (tTA controls 45 ± 0.4 nm, n=846; OE 45 ± 0.3 nm, n=1087), nor (E) PSD length (tTA controls 204 ± 3 nm, n=846; OE 200 ± 2 nm, n=1087). Distributions were identical by the Kolmogorov-Smirnov (KS) test (p=1 in C-E).
(F and G) Golgi staining in CA1 stratum radiatum at 5 months. (F) Apical secondary and tertiary dendrites of tTA controls (top) and SynCAM 1 OE (bottom). Arrowheads point to mushroom-type spines and arrows to thin spines, with examples enlarged. Scale bar, 10 μm. (G) Higher mushroom-type and thin spine density in SynCAM 1 OE. (tTA controls, n=422 spines, 3 male littermates; OE, n=757, 3 males)
(H-M) Electron microscopic analysis of synapses in CA1 stratum radiatum of SynCAM 1 KO mice at P28. (H) Micrograph of KO mice (magnification 26,500×) with one excitatory synapse enlarged. (I) SynCAM 1 KO mice have fewer excitatory synapses. (wild-type, n=120 images, 825 synapses, 2 male littermates; KO, n=180 images, 1110 synapses, 3 males) Lack of SynCAM 1 affects neither (I) inhibitory synapse density (wt, n=24 synapses; KO, n=40), (J) synaptic vesicle number per terminal (wt 14 ± 0.5, n=391 synapses; KO 13 ± 0.6, n=302), nor (K) PSD thickness (wt 46 ± 0.6 nm, n=391; KO 46 ± 0.7 nm, n=302; KS test p=1 in L, M). (L) SynCAM 1 KO shortens PSDs (wt 224 ± 4 nm, n=391 synapses; KO 182 ± 4 nm, n=302) and (M) active zones (wt 216 ± 6 nm, n=180; KO 184 ± 4 nm, n=236; KS test p<0.001 in L, M).
(N and O) Electron microscopy of synapses in CA1 stratum radiatum of SynCAM 1 KO mice at P14. (N) Lack of SynCAM 1 reduces excitatory synapse number already at P14. (wild-type, n=53 images, 211 synapses, 2 male littermates; KO, n=92 images, 293 synapses, 2 males) but does not affect inhibitory synapse density (wt, n=68 synapses; KO, n=117) (O) SynCAM 1 KO shortens PSDs already at P14 (wt 269 ± 5 nm, n=208 synapses; KO 243 ± 4 nm, n=306; KS test p<0.01).
We considered that our electron microscopic study was likely biased towards excitatory synapses on mushroom-type spines as these are most prominent and readily identifiable. For a comprehensive analysis of all spine types, we employed Golgi staining (Figure 2F) and classified spines of pyramidal neurons in CA1 stratum radiatum using described criteria (Knott et al., 2006). This demonstrated an increase in total spine density by 37 ± 10% in SynCAM 1 overexpressors. Morphometric scoring determined a 34 ± 10% increase in the density of mushroom-type spines per dendrite length, and a 4-fold increase in the number of the far less prominent thin spines (Figure 2G). The density of stubby spines and the small fraction of unclassifiable spine structures was unchanged (data not shown). These results agree with our electron microscopic analysis and additionally revealed an increased number of thin spines, which can correspond to sites of new synapses (Knott et al., 2006; Ziv and Smith, 1996).
Endogenous SynCAM 1 Regulates Excitatory Synapse Number and Structure
The effects of SynCAM 1 overexpression motivated us to analyze synapses in the brain of KO mice lacking SynCAM 1 to determine whether the organization of synapses is its endogenous function. The only previously known phenotype of SynCAM 1 KO neurons is their more exuberant growth cone morphology in early development (Stagi et al., 2010), while synaptic changes remained to be addressed. The one apparent phenotype of these KO mice is male infertility due to impaired spermatid adhesion (Fujita et al., 2006). Our electron microscopic analysis of the hippocampal CA1 stratum radiatum at P28 showed that the number of excitatory synapses in SynCAM 1 KO mice was significantly reduced by 10 ± 3% (Figure 2I), demonstrating that it is a biological function of SynCAM 1 to contribute to synapse organization. As in SynCAM 1 overexpressors, the number of inhibitory synapses was neither affected in the CA1 stratum radiatum of KO mice (Figure 2I) nor in the stratum pyramidale (Figure S2C and S2D). The PSD length was reduced in SynCAM 1 KO mice by 19 ± 2%, concomitant with a reduction in active zone length by 15 ± 3%, while other parameters of synapse ultrastructure were unchanged (Figures 2J-M). Electron microscopic analysis demonstrated that the presynaptic terminal area was unchanged in the KO (data not shown), indicating that these ultrastructural effects of SynCAM 1 loss result from impaired interactions across the synaptic cleft and are not due to a non-specific reduction of synapse size.
To address the developmental roles of SynCAM 1 at synapses, we analyzed KO mice at P14. Similar to the results at P28, the lack of SynCAM 1 reduced the number of excitatory synapses by 20 ± 6%, while inhibitory synapse density was unchanged (Figure 2N). PSD length was also shortened by 9 ± 2% (Figure 2O). SynCAM 1 therefore modulates excitatory synapse number at different stages of postnatal development. Moreover, our findings show that endogenous SynCAM 1 not only elevates synapse number but also plays a role in the structural organization of excitatory synapses.
We noted a higher density of excitatory synapses in wild-type controls of the KO mice compared to the transgenic controls containing the tTA transgene alone (Figure 2I and 2B). This likely reflects the different genetic backgrounds of the KO and transgenic mouse strains used in this study. A rescue of the SynCAM 1 KO by transgenic overexpression was not performed because the male infertility of the KO left only breeding strategies with a very low likelihood of obtaining litters that included offspring carrying the SynCAM 1 gene deletion and both transgenes encoding SynCAM 1flag and tTA, and the required littermate controls. Together, the reciprocal effects of overexpression and loss on synapse density in these mouse models demonstrate that SynCAM 1 promotes excitatory synapse numbers in vivo.
SynCAM 1-Induced Synapses are Functional
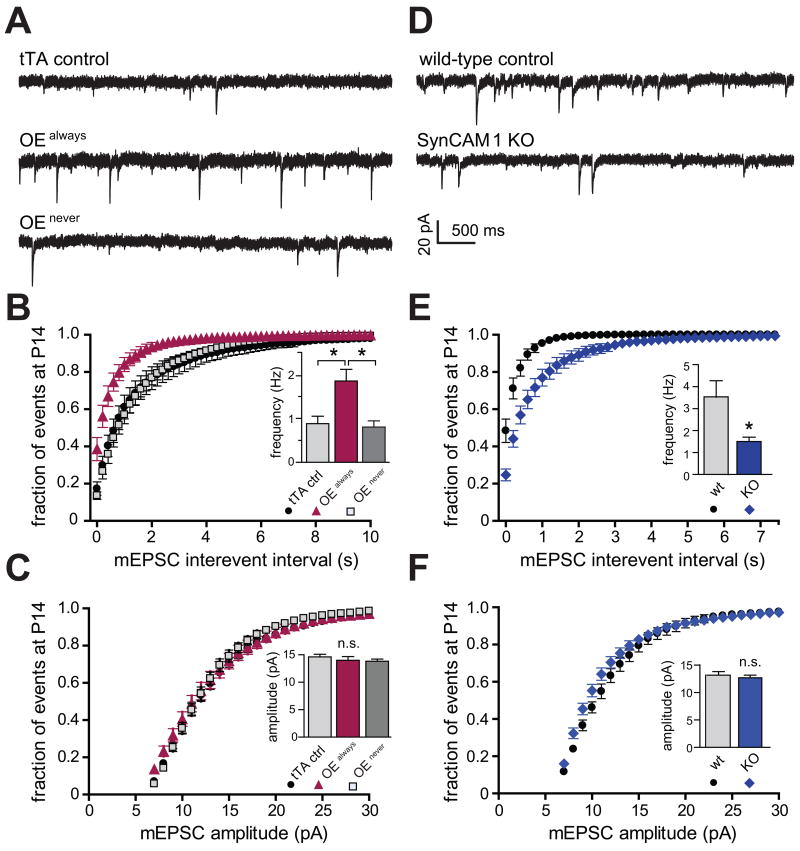
We next addressed whether the synapses gained by SynCAM 1 overexpression were functional. We used miniature excitatory postsynaptic current (mEPSC) frequency as a measure of synapse number, as an increase in the number of functional synapses would increase the frequency of mEPSCs. Recordings were obtained from acute hippocampal slices at P14. Similar to the morphological data, we observed strain-dependent mEPSC differences between overexpressor and KO controls. We therefore only compared relative differences of overexpressors and KOs to their respective littermate controls. Consistent with the increase in morphologically defined synapses, transgenic animals continuously overexpressing SynCAM 1 (OEalways) exhibited a strong 2.1-fold increase in mEPSC frequency compared to control littermates carrying only the tTA transgene (Figures 3A and 3B). mEPSC amplitude was not affected (Figure 3C). The transgenic design allowed us to continuously repress overexpression by administering doxycycline (see Figures 1C and 5B). These doxycycline-treated mice served as additional controls (OEnever) and their mEPSC frequencies and amplitudes were indistinguishable from those of tTA control littermates (Figures 3A-C).
Figure 3. SynCAM 1 Promotes Functional Excitatory Synapses.
Whole-cell mEPSC recordings from hippocampal CA1 pyramidal neurons at P15-19. (A-C) Analysis of mice continuously overexpressing SynCAM 1 (OEalways), mice in which SynCAM 1 overexpression was continuously repressed by doxycycline (OEnever), and tTA littermate controls. (A) Representative traces. (B) Cumulative distributions of mEPSC interevent intervals. mEPSC frequency is strongly increased in OEalways mice compared to tTA controls and OEnever mice. The inset shows average frequencies. (KS test p<0.001 for OEalways vs. tTA and OEalways vs. OEnever, p>0.1 for OEnever vs. tTA; tTA n=14; OEalways n=11; OEnever n=12) (C) mEPSC amplitudes are unaltered by SynCAM 1 overexpression. (tTA controls n=11; tTA controls +Dox n=11 (not depicted); OEalways n=14; OEnever n=12) n.s., not significant.
(D-F) mEPSC recordings from wild-type control and SynCAM 1 knockout littermates at P14-15. (D) Representative traces. Scale bar in (D) applies to (A, D). (E) Loss of SynCAM 1 decreases excitatory synapse number. Cumulative distributions of mEPSC interevent intervals show a strong reduction of mEPSC frequency in SynCAM 1 KO mice compared to wild-type littermates. (KS test p<0.001) The inset depicts average mEPSC frequencies. (wild-type n=16; KO n=8) Different mEPSC frequencies in tTA controls in (B) and controls in (E) are likely due to genetic background differences of these strains (see Supplemental Experimental Procedures). (F) mEPSC amplitudes of SynCAM 1 KO (n=16) mice are not significantly changed compared to wild-type littermates (n=8).
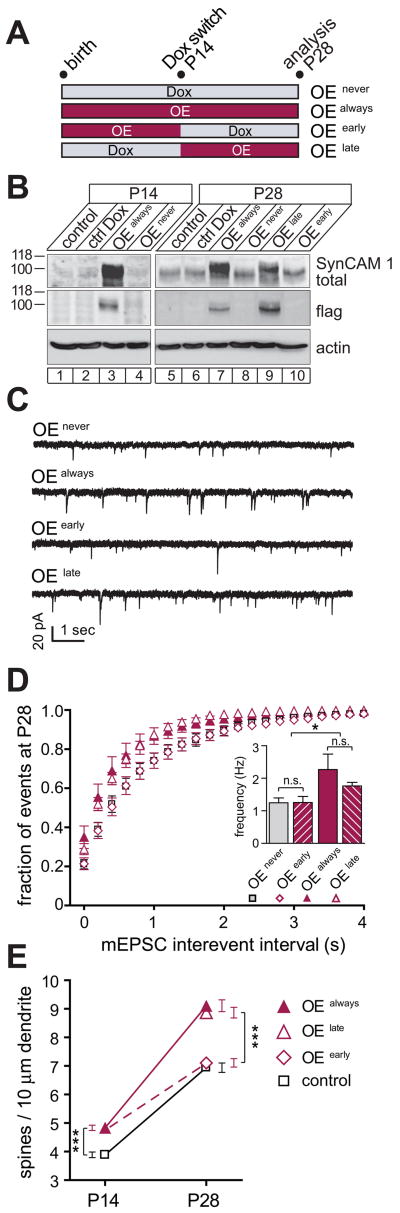
Figure 5. Continuous SynCAM 1 Presence is Required to Maintain Increased Synapse Number.
(A) Experimental design. SynCAM 1 overexpression was either continuously repressed by Dox treatment (OEnever), or occurred continuously until P28 (OEalways). Cohorts were treated with Dox from P14 to repress overexpression (OEearly), or were removed from Dox at P14 (OElate) to turn overexpression on.
(B) Hippocampal homogenates from animals treated as in (A) were probed by immunoblotting. At P14, SynCAM 1flag was only detected in OEalways mice. At P28, SynCAM 1flag is repressed to undetectable levels in both OEearly and OEnever conditions. SynCAM 1flag amounts reach maximum by P28 even when overexpression was only initiated from P14 on (OElate). Actin served as a loading control.
(C) Representative mEPSC traces from P28 hippocampal CA1 neurons after treatments as in (A).
(D) The SynCAM 1-induced increase in synapses requires its presence to be maintained. OEalways mice exhibit increased mEPSC frequency at P28 compared to OEnever controls. Overexpression of SynCAM 1 increases mEPSC frequencies at P14 (see Figure 3B), but subsequent repression returns frequencies to control levels by P28 (OEearly). When overexpression is turned on at P14 (OElate), mEPSC frequencies are indistinguishable from OEalways mice. mEPSC frequencies in OEnever mice are identical to littermate controls carrying only the tTA transgene (see Figures S3A and S3B). For statistical comparisons, see Figures S3C and S3F.
(E) Maintaining the SynCAM 1-induced spine increase requires its presence. Overexpression of SynCAM 1 until P14 increases spines densities over tTA littermate controls. At P28, OEalways mice similarly show increased spine densities over tTA controls. Turning SynCAM 1 overexpression on at P14 (OElate) results at P28 in spine densities that are identical to OEalways mice. Repression of SynCAM 1 overexpression from P14 (OEearly) reduces spine densities to control levels by P28 (dashed line). Spine densities in OEnever mice are identical to tTA littermate controls (see Figure S4A). For statistical comparisons, see Figures S4B.
Converse to the overexpression of SynCAM 1, its loss caused a strong reduction in mEPSC frequency by more than half compared to control wild-type littermates (Figures 3D and 3E). The unaltered mEPSC amplitude in the KO mice (Figure 3F) indicated that the density of AMPA receptors is not changed in their shortened PSD, as mEPSC amplitude reflects AMPA receptor density rather than total receptor number (Raghavachari and Lisman, 2004). A similar phenotype of reduced PSD length and unaltered mEPSC amplitude has also been observed in Shank1 KO mice (Hung et al., 2008). We noticed that the effect of SynCAM 1 on mEPSC frequency is more pronounced than on synapse number. One reason may be that synapses rendered non-functional by the absence of SynCAM 1 could appear normal on the morphological level such as reported for Munc-18-1 KO and neuroligin 1-3 KO mice (Varoqueaux et al., 2006; Verhage et al., 2000). Taken together, these electrophysiological data revealed that SynCAM 1 levels regulate the number of functional excitatory synapses.
Other Steady-State Synaptic Properties Are Unaffected by SynCAM 1
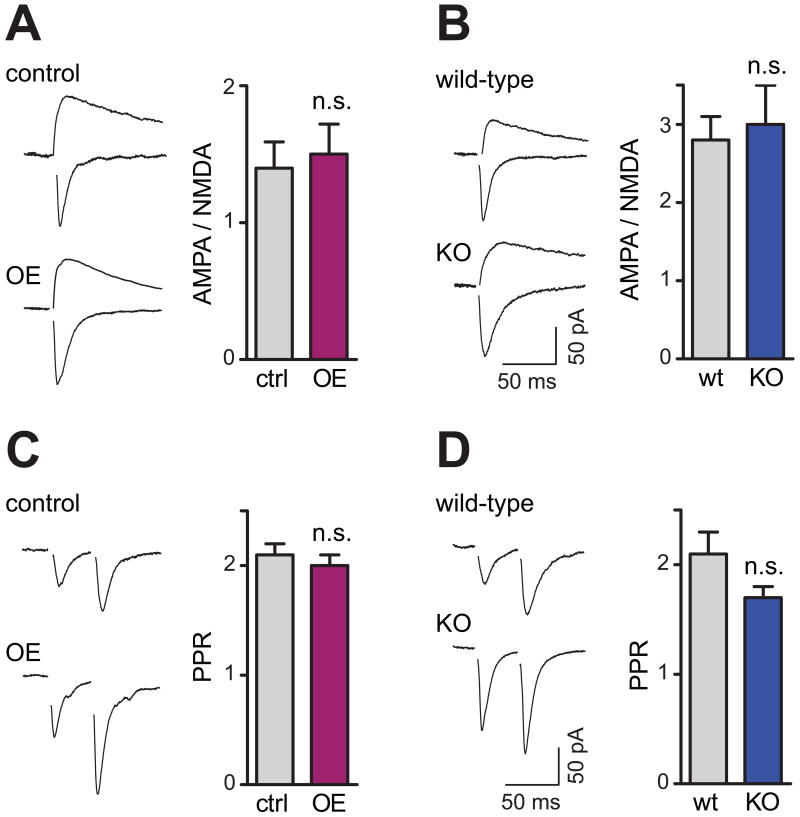
Does SynCAM 1 affect other functional synaptic properties? To assess synaptic strength, we analyzed the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor and N-methyl-D-aspartate (NMDA) receptor components of evoked excitatory postsynaptic currents (EPSC). Compared to their respective littermate controls, the AMPA/NMDA ratio was neither altered in SynCAM 1 overexpressors (Figure 4A) nor in KO animals (Figure 4B). Analyzing presynaptic properties, we found that the paired-pulse ratio (PPR), a measure of changes in the probability of transmitter release, was unchanged after SynCAM 1 overexpression or loss (Figures 4C and 4D). The increase in mEPSC frequency in SynCAM 1 overexpressors therefore likely reflects higher excitatory synapse numbers rather than an elevated release probability. Together with the ultrastructural analyses, these results showed that most structural and basic functional properties of synapses are intact under conditions of SynCAM 1 overexpression or loss, with the exception of the shortened PSD in the KO. This lack of changes in parameters associated with synapse maturation indicated a select effect of SynCAM 1 on synapse number.
Figure 4. SynCAM 1 Does not Alter Basal Synaptic Transmission.
(A and B) Left, representative traces of AMPA and NMDA currents from CA1 neurons at P15-19. Right, synaptic strength is unaffected by SynCAM 1 since AMPA/NMDA ratios are neither altered by SynCAM 1 overexpression (A; tTA controls n=7; OE n=8; p>0.5) nor its loss (B; wild-type n=12; KO n=13; p>0.6). Scale bar in (B) applies to (A, B).
(C and D) Paired-pulse ratio (PPR), a measure for short-term plasticity of presynaptic release, is neither altered by SynCAM 1 overexpression (C; tTA controls n=17; OE n=15; p>0.5) nor its absence (D; wild-type n=17; KO n=11; p>0.1). Scale bar in (D) applies to (C, D).
SynCAM 1 Sustains the Increase in Excitatory Synapse Number
We wanted to determine at which point in the lifetime of synapses SynCAM 1 acts and considered two hypotheses: Either SynCAM 1 functions at early stages of synapse development to increase synapse number, but is then dispensable. In this case, the SynCAM 1-mediated increase in synapse number would persist beyond a shutdown of its overexpression. Alternatively, SynCAM 1 could be continuously required to sustain excitatory synapses number, possibly by initially promoting excitatory synapse formation and then maintaining them. In that case, the gain in synapse number would be lost after ending SynCAM 1 overexpression.
To distinguish between these hypotheses, we utilized the temporal expression control afforded by our transgenic mouse model. SynCAM 1 overexpression effects on mEPSC frequency were compared at P14 and P28, i.e. during and after the peak of synaptogenesis. Three different experimental conditions were analyzed. Animals overexpressed SynCAM 1 either constitutively (OEalways), or only within the first two weeks of postnatal development until P14 (OEearly), or selectively from P14 - P28 (OElate) (Figure 5A). Immunoblotting of hippocampal lysates obtained after these treatments confirmed the intended repression and induced expression of SynCAM 1flag (Figure 5B). Three controls were analyzed in parallel. In the first cohort of controls, SynCAM 1 overexpression was continuously repressed by administering doxycycline (OEnever). A second and third cohort comprised mice carrying only tTA and lacking the SynCAM 1 transgene. This second group of controls remained untreated, while the third control group was treated with doxycycline to exclude nonspecific effects of this drug on synapse number. mEPSC frequencies and amplitudes were indistinguishable under all control conditions (Figure S3).
As observed at P14 (see Figure 3B), the continuous overexpression of SynCAM 1 until P28 caused a strong increase in mEPSC frequency (Figures 5C and 5D). Interestingly, when the overexpression of SynCAM 1 was repressed until P14 but switched on at P14 (OElate), we observed at P28 an increased mEPSC frequency that was statistically indistinguishable from continuous overexpression (Figure 5D). This indicates that SynCAM 1 can increase synapse number also at later stages of postnatal development. Importantly, when SynCAM 1 was overexpressed until P14, but was then shut down (OEearly), mEPSC recordings at P28 revealed that the increase in synapse number that had been observed at P14 was lost by P28 (Figures 5C and 5D). These results supported our second hypothesis that SynCAM 1 is required to sustain the increase in excitatory synapses it mediates.
Are these dynamic, SynCAM 1-dependent changes in functional excitatory synapse number also reflected on the morphological level? To address this, we analyzed Golgi-stained spines as measure of excitatory synapses. Following the temporally controlled expression employed for the mEPSC analysis, we determined that spine densities were indistinguishable under all control conditions at P28 (Figure S4). The overexpression of SynCAM 1 until P14 resulted in a significant increase in total spine density by 31 ± 3% compared to tTA littermate controls (Figure 5E), similar to the spine increase in adult OE mice (see Figure 2G). These SynCAM 1 overexpressing P14 mice also exhibited a 5-fold increase in the small fraction of thin spines over controls (data not shown). Similarly, overexpression of SynCAM 1 until P28 increased total spine density by 24 ± 2%. The same increase was determined in OElate mice (Figure 5E), demonstrating that SynCAM 1 can increase excitatory synapse number even after the peak of synaptogenesis has been reached around P14. Importantly, the spine gain caused by elevating SynCAM 1 until P14 was lost at P28 upon shutdown of overexpression in OEearly mice. Together, both the electrophysiological measurement of functional excitatory synapses and the Golgi staining of spines demonstrated that the continued elevation of SynCAM 1 is necessary to maintain the increase in excitatory synapses it drove in the first place.
Long-Term Depression is Regulated by SynCAM 1 Expression Levels
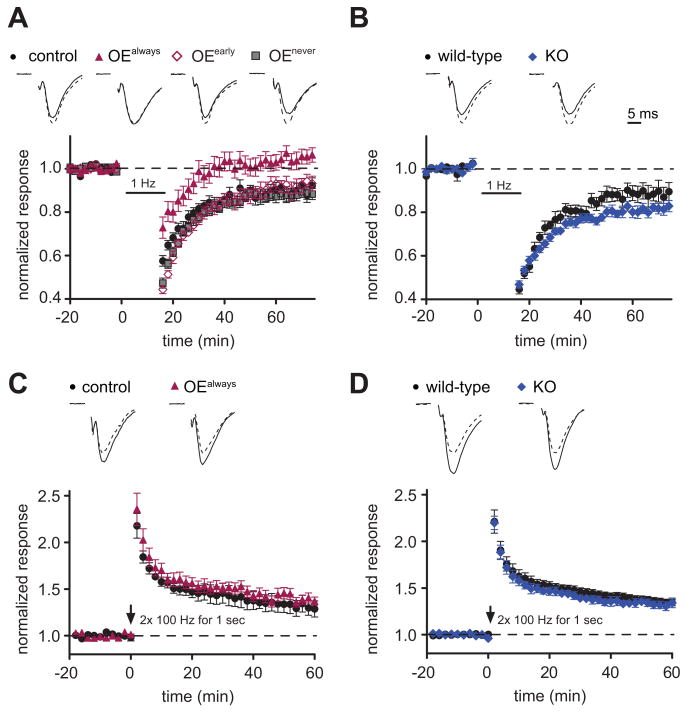
Trans-synaptic interactions may not only alter synapse formation and development but also synaptic plasticity. Long-term depression (LTD), a plasticity mechanism that decreases synaptic strength after low-frequency stimulation, correlates with spine shrinkage (Zhou et al., 2004). Considering the effects of SynCAM 1 on maintaining spine numbers, we tested whether SynCAM 1 expression modulates the activity-dependent weakening of synapses. Intriguingly, extracellular field potential recordings from the CA1 area of the hippocampus identified that mice continuously overexpressing SynCAM 1 (OEalways) failed to exhibit LTD (Figure 6A). We again used the temporal expression control of our transgenic mouse model and tested whether this impairment of LTD requires the continuous overexpression of SynCAM 1. Our results show that LTD was restored at P14 when SynCAM 1 overexpression was turned off from P8 (OEearly) (Figure 6A). As expected, continuously repressed animals (OEnever) did not exhibit a change in LTD.
Figure 6. SynCAM 1 Restricts Long-Term Depression.
(A and B) Extracellular field potential recordings (fEPSPs) were obtained before and after induction of LTD in SynCAM 1 overexpressors (A) and KO mice (B). Top, representative fEPSP traces show normalized average fEPSPs before (dashed line) and after LTD induction (solid line). (A) LTD is absent in SynCAM 1 overexpressors, while tTA control animals exhibit normal LTD. Shutting down SynCAM 1 overexpression continuously (OEnever) or after P8 (OEearly) rescues the loss of LTD observed in SynCAM 1 overexpressors (OEalways). Note that LTD recordings from rescue and control animals are indistinguishable (p > 0.1) and are superimposed in the graph. (tTA controls 0.90 ± 0.01, n=15; OEalways 0.99 ± 0.01, n=20; p < 0.001, OEearly 0.92 ± 0.02, n=7; OEnever 0.88 ± 0.01, n=19) (B) Loss of SynCAM 1 increases LTD. (wild-type littermate controls 0.88 ± 0.01, n=10; KO 0.82 ± 0.01, n=17; p < 0.001)
(C and D) Overexpression (C) or loss (D) of SynCAM 1 do not affect LTP. Top, representative fEPSP traces show normalized average fEPSPs before (dashed line) and after LTP induction (solid line). (C, tTA littermate controls 1.29 ± 0.09, n=6; OE 1.37 ± 0.05, n=10; p > 0.05; D, wild-type littermate controls 1.34 ± 0.06, n=4; KO 1.35 ± 0.04, n=10; p > 0.05) Scale bar in (B) applies to all panels.
As we detected opposite effects of SynCAM 1 overexpression and loss on synapse numbers, we hypothesized that SynCAM 1 KO mice may show increased LTD reciprocal to the lack of LTD in the overexpressors. Indeed, LTD was expressed more strongly in SynCAM 1 KO mice (Figure 6B). These findings indicate a direct modulatory effect of SynCAM 1 overexpression and loss on this plasticity mechanism.
To test whether SynCAM 1 also alters the ability to strengthen synaptic transmission in an activity-dependent manner, we measured long-term potentiation (LTP) using a robust tetanic stimulation protocol. We observed no effects of SynCAM 1 overexpression or SynCAM 1 loss on LTP (Figures 6C and 6D). Together, these experiments demonstrated that the weakening of synaptic connections by LTD is selectively and strongly affected by SynCAM 1.
SynCAM 1 Impacts Spatial Learning
As cognitive tasks involve synaptic plasticity (Nelson and Turrigiano, 2008; Neves et al., 2008), the synapse-organizing roles of SynCAM 1 may alter experience-dependent behaviors. We addressed this question first in the SynCAM 1 overexpressing mice that lacked LTD. Control experiments confirmed that their motor functions as well as vision were unaffected, permitting these behavioral studies (Figure 7A and Figure S5A-C). We tested these mice in the Morris water maze paradigm, a hippocampus-dependent task of spatial reference learning. The animals' motivation to reach a submerged, but visibly marked platform in a water tank was unaltered (Figure S6A and S6B). Notably, SynCAM 1 overexpressors failed to properly learn the target quadrant's location when the platform was hidden (Figure S6C), and correspondingly were unable to remember the correct maze quadrant when subjected to a probe trial (Figure 7B). This surprising impairment of spatial learning and memory was not due to a general hippocampal dysfunction, as these animals performed normally in a novel objection recognition task (Figure S6G). To extend this analysis beyond cognitive tasks, we also assessed anxiety-related behavior. Testing the SynCAM 1 overexpressing animals in the elevated plus maze identified no alteration in the number of entries into an exposed maze arm, indicative of unchanged anxiety levels (Figure S6H).
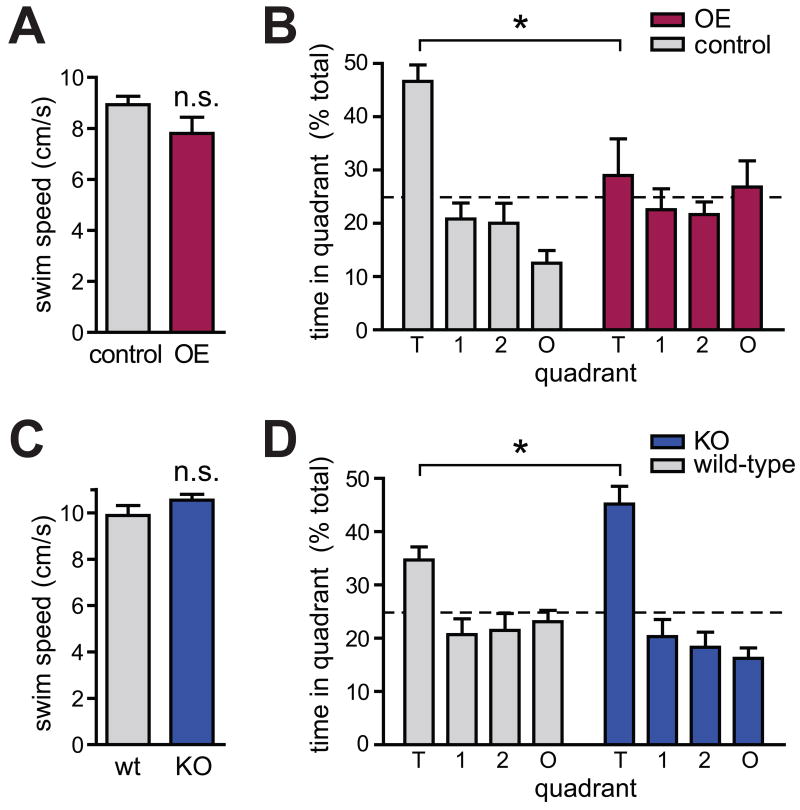
Figure 7. Spatial Learning is Impaired by SynCAM 1 Overexpression and Enhanced by its Loss.
(A) Locomotor activity, measured by swim speed, is not altered in SynCAM 1 overexpressors. Results were obtained from 11 tTA littermate control and 12 SynCAM 1 OE male mice at 3-5 months.
(B) Spatial reference memory is impaired in SynCAM 1 overexpressors. Following Morris water maze training, the time spent by mice in the target quadrant (T) was measured. Unlike controls, SynCAM 1 overexpressors exhibit no learned preference. Results were obtained from 11 tTA littermate control and 12 SynCAM 1 OE male mice at 4-5 months. O, opposite quadrant; 1, 2, adjacent quadrants. Dotted line indicates chance level.
(C) Swim speed is not altered in the SynCAM 1 KO. Results were obtained from 8 wild-type littermate control and 9 SynCAM 1 KO male mice at 6-12 months.
(D) SynCAM 1 KO mice exhibit better spatial reference memory. Following training, KO mice spend more time in the Morris water maze target quadrant than controls. Results were obtained from 7 wild-type littermate control and 9 SynCAM 1 KO male mice at 6-12 months. Differences in time spent in quadrant T by tTA controls in (B) and controls in (D) are likely due to their different age and genetic background.
In contrast to SynCAM 1 overexpressors, LTD was enhanced in SynCAM 1 KO animals. This led us to hypothesize that learning might be altered and possibly improved in these mice. To facilitate the detection of putative improvements, behavioral studies were performed in aged mice as older rodents exhibit impaired spatial memory (Gage et al., 1989), which is apparent in the high error rate of aged wild-type mice (see Figure S6F). Motor functions were normal in aged KO mice (Figure 7C and Figure S5D), and no changes in anxiety-related behavior were identified (Figure S6I). Furthermore, the animals' motivation to reach the visibly marked platform in a water tank was unaffected (Figures S6D and S6E). We were intrigued to observe that SynCAM 1 KO mice learned the location of the correct quadrant in the Morris water maze significantly faster than wild-type littermate controls (Figure S6F). Even more surprisingly, these trained SynCAM 1 KO mice exhibited significantly enhanced spatial memory (Figure 7D).
Discussion
Our results reveal that the synaptic adhesion molecule SynCAM 1 contributes to the regulation of excitatory synapses in the brain in two distinct ways, through altering their number as well as their plasticity. Consistent with its expression into adulthood, SynCAM 1 regulates excitatory synapse number both in the developing and adult brain as measured by electron microscopy, Golgi staining, and electrophysiology. SynCAM 1 not only increases excitatory synapse density but its expression is additionally required to maintain this increase as shown using temporally controlled overexpression. Moreover, the analysis of SynCAM 1 KO mice reveals that its loss results in fewer excitatory synapses with a shortened PSD. Our results in OE and KO mouse models demonstrate that the organization of excitatory synapses is a key developmental role of SynCAM 1 and indicate that its expression directly regulates synapse number. Notably, SynCAM 1 also changes synaptic plasticity through restricting LTD. These effects correlate with behavioral changes, and SynCAM 1 KO animals perform better than wild-types in spatial learning. In contrast, the overexpressing mice that lack LTD are unable to acquire this form of reference memory. Our findings demonstrate that SynCAM 1 organizes excitatory synapses by contributing to the regulation of both their number and synaptic plasticity. This distinguishes it from proteins such as neuroligins that serve in synapse maturation in vivo (Varoqueaux et al., 2006).
With respect to synapse development, this work provides new insights into trans-synaptic interactions by demonstrating that SynCAM 1 organizes synapses in the brain. Based on our results, and the contribution of adhesive SynCAM 1 assembly to axo-dendritic contact formation (Stagi et al., 2010), we propose that SynCAM 1 participates both in early steps of synaptogenesis and subsequently in maintaining this increase in excitatory synapse number. Mechanistically, SynCAM 1 adhesion may drive excitatory synapses by promoting the prolonged axo-dendritic interactions that precede excitatory, but not inhibitory, synapse formation (Wierenga et al., 2008). Here, an increase of homophilic SynCAM 1/1 complexes in transgenic overexpressors that is paralleled by the reduction in SynCAM 1/2 complexes may stabilize nascent synapses to increase synapse number. This molecular change in SynCAM adhesion complexes may therefore specifically impact synaptic differentiation.
Interestingly, the absence of SynCAM 1 already resulted in a significant reduction of synapse number, despite the continued expression of the three other members of this protein family. This demonstrated that its loss is not fully compensated by these or other synapse-organizing proteins. This was unexpected because SynCAM 1 is expressed at lower protein amounts than SynCAM 2, and may reflect a select role of SynCAM 1 in the adhesive stabilization of excitatory synapses. The acute loss of SynCAM 1 may impact the number of synapses even more strongly, but this could not be addressed as our studies were performed in a constitutive SynCAM 1 KO. Similarly, our analysis of the inducible transgenics showed that other synapse-organizing proteins did not compensate for the absence of overexpressed SynCAM 1 once it was shut down in OEearly mice. This resulted in the loss of the SynCAM 1-induced increase in synapse number we measured by electrophysiological recordings and Golgi staining of spines. These findings point to specific roles of SynCAM 1 compared to other synaptic adhesion molecules, and underline that SynCAM 1 acts in a partially non-redundant manner, unlike reported for neuroligins (Chih et al., 2005; Varoqueaux et al., 2006). These consequences of SynCAM 1 elevation and loss are reminiscent of the dose-dependent effects of the homophilic Ig adhesion molecule fasciclin II on synapse formation in Drosophila (Davis et al., 1997).
SynCAM 1 likely organizes excitatory synapses throughout development and into adulthood. This is indicated by the reduction of synapse number both during the peak of synapse formation at P14 and once most synapses have formed at P28 as observed by electron microscopy. Our physiological recordings in the developing brain of KO mice support this conclusion. Roles in the mature brain are consistent with the increase in synapse number even if SynCAM 1 is overexpressed only in later postnatal development, i.e. subsequent to the peak of synaptogenesis. Interestingly, functions of SynCAM 1 in the adult brain have been independently indicated by the upregulation of SynCAM 1 transcripts in the visual cortex after monocular deprivation (Lyckman et al., 2008) and in regenerating spinal cord axons (Zelano et al., 2009), two processes that require the formation of new synapses.
Moreover, this study demonstrates that SynCAM 1 not only impacts functional synapse number but also regulates the activity-dependent plasticity of synapses once they are formed. Specifically, SynCAM 1 overexpression occluded LTD and the loss of SynCAM 1 enhanced LTD. Furthermore, mice in which SynCAM 1 overexpression was shut down subsequently again exhibit normal LTD. These findings indicate that the impact of SynCAM 1 on LTD directly depends on its expression level and highlights an unexpected physiological role of this protein. This is the first report that an adhesion molecule can regulate LTD, extending the findings that LTP is affected by NCAM and Eph receptors (Gerrow and El-Husseini, 2006) and that the loss of neuroligin 1 impairs LTP (Blundell et al., 2010; Kim et al., 2008). Two mechanisms underlying the effect of SynCAM 1 on LTD are conceivable. The trans-synaptic interaction of SynCAM proteins might stabilize synaptic structures and thereby prevent the physical loss of synaptic contacts that occurs during LTD (Zhou et al., 2004). Also, SynCAM complexes may confine glutamate receptors to postsynaptic specializations, thereby preventing LTD. Future studies will have to test these possibilities.
Perhaps the most surprising finding of this study is that SynCAM 1 is the first adhesion molecule that can restrict a cognitive function, which extends previous molecular analyses of learning (Lee and Silva, 2009). This effect differs from the loss of neuroligin 1, which was reported to impair spatial learning (Blundell et al., 2010). SynCAM 1 may impact spatial learning through altering LTD, which plays roles in object exploration in space and spatial learning (Kemp and Manahan-Vaughan, 2004; Nicholls et al., 2008).
In summary, our studies of SynCAM 1 define for the first time the functions of a synapse-organizing protein during the development of synapses in vivo and show that it can promote both an increase in synapse number and the maintenance thereof, together with regulating plasticity.
Experimental Procedures
Transgenic and KO SynCAM 1 Mouse Models
To generate the transgenic cassette, a SynCAM 1 construct tagged within the middle of the cytosolic sequence with two flag epitopes was cloned into the pTRE vector (Clontech). This coding sequence preceded by a TRE element (Mansuy and Bujard, 2000) was used to generate a transgenic mouse line, which was crossed to mice carrying the CaMKII-tTA transgene (Mayford et al., 1996) to obtain double-heterozygotic SynCAM 1flag overexpressors. To temporally control SynCAM 1flag overexpression, doxycycline-containing water (1 g/l) was provided to the mice. Transgenic mice were maintained in a mixed BL6/SJLF1J background, and mice heterozygotic for the CaMKII-tTA transgene but lacking the SynCAM 1flag transgene served as controls. SynCAM 1 KO mice were generously provided by Dr. T. Momoi (National Institute for Neuroscience, Tokyo) (Fujita et al., 2006). This mouse line was maintained in a mixed BL6/129Sv background and homozygotic wild-type and KO littermates were compared to control for the same genetic background of analyzed mice.
Biochemical Procedures
Frozen tissue samples were rapidly homogenized using microtip-aided sonication. Brain homogenates were subfractionated by the method of Jones and Matus (Jones and Matus, 1974) with modifications (Biederer et al., 2002). For co-immunoprecipitation experiments, synaptosomes were prepared from forebrain, solubilized with 1% Triton X-100, and incubated with Protein G agarose-conjugated flag M2 antibodies or with anti-SynCAM 1 antibodies as described previously (Fogel et al., 2007). Quantitative immunoblotting was performed on an Odyssey Imaging System (Li-Cor, Lincoln, NE, USA) and signals were calibrated against purified epitopes (Fogel et al., 2007).
Immunohistochemistry and Electron Microscopy
Immunohistochemistry was performed on cryosections of P21 mouse brains, and stained sections were imaged with a Zeiss LSM 510 META laser scanning confocal microscope. Golgi staining of hippocampal pyramidal neurons was performed using the FD Rapid Golgi Stain Kit (FD NeuroTechnologies, Ellicott City, MD) according to the manufacturer's instructions. Differential interference contrast images of secondary and tertiary CA1 apical dendrites were acquired and spines of Golgi-stained sections were classified and quantitatively analyzed as described (Knott et al., 2006; Li et al., 2004). For electron microscopy, coronal sections of 100 μm thickness from the CA1 stratum radiatum of hippocampus were cut using a vibratome. Morphometric classification of synapses and analysis of ultrastructural parameters were performed as described (Rosahl et al., 1995).
Electrophysiological Recordings
Electrophysiological recordings from hippocampal slices were performed after preparing 400 μm slices and storing them in artificial cerebrospinal fluid (ACSF) gassed with carbogen. For whole cell recordings, pyramidal CA1 neurons were visualized using differential infrared video microscopy. Miniature EPSCs were recorded at a holding potential of -70 mV in ACSF supplemented with tetrodotoxin, picrotoxin, and trichlormethiazide. Statistical analyses of cumulative distributions were performed applying the Kolmogorov–Smirnov test. Field potentials were evoked by stimulating Schaffer collaterals and recorded in the CA1 stratum radiatum. LTP was induced by two trains of pulses at 100 Hz for 1 sec, and LTD by low frequency stimulation of 1 Hz for 15 min.
Behavioral Studies
Behavioral tests were performed using cohorts of male mice. Locomotor activity was controlled by video-tracking in an open field box, and swim speed and distance. Novel object exploration was scored as described (Gresack and Frick, 2004). Morris water maze training and probe trials were conducted in a water-filled circular tank, with visual cues mounted on the tank wall. Path length, time spent in each quadrant, and latency to find the escape platform were tracked by video as described (Rabenstein et al., 2005). Elevated plus maze studies were performed by measuring the frequency with which mice enter open and close maze arms as described (Lister, 1987).
Statistical analyses were performed using Student's t-test with errors corresponding to the standard error of mean and the Kolmogorov-Smirnov test as indicated. * denotes t-test p<0.05; ** p<0.01; *** p<0.001. Analyses were performed blinded to the genotype. In OE studies, mice carried one SynCAM 1flag and one tTA transgene, while their littermate controls carried only one tTA transgene. In KO studies, wild-type controls refer to homozygous littermates of KO mice.
Supplementary Material
Acknowledgments
We thank Ms Y. Lei, N. Meyer, and A. Gruschka for technical assistance and Drs. S. Chandra, L. Chen, R. Klein, and T. Mrsic-Flogel for discussions and comments on the manuscript. We are grateful to Dr. T. Momoi for generously providing the SynCAM 1/RA175 KO mouse line, Dr. R. Hammer for generating the transgenic line, and Dr. M. Picciotto for support of behavioral studies. E.M.R. performed histochemical and ultrastructural studies, protein expression and interaction analyses, and behavioral experiments, A.J.K. performed electrophysiological analyses, K.P. performed and analyzed ultrastructural studies, A.K.G. performed behavioral studies, A.I.F. quantified protein expression, A.B. initially bred transgenic mice and confirmed expression, T.B. and T.C.S. conceived in collaboration the transgenic approach and T.C.S. supervised A.B., V.S. conceived and supervised the physiological approaches and performed electrophysiological analyses, and T.B. conceived and supervised non-physiological approaches, performed protein expression and interaction analyses and histochemical studies, and wrote the manuscript. This work was supported by the National Institute of Health Grant R01 DA018928 (to T.B.), a March of Dimes Basil O'Connor Award (to T.B.), and a Scottish Rite Schizophrenia fellowship (to E.M.R).
Footnotes
Supplemental Data include 6 Figures, 1 Table, and detailed Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 2006;87:139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Biederer T, Stagi M. Signaling by synaptogenic molecules. Curr Opin Neurobiol. 2008;18:1–9. doi: 10.1016/j.conb.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, Kojima N, Senoo H, Toshimori K, Momoi T. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: “vertebrate” and “invertebrate” perspectives. Cold Spring Harb Perspect Biol. 2009;1:a003079. doi: 10.1101/cshperspect.a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Jones DH, Matus AI. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974;356:276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SY, Lee YK, Park S, Choi JS, Lee CJ, Kim HS, Choi YB, Scheiffele P, Bailey CH, et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci U S A. 2008;105:9087–9092. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lyckman AW, Horng S, Leamey CA, Tropea D, Watakabe A, Van Wart A, McCurry C, Yamamori T, Sur M. Gene expression patterns in visual cortex during the critical period: synaptic stabilization and reversal by visual deprivation. Proc Natl Acad Sci U S A. 2008;105:9409–9414. doi: 10.1073/pnas.0710172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM, Bujard H. Tetracycline-regulated gene expression in the brain. Current Opinion in Neurobiology. 2000;10:593–596. doi: 10.1016/s0959-4388(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, Kandel ER. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng LH, Colman DR, Miki N. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci. 2005;25:2138–2145. doi: 10.1523/JNEUROSCI.3530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Südhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Stagi M, Fogel AI, Biederer T. SynCAM 1 participates in axo-dendritic contact assembly and shapes neuronal growth cones. Proc Natl Acad Sci U S A. 2010;107:7568–7573. doi: 10.1073/pnas.0911798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Akins MR, Biederer T. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J Comp Neurol. 2008;510:47–67. doi: 10.1002/cne.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11:1044–1052. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Zelano J, Berg A, Thams S, Hailer NP, Cullheim S. SynCAM1 expression correlates with restoration of central synapses on spinal motoneurons after two different models of peripheral nerve injury. J Comp Neurol. 2009;517:670–682. doi: 10.1002/cne.22186. [DOI] [PubMed] [Google Scholar]
- Zhiling Y, Fujita E, Tanabe Y, Yamagata T, Momoi T, Momoi MY. Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. Biochem Biophys Res Commun. 2008;377:926–929. doi: 10.1016/j.bbrc.2008.10.107. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.