Abstract
Gonadal steroids regulate appetite and thus body weight. In addition, continuous exposure to stressors negatively influences appetite through circuits likely distinct from those of gonadal steroids. The occurrence of adverse metabolic consequences due to chronic exposure to psychosocial stressors is twice as frequent in women as men, implicating a role for ovarian hormones, estradiol (E2) and progesterone (P4), in modulating stress-induced changes in appetite. Using social subordination in female macaques as a model of social stress, the current study tested the hypothesis that subordinate females would lose more weight during E2 treatment and gain less weight during P4 administration than dominant females. Because polymorphisms in the gene encoding the serotonin transporter (5HTT; SCL6A4) are known to alter responsivity to stress, we hypothesized that weight loss during E2 administration would be greatest in females with the short variant (s-variant) allele of 5HTT. Dominant females were significantly heavier than subordinate animals throughout the study, a result consistent with previous accounts of food intake when animals are fed a low-fat, high-fiber diet. Females with the s-variant 5HTT genotype weighed significantly less than l/l animals. Dominant animals lost significantly more weight than subordinate animals during E2 treatment. Administration of P4 blocked the weight-reducing effects of E2 in all females, regardless of social status. These data provide evidence that social subordination modulates the influence of ovarian steroid hormones on body weight in female rhesus monkeys independent of 5HTT genotype. Given the prosocial effects of these steroids, future studies are necessary to determine whether status differences in E2-induced weight loss are due to diminished food intake and or increases in energy expenditure and how the change in energy availability during E2 treatments relates to a female’s motivation to interact with conspecifics.
Keywords: social subordination, psychosocial stress, body weight, estrogen, progesterone, metabolism
Introduction
Rhesus macaque females, when housed socially, organize themselves into a linear dominance hierarchy that is enforced by the constant harassment and threat of aggression towards subordinate members by more dominant animals [1, 2]. Subordinate females show reductions in body weight and metabolic hormones [3, 4], including reduced fat mass and circulating leptin concentrations in comparison to dominant animals [5] when fed a standard low-calorie monkey chow [6]. These metabolic alterations are accompanied by disruptions in limbic-hypothalamic-pituitary-adrenal axis (LHPA) activity, as subordinate females are hypercortisolemic due to diminished glucocorticoid negative feedback [4, 7, 8]. Heightened activity of corticotropin-releasing hormone (CRH) in rodents mediates the anorectic effects of exposure to chronic stressors [9–11] and could be the underlying cause of reductions in body weight seen in subordinate animals.
The occurrence of adverse metabolic consequences due to chronic psychosocial stressors is more prevalent in women than in men, as women are twice as susceptible than men to developing eating disorders and altered metabolism [12–14]. This increased vulnerability in women implicates the role of the ovarian steroid hormones, estradiol (E2) and progesterone (P4), in affecting body weight and metabolism. Indeed, E2 replacement and high estrogen levels associated with stages of the ovarian cycle are linked to decreases in body weight [15] and food intake [16–19] in rodents and humans. Periods of elevated P4 coincide with increases in food intake and body weight in women [15, 19–21] and synthetic progestins are known to increase body weight [22]. While the effects of E2 and P4 on body weight are well described, it is unclear how the effects of social stress interact with those of gonadal steroids to regulate body weight.
Individual vulnerability to adverse health outcomes associated with chronic exposure to stressors is likely affected by a number of genetic factors. In particular, polymorphisms in the gene (SCL6A4) that encodes the serotonin transporter (5HTT) [23] are associated with greater vulnerability to environmental stressors in monkeys and in people. The short promoter length variant (s-variant) of 5HTT has reduced transcriptional activity in humans [24] and individuals with this short promoter allele have a higher incidence of psychopathology [23] than individuals homozygous for the long 5HTT allele (l/l). Rhesus monkeys also show homologous polymorphisms in 5HTT [25, 26] and the s-variant allele increases individual susceptibility to adverse effects of social subordination on reproduction and metabolism [5, 27].
The present study was designed to determine how social stress, resulting from social subordination, interacts with gonadal steroids to influence body weight in females. Using adult ovariectomized rhesus monkeys, we hypothesized that replacement therapy with E2 would produce the greatest decrements in body weight in subordinate compared with more dominant animals and that this effect would be exacerbated by the short promoter length allele in the 5HTT gene. Because progestins promote weight gain, the study determined whether administration of P4 would attenuate the effects of E2.
Methods
Animals
Subjects included 39 ovariectomized adult female rhesus monkeys (13–17 years old) socially housed in indoor-outdoor runs in small social groups without males (n = 4 or 5 per group) as previously described [5]. Animals were fed standard monkey chow (low-fat, high-fiber diet from PMI Lab Diets, #5038, St. Louis MO) ad libitum twice-daily via two chow bins from which multiple animals could access food simultaneously. We observe no evidence of more dominant monkeys restricting subordinate females from access to food under these ad libitum conditions, even when more preferred diets are available [4]. In addition, animals had continuous access to water and received daily supplementation with seasonal fresh fruit and vegetables. All females had been previously ovariectomized 18 months prior to initiation of the study and genotyped for 5HTT gene polymorphism, either having two copies of the long promoter length alleles (l/l) or having at least one short promoter length allele (s-variant) [28]. Groups were homogeneous for genotype such that four groups were comprised of all females with the l/l genotype and 4 groups entirely of the s-variant genotype [5]. Dominance status was assessed by performing 30 min group scans of aggressive and submissive behaviors twice weekly throughout each of the 4-week treatments. Subordinate status was determined by the unequivocal submissive behavior emitted by a female to a group mate [29]. Data were recorded using a Palm PDA and the “Hands Obs” program developed by the Center for Behavioral Neuroscience [30]. Inter-observer reliability exceeded 90%. Females ranked 1 and 2 were considered as dominant and females ranked 3–5 were classified as subordinate [7]. Total numbers of animals classified by status and genotype were: 8 dominant, l/l; 8 dominant, s-variant; 11 subordinate, l/l, and 12 subordinate, s-variant. Groups had been formed for 12 months prior to the initiation of this study. All procedures were approved by the Emory University Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
Treatment Conditions
Animals were subjected to four different hormonal manipulations each lasting four weeks in duration. The four treatment conditions were control (C) followed immediately by E2 replacement (E2), and progesterone replacement (P4) followed immediately by E2 plus P4 (E2 + P4). The order (C – E2) and P4 – E2+P4) was randomized among the females but counterbalanced to ensure half the females received the C condition first and the other half the P4 condition first. A two-week washout period separated the first treatment sequence from the second. E2 and P4 replacement was attained via Silastic capsules implanted subcutaneously between the scapulae as previously described [31]. Analyses of blood samples collected on days 5 and 26 of each of the four treatment conditions showed that, compared to the control condition, hormone replacement achieved mid-follicular phase levels of E2 (49.7 ± 15.8 vs. <5.0 pg/ml) and typical luteal phases concentrations for P4 (4.29 ± 0.11 vs. 0.61 ± 0.03 ng/ml).
Outcome measures
All blood samples were taken within 10 minutes from disturbance time via conscious venipuncture using previously validated methods to minimize arousal [32, 33]. Fasted blood samples were collected at the end of each four-week treatment period between 0800 and 0900 hours, some 10 hours after food have been removed from their food bins. Blood was centrifuged and serum frozen for subsequent analyses. Leptin was assessed as a peripheral index of adiposity and acute energy balance [34, 35]. Peripheral insulin and glucose were measured as signals of glucose regulation [36]. Weekly body weights were collected and sagittal abdominal circumference (SAC) [37] was taken at the end of each four-week treatment period. SAC was determined by measuring the circumference of the abdomen at the level of the navel while animals were in the supine position during anesthesia with ketamine (10 mg, kg, IM) [5]. Two independent measures of SAC were measured and the mean value calculated for each time point.
Hormone assays
All assays were performed in the Biomarkers Core Lab at the Yerkes National Primate Research Center (YNPRC). Selected samples were assayed for E2 to verify Silastic capsule efficacy using a modification of a previously validated commercial RIA from Diagnostics Products Corporation (DPC, Los Angeles, CA) [38]. Using 200 µl of serum, the assay has a sensitivity of 5 pg/ml and an intra- and inter-assay coefficient of variation (CV) of 5.2% and 11.1%, respectively. Progesterone levels were determined using a modification of a previously described RIA using a commercially available kit (Siemens) [39]. Briefly, 125 µl of sample are extracted with anesthesia grade ether and the organic layer evaporated off by a stream of N2. The sample is reconstituted in 125 µl of the assay buffer and replicates are assayed following the kit protocol. The sensitivity of the assay is 0.10 ng/ml with an inter- and intra-assay CV of 8.14% and 7.73%, respectively. Serum leptin was measured by a RIA using a commercially available kit (Millipore, St. Louis MO). Assaying 100 µl, the assay has a range of 0.5 to 100 ng/ml. Intra-assay CVs were 6.84% and inter-assay were 7.24% [39]. Serum insulin was assayed with a RIA kit from Siemens having a sensitivity of 3 to 372 IU/L and an inter-and intra-assay CV of 9.02%and 5.87%, respectively [5]. Serum glucose was determined by a commercially available colorimetric enzyme assay (Stanbio Laboratory, Boerne TX), having a range from 0 to 27 mmol/L and inter-and intra-assay CVs of 2.12% and 4.21%, respectively [5].
Statistical analyses
Data were summarized as mean ± standard error of the mean (SEM) using PASW SPSS v18 (IBM, Somers NY). An analysis of variance for repeated measures was used to assess the main effects of the between-subject factors status (dominant vs. subordinate) and 5HTT genotype (l/l vs. s-variant), and the within-subject factor of the four treatment conditions (C, E2, P4, and E2+P4), as well as their interactions on overall body weight, SAC, leptin, glucose and insulin. A similar analysis was undertaken to assess the change in body weight and SAC over the course of each 4-wk hormonal condition (final value minus initial value on each hormone condition). If the main effect of treatment or status - genotype interactions with treatment were significant, simple main effects were evaluated with the Newman-Keuls test to isolate specific group or treatment effects. Such corrections were unnecessary for main effects of status and genotype as each had only two levels.
Results
Social Status Categorization
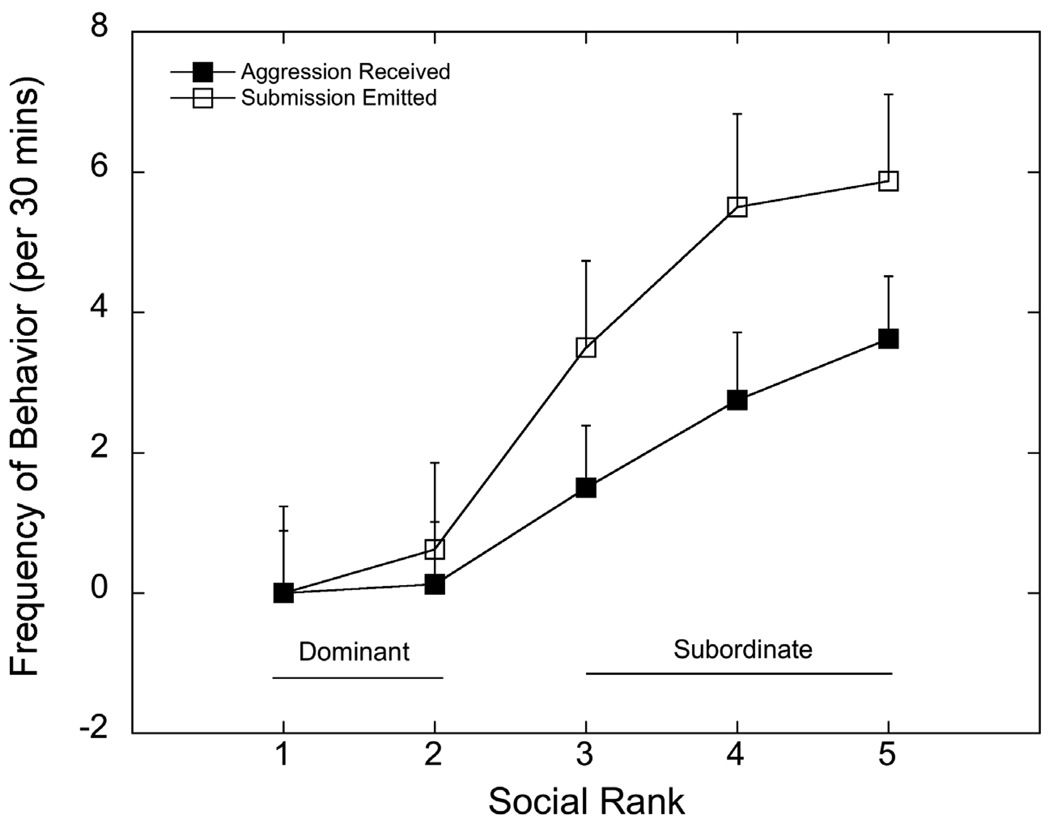
Figure 1 shows rates of aggression received and submissive behavior emitted by monkeys at each social dominance rank position, independent of 5HTT genotype. These data were derived from two-30 min group scans of aggressive and submissive behaviors obtained each week of the four treatment conditions. Hormone treatments did not alter the dominance hierarchy in any of the groups. As expected, subordinate females (ranks 3 – 5) received significantly more aggression from higher ranking cage mates (F 1, 8 = 10.6, p =0.003) and, consequently, emitted significantly more submissive behaviors (F 1, 8 = 17.9, p<0.001).
Figure 1.
Mean ± SEM rates of aggressive behavior received and submissive behavior emitted by females at each social dominance rank. Rates of aggression received and submission emitted were significantly higher (p < 0.002) in animals categorized as subordinate females (ranks 3 – 5) compared with those categorized as dominant (ranks 1 and 2).
Body Weight and Sagittal Abdominal Circumference
Body weight and SAC throughout the 16-week study were consistently greater in dominant females than subordinate animals (F 1, 35 = 5.59, p=0.02 and F 1, 35 = 6.60, p=0.02 respectively) (Table 1). There was also a significant main effect of genotype, as body weights (F 1, 35 = 4.91, p=0.03) and SAC (F 1, 35 = 5.59, p=0.02) were significantly greater in l/l compared with s-variant females (Table 1). In addition, the effect of social status on SAC, but not on body weight (F 1, 35 = 2.32, p=0.14), was modified significantly by 5HTT genotype (F 1, 35 = 5.10, p=0.03). Post hoc analyses revealed that dominant l/l females had significantly greater SAC (p < 0.03) than any of the other groups of females who did not differ from one another (p > 0.05; Table 1).
Table 1.
Mean ± SEM for body weight (kg) and sagittal abdominal circumference (cm) collapsed across treatments over the course of the 16-week study as a function of dominance status and 5HTT genotype. Letters indicate significant main effects of status while numbers indicate significant main effects of genotype. Post hoc analyses of the significant status by genotype interaction on sagittal circumference revealed that dominant, l/l females had greater abdominal adiposity than all other groups as indicated by an asterisk.
| Status | Body Weight (kg) | Sagittal Abdominal Circumference (cm) | ||||
|---|---|---|---|---|---|---|
| 5HTT Genotype | 5HTT Genotype | |||||
| l/l | s-variant | l/l | s-variant | |||
| 8.33 ± 0.301 | 7.37 ± 0.322 | 39.50 ± 1.231 | 35.13 ± 1.212 | |||
| Dominant | 8.35 ± 0.33a | 9.16 ± 0.46 | 7.54 ± 0.46 | 39.54 ± 1.33a | 43.68 ± 1.88* | 35.33 ± 1.60 |
| Subordinate | 7.35 ± 0.27b | 7.51 ± 0.40 | 7.19 ± 0.38 | 35.09 ± 1.12b | 35.40 ± 1.88 | 34.86 ± 1.53 |
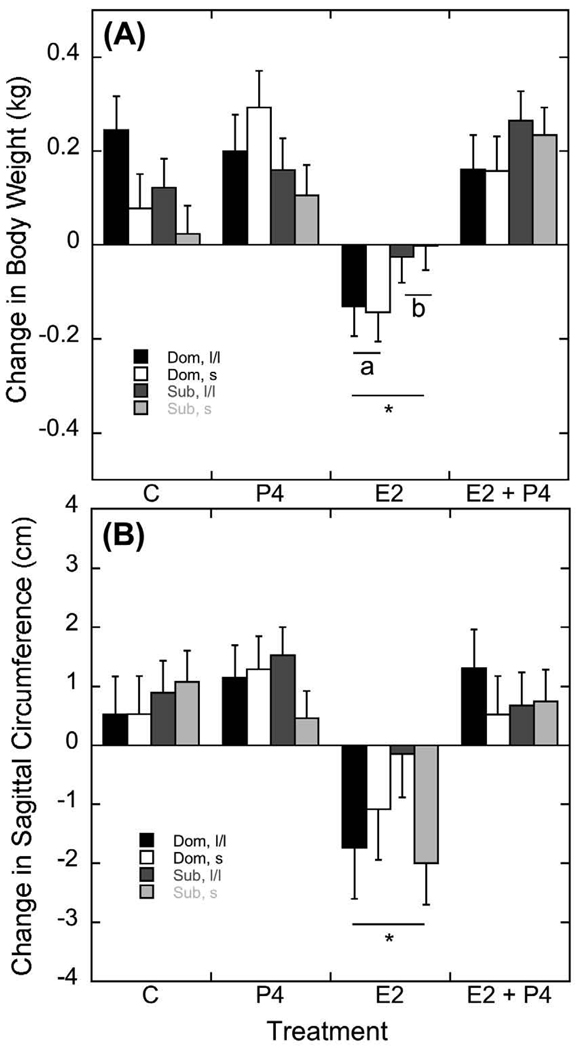
To assess the effect of treatment on body weight, the change in weight from day 0 to day 28 of each treatment condition was calculated. As illustrated in Figure 2A, the change in body weight was significantly affected by treatment (F 3, 105 = 15.68, p<0.001). Post hoc analyses revealed this main effect of treatment was due to a significant loss of body weight during E2 administration compared to an increase in body weight during the other three treatments (p < 0.001). However, administration of E2 in combination with P4 did not decrease body weight, as weights were similar to the P4 alone and control treatments (p > 0.05).
Figure 2.
Mean ± SEM of changes in body weight (A) and sagittal abdominal circumference (SAC; B) from the beginning to the end of each 4-week hormonal replacement condition. Estradiol significantly attenuated body weight and SAC compared to all other treatments, depicted by an asterisk. Letters in panel (A) highlight the status difference in E2’s ability to decrease body weight.
This effect of treatment on the change in body weight was modified by the interaction of social status (Figure 2A; F 3, 105 = 3.46, p=0.02). Post hoc analyses revealed that dominant animals lost significantly more weight than subordinate animals on E2 alone (F1, 35 = 4.45, p=0.04). Body weight changes during the control, P4, or E2 + P4 conditions were unaffected by status (p > 0.05). 5HTT genotype did not interact with treatment (F 1, 35 = 0.76, p=0.39) or status and treatment (F 1, 35 = 0.06, p=0.81) to affect the change in body weight.
Parallel to these changes in body weight were changes in waist circumference as illustrated in Figure 2B. The significant effect of treatment on changes in SAC (F 3, 105 = 10.49, p<0.001) was again accounted for by E2 administration. Post hoc analyses revealed SAC decreased significantly during E2 compared with the other treatment conditions (p < 0.01). The increase in SAC during control, P4, and E2+P4 did not vary significantly (p > 0.05). Finally, these treatment-dependent changes in SAC were not influenced by status (F 1, 35 = 0.12, p=0.73) or 5HTT genotype (F 1, 35 = 1.52, p=0.23).
Metabolic measures
Dominant females had higher overall serum leptin levels than subordinate females throughout the duration of the study (F 1, 35 = 4.46, p=0.04, Table 2). Leptin levels did not vary significantly with 5HTT genotype or by a status by genotype interaction (F 1, 35 = 1.59, p=0.22 and F 1, 35 = 0.16, p=0.69 respectively). Even though changes in SAC were influenced by E2 treatment, there were no significant effects on these hormonal manipulations on changes in serum leptin levels (F 3, 105 = 1.08, p = 0.36). However, treatment condition significantly affected serum insulin (F 3, 105 = 6.70, p<0.001) but not serum glucose (F 3, 105 = 2.09, p=0.11). Post hoc analyses revealed that E2 administration significantly reduced serum insulin compared to the other treatment conditions (p=0.03). While it appeared that administration of P4 increased serum insulin compared to other conditions, the difference was not significant (p>0.05). These treatment effects on serum insulin were not affected by status (F 3, 105 = 0.33, p=0.81) or genotype (F 3, 105 = 0.47, p=0.70). Similarly, serum glucose was also unaffected by status (F 3, 105 = 2.07, p=0.159) or genotype (F 3, 105 = 1.024, p=0.318; Table 2).
Table 2.
Metabolic hormone concentrations (mean ± SEM) at the end of each treatment phase. Dominant females had significantly higher levels of serum leptin compared to subordinates, regardless of treatment (p = 0.05). E2 decreased serum insulin concentrations, as indicated by different letters for each of the four treatment conditions.
| Dominant | Subordinate | ||||
|---|---|---|---|---|---|
| Metabolic measure |
Treatment | l/l 5HTT genotype |
s-variant 5HTT genotype |
l/l 5HTT genotype |
s-variant 5HTT genotype |
| Leptin (ng/ml) | C | 34.88 ± 4.19 | 41.03 ± 4.19 | 27.67 ± 3.57 | 30.98 ± 3.42 |
| E2 | 37.05 ± 5.79 | 47.03 ± 5.79 | 28.47 ± 4.94 | 32.52 ± 4.73 | |
| P4 | 41.28 ± 5.01 | 43.06 ± 5.01 | 29.66 – 4.23 | 32.14 ± 4.09 | |
| E2 + P4 | 32.94 ± 5.33 | 41.85 ± 5.33 | 30.45 ± 4.55 | 35.56 ± 4.35 | |
| Insulin (µU/ml) | Cb | 92.37 ± 19.76 | 79.83 ± 19.76 | 77.21 ± 16.85 | 55.27 ± 16.13 |
| E2a | 67.17 ± 14.42 | 46.27 ± 14.42 | 41.08 ± 12.30 | 65.20 ± 11.77 | |
| P4b | 124.2 ± 33.52 | 79.66 ± 33.52 | 87.54 ± 28.58 | 109.2 ± 27.37 | |
| E2 + P4b | 125.9 ± 28.36 | 60.51 ± 28.36 | 66.27 ± 24.18 | 88.91 ± 23.15 | |
| Glucose (mg/dl) | C | 89.99 ± 9.81 | 74.76 ± 9.81 | 71.85 ± 8.36 | 66.07 ± 8.01 |
| E2 | 78.10 ± 6.91 | 71.87 ± 6.91 | 63.11 ± 5.89 | 65.37 ± 5.64 | |
| P4 | 86.81 ± 8.93 | 71.11 ± 8.93 | 68.80 ± 7.62 | 73.73 ± 7.29 | |
| E2 + P4 | 82.79 ± 7.70 | 68.25 ± 7.70 | 70.39 ± 6.57 | 64.61 ± 6.29 | |
Discussion
Subordinate females, receiving more aggression and emitting more submissive behavior, maintained lower body weights, abdominal adiposity, and peripheral leptin than dominant females. Furthermore, as observed previously [5], dominant females with an l/l 5HT genotype were heavier than dominant s-variant females throughout the study period. These status differences is body weight are consistent with previous reports of status-dependent body weight differences [5, 40] due to diminished food intake in female rhesus monkeys [6]. Importantly, E2 administration significantly lowered body weight and estimates of body fat in all females but the effect on body weight was modulated by social status, as dominant females, regardless of 5HTT genotype, lost more body weight than did subordinate females. However, co-administration of P4 with E2 blocked the effects of E2 on body weight and fat. Taken together, these data indicate that social status modulates the effects of E2 on body weight.
Even though food intake and energy expenditure were not monitored in the current study, the overall status difference in body weights and abdominal adiposity is consistent with our previous observation that subordinate animals consume fewer calories daily than dominant animals when only a standard low-calorie diet is available [6]. Disruptions of food intake due to heightened activity of the LHPA axis that is characteristic of chronic stress conditions in humans and rodents, as well as social subordination in macaques, are dependent upon diet type and availability [4, 9, 10, 13, 14, 41]. Chronic LHPA activity in rodents and monkeys decreases food intake only when a low-calorie standard chow is available, an effect due to the anorectic action of CRH – urocortin system [5, 10, 42–44].
The administration of ovarian steroid hormones differentially altered body weight and sagittal adiposity over the course of a four-week treatment. As expected, replacement of E2 alone decreased body weight and sagittal adiposity in all groups of females. The decrease in body weight seen during E2 replacement corroborates previous reports that E2 attenuates body weight in monkeys and rodents [17, 18] and the inverse correlation between E2 and body weight over the course of the menstrual cycle in women [15]. Even though we did not assess food intake during the course of this study, ample literature suggests that E2 is anorectic [19] and attenuates body weight primarily by reducing meal size [45]. E2 modulates the expression of molecules crucial to feeding behavior at the level of the hypothalamus by decreasing the orexigenic effects of melanin-concentrating hormone (MCH, [46]), neuropeptide Y (NPY [47]), and ghrelin [48]. E2 also acts at the level of the nucleus of the solitary tract to have anorexic effects via estrogen receptor alpha (ERα) activation [19, 49].
We had hypothesized that E2 would interact with status to produce a greater loss in subordinates. While E2 decreased body weight in all females, dominant females lost significantly more weight than did subordinates. Despite the greater weight loss by dominant females under E2 administration, subordinate animals nonetheless still weighed significantly less. These data imply that the anorectic actions of E2 are related to preexisting energy stores represented by body weight and waist circumference. Without measuring food intake [19] or energy expenditure [50], we can only hypothesize that dominant females are more sensitive to these anorectic actions of estradiol. The mechanisms that would reduce the anorectic action of E2 in females with reduced body weights, which in the present study are subordinate females, are unknown and we are unaware of any studies that have assessed this relation. It is possible that signals from the LHPA axis attenuate the action of E2. For example, stressor exposure [51] or CRH over-expression in the amygdala [52] attenuate E2 activation of female sexual behavior. In any event, it is possible that even the smaller weight loss in subordinate females due to E2 has put even greater demands on their energy balance that could affect other homeostatic processes. Indeed, previous studies of female rhesus monkeys show subordinates are hypersensitive to the negative feedback action of low dose E2 treatment on LH (luteinizing hormone) secretion [53], an observation that could be due to the direct effects of E2 on the GnRH (gonadotropin-releasing hormone) – gonadotropic axis or to deficits in metabolic energy necessary to support reproductive function. Thus, although the weight loss induced by E2 may be less in subordinate females, the functional consequences may nonetheless be greater compared with dominant animals.
Administration of P4 in combination with E2 negated E2-induced weight loss and increased body weight in all females compared to the E2 alone condition. Periods of high P4, including the luteal phase of the menstrual cycle and pregnancy, coincide with increases in food intake and body weight [15, 19–21]. Changes in orexigenic signals such as NPY and agouti-related protein (AgRP) are expressed at these times of maximal P4 circulation [54–56]. The ability of P4 to increase food intake could be due to changes in gene expression mediated by activation of the progesterone receptor (PR) [57] or P4’s neuroactive metabolite, allopregnanolone [58, 59]. Both mechanisms are capable of increasing NPY expression, as PR is co-localized with NPY in hypothalamic neurons [60] and administration of allopregnanolone induces feeding in rodents [61, 62] and increases expression of NPY [63]. We cannot discern from the current study if either or both of these P4 mechanisms are responsible for the weight gain upon P4 treatment.
Alterations in abdominal adiposity were consistent with the changes in body weight, as E2 decreased and addition of P4 to the E2 treatment increased sagittal abdominal circumference. Hormone effects on adiposity are likely mediated through changes in gene expression in adipocytes, as these cells express both E2 and P4 receptors [64–66]. E2 decreased abdominal circumference in all females, regardless of social status, an effect in harmony with the observation that E2 decreases white adipose tissue (WAT) mass in rodents and women [67, 68] via activation of ERα [69]. Interestingly, even though subordinate animals did not lose as much weight as dominant animals on E2 treatment, they did lose abdominal adiposity, suggesting that their weight was redistributed on this hormonal condition possibly via increased energy expenditure [70]. There were no social status differences in serum insulin and glucose levels that corresponded with increased body weight and SAC in dominant animals. However, E2 replacement significantly lowered serum insulin without affecting glucose in all females, supporting data that E2 improves insulin sensitivity in a number of species and models [71, 72]. In contrast, P4 in combination with E2 increased abdominal circumference and circulating insulin levels in all females, an effect consistent with the fact that P4 increases WAT [73, 74].
Our data support previous observations that dominant females had significantly higher serum leptin compared with subordinates [5, 40]. This difference was maintained through each of the four different treatment conditions, despite significantly greater weight loss in dominant females during E2 replacement. Knowing E2-induced weight loss occurs independent of leptin [75], we expected that serum leptin would be decreased by the weight loss induced by E2 administration but this hypothesis was not supported. The data suggest that any decrease in leptin resulting from a loss of body fat during E2 treatment was compensated for by a direct effect of E2 increasing leptin synthesis and release. However, data supporting an E2-induced increase in leptin in rodents or in vitro models are equivocal [76–80]. Other approaches also report mixed results, as leptin may vary across the ovulatory cycle but is not necessarily lower during the periovulatory phase in cycling women [81, 82], and exogenous E2 administration in postmenopausal women is shown to increase [83, 84] and decrease plasma leptin [85]. The weight loss induced by E2 in obese mice is accompanied by reduced leptin gene expression and lower serum levels [78]. However, studies of non-obese, ovariectomized rats [86] or women [87] show that serum leptin levels do not decrease following E2 replacement despite a significant loss of body weight. Thus, it appears that the regulation of leptin synthesis following E2-induced weight loss in obese versus non-obese females may be different, but the mechanisms are undefined.
The effects of social status on body weight, SAC, and the response to E2 administration were not affected by 5HTT. We did however corroborate our previous finding that females with an l/l 5HTT genotype were overall larger than females with s- variant of the gene and that dominant l/l females had higher sagittal adiposity then all other groups of females [5, 28]. The overall greater body size associated with the l/l 5HTT genotype is seemingly contradictory to the reports in humans linking the s-variant 5HTT allele to higher rates of obesity [88, 89]. However, our effect of genotype is observed in monkeys maintained on a low-fat diet while humans are likely eating a variety of obesigenic diets. The question of whether 5HTT genotype modifies stress-induced changes in consumption of high caloric diets remains to be addressed.
In conclusion, the results of the present study show that hormonal replacement to ovariectomized female monkeys fed a low-fat, high-fiber diet affects body weight, estimates of body fat, and circulating insulin, and that these effects are modified by the social stress of subordination. Our data show that while E2 decreases body weight in all females, it does so less in females under chronic social stress who are smaller to begin with. It would seem these results are more directly applicable to postmenopausal women or premenopausal women whose ovaries have been removed than premenopausal women. The prevalence of abdominal obesity increases during the peri-menopause and early postmenopausal period [90, 91] supports a role of E2 in regulating food intake and body weight [19]. What is unknown is how social stress modulates postmenopausal weight gain in women eating a typical American diet, quite unlike the diet fed the monkeys, and whether this is influenced by E2 replacement. The other question is whether these results are relevant for premenopausal women or monkeys. Chronic exposure to psychosocial stressors in females can often produce anovulation and low but measureable levels of E2 [7, 92]. It is unclear how this noncyclic pattern of E2 affects body weight regulation in this population. While E2 does produce cyclic changes in body weight in ovulatory females [93, 94], it is unlikely these changes in body weight put females at metabolic risk. Rather, the elevated levels of E2 associated with a decrease in body weight and food intake occur in the context of increased activity and arousal [50], improve peripheral and central glucose utilization [95], and stimulate more prosocial behaviors [96], all functioning to facilitate mate selection and reproduction.
Acknowledgements
The study was conducted with the invaluable expert technical assistance of Jennifer Whitley, Holly Jarrell, Dr. Jacquelyn Hoffman, Marta Checchi and Jeff Fisher. The study was supported by NIH grants HD46501 and RR00165, and F31MH085445 (VM). The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- 2.Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JR, Adams M, Simon N, Wagner J, Franke AA. Monkeys exhibit sex differences in behavioral and physiological responses to a high-isoflavone, soy based diet. 6th International Symposium on the Role of Soy in Preventing and Treating Chronic Disease. 2005 (abstract) [Google Scholar]
- 4.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michopoulos V, Shepard KN, Arce M, Whitley JA, ME W. Society for the Study of Ingestive Behavior. Portland, OR: 2009. Food history and social status affect food intake in monkeys; p. 848. [Google Scholar]
- 7.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female 'protection' among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 8.Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Annals of the New York Academy of Sciences. 1997;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin releasing factor, corticosteroids, stress, and sugar: energy balance the brain and behavior. In: Pfaff DW, editor. Hormones, Brain, and Behavior. San Diego: Academic Press; 2002. pp. 571–631. [Google Scholar]
- 10.Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. European journal of pharmacology. 2002;440:189–197. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- 11.Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. The American journal of physiology. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 12.Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 13.Barry D, Pietrzak RH, Petry NM. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Ann Epidemiol. 2008;18:458–466. doi: 10.1016/j.annepidem.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiskanen TH, Niskanen LK, Hintikka JJ, Koivumaa-Honkanen HT, Honkalampi KM, Haatainen KM, et al. Metabolic syndrome and depression: a cross-sectional analysis. The Journal of clinical psychiatry. 2006;67:1422–1427. doi: 10.4088/jcp.v67n0913. [DOI] [PubMed] [Google Scholar]
- 15.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiology & behavior. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 16.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. The Journal of clinical endocrinology and metabolism. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 17.Czaja JA, Bielert C. Female rhesus sexual behavior and distance to a male partner: relation to stage of the menstrual cycle. Archives of sexual behavior. 1975;4:583–597. doi: 10.1007/BF01544267. [DOI] [PubMed] [Google Scholar]
- 18.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. Journal of comparative and physiological psychology. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 19.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical transactions of the Royal Society of London. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur G, Kulkarni SK. Subchronic studies on modulation of feeding behavior and body weight by neurosteroids in female mice. Methods and findings in experimental and clinical pharmacology. 2001;23:115–119. doi: 10.1358/mf.2001.23.3.627942. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz B, Cumming DC, Riordan E, Selye M, Yen SS, Rebar RW. Exercise-associated amenorrhea: a distinct entity? American journal of obstetrics and gynecology. 1981;141:662–670. doi: 10.1016/s0002-9378(15)33308-1. [DOI] [PubMed] [Google Scholar]
- 22.Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA) Journal of pediatric and adolescent gynecology. 2004;17:109–115. doi: 10.1016/j.jpag.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, N.Y. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 24.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, N.Y. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 25.Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, et al. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta) Archives of general psychiatry. 1993;50:615–623. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- 26.Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- 27.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social Subordination and Polymorphisms in the Gene Encoding the Serotonin Transporter Enhance Estradiol Inhibition of Luteinizing Hormone Secretion in Female Rhesus Monkeys. Biology of reproduction. 2009 doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in the adult female rhesus macaque (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 30.Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Hormones and behavior. 2006;49:233–236. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Mook D, Felger J, Graves F, Wallen K, Wilson ME. Tamoxifen fails to affect central serotonergic tone but increases indices of anxiety in female rhesus macaques. Psychoneuroendocrinology. 2005;30:273–283. doi: 10.1016/j.psyneuen.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- 33.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 34.Wynne K, Stanley S, Bloom S. The gut and regulation of body weight. The Journal of clinical endocrinology and metabolism. 2004;89:2576–2582. doi: 10.1210/jc.2004-0189. [DOI] [PubMed] [Google Scholar]
- 35.Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay A. Dietary fat and body weight set point. Nutr Rev. 2004;62:S75–S77. doi: 10.1111/j.1753-4887.2004.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 37.Riserus U, Arnlov J, Brismar K, Zethelius B, Berglund L, Vessby B. Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care. 2004;27:2041–2046. doi: 10.2337/diacare.27.8.2041. [DOI] [PubMed] [Google Scholar]
- 38.Pazol K, Kaplan JR, Abbott DH, Wilson ME. Practical Measurement of total and bioavailable estradiol in female macaques. Clinica Chimica Acta. 2004;340:117–126. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, et al. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. The Journal of clinical endocrinology and metabolism. 2003;88:4874–4883. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- 40.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- 41.Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Meerlo P, Overkamp GJ, Koolhaas JM. Behavioural and physiological consequences of a single social defeat in Roman high- and low-avoidance rats. Psychoneuroendocrinology. 1997;22:155–168. doi: 10.1016/s0306-4530(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 44.Sachser N, Lick C. Social stress in guinea pigs. Physiology & behavior. 1989;46:137–144. doi: 10.1016/0031-9384(89)90246-1. [DOI] [PubMed] [Google Scholar]
- 45.Gray JM, Greenwood MR. Time course of effects of ovarian hormones on food intake and metabolism. The American journal of physiology. 1982;243:E407–E412. doi: 10.1152/ajpendo.1982.243.5.E407. [DOI] [PubMed] [Google Scholar]
- 46.Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 49.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain research. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 50.Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and biobehavioral reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Uphouse L, Selvamani A, Lincoln C, Morales L, Comeaux D. Mild restraint reduces the time hormonally primed rats spend with sexually active males. Behavioural brain research. 2005;157:343–350. doi: 10.1016/j.bbr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ajala MO, Oladipo OO, Afonja OA. Hypothalamic neuropeptide Y mRNA in pregnant, lactating and suckling rats. The Nigerian postgraduate medical journal. 2001;8:16–21. [PubMed] [Google Scholar]
- 55.Oberto A, Mele P, Zammaretti F, Panzica G, Eva C. Evidence of altered neuropeptide Y content and neuropeptide Y1 receptor gene expression in the hypothalamus of pregnant transgenic mice. Endocrinology. 2003;144:4826–4830. doi: 10.1210/en.2003-0197. [DOI] [PubMed] [Google Scholar]
- 56.Rocha M, Bing C, Williams G, Puerta M. Pregnancy-induced hyperphagia is associated with increased gene expression of hypothalamic agouti-related peptide in rats. Regulatory peptides. 2003;114:159–165. doi: 10.1016/s0167-0115(03)00119-8. [DOI] [PubMed] [Google Scholar]
- 57.Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- 58.Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. The Journal of physiology. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science (New York, N.Y. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 60.Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC. Progesterone-receptive dopaminergic and neuropeptide Y neurons project from the arcuate nucleus to gonadotropin-releasing hormone-rich regions of the ovine preoptic area. Neuroendocrinology. 2005;82:21–31. doi: 10.1159/000090122. [DOI] [PubMed] [Google Scholar]
- 61.Chen SW, Rodriguez L, Davies MF, Loew GH. The hyperphagic effect of 3 alpha-hydroxylated pregnane steroids in male rats. Pharmacology, biochemistry, and behavior. 1996;53:777–782. doi: 10.1016/0091-3057(95)02142-6. [DOI] [PubMed] [Google Scholar]
- 62.Reddy DS, Kulkarni SK. The role of GABA-A and mitochondrial diazepam-binding inhibitor receptors on the effects of neurosteroids on food intake in mice. Psychopharmacology. 1998;137:391–400. doi: 10.1007/s002130050635. [DOI] [PubMed] [Google Scholar]
- 63.Ferrara G, Serra M, Zammaretti F, Pisu MG, Panzica GC, Biggio G, et al. Increased expression of the neuropeptide Y receptor Y(1) gene in the medial amygdala of transgenic mice induced by long-term treatment with progesterone or allopregnanolone. Journal of neurochemistry. 2001;79:417–425. doi: 10.1046/j.1471-4159.2001.00559.x. [DOI] [PubMed] [Google Scholar]
- 64.Lacasa D, Garcia Dos Santos E, Giudicelli Y. Site-specific control of rat preadipocyte adipose conversion by ovarian status: Possible involvement of CCAAT/enhancer-binding protein transcription factors. Endocrine. 2001;15:103–110. doi: 10.1385/endo:15:1:103. [DOI] [PubMed] [Google Scholar]
- 65.Lacasa D, Le Liepvre X, Ferre P, Dugail I. Progesterone stimulates adipocyte determination and differentiation 1/sterol regulatory element-binding protein 1c gene expression. potential mechanism for the lipogenic effect of progesterone in adipose tissue. The Journal of biological chemistry. 2001;276:11512–11516. doi: 10.1074/jbc.M008556200. [DOI] [PubMed] [Google Scholar]
- 66.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 67.Tchernof A, Poehlman ET. Effects of the menopause transition on body fatness and body fat distribution. Obesity research. 1998;6:246–254. doi: 10.1002/j.1550-8528.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 68.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. International journal of obesity. 1985;9 Suppl 1:83–92. [PubMed] [Google Scholar]
- 69.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner JD, Thomas MJ, Williams JK, Zhang L, Greaves KA, Cefalu WT. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. The Journal of clinical endocrinology and metabolism. 1998;83:896–901. doi: 10.1210/jcem.83.3.4628. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez C, Alonso A, Diaz F, Patterson AM. Dose- and time-dependent effects of 17beta-oestradiol on insulin sensitivity in insulin-dependent tissues of rat: implications of IRS-1. The Journal of endocrinology. 2003;176:367–379. doi: 10.1677/joe.0.1760367. [DOI] [PubMed] [Google Scholar]
- 73.Nava MP, Abelenda M, Puerta ML. Cold-induced and diet-induced thermogenesis in progesterone-treated rats. Pflugers Arch. 1990;415:747–750. doi: 10.1007/BF02584015. [DOI] [PubMed] [Google Scholar]
- 74.Steingrimsdottir L, Brasel J, Greenwood MR. Hormonal modulation of adipose tissue lipoprotein lipase may alter food intake in rats. The American journal of physiology. 1980;239:E162–E167. doi: 10.1152/ajpendo.1980.239.2.E162. [DOI] [PubMed] [Google Scholar]
- 75.Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obesity research. 2004;12:716–724. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- 76.Kristensen K, Pedersen SB, Richelsen B. Regulation of leptin by steroid hormones in rat adipose tissue. Biochemical and biophysical research communications. 1999;259:624–630. doi: 10.1006/bbrc.1999.0842. [DOI] [PubMed] [Google Scholar]
- 77.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 78.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 80.Pelleymounter MA, Baker MB, McCaleb M. Does estradiol mediate leptin's effects on adiposity and body weight? The American journal of physiology. 1999;276:E955–E963. doi: 10.1152/ajpendo.1999.276.5.E955. [DOI] [PubMed] [Google Scholar]
- 81.Wunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, Birkhauser MH. Serum leptin and C-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155:137–142. doi: 10.1530/eje.1.02178. [DOI] [PubMed] [Google Scholar]
- 82.Cella F, Giordano G, Cordera R. Serum leptin concentrations during the menstrual cycle in normal-weight women: effects of an oral triphasic estrogen-progestin medication. European journal of endocrinology / European Federation of Endocrine Societies. 2000;142:174–178. doi: 10.1530/eje.0.1420174. [DOI] [PubMed] [Google Scholar]
- 83.Lavoie HB, Taylor AE, Sharpless JL, Anderson EJ, Strauss CC, Hall JE. Effects of short-term hormone replacement on serum leptin levels in postmenopausal women. Clin Endocrinol (Oxf) 1999;51:415–422. doi: 10.1046/j.1365-2265.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 84.Konukoglu D, Serin O, Ercan M. Plasma leptin levels in obese and non-obese postmenopausal women before and after hormone replacement therapy. Maturitas. 2000;36:203–207. doi: 10.1016/s0378-5122(00)00153-5. [DOI] [PubMed] [Google Scholar]
- 85.Di Carlo C, Tommaselli GA, Pisano G, Nasti A, Rossi V, Palomba S, et al. Serum leptin levels in postmenopausal women: effects of transdermal hormone replacement therapy. Menopause. 2000;7:36–41. doi: 10.1097/00042192-200007010-00007. [DOI] [PubMed] [Google Scholar]
- 86.Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP. Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci. 1999;64:2299–2306. doi: 10.1016/s0024-3205(99)00181-2. [DOI] [PubMed] [Google Scholar]
- 87.Nar A, Demirtas E, Ayhan A, Gurlek A. Effects of bilateral ovariectomy and estrogen replacement therapy on serum leptin, sex hormone binding globulin and insulin like growth factor-I levels. Gynecol Endocrinol. 2009;25:773–778. doi: 10.3109/09513590903159532. [DOI] [PubMed] [Google Scholar]
- 88.Sookoian S, Gemma C, Garcia SI, Gianotti TF, Dieuzeide G, Roussos A, et al. Short allele of serotonin transporter gene promoter is a risk factor for obesity in adolescents. Obesity (Silver Spring) 2007;15:271–276. doi: 10.1038/oby.2007.519. [DOI] [PubMed] [Google Scholar]
- 89.Sookoian S, Gianotti TF, Gemma C, Burgueno A, Pirola CJ. Contribution of the functional 5-HTTLPR variant of the SLC6A4 gene to obesity risk in male adults. Obesity (Silver Spring) 2008;16:488–491. doi: 10.1038/oby.2007.64. [DOI] [PubMed] [Google Scholar]
- 90.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 91.Okosun IS, Boltri JM, Anochie LK, Chandra KM. Racial/ethnic differences in prehypertension in American adults: population and relative attributable risks of abdominal obesity. J Hum Hypertens. 2004;18:849–855. doi: 10.1038/sj.jhh.1001771. [DOI] [PubMed] [Google Scholar]
- 92.Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr Clin North Am. 1989;12:105–116. [PubMed] [Google Scholar]
- 93.Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm Behav. 1989;23:235–250. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 94.Geary N. Estradiol and appetite. Appetite. 2000;35:273–274. doi: 10.1006/appe.2000.0362. [DOI] [PubMed] [Google Scholar]
- 95.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 96.Wallen K. Sex and context: hormones and primate sexual motivation. Hormones and behavior. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]