Abstract
Disturbed information processing observed in neuropsychiatric disorders is reflected by deficient sensorimotor gating, measured as prepulse inhibition (PPI) of the acoustic startle response (ASR). Long-term, higher-dose methamphetamine (METH) abuse patterns are associated with cognitive impairments, mania and/or schizophrenia-like psychosis. The present study investigated in rats METH-induced impairment of sensorimotor gating using an intravenous self-administration (IVSA) escalating dose procedure. In this procedure, rats escalated drug intake during weekly extended access periods to METH IVSA (1, 3, and 6-h), where PPI was assessed after each access period and thus at various times of drug exposure. Despite increased drug intake over the course of extended access to METH, disruption of sensorimotor gating was only seen after the access period of 6-h. The data suggest that METH-induced impairment of sensorimotor gating in IVSA-tasks is rather attributed to continuous and higher-dose exposure than to actual amounts of drug present at the time of testing. IVSA procedures, comprising stepwise stimulant escalation may serve as a useful translational model in rats that approximate important aspects of human abuse pattern in the context of stimulant-induced cognitive and behavioral deficits.
Keywords: methamphetamine, self-administration, escalation, sensorimotor gating
1 INTRODUCTION
Methamphetamine (METH) is a highly addictive psychostimulant, and its use may lead to deleterious psychiatric symptoms, including cognitive impairments and psychosis, particularly with long-term, high-dose abuse patterns [23, 22, 25]. The factors contributing to these impairments have not been fully delineated but some evidence suggests that the duration and pattern of METH exposure are contributing factors [28, 9]. Attempts to model the pattern and outcomes of human METH abuse in animals have been made, using both non-contingent and contingent drug administration procedures, and evidence suggests that the neurobiological consequences of these procedures are different [13]. Indeed, commonly used non-contingent administration procedures do not provide the opportunity to investigate the relationship between drug-induced dysfunctions and motivational aspects of METH abuse [25], whereas METH self-administration procedures in rodents clearly exhibit greater face and construct validity with respect to human abuse patterns from the perspective of drug taking and seeking. However, the potential significance of these differences remains controversial [29, 15, 41, 17, 30].
Psychotic states induced by stimulants, such as METH, closely resemble symptoms observed in patients with idiopathic schizophrenia [10]. Disruptions in measures of attention and cognition have been seen in patients as well, whereas these deficits may be linked to impairments in sensorimotor gating [12]. (One operational measure of sensorimotor gating is prepulse inhibition (PPI) of the acoustic startle response (ASR), i.e. the reduction of the startle response to an intense acoustic stimulus when shortly preceded by a weaker stimulus (prepulse). The measurement of PPI uses almost identical procedures in humans and rodents and is considered to reflect the ability of organisms to gate out irrelevant sensory information [18, 35, 36, 2]. Patients with schizophrenia exhibit deficits in PPI and similar deficits can be produced in rats experimentally via pharmacological or developmental manipulations. While such induced PPI deficits are clearly not animal models of schizophrenia per se, they may serve as endophenotypes or physiological markers that are associated with certain psychiatric disorders. Huntington’s disease, obsessive-compulsive disorder and mania are also accompanied by disturbed information processing and probably have dysfunctions of cortico-striato-pallidal loops similar to schizophrenia [32, 33, 34, 12, 4, 19]. Although it is well established in experimental animals that PPI can be disrupted by a variety of drugs, e.g. direct or indirect dopamine (DA) receptor agonists including apomorphine and amphetamine (AMPH) [14, 12, 33], little is known about the possible effects of continuous, escalated METH self-administration on sensorimotor gating processes.
Since animal models are important paradigms for the understanding and treatment of neuropsychiatric disorders this study tried to combine certain aspects of human METH abuse such as motivation or intake pattern in rats using a self-administered escalating dose procedure. To analyze whether and how stepwise extended access to METH self-administration may induce disruption in sensorimotor gating, PPI of the ASR was assessed after various exposure times over this stimulant escalation course.
2 METHODS
2.1 Subjects
Adult male Sprague Dawley rats (350-450 g) were purchased from Harlan Labs (Gilroy, CA) and were single-housed with ad libitum access to food and tap water. The vivarium was temperature- (20°C) and humidity- (55 ± 5%) controlled and maintained on a reverse 12 h/12 h light/dark cycle (lights off 8:00 am to 8:00 pm) to allow the experiments to be performed during the normal active phase of the rats’ awake/sleep cycle. During the dark period, the facilities were illuminated with red light to facilitate observation of the animals. All animal facilities, surgical and experimental procedures were in accordance with the National Institutes of Health and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the Institutional Animal Care and Use Committee.
2.2 Surgery
After one week of acclimation, animals were anesthetized with an isoflurane/oxygen mixture (1-3% isoflurane), and chronic indwelling catheters were implanted into the right jugular vein as described previously [5] with modifications. Briefly, catheters were constructed by fitting silastic tubing to a guide cannula, bent at a right angle. The guide cannula was embedded in dental cement, attached to a 1-inch circle of marlex mesh, and mounted on the animal’s back. The silastic tubing was passed subcutaneously from the back of the rat to the right external jugular vein. To maintain a closed system, a Tygon cap was inserted over the guide cannula. After surgery, animals were single-housed and catheters were flushed daily with approximately 0.15 ml of sterile saline solution containing heparin (30 USP units/ml) and timentin (3.75 mg). Similarly, catheters were flushed prior to self-administration sessions to test their patency. When the rats were not connected to infusion pumps, a Tygon cap was inserted over the guide cannula to minimize contamination.
2.3 Methamphetamine self-administration
After a 5-day recovery period from surgery, intravenous self-administration (IVSA) of METH was initiated in custom-made experimental chambers (30 × 30 × 38 cm) placed within ventilated sound-attenuating boxes. Each chamber was maintained at constant temperature (20° C) and humidity (55 ± 5%). One wall of the chamber was equipped with two retractable levers (active and inactive, 6 cm apart) located 6 cm above the metal grid floor of the chamber. A cue light was centered approximately 1 cm above the levers. A counterbalanced arm above the ceiling of the chamber held a fluid swivel whose inlet port was attached by Tygon tubing to a syringe mounted on a motor-driven syringe pump located outside of the chamber. The outlet port of the swivel was connected to the animal’s catheter by a length of Tygon tubing encased in a stainless steel spring. The swivel system allowed free movement of the animal in the chamber. A lever press (active lever) resulted in an infusion of METH at a dose of 0.05 mg/kg in a volume of 0.05 ml over a period of 1.5 s. Responses on the other lever (inactive lever) were recorded but had no consequences. The delivery of METH was paired with the activation of the cue light located above the levers. The cue light remained illuminated throughout a 20 s timeout (TO) period, during which the lever became inactive. Lever pressing for METH IVSA was reinforced on a continuous reinforcement fixed-ratio 1 (FR1) schedule, and the levers retracted immediately at the end of the session. Based on preliminary observations in our laboratory, food restriction (14 g/rat/day for 3 days before starting training) enhanced exploratory activity in the operant chamber and facilitated acquisition of drug self-administration. The sessions were initiated with the introduction of the levers. For acquisition of drug intake (baseline sessions), animals had access to METH IVSA (0.05 mg/kg/infusion) for 1-h on an FR1 TO 20 s schedule. The rat’s performance was considered stable when it pressed the active lever at least 10 times and its baseline performance showed less than 10% variation over three days. It took the animals about three to five sessions to learn the task and thus reach baseline performance. After acquisition of stable responding, the access time for IVSA was progressively extended during successive six day periods, from 1-h to 3-h and finally to 6-h access. Between the different access periods, animals always had two drug-free days. All data collection and test session functions were controlled by computers and MED-PC IV software (MED Associates, St. Albans, VT). The weights of the animals remained relatively stable throughout the escalation periods and throughout the experiment, none of the animals exhibited evidence of METH-induced hyperthermia (e.g., excess salivation, nasal discharge, dehydration, or lying prone). No METH-treated animal died during the experiment.
2.4 Drugs
Methamphetamine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline and administered intravenously. The dose represents its base.
2.5 Prepulse inhibition of the acoustic startle response
PPI was measured using a three-unit automated SRLab startle system (San Diego Instruments, San Diego, CA, USA). The startle boxes consisted of non-restrictive Plexiglas cylinders (9 cm in diameter) resting on a platform, mounted above a sensitive piezoelectric sensor, inside a sound-attenuated, ventilated chamber. Vibrations of the cylinder caused by the whole-body ASR were transduced into analog signals and digitized and stored on a computer using SRLab software. At the beginning of each session, animals were placed into the startle chambers and, during a 5 min acclimatization period, they were exposed to 70 dB white background noise delivered through speakers in the ceiling of the chamber above the animal. The background noise continued throughout the session. A test session consisted of five different blocks of trials presented in a pseudo-random order. Blocks A and E consisted of five successive 40 ms pulse-alone white noise trials with a 120 dB intensity sound pressure level (SPL). The trials in Block B were presented 10 times and consisted of a pulse alone (120 dB) and a prepulse followed by pulse trials (three trial types: 73+120 dB, 76+120 dB, and 82+120 dB). Block C was presented four times and comprised pulse-alone stimuli presented in a random order (75, 80, 85, 90, 95 and 100 dB SPL). Inter stimulus intervals (ISI) were presented in Block D in a random order (25, 50, 100, 200 and 500 ms). Each block (B-D) included two randomly distributed 120 dB pulse-alone stimuli. Each trial was separated by a non-stimulus trial (NOSTIM), and the inter-trial interval (ITI) was an average of 7.5 s (minimum, 3 s; maximum, 12 s). Since stimuli were presented every second trial, the actual ITI can be considered to be an average of 15 s. The pulse-alone trials consisted of a 40 ms pulse of broadband noise at given intensities. The prepulse + pulse trials consisted of a 20 ms noise prepulse, a 100 ms delay and a 40 ms 120 dB startle stimulus (100 ms onset-to-onset interval). The NOSTIM trials consisted of background noise only and allowed the assessment of general activity in the startle chamber when no acoustic stimuli were present. The percentage of PPI induced by each of the three prepulse intensities was calculated as 100 — (startle amplitude on pre-pulse trial)/(startle amplitude on pulse-alone trial). Startle sessions were ~35 min in duration.
2.6 Experimental design
To better simulate motivational aspects and abuse patterns in humans, rats were exposed to METH IVSA, while access to the drug was extended stepwise for successive six-day periods of 1, 3, and 6-h. For analyzing the impact of this drug-escalation course on sensorimotor gating, rats were disconnected from the catheters and immediately tested for ASR and PPI performance always on the last day of each progressively extended IVSA period. A control group of age-matched, drug-free naive rats, which was neither implanted with catheters nor received sham surgeries, was tested for ASR and PPI performance three times in the same time cycle as the drug-exposed animals.
2.7 Statistical analyses
The descriptive statistics are based on means, and variance is indicated by the standard error of the mean (SEM). Statistical analyses were conducted using SigmaStat version 2.0 software (SPSS, Chicago, IL). Values of p < 0.05 were considered statistically significant. Drug intake during IVSA (first hour and total intake) was analyzed using one-way analysis of variance (ANOVA) for access time comparisons. The effects of progressively extended METH IVSA on startle, PPI and NOSTIM trials were evaluated using two-way ANOVAs. The actual amount of drug (mg/kg METH) consumed within the last 30 min of the different IVSA sessions before testing PPI was analyzed using a one-way ANOVA. All statistical analyses were followed by Tukey’s post hoc t-test for pairwise comparisons.
3 RESULTS
3.1 Methamphetamine self-administration
Active lever responding and thus total METH intake (mg/kg/day) is shown in Fig. 1A. ANOVA revealed an effect of access time on total active lever pressing [F(17,119)= 175.668, p < 0.001]. Lever pressing was significantly greater over the extended 3 and 6-h periods compared to the last day of the limited 1-h access period (p < 0.001). To further analyze the nature of escalation, drug intake and active lever pressing during the first hour was compared (ANOVA; [F(17,119)= 12.295, p < 0.001], Fig. 2). In contrast to the last day of the 1-h access period, first hour active lever pressing was significantly increased on the last three days of the extended 6-h period (post hoc comparisons, p < 0.001).
Fig. 1.
Course of escalation of METH intake following extended drug availability at a dose of 0.05 mg/kg/infusion. Active lever pressing and total METH intake (mg/kg) during the different extended access sessions is presented in (A), active lever pressing and METH intake (mg/kg) during the first hour is depicted in (B). Data are expressed as means ± SEM, whereas each dot represents lever pressing and METH intake (mg/kg) at the respective time points. Differences in active lever pressing between long-term access (3, 6-h) and the last session of the short-term (1-h) access period are indicated as asterisks (ANOVA, Tukey’s post hoc t-test, p < 0.05).
Fig. 2.
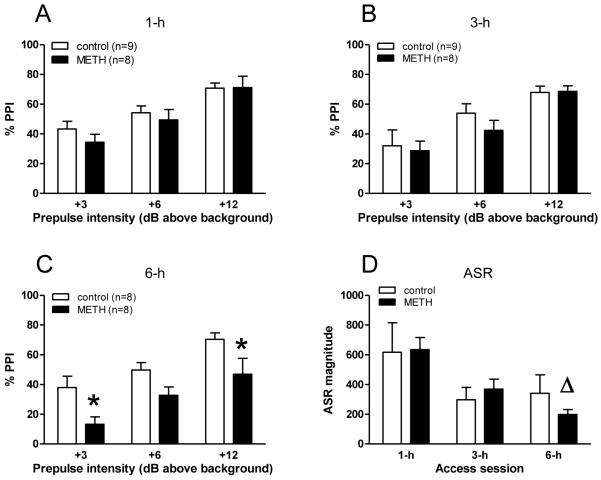
PPI of the ASR in rats after extended access to METH IVSA. Animals were always tested after six days of different access periods. Data are expressed as mean ± SEM. Animal numbers of the access/control group for ASR testing correspond to the ones of the PPI testing (see A-C). Differences in PPI between the METH group and controls are indicated by asterisks (A-C), differences in ASR between access times within the METH group by triangles (D) (ANOVA, Tukey’s post hoc t-test, p < 0.05).
3.2 Prepulse inhibition and acoustic startle response after extended access to METH
Animals were tested for PPI after six days of access to different periods of METH IVSA. There were no differences in PPI after six days of 1 or 3-h daily access to METH (Fig. 2A, B). However, 6-h daily access to METH significantly reduced PPI [(F(1,42) = 15.427, p < 0.001)], and post hoc comparisons revealed significant differences at the prepulse intensities of +3 dB (p < 0.05) and +12 dB (p < 0.05) compared to controls (Fig. 2C). In addition, although ASR was not significantly affected in the METH-treated animals compared with controls, an ANOVA revealed a significant difference in ASR among the different extended access periods [F(2,44) = 5.476, p < 0.008)]. Post hoc comparisons indicated that daily extended access to METH for 6-h reduced the ASR compared to 1-h access (p < 0.05, Fig. 2D).
NOSTIM trials were used to measure gross motor activity during the PPI testing sessions [37]. As shown in Fig. 3, ANOVA revealed a significant difference in NOSTIM activity after METH IVSA [F(1,44) = 5.640, p < 0.022)]. Compared to controls gross locomotor activity was increased after 1-h limited access sessions (post hoc comparisons, p < 0.05).
Fig. 3.

Effects of METH on activity readings between startle trials (“Nostim” levels) during limited and extended access to METH self-administration. Data are expressed as means ± SEM. Difference in NOSTIM activity between the METH group and controls is indicated by asterisk (ANOVA, Tukey’s post hoc t-test, p < 0.05).
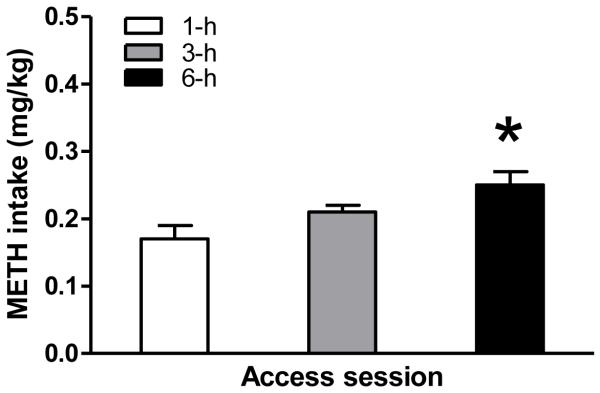
3.3 Estimated drug levels
Because of the relatively short half-life of METH in rats [< 1-hr] (Melega et al., 1995; Rivie‘re et al., 1999), we reasoned that an evaluation of the number of active lever presses during the last 30 min prior to PPI testing would more accurately reflect an approximation of the relative drug levels at the time of testing (Fig. 4). ANOVA revealed a significant difference in drug intake (mg/kg) during the last 30 min before testing PPI [F(2,14) = 5.023, p < 0.023]. Access to 6-h extended METH IVSA increased drug intake compared to limited access of 1-h (post hoc comparisons, p < 0.05). However, there was no difference in drug intake between the 3-h and 6-h sessions during the last 30 min prior to PPI testing.
Fig. 4.
Estimated METH intake during the last 30 min before PPI and ASR testing. Data are expressed as means ± SEM. Difference in drug intake between short- (1-h) and long-term access (6-h) is indicated by asterisk. (ANOVA, Tukey’s post hoc t-test, p < 0.05).
4 DISCUSSION
The present results indicate that extending access to METH from 1 to 6-h leads to progressively increased METH consumption, consistent with previously reported observations [16]. Increased consumption was evident in both overall daily drug intake (increased from near 0.5 mg/kg /day to about 3.4 mg/kg/day) as well as in the amount of drug taken during the first hour of exposure (0.5 mg/kg to about 1.1 mg/kg) (Fig 1A, B). Thus, the extended access approach promotes a drug consumption pattern resembling human abuse patterns, where escalation in dosage typically precedes higher dose maintenance use [28].
Secondly, extended access to METH IVSA resulted in a disruption of sensorimotor gating. This observation is consistent with previous findings, that reduced PPI in rats is seen after acute and long-term treatment with amphetamines [e.g. 20, 10, 3, 38, 39], or after direct infusion of DA into the mesolimbic DA system [11]. Following extended access to METH IVSA, a significant disruption in PPI was only evident after exposure to the 6-h period (Fig. 2C). Although the daily drug exposure was considerably greater during the 6-h sessions, this amount of METH does not provide an accurate representation of the amount of drug “on board” at the time of PPI testing, because the half-life of METH is less than one hour. Rather, a more appropriate estimate of drug levels at the time of PPI testing could be made based on lever presses accumulated during the 30-min interval immediately prior to testing (Fig. 4). Because estimated plasma METH concentrations were not significantly different at the time of testing following the 3-h and 6-h periods (Fig. 3), we conclude that the duration of exposure, rather than simply the dose may play a critical role in the observed PPI deficit. But, in this regard, it is difficult to compare results achieved with relative, estimated amounts of self-administered METH with effects obtained in studies using non-contingent METH or AMPH administration. That is, most non-contingent studies typically used either the subcutaneous (s.c.) or intraperitoneal (i.p.) route of high, single dose drug administration.
A gradual emergence of psychotic reactions to stimulants is suggested to be accompanied by neuroadaptations that progressively increase an individual’s sensitivity to the drug’s psychotogenic properties. Similar neuroadaptations are hypothesized to be responsible for the development of idiopathic psychosis [10], and chronic stimulant intake results in long-term changes in the function of a variety of neurotransmitter systems [40, 6]. For example, Schwendt and colleagues [26] observed a persistent decrease in DA transporter protein levels in the prefrontal cortex and dorsal striatum after extended access to 6-h METH IVSA in rats. We therefore hypothesize that the prolonged exposure to the drug with extended access periods in the present study was also associated with compensatory neuroadaptations in certain brain areas that probably contribute to the disruption of PPI.
The course of extended access to METH IVSA was accompanied by a non-significant decrease in startle amplitude at least between the control and the METH group (Fig. 2D). Indeed, the finding of a decreased startle is somewhat novel because psychomotor stimulants usually lead to an increase in ASR [e.g. 3, 38, 39] and overall activity in general, while depressant drugs that generally decrease activity typically decrease the ASR [see 18]. Since an increase in NOSTIM values of self-administering animals during PPI testing was observed in the METH exposed animals (Fig. 3), it seems that the diminished ASR did not result from inhibition of overall activity or acute exhaustion, effectuated by METH. Previous reports revealed that an increase in NOSTIM values during PPI/ASR testing is consistent with enhanced motor activity [e.g. 37]. Alternatively, the increase in NOSTIM values, at least after the extended access period of 6-h (although it did not reach level of significance), may reflect the presence of stereotyped behaviors, since stereotypy was prominent during this period (data not shown). On the other hand, a trend towards attenuated startle reactivity was also evident in the naive control group, possibly reflecting within-session habituation, while PPI was unaffected (Fig. 2). Admittedly, this finding and the notion that PPI and ASR have been shown to be generally independent dissociable measures [for example, 7, 8] makes it difficult to distinguish, whether the observed PPI disruption was due to the escalation course of METH IVSA or rather attributed to simply a METH induced startle attenuation, possibly elicited by stereotyped behaviors.
Taken together, psychotic states induced by METH in humans are associated with long-term, high-dose abuse patterns [1, 23, 22, 25] and closely resemble psychosis observed in patients with idiopathic schizophrenia [10]. Deficient sensorimotor gating, measured by PPI of the ASR is suggested to have direct relevance to psychosis [2, 31], and stimulant-induced psychotic states most likely develop during sustained intake of relatively high doses of drug over an extended period of time [27]. The results of the present study demonstrate that rats escalate drug intake following stepwise extended access to METH IVSA up to 6-h. Importantly, this self-administered escalating dose procedure resulted in disrupted sensorimotor gating processes similar to those seen during psychotic states in humans, whereupon this disruption rather seems to be caused by continuous higher-dose intake of METH than by the actual drug level at the time of testing.
IVSA procedures, comprising stepwise stimulant escalation may serve as a useful translational model in rats that approximate important aspects of human abuse pattern in the context of stimulant-induced cognitive and behavioral deficits.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grant DA01568-30 from the National Institute on Drug Abuse to Ronald Kuczenski. The authors would like to thank Mr. Michael Arends for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Martin Hadamitzky and Ronald Kuczenski declare no conflicts of interest. Athina Markou declares that she has received contract research support from Intracellular Therapeutics, Inc., Lundbeck Research USA, Inc., Bristol-Myers Squibb Co., and F. Hoffman-La Roche Inc., Pfizer and Astra-Zenca, and honorarium/consulting fees from Abbott GmbH, AstraZeneca, and Pfizer during the past 3 years.
REFERENCES
- [1].Angrist B. Amphetamine psychosis: clinical variations of the syndrome. In: Cho AK, Segal DS, editors. Amphetamine and its analogues. Academic Press; San Diego: 1994. pp. 387–414. [Google Scholar]
- [2].Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- [3].Brunell SC, Spear LP. Effects of acute ethanol or amphetamine administration on the acoustic startle response and prepulse inhibition in adolescent and adult rats. Psychopharmacology. 2006;186:579–586. doi: 10.1007/s00213-006-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL. Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? Am. J. Psychiatry. 2002;159:869–871. doi: 10.1176/appi.ajp.159.5.869. [DOI] [PubMed] [Google Scholar]
- [5].Caine B, Lintz R, Koob GF. Intravenous drug-self-administration techniques in animals. In: Sahgal A, editor. Behavioural neuroscience: a practical approach. Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- [6].Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. 2007. [DOI] [PubMed] [Google Scholar]
- [7].Cilia J, Reavill C, Hagan JJ, Jones DNC. Long-term evaluation of isolation-reared prepulse inhibition deficits in rats. Psychopharmacology. 2001;156:327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- [8].Cilia J, Hatcher P, Reavill C, Jones DN. (+/−) Ketamine-induced prepulse inhibition deficits of an acoustic startle response in rats are not reversed by antipsychotics. J Psychopharmacol. 2007;21:302–311. doi: 10.1177/0269881107077718. [DOI] [PubMed] [Google Scholar]
- [9].Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- [10].Druhan JP, Geyer MA, Valentino RJ. Lack of sensitization to the effects of d-amphetamine and apomorphine on sensorimotor gating in rats. Psychopharmacology. 1998;135:296–304. doi: 10.1007/s002130050513. [DOI] [PubMed] [Google Scholar]
- [11].Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [12].Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- [13].Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- [14].Hutchison KE, Swift R. Effect of d-amphetamine on prepulse inhibition of the startlereflex in humans. Psychopharmacology. 1999;143:394–400. doi: 10.1007/s002130050964. [DOI] [PubMed] [Google Scholar]
- [15].Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- [16].Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- [17].Kiyatkin EA, Brown PL. Brain temperature fluctuations during passive vs. active cocaine administration: clues for understanding the pharmacological determination of drug-taking behavior. Brain Res. 2004;1005:101–116. doi: 10.1016/j.brainres.2004.01.038. [DOI] [PubMed] [Google Scholar]
- [18].Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- [19].Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am. J. Psychiatry. 2005;162:1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- [20].Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1998;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- [21].Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- [22].Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- [23].Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- [24].Riviere GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- [25].Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Segal DS, Schuckit MA. Animal models of stimulant-induced psychosis. In: Creese I, editor. Stimulants: neurochemical, behavioral, and clinical perspectives. Raven Press; New York: 1983. pp. 131–167. [Google Scholar]
- [28].Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- [29].Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- [30].Stuber GD, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- [31].Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizoph Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- [32].Swerdlow NR, Benhow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- [33].Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- [34].Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think to know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- [36].Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- [37].Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL. Reduced startle gating after D1 blockade: effects of concurrent D2 blockade. Pharmacol Biochem Behav. 2005;82:293–299. doi: 10.1016/j.pbb.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizoph Res. 2003;64:103–114. doi: 10.1016/s0920-9964(03)00009-4. [DOI] [PubMed] [Google Scholar]
- [39].Tenn CC, Kapur S, Fletcher PJ. Sensitization to amphetamine, but not phencyclidine, disrupts prepulse inhibition and latent inhibition. Psychopharmacology. 2005;180:366–376. doi: 10.1007/s00213-005-2253-z. [DOI] [PubMed] [Google Scholar]
- [40].Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- [41].Winsauer PJ, Moerschbaecher JM, Molina PE, Roussell AM. Contingent and concontingent cocaine administration in rhesus monkeys: a comparison of the effects on the acquisition and performance of response sequences. Behav Pharmacol. 2003;14:295–306. doi: 10.1097/01.fbp.0000081785.35927.08. [DOI] [PubMed] [Google Scholar]