Abstract
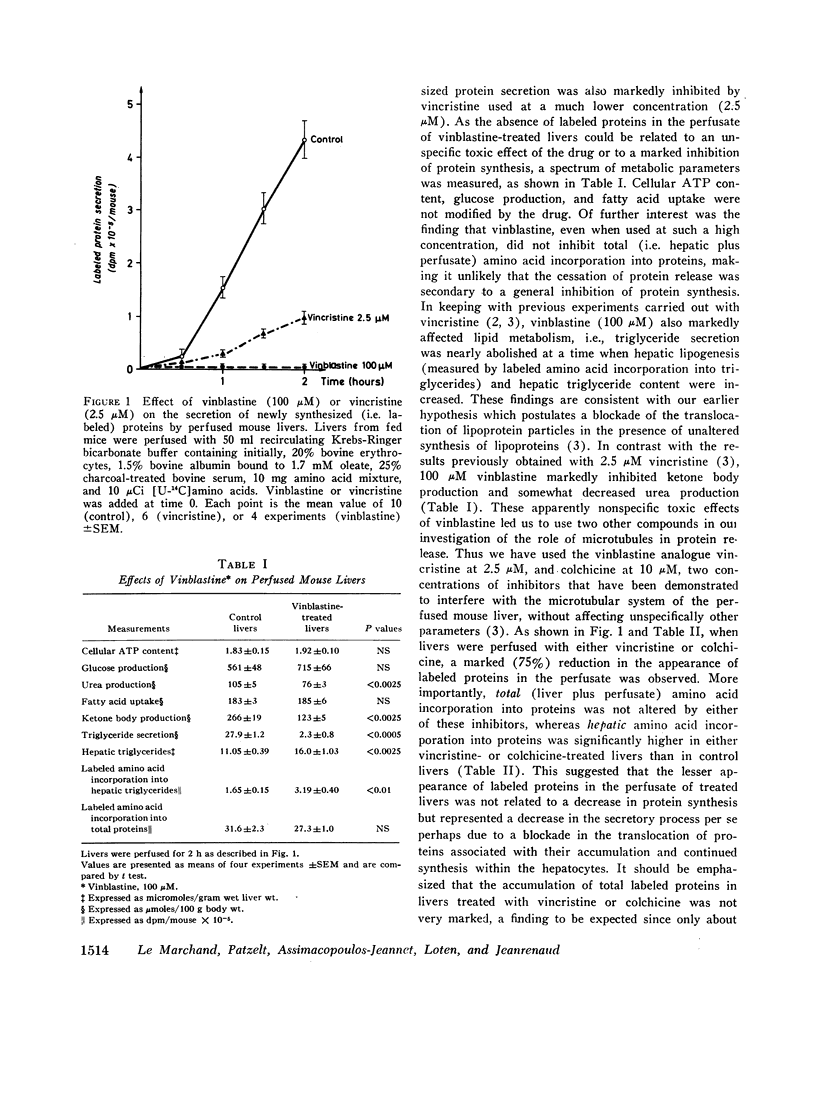
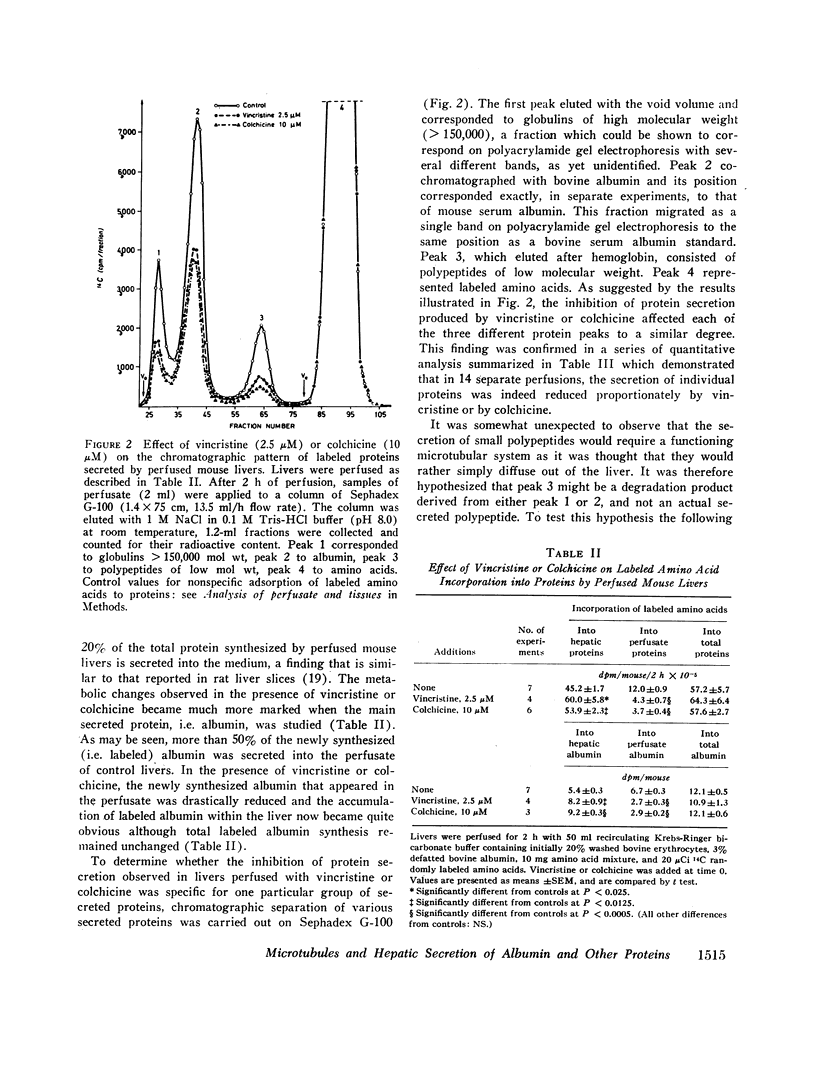
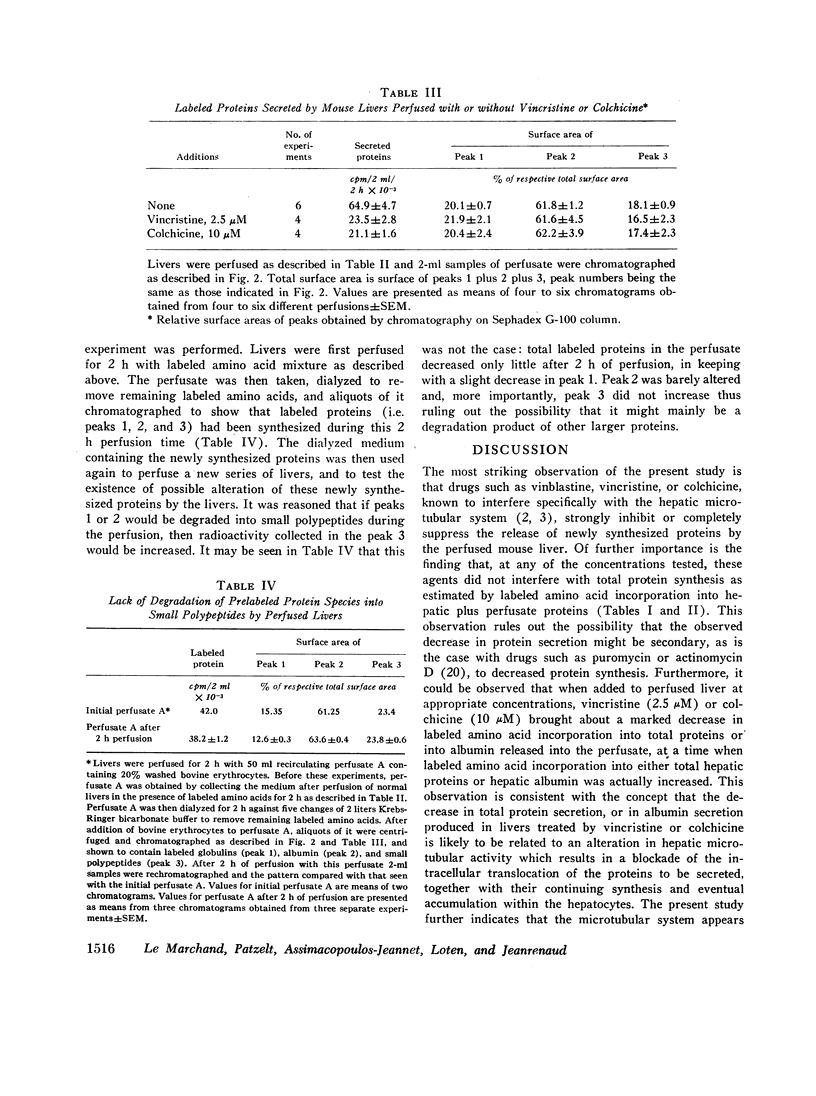
Livers of normal mice were prefused in situ and the secretion of newly synthesized (i.e. labeled) proteins into the perfusate were measured. In control livers, the secretion of newly synthesized proteins was found to be linear with time. In marked contrast, when livers were perfused with vinblastine, vincristine, or colchicine, drugs known to interfere with the hepatic microtubular system, the release of newly synthesized proteins was either strongly inhibited or completely suppressed although total hepatic protein synthesis (estimated by the incorporation of labeled amino acids into hepatic plus perfusate proteins) remained unaltered. Chromatographic separation of the various secreted proteins showed that the release of albumin, globulins, and small polypeptides was decreased to a similar extent by vincristine or colchicine. In the particular case of albumin, it was further observed that total (i.e. liver plus perfusate) labeled amino acid incorporation into albumin was not altered by either vincristine or colchicine, whereas the incorporation of these amino acids into liver albumin was markedly increased but incorporation into perfusate albumin was decreased, suggesting that the translocation of this particular protein from the liver to the perfusate had been affected by the presence of these drugs. It is proposed that the functional integrity of microtubules is necessary for the intracellular movement and eventual release of albumin and other proteins by the liver, and suggested that microtubules might possibly be a site of regulation of hepatic protein secretion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. A., Peters T., Jr Electron microscopic radioautographic detection of sites of protein synthesis and migration in liver. J Cell Biol. 1969 Nov;43(2):237–249. doi: 10.1083/jcb.43.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., Exton J. H., Jeanrenaud B. Control of gluconeogenesis and glycogenolysis in perfused livers of normal mice. Am J Physiol. 1973 Jul;225(1):25–32. doi: 10.1152/ajplegacy.1973.225.1.25. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C., Porter K. R. The Fine Structure of the Parenchymal Cell of the Normal Rat Liver: I. General Observations. Am J Pathol. 1965 May;46(5):691–755. [PMC free article] [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Ericsson J. L. Evidence for the participation of the Golgi apparatus in the intracellular transport of nascent albumin in the liver cell. J Cell Biol. 1970 Dec;47(3):555–567. doi: 10.1083/jcb.47.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. W., Miller L. L. Influence of actinomycin D and puromycin on net synthesis of plasma albumin and fibrinogen by the isolated perfused rat liver. J Biol Chem. 1966 Nov 10;241(21):4817–4824. [PubMed] [Google Scholar]
- John D. W., Miller L. L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969 Nov 25;244(22):6134–6142. [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- MILLER L. L., BALE W. F. Synthesis of all plasma protein fractions except gamma globulins by the liver; the use of zone electrophoresis and lysine-epsilon-C14 to define the plasma proteins synthesized by the isolated perfused liver. J Exp Med. 1954 Feb;99(2):125–132. doi: 10.1084/jem.99.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Sato H. Vinblastine produces uniaxial, birefringent crystals in starfish oocytes. J Cell Biol. 1969 Aug;42(2):596–599. doi: 10.1083/jcb.42.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS T., Jr The biosynthesis of rat serum albumin. II. Intracellular phenomena in the secretion of newly formed albumin. J Biol Chem. 1962 Apr;237:1186–1189. [PubMed] [Google Scholar]
- Peters T., Jr Biosynthesis of rat serum albumin. VII. Effects observed in liver slices. Am J Physiol. 1973 Jun;224(6):1363–1368. doi: 10.1152/ajplegacy.1973.224.6.1363. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Fleischer B., Fleischer S. The biosynthesis of rat serum albumin. IV. Apparent passage of albumin through the Golgi apparatus during secretion. J Biol Chem. 1971 Jan 10;246(1):240–244. [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Mongelli J., Schreiber S. S. Effects of a short-term fast on albumin synthesis studied in vivo, in the perfused liver, and on amino acid incorporation by hepatic microsomes. J Clin Invest. 1968 Dec;47(12):2591–2599. doi: 10.1172/JCI105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. Colchicine-induced inhibition of very low density lipoprotein release by rat liver in vivo. Biochim Biophys Acta. 1973 Apr 13;306(1):142–147. doi: 10.1016/0005-2760(73)90219-1. [DOI] [PubMed] [Google Scholar]


