Abstract
Live, attenuated strains of many bacteria that synthesize and secrete foreign antigens are being developed as vaccines for a number of infectious diseases and cancer. Bacterial-based vaccines provide a number of advantages over other antigen delivery strategies including low cost of production, the absence of animal products, genetic stability and safety. In addition, bacterial vaccines delivering a tumor-associated antigen (TAA) stimulate innate immunity and also activate both arms of the adaptive immune system by which they exert efficacious anti-tumor effects. Listeria monocytogenes and several strains of Salmonella have been most extensively studied for this purpose. A number of attenuated strains have been generated and used to deliver antigens associated with infectious diseases and cancer. Although both bacteria are intracellular, the immune responses invoked by Listeria and Salmonella are different due to their sub-cellular locations. Upon entering antigen-presenting cells by phagocytosis, Listeria is capable of escaping from the phagosomal compartment and thus has direct access to the cell cytosol. Proteins delivered by this vector behave as endogenous antigens, are presented on the cell surface in the context of MHC class I molecules, and generate strong cell-mediated immune responses. In contrast, proteins delivered by Salmonella, which lacks a phagosomal escape mechanism, are treated as exogenous antigens and presented by MHC class II molecules resulting predominantly in Th2 type immune responses. This fundamental disparity between the life cycles of the two vectors accounts for their differential application as antigen delivery vehicles. The present paper includes a review of the most recent advances in the development of these two bacterial vectors for treatment of cancer. Similarities and differences between the two vectors are discussed.
Key words: Listeria, Salmonella, cancer, immunotherapy, vaccine, bacteria
Introduction
Earliest attempt for using bacterial preparations for the treatment of cancer dates back to the nineteen century when William B. Coley witnessed for the first time the regression of a malignant tumor in one of his patients after a bacterial infection.1 Coley developed the first bacterial-based cancer treatment, which was derived from killed gram-positive bacteria Streptococci combined with gram-negative Serratia marcescens injected directly into tumors.2 These bacterial mixtures known as ‘Coley's toxins’ were also injected systematically in the gluteus maximus or pectoral muscles in addition to the intratumoral injections. Coley managed to achieve 10% success rate, which was significant based on the fact that these patients had advanced cancer.3 While the knowledge of immune system was limited at that time, this amazing observation of Coley forms the basis of recent advancement of using bacteria as vaccine vectors for tumor immunotherapy.
Our current vast knowledge in molecular biology, bacterial genetics and immunology has significantly accelerated the progress in bioengineering and use of these intriguing microorganisms as vaccine vectors. Attenuated strains of many otherwise pathogenic bacteria are now available and the ease of manipulation for generating recombinant strains provides a means for using bacteria as efficacious delivery vehicles for a number of foreign proteins such as antigens associated with infectious diseases and cancer. The most commonly used bacterial vectors include microbes such as Listeria monocytogenes,4 Escherichia coli,5 different strains of Salmonella6 and Shigella.7,8 Other less commonly used bacteria, which have been investigated as delivery vectors include Lactobacillus9,10 and Yersinia.11 A literature search using terms ‘bacterial vaccine vectors’ resulted in more than a thousand publications. The present paper is an attempt to summarize some of the most recent advances in using two of the bacterial vectors, L. monocytogenes and Salmonella, and assessment of their characteristics as vaccine vectors in cancer treatment is discussed.
Recombinant Listeria monocytogenes as a Vaccine Vector
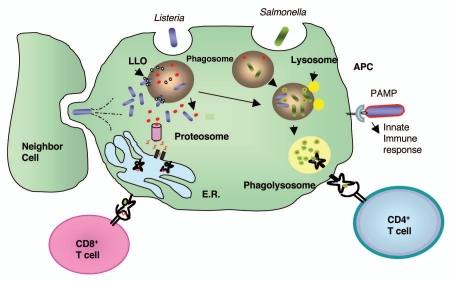
L. monocytogenes is a gram-positive bacterium that causes listeriosis in both animals and humans, of whom the most commonly infected individuals are either immune-compromised or immunesuppressed. L. monocytogenes is able to infect both phagocytic and non-phagocytic cells. Soon after infection of phagocytic cells such as macrophages or dendritic cells with L. monocytogenes, the vast majority of the bacteria are killed and degraded within the phago-lysosome although less than 10% escape into the cell cytosol. This phagosomal escape is mediated by the action of the poreforming hemolysin, listeriolysin O (LLO) and phospholipases.12 Once the bacterium enters the cell cytosol, expression of Actin nucleator A (ActA) protein is switched on, which allows for the polymerization of the host cell actin and gives the bacteria ability to spread from cell to cell.13 Through this mechanism of direct cell to cell transfer, L. monocytogenes can spread without being exposed to the host extracellular defenses. The majority of the virulence genes that are important in this life cycle are regulated by a pluri-potential transcription factor (PrfA), which is encoded by prfA gene.14 This unique life cycle of L. monocytogenes makes it an ideal vector for delivery of suitably expressed and secreted antigens in an antigen presenting cell.15,16 As a consequence of the dual intracellular location of L. monocytogenes, antigens secreted by the bacterium are targeted for both MHC class I and II presentation on the cell surface (Fig. 1).
Figure 1.
Comparison of L. monocytogenes and Salmonella life cycles. After infecting a host, both L. monocytogenes and salmonella are taken up by the antigen presenting cells (APC) by phagocytosis. However, a portion of L. monocytogenes can escape from the phagosome by the action of poreforming LLO, whereas Salmonella is trapped in the phagosome. L. monocytogenes proliferates in the cell cytosol and proteins secreted by it are degraded by the proteosomes, enter the endoplasmic reticulum (E.R.) and are presented to the immune system preferentially by MHC class I molecules. A portion of L. monocytogenes remains in the phagosome and shares the Salmonella life cycle which proliferates in the phagosome. Proteins secreted by the phagosomal bacteria are trapped and degraded in this compartment and presented on the APC's cell surface by MHC class II molecules. Cytosolic L. monocytogenes polymerized actin filaments which mediate its intracellular movement and direct transfer to a neighbor cell without exiting the host cells.
L. monocytogenes infection results in strong stimulation of both innate and adaptive immune responses. As a first line defense against bacterial invasion, interaction of L. monocytogenes with the host pattern recognition receptors triggers a cascade of cytokine and chemokine secretion that activates several components of the innate immune system.17–19 L. monocytogenes has a number of toll-like receptor ligands (TLR-L) such as peptidoglycan, lipoprotein and lipotechoic acid and nucleotide-binding oligomerization domain (NOD) which may trigger the secretion of pro-inflammatory cytokines.20–23 TLRs play a major role in the recognition of L. monocytogenes by the innate immune system. They bind to conserved molecular structures of the bacterial surface and trigger a signaling cascade through adaptor molecules, such as MyD88 which induces the transcription of several proinflammatory genes through NFκB activation. In particular, TLR2 which recognizes bacterial cell wall components such as peptidoglycan, lipoteichoic acid and lipoproteins, might play an important role during listerial infection, because mice deficient in TLR2 are more susceptible to listeriosis than their wild type counterparts.24 TLR5, which recognizes bacterial flagellin, may also be involved in L. monocytogenes-mediated innate immune responses. However, flagellin expression is downregulated at 37°C in most L. monocytogenes isolates and thus the role of TLR5 in listeriosis is unclear.25 NOD-like receptors also recognize peptidoglycans present in the bacterial cell wall. In activated macrophages, degraded L. monocytogenes in the phagolysosome, but not intact bacteria, can induce IFNβ mediated transcriptional responses which are mediated by the interaction of these ligands with NOD1, independently from TLR engagement.26 In vitro experiments with human endothelial cells demonstrate that NOD1 is also critically involved in IL-8 production and NFκB activation initiated by L. monocytogenes.27 Although TLRs are important in bacterial recognition, a single TLR has not been shown to be essential in the innate immune responses toward L. monocytogenes. In contrast, the adaptor molecule MyD88, which is a shared component of all TLR-associated signal transduction pathways besides IL-1 and IL-18, is critical to the defenses against L. monocytogenes.28 Consequently, Listeria infection is lethal in MyD88-deficient mice, which are unable or severely impaired in producing IL-12, IFNγ, TNFα and nitric oxide (NO) following the bacterial infection. In summary, L. monocytogenes infection generates strong innate immune responses, which may directly or indirectly contribute to the adaptive immune responses that are generated by this vaccine vector.
The use of live recombinant strains of L. monocytogenes to deliver tumor associated antigens (TAA) originated at the University of Pennsylvania, with the pioneering work of Dr. Yvonne Paterson.29,30 The results from Dr. Paterson's research demonstrated that a recombinant L. monocytogenes that expressed and secreted the model antigen influenza virus nucleoprotein (NP), could efficiently infect antigen presenting cells and mediate presentation of NP by both MHC class I and II pathways. Lm-NP vaccine could not only protect mice against lethal challenge with NP expressing tumors, but also caused regression of established macroscopic tumors in an antigen-specific and T cell-dependent manner. Since then L. monocytogenes platform has been rapidly evolved to become one of the most efficacious approaches for antigen delivery.
In most L. monocytogenes vaccine strains the antigen of interest is delivered in form of a multicopy plasmid in which the TAA is expressed under the control of a L. monocytogenes promoter. Several studies have demonstrated that T cell mediated immune responses against these antigens could be significantly enhanced when they were fused to a truncated non-hemolytic fragment of LLO.31,32 The immunogenic nature of LLO has been associated with the presence of PEST containing sequences at its aminoterminus that may target the protein for rapid ubiquitin-mediated proteosome degradation.33–35 In addition, LLO has shown to mediate a number of immuno-stimulatory effects by inducing the secretion of cytokines such as IL-1α, IL-12, IL-18, TNFα36,37 and IFNγ from NK cells;38 increasing monocyte recruitment and activation of CCR2, MCP-1 and 3 in a MyD88-independent manner; and causing the maturation of dendritic cells derived from these monocytes.38
In order to make a more stable vaccine platform, chromosomal integration techniques have also been utilized for making recombinant vaccine strains. One such method uses a phage-based system with a site-specific integrase to integrate a gene at a specific location in the bacterial genome.39 Homologous recombination using allelic exchange, can also be used to integrate a gene into a known chromosomal locus.40 Nevertheless, the use of a multicopy plasmid for antigen delivery might be more advantages over a chromosomally integrated antigen, as the former provides higher expression levels than a single copy chromosomal gene. The latest generations of L. monocytogenes based vaccine strains have been specifically designed for human use as they express TAA using plasmids which lack antibiotic resistance markers.41,42
Presently, a number of live, attenuated strains of L. monocytogenes have been generated that express and secrete a wide range of viral and tumor antigens including human papillomavirus (HPV)-16 E7 associated with cervix, head and neck cancer,31,43 Her-2/neu that is overexpressed by ∼30% of breast cancer,32,44 high molecular weight-melanoma associated antigen involved with tumor angiogenesis,45 and prostate specific antigen (PSA).44,46 Extensive preclinical testing of these strains using mouse models for cancer has shown that these vaccines are immunogenic and can cause regression of established tumors that express the target antigen. In addition, L. monocytogenes based vaccines can break the immunological tolerance toward self-antigens such as Her-2/neu or HPV-16 E7 as demonstrated by the data obtained in Her-2/neu or HPV-16 transgenic animals, respectively.47,48 More recent L. monocytogenes based vaccine strains target tumor vasculature and angiogenesis44,45 and show that immunization with these recombinant cancer vaccines can result in epitope spreading of tumor antigens.44
In recent years L. monocytogenes vaccine technology has advanced from the preclinical phase research to human trials using a recombinant Lm-HPV16 E7 (ADXS11-001) that expresses and secretes the tumor antigen E7, in a phase I clinical trial for cervical cancer.43 The trial was conducted in late stage metastatic cervical cancer patients who had a historical median survival of 180 days and a one-year survival rate of approximately 5%. These patients had failed prior cytotoxic treatment. The endpoint for this clinical trial was the determination of the safety and tolerability of the vaccine at escalating doses. Fifteen patients in 3 groups of 5 received one of 3 doses of ADXS11-001: 1 × 109, 3.3 × 109 or 1 × 1010 colony forming units administered twice at a 3 week interval and followed after 5 days with ampicillin. Adverse events (AE) following intravenous infusion of ADXS11-001 were comprised of a flu-like syndrome, with fever-related hypotension considered as the dose limiting AE observed at the highest dose (1 × 1010 CFU). Antibiotics were not necessary to relieve the AE indicating they were likely due to cytokine release rather than bacterial disease. Rapid clearance from the blood was seen, and no bacterial shedding in the stools or urine was observed. Of the 13 patients evaluable for efficacy 5 had progressive disease, 7 had stable disease and 1 was classified as objective responder. Tumor reductions were observed in 30% of evaluable patients, including 2 with lesions that disappeared. Median survival for treated patients was 347 days, one year survival was 53%, and 3 patients are still alive at 3+ years. These data clearly suggest the potential safety and efficacy of this treatment for cervical cancer or the precancerous stages of this malignancy (cervical intraepithelial neoplasia, CIN).
Salmonella as a Vaccine Vector
Salmonella is a gram-negative bacterium and the causal agent for salmonellosis. Two Salmonella serovars have been used as vaccines vectors, S. typhimurium in mice and S. typhi in humans. S. typhimurium causes gastroenteritis in a broad host range, including humans. S. enterica serovar typhi and S. enterica serovar paratyphi are exclusive human pathogens which cause systemic infection and typhoid fever.49 Infection of mice by S. typhimurium produces a systemic disease similar to human typhoid fever.50 In natural infections, the bacteria enters the host by the oral route, invade specialized antigen-transporting membranous cells (M cells) within the follicle-associated epithelium, colonize the Peyer's patches of the small intestine, gain access to the gut-associated lymphoid tissue, migrate to the mesenteric lymph nodes (MLN) and disseminate to the liver and spleen (reviewed by Jones and Falkow, 1996).51 Within the lymphoid organs, Salmonella resides in intracellular compartments.52,53 Intracellular survival and replication in host cells, including macrophages, is critical for bacterial pathogenesis and the development of serious systemic disease, since mutant strains that fail to replicate intracellularly are avirulent.54 Attenuation due to defective intramacrophage replication can be due to two types of mutations, those that alter the metabolic/structural integrity of the bacteria54–56 or the ones that affect the expression of specific virulence traits mediating host-pathogen interactions. For example, intracellular survival of S. typhimurium depends on the two-component regulatory system PhoP/PhoQ,57,58 which regulates genes involved in resistance to antimicrobial peptides, nutrient scavenging and LPS modification.59,60
Salmonella triggers a wide range of innate immune responses, mostly due to the expression of PAMPs such as flagellin,61,62 and lipopolysaccharides (LPS).63 While flagellin binds and activates toll-like receptor 5 (TLR5), LPS is a ligand for TLR4. Engagement of TLR5 by flagellin results in activation of NFκB and MAPK pathways leading to the secretion of many cytokines, including IL-6, IL-12 and TNFα.64 In addition, recognition of LPS by TLR4 on the surface of macrophages results in the release of cytokines such as IL-6 and IFNβ.64,65 After binding to LPS, and in association with the proteins MD2 and CD14, TLR4 dimerizes and undergoes a conformational change which is required for the recruitment of downstream Toll/IL-1 receptor (TIR) domain-containing adaptor molecules to activate both NFκB and MAPKs.66 TLR4 triggers an early response to Salmonella and together with TLR2, they are required for efficient macrophage activation which is essential for clearaning of Salmonella infection.8 Other immuno-stimulatory factors include lipoproteins and lipoteichoic acid, which are recognized by TLR2,65,67 probably in association with TLR6 and/or TLR1,68,69 and unmethlyated CpG motifs detected by TLR9.70 Upon infection TLR1, TLR2 and TLR9 are upregulated, while TLR6 is downregulated, accounting for the plateau phase observed during sublethal S. typhimurium infection.70 Thus, in addition to TLR4, the TLR2-TLR1 complex and TLR9 may play a role in controlling a Salmonella infection, particularly in the later stages when the bacterial growth is suppressed by the adaptive phase of the immune response.70 Salmonella infections are thus cleared from the host by a combination of the first line innate immunity and the later occurring adaptive cellular and humoral immune responses against Salmonella antigens.71
Live attenuated Salmonella strains have been extensively studied to elicit mucosal as well as systemic immune responses against antigens from other infectious bacteria such as E. coli, Shigella sonnie, Plasmodium falciparum, Bacillus anthracis, Helicobacter pylori and L. monocytogenes;72–74 viruses such as HPV-16 and hepatitis B;75 tumor antigens such as PSA,76 survivin,77,78 tyrosine hydroxylase,79,80 vascular endothelial growth factor receptor-2, transcription factor Fos-related antigen-1; Legumain, an asparaginyl endopeptidase which is specifically overexpressed on tumor-associated macrophages;81 proteins involved in angiogenesis; and endothelial cell markers and proinflammatory factors such as cytokines or chemokines.82 Recently, oral delivery of an attenuated Salmonella strains targeting tumor vasculature antigen Endoglin (CD105) was shown to be able to inhibit angiogenesis and vasculature formation in various types of tumors, ultimately resulting in tumor regression.83,84 A similar approach was taken by Ruan et al. who reported that a S. typhimurium carrying a xenogenic DNA vaccine encoding a cell surface protein TEM8, which is dominantly expressed by tumor endothelium could overcame peripheral immune tolerance and generate anti-TEM8 CD8+ cytotoxic T-cell responses and had anti-tumor effects.85,86
Delivery of immuno-modulators by attenuated Salmonella has also been investigated by different researchers. Loeffler et al. created a S. typhimurium expressing a chemokine, CCL21 which could inhibit the growth of primary tumors and pulmonary metastases in a CD4+ and CD8+ T-cell-dependent manner in a preclinical model of multi-drug-resistant murine carcinoma.82 This response was associated with inflammatory cell infiltration and high levels of downstream cytokines and chemokines induced by CCL21 such as INFγ, CXCL9 and CXCL10. As a vehicle for sustained delivery of cytokines into tumors, recombinant strains have been generated which carry eukaryotic expression vectors encoding the IL-4 or IL-18 proteins. Pre-clinical studies in mice show that a single oral dose of these recombinants can exert anti-tumor effects and result in the release of IFNγ.87 In addition, strategies combining pro-inflammatory factors and anti-angiogenic targets have been reported. Lu et al. described a S. typhimurium vaccine which delivered the murine vascular endothelial growth factor receptor-2. When mice implanted with melanoma tumors were treated with a combination of this vaccine and direct intratumoral injection of a plasmid DNA encoding the murine IP-10 (CXCL10) gene, significant synergistic effects against tumors were detected. The combination strategy showed improved immunogenicity, a strong anti-tumor effect, apoptosis of tumor cells, reduced neovascularization, and cell proliferation.71
A novel approach using Salmonella as an antigen carrier, mediated antigen expression only in tumor cells by controlling their expression with human telomerase reverse transcriptase (hTert) promoter, which is overactive in tumor—but not in normal cells. This Salmonella strain delivered a eukaryotic expression vector that contained mitochondria derived activator of caspases (Smac) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) genes caused enhanced TRAIL-induced apoptosis of tumor cells and consequently demonstrated strong antitumoral effect in vivo.71
One strategy used to improve antigen delivery and tumor specificity of Salmonella-based vaccines includes the use of type III secretion system (TTSS)88 that can mediate efficient delivery of heterologous antigens directly to the cytosol of antigen-presenting cells. In vitro studies using a recombinant S. typhimurium vaccine strain secreting the NY-ESO-I antigen through this system showed that antigen-specific CD8+ and CD4+ T cell responses were obtained in peripheral blood lymphocytes of cancer patients. Oral administration of this vaccine to mice resulted in the regression of established NY-ESO-1-expressing tumors and its intratumoral injection invoked epitope spreading.89 In another study, an attenuated Salmonella strain, VNP20009, was bioengineered to specifically target carcinoembryonic antigen (CEA) expressing tumors, by expression of an anti-CEA single chain antibody fragments on the bacterial cell surface. The recombinant vaccine strain accumulated in CEA-expressing tumors and inhibited their growth.90
Despite the vast preclinical testing of Salmonella strains for cancer associated antigen delivery, very few recombinants have been advanced to human clinical trials. In 2002 Toso et al. conducted a phase I clinical study infusing a ΔmsbB/Δpurl strain of S. typhimurium intravenously in 24 patients with metastatic cancer.17 The chromosomal deletion of purI and msbB genes increased its safety and lowered the toxicity. The purI deletion created a requirement for an external source of adenine, whereas the deletion of the msbB gene reduced the toxicity associated with lipopolysaccharide (LPS) by preventing the addition of a terminal myristyl group to the lipid A domain. The msbB mutation in Salmonella resulted in lower toxicity in mice by reducing the induction of proinflammatory cytokines and nitric oxide synthase compared with the parental Salmonella. These deletions increased the median lethal dose by 10,000-fold in mice. Dose-limiting toxicity was observed in patients receiving 1 × 109 CFU/m2. The side effect profile included thrombocytopenia, anemia, persistent bacteremia, hyperbilirubinemia, diarrhea, vomiting, nausea, elevated alkaline phosphatase, and hypophosphatemia. The vaccine also induced a dose-related increase in serum proinflammatory cytokines, such as interleukin IL-1β, TNFα, IL-6 and IL-12. None of the patients experienced objective tumor regression, including those patients with colonized tumors. In 2003, a pilot trial in refractory cancer patients was conducted with the same vector modified to express the E. coli cytosine deaminase gene. When administered in combination with 5-fluorocytosine, conversion of 5-fluorocytosine to 5-fluorouracil as a result of cytosine deaminase expression, was demonstrated in two out of three patients. However, no clinical efficacy with this vaccine was reported.91
Comparison of L. monocytogenes and Salmonella as Vaccine Vectors
L. monocytogenes and Salmonella are both intracellular bacteria. Neverthless, there are some differences in their life cycle, which might account for the differences observed in their immunological properties. In vitro, Salmonella grows almost twice as fast as Listeria.92 However, in vivo L. monocytogenes infection peaks more quickly on days 2–3 post-infection when compared with Salmonella which continues to grow for up to 10–14 days postinfection. Attenuated L. monocytogenes is cleared from experimental animals much faster (∼2–5 days) than Salmonella which can cause a chronic infection lasting for up to two months.92 Some of these events are due to the differences between the ways that these two bacteria infect and proliferate in a host cell (Fig. 1). While both are taken up by the cells through phagocytosis, L. monocytogenes is able to escape from the phagosome, whereas Salmonella is confined in the phagolysosomes,93 having to proliferate in this compartment under hostile and changing environment, in the absence of nutrients, and with the presence of lysosomal enzymes and low pH.51 This might explain why in vivo the growth rate of Salmonella is lower than L. monocytogenes. As a consequence of its intracellular location, proteins delivered by Salmonella are trapped in the phagolysosome and presented to the immune system preferentially in the context of MHC class II molecules.94 In contrast, as mentioned before, proteins delivered by L. monocytogenes have direct access to cell cytosol and consequently to the MHC class I presentation pathway.
There have been many approaches in the literature for bioengineering Salmonella strains, which could deliver antigens directly to the intracellular compartment instead of the phagosome. For instance, Panthel et al. used a type 3 secretion system (TTSS) to mediate translocation of heterologous antigens to the cytosol of antigen-presenting cells and in this way achieved prominent CD8+ T-cell priming.95 A different approach for accessing the MHC class I antigen presentation pathway using Salmonella was to create strains that can secrete LLO, which assists the bacteria to escape from the phagosome.94
The availability of a murine model for oral infection by Salmonella i.e., S. typhymurium, has significantly facilitated the research and development of oral Salmonella-based vaccines for human use. The mouse model has played an invaluable role in identifying the bacterial virulence genes and different strategies for generating attenuated strains. As reviewed by Pasetti et al. among the attenuating mutations first identified in the mouse model and later used to design S. typhi based vaccines for human use, are those introduced into genes required for biosynthesis of bacterial components (galE, LPS), nutrients (purD, aroA) and regulatory system (phoP/phoQ).96 In contrast L. monocytogenes does not establish infection in mice via oral route. Lecuit et al. identified a single amino acid in E-cadherin to be responsible for L. monocytogenes host specificity.97 E-cadherin promotes entry of L. monocytogenes into epithelial cells by interacting with internalin (InlA), a bacterial surface protein. Because of this single amino acid difference, mouse E-cadherin does not bind InlA. This has been a major obstacle for the pre-clinical development of oral L. monocytogenes based vaccines. Despite this, Paterson et al. showed that oral delivery of L. monocytogenes at high doses in mice is possible and can result in induction of cellular and humoral immune responses toward L. monocytogenes and heterologous antigens.98–100
Salmonella vaccine strains contain high levels of lipopolysaccharide (LPS), a strong inducer of TNFα which at high doses is associated with septic shock. Deletion of the waaN gene product, which is involved in synthesizing the lipid A component of the LPS results in a less virulent strain and a lesser ability to induce TNFα, IL-1β and iNOS,63,101 although mutants harboring the modified LPS retain their ability to target and accumulate within the tumors and can still suppress tumor growth. On the other hand L. monocytogenes lacks LPS102 but it has other PAMPs that activate TLRs and NOD receptors to release a variety of inflammatory cytokines. Therefore, both of these bacteria possess intrinsic characteristics to strongly stimulate innate immune responses.
The inherent characteristics listed above for each of these bacteria may account for the type and duration of the immune responses obtained by them as vaccine vectors. For instance, L. monocytogenes has been shown to generate an earlier immune response than Salmonella, with a peak CD8+ T-cell response between days 4–7 after immunizations. In contrast, Salmonella infection induces a delayed CD8+ T-cell response peaking on day 21.92 Similarly, acute immune responses against L. monocytogenes are drastically contracted after the first week of infection, whereas those elicited by Salmonella increase for up to three weeks postinfection, followed by a gradual reduction thereafter.92 Unlike L. monocytogenes, which rapidly generates activated effector T cells (CD62LlowCD44high), followed by their rapid conversion to a central memory phenotype (CD62LhighCD44high), Salmonella causes a delayed T cell activation persisting in the same state for up to 90 days. Although T cells stimulated by both vectors are able to secrete IFNγ in response to antigen pulsing, only CD8+ T cells from L. monocytogenes immunized animals are able to efficiently lyse cells expressing a target antigen. Salmonella-induced CD8+ T cells seem to be less lytic than those induced by the L. monocytogenes vector.92 The differences in the immunology of the two vectors have profound implications in their efficiency as carriers for foreign antigens. This can be noted from their anti-tumor activity, when delivering a TAA. In a tumor model using OVA as a target antigen, only L. monocytogenes expressing OVA could protect mice against OVA expressing tumors.103
Attenuated Salmonella vaccines have been demonstrated to induce high levels of antibody responses against Salmonella antigens such as LPS as well as foreign antigens delivered by the vector.104 On the other hand, the elicitation of humoral responses against L. monocytogenes based vaccines is low.105 In accordance with this, our studies using a recombinant L. monocytogenes expressing a fusion of LLO to PSA, showed that this vaccine did not stimulate any antibody responses toward the target antigen, PSA (Shahabi et al. unpublished data).
Another mechanism that has been demonstrated to play a role in the anti-cancer properties of the two vectors is attributed to the ability of both Salmonella and L. monocytogenes to colonize tumors.106,107 Pawelek and colleagues reported that when Salmonella auxotrophs are injected into tumor bearing mice they preferentially replicate within tumors achieving tumor: liver ratios in the excess of 1,000:1,108 and the infected tumor cells have shown to become the target of Salmonella specific T cells.109 The ability of L. monocytogenes to colonize tumor cells both in vivo and in vitro has also been demonstrated and the data shows that L. monocytogenes infected tumor cells are killed through the generation of high levels of ROS and oxidative stress.110
Conclusions
Intracellular bacterial vectors show great potential as antigen delivery vehicles for cancer-associated genes and proteins. Bacterial vectors offer multiple advantages over other vaccine delivery strategies including their easy bioengineering methods, low cost of production at large scale and more importantly broad diversity of their effects on the immune system. Both classes of bacteria have profound immune-stimulatory effects that augment their efficacy as a delivery system for foreign antigens which can be either expressed by the vector or delivered as a genetic entity and expressed by the host cell. Either way, these vectors have shown to activate different components of the immune system including components of the innate immune system through TLR binding, secretion of inflammatory cytokines and chemokines, upregulation of co-stimulatory molecules, stimulation of antigen specific CD4+ and CD8+ T cells, and suppression of regulatory T cells. Both classes of vectors are accumulated in tumors, a property that might enhance their inhibitory effect on tumor growth. Some of the biological differences between these bacteria might explain their efficacy as vectors. In particular, L. monocytogenes vectors with access to both phagosomal and cytosolic compartments have advantage over vectors such as Salmonella, which are trapped within the phagolysosomes. In preclinical studies, L. monocytogenes based vectors carrying tumor antigens have been proven to be extremely efficacious in regressing established tumors. In addition, clinical data suggest that such vectors might be used safely in humans. Based on the extensive pre-clinical data and accumulated knowledge about these vectors in the past few decades, bacterial vectors have become a potential option for development of cancer vaccines. Future clinical trials are however warranted to better establish their efficacy.
Acknowledgements
We would like to thank Dr. John Rothman and Dr. Sandra Wadsworth for the review of the paper and their helpful advices.
Abbreviations
- AE
adverse event
- CCL
chemokine (C-C motif) ligand
- CD
cluster of differentiation
- CEA
carcino-embryonic antigen
- CFU
colony forming units
- CXCL
chemokine (C-X-C motif) ligand
- DNA
deoxyribonucleic acid
- E. coli
Escherichia coli
- HPV
human papilloma virus
- hTert
human telomerase reverse transcriptase
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IP-10
interferon-inducible protein 10
- LLO
listeriolysin O
- LPS
lipopolysaccharide
- MAPK
mitogen activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MHC
major histocompatibility complex
- MLN
mesenteric lymph node
- MyD88
myeloid differentiation primary response gene (88)
- NFκB
nuclear factor-kappaB
- NK
natural killer
- NOD
nucleotide-binding oligomerization domain
- NP
nucleoprotein
- OVA
ovalbumin
- PAMPs
pathogen associated molecular patterns
- PSA
prostate specific antigen
- TAAs
tumor associated antigens
- Th1
helper T cell
- TLR
toll-like receptor
- TLR-L
toll-like receptor ligand
- TEM8
tumor endothelium marker-8
- TNF
tumor necrosis factor
- TRAIL
(TNF)-related apoptosis inducing ligand
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/11243
References
- 1.Coley WB., II Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coley WB. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc R Soc Med. 1910;3:1–48. doi: 10.1177/003591571000301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsung K, Norton JA. Lessons from Coley's Toxin. Surg Oncol. 2006;15:25–28. doi: 10.1016/j.suronc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Wallecha A, Carroll KD, Maciag PC, Rivera S, Shahabi V, Paterson Y. Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Adv Appl Microbiol. 2009;66:1–27. doi: 10.1016/S0065-2164(08)00801-0. [DOI] [PubMed] [Google Scholar]
- 5.Dai MS, Nitcheu-Tefit J, Alcock S, Ramirez-Jimenez F, Chao TY, Baril P, et al. Development of an Escherichia coli Expressing Listeriolysin-O Vaccine Against Wilms Tumor Gene 1-expressing Tumors. J Immunother. 2009;32:845–855. doi: 10.1097/CJI.0b013e3181aee259. [DOI] [PubMed] [Google Scholar]
- 6.Krul MR, Tijhaar EJ, Kleijne JA, Van Loon AM, Nievers MG, Schipper H, et al. Induction of an antibody response in mice against human papillomavirus (HPV) type 16 after immunization with HPV recombinant Salmonella strains. Cancer Immunol Immunother. 1996;43:44–48. doi: 10.1007/s002620050302. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich G, Spreng S, Favre D, Viret JF, Guzman CA. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr Opin Mol Ther. 2003;5:10–19. [PubMed] [Google Scholar]
- 8.Loessner H, Weiss S. Bacteria-mediated DNA transfer in gene therapy and vaccination. Expert Opin Biol Ther. 2004;4:157–168. doi: 10.1517/14712598.4.2.157. [DOI] [PubMed] [Google Scholar]
- 9.Gerritse K, Posno M, Schellekens MM, Boersma WJ, Claassen E. Oral administration of TNP-Lactobacillus conjugates in mice: a model for evaluation of mucosal and systemic immune responses and memory formation elicited by transformed lactobacilli. Res Microbiol. 1990;141:955–962. doi: 10.1016/0923-2508(90)90135-d. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt EG, Claesson MH, Jensen SS, Ravn P, Kristensen NN. Antigen-presenting cells exposed to Lactobacillus acidophilus NCFM, Bifidobacterium bifidum BI-98, and BI-504 reduce regulatory T cell activity. Inflamm Bowel Dis. 2010;16:390–400. doi: 10.1002/ibd.21068. [DOI] [PubMed] [Google Scholar]
- 11.Gentschev I, Dietrich G, Spreng S, Kolb-Maurer A, Daniels J, Hess J, et al. Delivery of protein antigens and DNA by virulence-attenuated strains of Salmonella typhimurium and Listeria monocytogenes. J Biotechnol. 2000;83:19–26. doi: 10.1016/s0168-1656(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilney LG, Portnoy DA. Actin filaments and the growth, movement and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berche P, Gaillard JL, Geoffroy C, Alouf JE. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987;139:3813–3821. [PubMed] [Google Scholar]
- 16.Portnoy DA, Schreiber RD, Connelly P, Tilney LG. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989;170:2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci USA. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 19.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 21.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 23.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Torres D, Barrier M, Bihl F, Quesniaux VJ, Maillet I, Akira S, et al. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72:2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, et al. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 26.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opitz B, Puschel A, Beermann W, Hocke AC, Forster S, Schmeck B, et al. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176:484–490. doi: 10.4049/jimmunol.176.1.484. [DOI] [PubMed] [Google Scholar]
- 28.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 29.Weiskirch LM, Pan ZK, Paterson Y. The tumor recall response of antitumor immunity primed by a live, recombinant Listeria monocytogenes vaccine comprises multiple effector mechanisms. Clin Immunol. 2001;98:346–357. doi: 10.1006/clim.2000.4987. [DOI] [PubMed] [Google Scholar]
- 30.Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med. 1994;180:2209–2218. doi: 10.1084/jem.180.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 33.Sewell DA, Shahabi V, Gunn GR, 3rd, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–8825. doi: 10.1158/0008-5472.CAN-04-1958. [DOI] [PubMed] [Google Scholar]
- 34.Schnupf P, Portnoy DA, Decatur AL. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell Microbiol. 2006;8:353–364. doi: 10.1111/j.1462-5822.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 35.Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 36.Kimoto T, Kawamura I, Kohda C, Nomura T, Tsuchiya K, Ito Y, et al. Differences in gamma interferon production induced by listeriolysin O and ivanolysin O result in different levels of protective immunity in mice infected with Listeria monocytogenes and Listeria ivanovii. Infect Immun. 2003;71:2447–2454. doi: 10.1128/IAI.71.5.2447-2454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura T, Kawamura I, Tsuchiya K, Kohda C, Baba H, Ito Y, et al. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect Immun. 2002;70:1049–1055. doi: 10.1128/IAI.70.3.1049-1055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishibori T, Xiong H, Kawamura I, Arakawa M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996;64:3188–3195. doi: 10.1128/iai.64.8.3188-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mata M, Yao ZJ, Zubair A, Syres K, Paterson Y. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine. 2001;19:1435–1445. doi: 10.1016/s0264-410x(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 41.Verch T, Pan ZK, Paterson Y. Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines. Infect Immun. 2004;72:6418–6425. doi: 10.1128/IAI.72.11.6418-6425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin Vaccine Immunol. 2009;16:96–103. doi: 10.1128/CVI.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Seavey MM, Maciag PC, Al-Rawi N, Sewell D, Paterson Y. An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol. 2009;182:5537–5546. doi: 10.4049/jimmunol.0803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–8075. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1313. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26:5315–5320. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56:927–938. doi: 10.1007/s00262-006-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 50.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 52.Dunlap NE, Benjamin WH, Jr, McCall RD, Jr, Tilden AB, Briles DE. A ‘safe-site’ for Salmonella typhimurium is within splenic cells during the early phase of infection in mice. Microb Pathog. 1991;10:297–310. doi: 10.1016/0882-4010(91)90013-z. [DOI] [PubMed] [Google Scholar]
- 53.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 56.De Groote MA, Testerman T, Xu Y, Stauffer G, Fang FC. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 57.Belden WJ, Miller SI. Further characterization of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect Immun. 1994;62:5095–5101. doi: 10.1128/iai.62.11.5095-5101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groisman EA, Chiao E, Lipps CJ, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, Miller SI. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 60.Soncini FC, Groisman EA. Two-component regulatory systems can interact to process multiple environmental signals. J Bacteriol. 1996;178:6796–6801. doi: 10.1128/jb.178.23.6796-6801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciacci-Woolwine F, McDermott PF, Mizel SB. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect Immun. 1999;67:5176–5185. doi: 10.1128/iai.67.10.5176-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyant TL, Tanner MK, Sztein MB. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan SA, Everest P, Servos S, Foxwell N, Zahringer U, Brade H, et al. A lethal role for lipid A in Salmonella infections. Mol Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 64.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 66.Mahieu T, Libert C. Should we inhibit type I interferons in sepsis? Infect Immun. 2007;75:22–29. doi: 10.1128/IAI.00829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 68.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 70.Totemeyer S, Kaiser P, Maskell DJ, Bryant CE. Sublethal infection of C57BL/6 mice with Salmonella enterica Serovar Typhimurium leads to an increase in levels of Toll-like receptor 1 (TLR1), TLR2 and TLR9 mRNA as well as a decrease in levels of TLR6 mRNA in infected organs. Infect Immun. 2005;73:1873–1878. doi: 10.1128/IAI.73.3.1873-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aravalli RN, Hu S, Lokensgard JR. Inhibition of toll-like receptor signaling in primary murine microglia. J Neuroimmune Pharmacol. 2008;3:5–11. doi: 10.1007/s11481-007-9097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baillie LW, Rodriguez AL, Moore S, Atkins HS, Feng C, Nataro JP, et al. Towards a human oral vaccine for anthrax: the utility of a Salmonella typhi Ty21a-based prime-boost immunization strategy. Vaccine. 2008;26:6083–6091. doi: 10.1016/j.vaccine.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bumann D, Hueck C, Aebischer T, Meyer TF. Recombinant live Salmonella spp. for human vaccination against heterologous pathogens. FEMS Immunol Med Microbiol. 2000;27:357–364. doi: 10.1111/j.1574-695X.2000.tb01450.x. [DOI] [PubMed] [Google Scholar]
- 74.DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18:449–459. doi: 10.1016/s0264-410x(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 75.Echchannaoui H, Bianchi M, Baud D, Bobst M, Stehle JC, Nardelli-Haefliger D. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect Immun. 2008;76:1940–1951. doi: 10.1128/IAI.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fensterle J, Bergmann B, Yone CL, Hotz C, Meyer SR, Spreng S, et al. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 2008;15:85–93. doi: 10.1038/sj.cgt.7701109. [DOI] [PubMed] [Google Scholar]
- 77.Fest S, Huebener N, Bleeke M, Durmus T, Stermann A, Woehler A, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 78.Fest S, Huebener N, Weixler S, Bleeke M, Zeng Y, Strandsby A, et al. Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 2006;66:10567–10575. doi: 10.1158/0008-5472.CAN-06-1158. [DOI] [PubMed] [Google Scholar]
- 79.Huebener N, Fest S, Strandsby A, Michalsky E, Preissner R, Zeng Y, et al. A rationally designed tyrosine hydroxylase DNA vaccine induces specific antineuroblastoma immunity. Mol Cancer Ther. 2008;7:2241–2251. doi: 10.1158/1535-7163.MCT-08-0109. [DOI] [PubMed] [Google Scholar]
- 80.Huebener N, Lange B, Lemmel C, Rammensee HG, Strandsby A, Wenkel J, et al. Vaccination with minigenes encoding for novel ‘self’ antigens are effective in DNA-vaccination against neuroblastoma. Cancer Lett. 2003;197:211–217. doi: 10.1016/s0304-3835(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 81.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222:117–128. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 82.Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother. 2009;58:769–775. doi: 10.1007/s00262-008-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, et al. Upregulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1:1623–1634. [PubMed] [Google Scholar]
- 84.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–1044. [PubMed] [Google Scholar]
- 85.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, et al. TEM8 interacts with the cleaved C5 domain of collagen alpha3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 86.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, et al. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 87.Agorio C, Schreiber F, Sheppard M, Mastroeni P, Fernandez M, Martinez MA, et al. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med. 2007;9:416–423. doi: 10.1002/jgm.1023. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JW, Nickerson CA. Cloning of a functional Salmonella SPI-1 type III secretion system and development of a method to create mutations and epitope fusions in the cloned genes. J Biotechnol. 2006;122:147–160. doi: 10.1016/j.jbiotec.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bereta M, Hayhurst A, Gajda M, Chorobik P, Targosz M, Marcinkiewicz J, et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine. 2007;25:4183–4192. doi: 10.1016/j.vaccine.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Human Gene Ther. 2001;12:1594–1596. [PubMed] [Google Scholar]
- 92.Luu RA, Gurnani K, Dudani R, Kammara R, van Faassen H, Sirard JC, et al. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J Immunol. 2006;177:1516–1525. doi: 10.4049/jimmunol.177.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaufmann SH, Raupach B, Finlay BB. Introduction: microbiology and immunology: lessons learned from Salmonella. Microbes Infect. 2001;3:1177–1181. doi: 10.1016/s1286-4579(01)01498-8. [DOI] [PubMed] [Google Scholar]
- 94.Kaufmann SH, Hess J. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett. 1999;65:81–84. doi: 10.1016/s0165-2478(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 95.Panthel K, Meinel KM, Sevil Domenech VE, Trulzsch K, Russmann H. Salmonella type III-mediated heterologous antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int J Med Microbiol. 2008;298:99–103. doi: 10.1016/j.ijmm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Pasetti MF, Levine MM, Sztein MB. Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors. Vaccine. 2003;21:401–418. doi: 10.1016/s0264-410x(02)00472-3. [DOI] [PubMed] [Google Scholar]
- 97.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 99.Peters C, Peng X, Douven D, Pan ZK, Paterson Y. The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J Immunol. 2003;170:5176–5187. doi: 10.4049/jimmunol.170.10.5176. [DOI] [PubMed] [Google Scholar]
- 100.Schafer R, Portnoy DA, Brassell SA, Paterson Y. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J Immunol. 1992;149:53–59. [PubMed] [Google Scholar]
- 101.Watson PR, Benmore A, Khan SA, Jones PW, Maskell DJ, Wallis TS. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect Immun. 2000;68:3768–3771. doi: 10.1128/iai.68.6.3768-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hether NW, Campbell PA, Baker LA, Jackson LL. Chemical composition and biological functions of Listeria monocytogenes cell wall preparations. Infect Immun. 1983;39:1114–1121. doi: 10.1128/iai.39.3.1114-1121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stark FC, Sad S, Krishnan L. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 2009;69:4327–4334. doi: 10.1158/0008-5472.CAN-08-3160. [DOI] [PubMed] [Google Scholar]
- 104.Dunstan SJ, Simmons CP, Strugnell RA. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect Immun. 1998;66:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hahn H. Antibacterial defence mechanisms. Infection. 1983;11:112–118. doi: 10.1007/BF01645301. [DOI] [PubMed] [Google Scholar]
- 106.Riedel CU, Monk IR, Casey PG, Morrissey D, O'Sullivan GC, Tangney M, et al. Improved luciferase tagging system for Listeria monocytogenes allows real-time monitoring in vivo and in vitro. Appl Environ Microbiol. 2007;73:3091–3094. doi: 10.1128/AEM.02940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 108.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 109.Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 110.Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]