Abstract
Growth and polymer synthesis were studied in a recombinant E. coli strain carrying phaBAC and phaP of Azotobacter sp. strain FA8 using different carbon sources and oxygen availability conditions. The results obtained with glucose or glycerol were completely different, demonstrating that the metabolic routes leading to the synthesis of the polymer when using glycerol do not respond to environmental conditions such as oxygen availability in the same way as they do when other substrates, such as glucose, are used. When cells were grown in a bioreactor using glucose the amount of polymer accumulated at low aeration was reduced by half when compared to high aeration, while glycerol cultures produced at low aeration almost twice the amount of polymer synthesized at the higher aeration condition. The synthesis of other metabolic products, such as ethanol, lactate, formate and acetate, were also affected by both the carbon source used and aeration conditions. In glucose cultures, lactate and formate production increased in low agitation compared to high agitation, while poly(3-hydroxybutyrate) synthesis decreased. In glycerol cultures, the amount of acids produced also increased when agitation was lowered, but carbon flow was mostly redirected towards ethanol and poly(3-hydroxybutyrate). These results indicated that carbon partitioning differed depending on both carbon source and oxygen availability, and that aeration conditions had different effects on the synthesis of the polymer and other metabolic products when glucose or glycerol were used.
Key words: poly(3-hydroxybutyrate), Escherichia coli, glucose, glycerol, aeration, oxygen availability, carbon partitioning
Polyhydroxyalkanoates (PHAs) are accumulated as intracellular granules by many bacteria under unfavorable conditions.1,2 These polymers are carbon and energy reserves and also act as electron sinks, enhancing the fitness and stress resistance of bacteria, and contributing to redox balance.2–4 Growing concern about environmental pollution and dwindling petroleum supplies has renewed in the last decade the interest in PHAs, which have thermoplastic properties, are totally biodegradable by microorganisms present in most environments, and can be produced from different renewable carbon sources.5
Escherichia coli is the best known bacterial species. The physiology, biochemistry and genetics of this microorganism have been studied in great detail, and high cell density cultivation strategies for numerous E. coli strains have been established.6 It is a suitable host for the heterologous expression of foreign genes, that can be easily manipulated and improved by means of recombinant DNA methodologies. It grows fast and offers a well defined physiological environment for the construction and manipulation of various metabolic pathways to produce different bioproducts, such as poly(3-hydroxybutyrate) (PHB) from cost-effective carbon sources. This facultative aerobe adjusts its metabolism to optimize cell growth in each environmental condition by using different combinations of metabolic pathways. As a result, the metabolic product distribution varies according to growth conditions, such as carbon source and terminal electron acceptor availability. There is an intimate association between carbon and electron flow, as carbon will be directed towards the synthesis of more reduced or more oxidized products according to intracellular redox conditions. This has to be considered when designing efficient processes for the synthesis of compounds derived from central catabolic pathways, as the amounts of the metabolites produced can vary dramatically according to growth conditions.
In this work we focused on the heterologous synthesis of PHB, that consumes both acetyl-coenzyme A (acetyl-CoA) and reducing power, and is thus directly affected by carbon flow and reducing equivalents availability. Both aeration conditions and carbon source have been observed to influence the synthesis of the different metabolic products in the recombinants, including PHB. The E. coli strain used in this work, K24KP, carries phaBAC, the structural genes responsible for PHB synthesis, from Azotobacter sp. strain FA8.7 It also carries phaP, encoding a granule associated protein that has been shown to promote growth and polymer synthesis in the recombinants.
In recent years, a significant increase in the production of biodiesel has caused a sharp fall in the cost of glycerol, the main by-product of biodiesel synthesis. As a result, glycerol has become a very attractive substrate for bacterial fermentations, especially for reduced products, such as PHB.8 However, only a few studies have focused on the metabolism of PHB synthesis from glycerol in recombinant E. coli. Previous reports have described the use of glycerol for the synthesis of PHAs using different bacteria. In these studies, the Mr of PHAs obtained using glycerol was found to be significantly lower than those obtained from other substrates, typically less than 1 MDa. A low Mr is undesirable for industrial processing of the polymer, so the results available in the literature pointed to a drawback in the use of glycerol as a convenient substrate for the microbial production of PHAs. This prompted us to investigate the physical properties of PHB produced by strain K24KP in bioreactor cultures grown in different conditions using glycerol. Cells from 24 h cultures stirred at 125 rpm reached a PHB content of 30.1% (wt/wt) with a Mr of 1.90 MDa, while those stirred at 500 rpm attained a polymer content of 16.9% (wt/wt) with a Mr of 1.71 MDa. The Mr of PHB produced in a 48 h fermentation, in which cells accumulated 51.9% (wt/wt) polymer,9 was even higher (2.04 MDa). These results indicate that it is possible to obtain PHB from glycerol with Mrs similar to those of the polymer obtained from other carbon sources, such as glucose or lactose, by using adequate bacterial strains and culture conditions. Additionally, these data suggest a possible correlation between the amount of polymer accumulated and its Mr, as the values were slightly higher in cultures accumulating more PHB. Previous results that analyzed PHB formation from glycerol proposed that this compound affects the elongation of the polymer by a process termed end capping, that results in premature chain termination.10 The Mr of the PHAs can be affected by numerous factors, such as the bacterial strain and carbon source used,11 and the presence of granule associated proteins such as PhaP.9 In experiments performed using glucose, for instance, variations in the pH of the medium were observed to affect PHB Mr.12 Our results suggest that the Mr of the polymer obtained from glycerol is affected by other parameters apart from the carbon source. In particular, the findings open the possibility of further increasing the Mr of the polymer obtained from glycerol by optimizing PHB yields.
The synthesis of PHB not only consumes acetyl-CoA but also reducing power. The reducing power scavenging nature of PHB synthesis in E. coli has been demonstrated in arcA mutants, that are unable to inhibit NAD(P)H generating pathways when the availability of terminal electron acceptors decreases. The use of excess reducing power for the synthesis of PHB enabled these redox mutants to grow in the presence of toluidine blue, a redox dye which inhibits growth of the mutants by increasing the production of reactive oxygen species.4 High aeration conditions, which increase reducing power availability, are known to favor the synthesis of PHB.13,14 However, recent research has been done in several E. coli strains demonstrating that the polymer can be synthesized in E. coli under reduced aeration conditions15,16 using either glucose or glycerol as substrates. In some of these strains, both mutations in the redox regulator arcA, resulting in augmented reducing power generation, and constitutive mutations in the carbon regulator creC, that increase carbon consumption, enhanced polymer production from glucose and glycerol in microaerobiosis, conditions in which the wild-type strains were only able to produce minimal amounts of PHB.17
The majority of studies on PHB production use glucose or other sugars such as lactose as a substrate. High aeration conditions normally favor high polymer accumulation from these carbon sources, as low aeration promotes the synthesis of other metabolic products derived from fermentation pathways, such as acetate13,14 (Fig. 1). While studying the accumulation of PHB in strain K24KP using glycerol or glucose in shaken-flask experiments, we observed that the relationship between oxygen availability and relative PHB content was not always the same, and depended on the carbon source used.18 When cells were grown at four different aeration conditions, we observed that while there was a direct relationship between growth and aeration, increased aeration was followed by an increase in PHB content only in the glucose cultures. In the glycerol cultures, a correlation between aeration and polymer accumulation was only observed at relatively low aeration, but the amount of PHB accumulated surprisingly decreased in more aerated conditions.
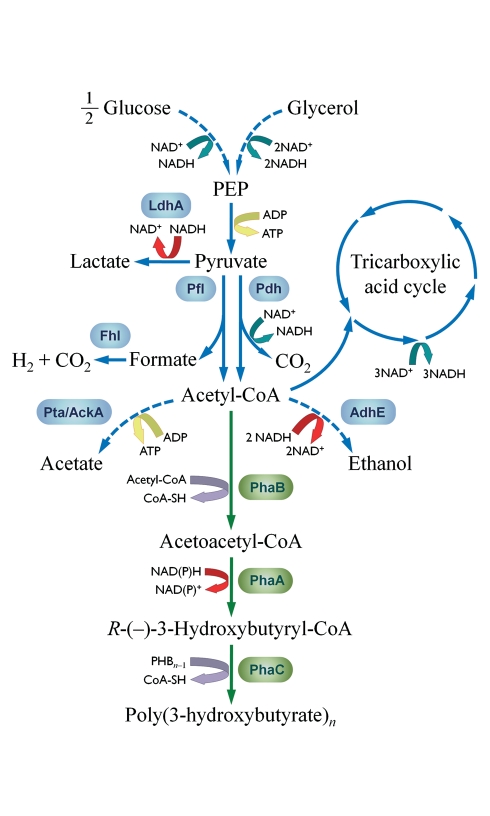
Figure 1.
Carbon and electron flow in E. coli K24KP. Main metabolic pathways involved in glucose and glycerol catabolism for recombinant strain K24KP, carrying phaBAC and phaP from Azotobacter sp. strain FA8. Relevant native biochemical pathways and enzymes are shown by blue arrows and boxes, respectively. The heterologous pathway for PHB synthesis and enzymes involved are depicted in green. Dashed lines represent more than one biochemical step. Pathways that generate ATP are indicated by yellow arrows, while reduction/oxidation of adenine dinucleotides are represented by green and red arrows, respectively. Abbreviations used are as follows: PEP, phosphoenolpyruvate; CoA, coenzyme A; LdhA, D-lactate dehydrogenase; Pfl, pyruvate-formate lyase; Pdh, pyruvate dehydrogenase; Pta/AckA, phosphotransacetylase/acetate kinase; AdhE, acetaldehyde/alcohol dehydrogenase; PhaB, 3-ketoacyl-CoA thiolase; PhaA, acetoacetyl-CoA reductase; PhaC, poly(3-hydroxyalkanoate) synthase.
This unexpected finding prompted us to investigate this effect further in bioreactor cultures, in which higher biomass and polymer accumulation levels can be achieved. Cells were grown in a bioreactor in different aeration conditions (125 and 500 rpm) using glucose or glycerol as carbon sources. Growth was higher at higher aeration rates, and the maximum biomass production was observed at the highest aeration condition using both substrates, as expected. PHB accumulation was observed to accompany growth in glucose cultures, as the highest values were observed for the most aerated cultures. However, in glycerol-containing medium, the highest PHB content was obtained at the low aeration condition. Cultures using glucose produced 1.8 times more polymer at 500 rpm than at 125 rpm, while those grown on glycerol produced 1.8 times more polymer at the lower aeration condition. These results indicated that carbon partitioning differed depending on both carbon source and oxygen availability, affecting growth and polymer accumulation from the two substrates used.
To further characterize these differences we measured the amounts of other major metabolic products: ethanol, acetate, lactate and formate (Fig. 2). Both the carbon sources and the aeration conditions used were observed to affect carbon distribution among the different metabolic products assayed. Lactate and formate concentrations were higher in the bioreactor cultures stirred at low speed than in strongly agitated cultures with both carbon sources, in accordance with results obtained in other studies,19 and higher with glucose, the more oxidized carbon source, in each aeration condition. The increase in lactate was more pronounced, probably due to the fact that its formation consumes reducing power, while formate synthesis does not.20 Accordingly, glucose cultures produced more formate than glycerol cultures.
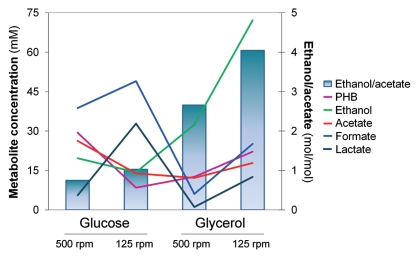
Figure 2.
Metabolic product distribution for E. coli K24KP growing on glucose or glycerol at 125 or 500 rpm. Final concentrations of the metabolic products (PHB, ethanol, acetate, formate and lactate) assayed in 24-h bioreactor cultures are shown as a function of the different carbon sources and agitation conditions used. The ethanol to acetate ratio, that reflects the internal redox state of the cells, is also shown.
In cultures grown on glucose, it was observed that in low agitation the amount of PHB and ethanol decreased while lactate increased; and the production of acetate, that does not consume reducing power, decreased. The highest agitation speed elicited a mostly oxidative metabolism in glycerol cultures, with low amounts of fermentation products, except for ethanol. The reduction in oxygen availability favored the formation of PHB and ethanol, which were increased 2.6 and 2.2 times respectively. In these cultures, carbon flow was redirected towards fermentation acids, as observed for the glucose cultures, but also towards ethanol and PHB. A dramatic increase in lactate formation was also observed. This can be due to the fact that poorly agitated cells growing on glycerol resort to lactate synthesis to dispose of the excess reducing power. However, the overall amount of acids produced from glycerol was lower than in cultures grown on glucose.
Carbon in glycerol has a lower oxidation state (−2) than glucose (0). As a consequence, glycerol catabolism produces more reducing equivalents (Fig. 1). This has a significant effect on the intracellular NADH/NAD+ ratio, that causes the cells to direct carbon flow towards the synthesis of more reduced products when using glycerol compared to glucose in order to achieve redox balance.21 On the other hand, the two different agitation speeds provided the cultures with different oxygen availability, which also affected the oxidation state of the cells, resulting in variations in the product pattern. The ratio ethanol/acetate can be used as an indicator of the redox state of the cells.21,22 An increase in ethanol production relative to a small decrease in acetate in cultures grown in glycerol at 125 rpm resulted in a high ethanol to acetate ratio, reflecting a reduced internal state. These cultures had the highest values (4.04 mol/mol), followed by cultures grown in glycerol at 500 rpm (2.66), and cells grown in glucose at 125 rpm (1.03) (Fig. 2). The amount of ethanol produced in the cultures grown in glucose did not suffer major changes, but acetate concentration decreased at lower agitation. As a result, the lowest ethanol to acetate ratio (0.75) corresponded to the cultures grown with the more oxidized carbon source (glucose) at the highest agitation speed (500 rpm). Cultures growing on glycerol produced much more ethanol than those using glucose, and significant amounts of ethanol were accumulated in the glycerol cultures even at high aeration.
These results show that the effects of using two different agitation speeds in recombinant E. coli cultures were totally different with the two carbon sources. Reduced aeration favored the formation of PHB and ethanol in the glycerol cultures, probably as a result of redirecting carbon metabolism to increase the consumption of reducing power. The behavior of the glucose cultures was clearly different, as carbon flow was mostly directed towards lactate and formate when oxygen availability was lower, as previously described,21 resulting in a decrease in PHB synthesis. The internal redox state of the cells is one of the main signals driving the metabolic changes that result in the differences in metabolic product distribution. Cells growing on glycerol are in a more reduced intracellular state than when glucose is used in similar conditions of oxygen availability. As a result, cultures using these substrates will respond to the same aeration conditions with different metabolic profiles in order to tune their metabolism, adjusting the consumption of reducing equivalents. This occurs because cells growing on glycerol will switch to high reducing power consuming metabolism in conditions in which glucose cultures are still able to sustain an oxidative metabolism.
In view of the results obtained in this work, it can be concluded that even small variations in oxygen availability, such as the ones used in this study, can lead to significant changes in the metabolite distribution of E. coli cultures. These changes vary when using carbon sources with different oxidation states, for instance glucose and glycerol, reflecting the metabolic adjustments that take place in order to optimize cell growth. Most metabolic studies are performed using glucose, but many of the effects observed in cultures using this substrate cannot be extrapolated to other carbon sources. Because of this, the effect of different culture conditions, such as oxygen availability, must be carefully assessed in order to optimize the production of desired metabolites from other substrates, such as glycerol. Moderate agitation led to the highest amount of PHB and ethanol from glycerol in the E. coli recombinants, which is desirable in bioprocesses in order to reduce aeration related problems and associated costs, and opens the possibility for the joint production of these high value biochemicals.
Acknowledgements
Financial funding by Universidad de Buenos Aires (Project X173) is gratefully acknowledged. P.I.N. and M.J.P. are researchers from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). A.D.A. holds a post-doctoral fellowship from CONICET.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/12103
References
- 1.Dawes EA, Senior PJ. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 2.Madison LL, Huisman GW. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63:21–53. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López NI, Floccari ME, Steinbüchel A, García AF, Méndez BS. Effect of poly(3-hydroxybutyrate) (PHB) content on the starvation-survival of bacteria in natural waters. FEMS Microbiol Ecol. 1995;16:95–112. [Google Scholar]
- 4.Ruiz JA, Fernández RO, Nikel PI, Méndez BS, Pettinari MJ. dye (arc) mutants: insights into an unexplained phenotype and its suppression by the synthesis of poly(3-hydroxybutyrate) in Escherichia coli recombinants. FEMS Microbiol Lett. 2006;258:55–60. doi: 10.1111/j.1574-6968.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 5.Taidi B, Anderson AJ, Dawes EA, Byrom D. Effect of carbon source and concentration on the molecular mass of poly(3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus. Appl Microbiol Biotechnol. 1994;40:786–790. [Google Scholar]
- 6.Shiloach J, Fass R. Growing E. coli to high cell density—A historical perspective on method development. Biotechnol Adv. 2005;23:345–357. doi: 10.1016/j.biotechadv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Nikel PI, de Almeida A, Melillo EC, Galvagno MA, Pettinari MJ. New recombinant Escherichia coli strain tailored for the production of poly(3-hydroxybutyrate) from agroindustrial by-products. Appl Environ Microbiol. 2006;72:3949–3954. doi: 10.1128/AEM.00044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solaiman DK, Ashby RD, Foglia TA, Marmer WN. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates) Appl Microbiol Biotechnol. 2006;71:783–789. doi: 10.1007/s00253-006-0451-1. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida A, Nikel PI, Giordano AM, Pettinari MJ. Effects of granule-associated protein PhaP on glycerol-dependent growth and polymer production in poly(3-hydroxybutyrate)-producing Escherichia coli. Appl Environ Microbiol. 2007;73:7912–7916. doi: 10.1128/AEM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madden LA, Anderson AJ, Shah DT, Asrar J. Chain termination in polyhydroxyalkanoate synthesis: involvement of exogenous hydroxy-compounds as chain transfer agents. Internatl J Biol Macromol. 1999;25:43–53. doi: 10.1016/s0141-8130(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 11.Agus J, Kahar P, Abe H, Doi Y, Tsuge T. Molecular weight characterization of poly[(R)-3-hydroxybutyrate] synthesized by genetically engineered strains of Escherichia coli. Polym Degrad Stab. 2006;91:1138–1146. [Google Scholar]
- 12.Choi JI, Lee SY. High level production of supra molecular weight poly(3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol Bioprocess Eng. 2004;9:196–200. [Google Scholar]
- 13.Ahn WS, Park SJ, Lee SY. Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol. 2000;66:3624–3627. doi: 10.1128/aem.66.8.3624-3627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SY, Choi J, Wong HH. Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review. Internatl J Biol Macromol. 1999;25:31–36. doi: 10.1016/s0141-8130(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 15.Nikel PI, Pettinari MJ, Galvagno MA, Méndez BS. Poly(3-hydroxybutyrate) synthesis by recombinant Escherichia coli arcA mutants in microaerobiosis. Appl Environ Microbiol. 2006;72:2614–2620. doi: 10.1128/AEM.72.4.2614-2620.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikel PI, Pettinari MJ, Galvagno MA, Méndez BS. Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl Microbiol Biotechnol. 2008;77:1337–1343. doi: 10.1007/s00253-007-1255-7. [DOI] [PubMed] [Google Scholar]
- 17.Nikel PI, de Almeida A, Pettinari MJ, Méndez BS. The legacy of HfrH: mutations in the two-component system CreBC are responsible for the unusual phenotype of an Escherichia coli arcA mutant. J Bacteriol. 2008;190:3404–3407. doi: 10.1128/JB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Almeida A, Giordano AM, Nikel PI, Pettinari MJ. Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76:2036–2040. doi: 10.1128/AEM.02706-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalel-Levanon S, San KY, Bennett GN. Effect of oxygen on the Escherichia coli ArcA and FNR regulation systems and metabolic responses. Biotechnol Bioeng. 2005;89:556–564. doi: 10.1002/bit.20381. [DOI] [PubMed] [Google Scholar]
- 20.Clark DP. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;5:223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 21.San KY, Bennett GN, Berríos-Rivera SJ, Vidali RV, Yang YT, Horton RE, et al. Metabolic engineering through cofactor manipulation and its effect on metabolic flux redistribution in Escherichia coli. Metab Eng. 2002;4:182–192. doi: 10.1006/mben.2001.0220. [DOI] [PubMed] [Google Scholar]
- 22.Nikel PI, Pettinari MJ, Ramirez MC, Galvagno MA, Méndez BS. Escherichia coli arcA mutants: metabolic profile characterization of microaerobic cultures using glycerol as a carbon source. J Mol Microbiol Biotechnol. 2008;15:48–54. doi: 10.1159/000111992. [DOI] [PubMed] [Google Scholar]