Abstract
Declines in heart disease and stroke mortality rates are conventionally attributed to reductions in cigarette smoking, recognition and treatment of hypertension and diabetes, effective medications to improve serum lipid levels and to reduce clot formation, and general lifestyle improvements. Recent evidence implicates these and other cerebrovascular factors in the development of a substantial proportion of dementia cases. Analyses were undertaken to determine if corresponding declines in age-specific prevalence and incidence rates for dementia and cognitive impairment have occurred in recent years. Data spanning 1 or 2 decades were examined from community-based epidemiologic studies in Minnesota, Illinois, and Indiana, and from the Health and Retirement Study, a national survey. Although a marginal decline was observed in the Minnesota cohort, no clinically significant trends were apparent in the community studies. A significant reduction in cognitive impairment measured by neuropsychological testing was identified in the national survey. Cautious optimism appears justified.
1. Introduction
Life expectancy has increased dramatically over the past century across the globe [1]. This salutary trend unfortunately also has a negative aspect in that common chronic diseases of older people are becoming much more prevalent. The number of people affected by Alzheimer’s disease (AD) was 26.6 million worldwide in 2006, and it was estimated that $156 billion is spent annually to care for dementia patients worldwide [2]. By 2050, the prevalence is expected to quadruple, so that 1 in 85 persons will be living with the disease [3], and 43.0% of them are expected to need a high level of care (e.g., a nursing home). Although the prevalence of dementia and its associated disability increases exponentially with age [4–6], the focus of research has recently shifted towards younger persons and the early stages of cognitive decline and mild cognitive impairment (MCI). The hope is to discover pathogenetic mechanisms underlying dementia and to delay the conversion of cognitive decline and MCI to full dementia [7]. Indeed, if interventions could delay disease onset or progression by as little as 1 year, nearly 9.2 million fewer AD patients would be expected by the year 2050 [3].
Dementia and AD represent a significant public health challenge for US society and one that is only likely to increase as the population ages. For example, there has been a large increase in diagnosis of AD on US death certificates in the last 10 years. However, it is important to separate increases in death certificate diagnoses due to growing awareness of the disease from increases due to changes in disease occurrence or mortality. Epidemiologists have attempted to uncover a wide range of risk and protective factors for dementia. Advocacy and public health organizations have also made efforts over the last 3 decades to increase the awareness of dementia and its known risk factors among both the public and practicing physicians. Thus far, these efforts have likely altered the risk of dementia only modestly, if at all. Society’s investment in dementia research must eventually be justified by progress made towards reducing the incidence of dementia or at least its associated disability.
Based on the time trends in potential risk and protective factors for cognitive impairment, there are good reasons to expect a decline in the incidence rates of cognitive impairment over time. However, there are also some trends in cardiovascular risk factors that would suggest an expected increase. A cluster of demographic, lifestyle, and medical factors have been identified which appear to alter the risk of developing dementia [8,9]. Prominent amongst these are vascular risk factors, including hypertension and diabetes [10,11]. Because cardiovascular disease and cerebrovascular disease contribute to the risk of dementia, and because there has been a substantial decline in the incidence of stroke in the last 50 years, some decline also in the risk of dementia might be expected. Alternatively, improved survival after stroke, or an increased prevalence of subclinical vascular disease in the absence of overt stroke, might result in more individuals in the population with increased risk of dementia.
New medications and other therapies for cardiovascular disease introduced since the early 1990s (e.g., wider use of antihypertensive and statin medications) may have contributed to a reduction in myocardial infarction and stroke over the past 20 years [12,13]. While the likelihood of treating hypertension and diabetes has increased, so also has the prevalence of these 2 conditions. From 1994 to 2002, in African-Americans over age 65 years, the prevalence of hypertension climbed from 73.0% to 83.0%, and the prevalence of diabetes rose from 26.0% to 36.0% [14,15]. The increased prevalence of hypertension, diabetes, and obesity may have contributed to an increased risk for cognitive decline and dementia, although the negative impact on brain health of diabetes and obesity may not become evident until future decades.
The increasing level of education among older adults over the past 20 years may have influenced the prevalence and outcomes of dementia. The proportion of adults age 65 years or older with a high school diploma increased from 53.0% in 1990 to 72.0% in 2003, whereas the proportion with a college degree increased from 11.0% to 17.0% during this same time period [16]. More years of formal education is associated with a reduced risk of dementia [17], likely through multiple causal pathways, including a direct effect on brain development and function (i.e., the building of “cognitive reserve”), better health behaviors, and the general health advantages of having more wealth and social opportunities [18].
The wealth of older adults has also increased significantly, with median household net worth for those age 65 years or older increasing from $119,000 in 1989 to $196,000 in 2005 (in constant 2005 dollars) [18]. Like education, greater wealth is associated with lower levels of disability throughout the life course and may have contributed to declining levels of dementia over the past 20 years. In addition, there has been a widespread general increase in Intelligence Quotient (IQ) score, known as the Flynn effect. Mean IQ score in the United States was estimated to have risen 13.8 points in the 46 years from 1932 to 1978. Changes in environmental factors, education, and socioeconomic status have been offered as possible explanations, however, the precise mechanisms underlying the trend in IQ remain unknown [19].
In existing projections of future prevalence of AD in the US population, the risk of AD is presumed to remain constant [20,21]. The increase in numbers is attributable to the continued increase in size of the older population, especially the oldest old (those age 85 years or older). The research reported here examines whether there is also a trend in the underlying incidence or prevalence which could alter estimates of the number of subjects affected by AD, dementia, or cognitive impairment in the future. Trends in prevalence may indicate changes in either incidence or survival. Trends in incidence, on the other hand, would suggest changes in risk. Existing studies of trends in the incidence or prevalence of dementia and AD reported conflicting results. Rorsman et al. found no increased incidence of dementia and AD between the period from 1947 through 1957 and the period from 1957 through 1972 in a Swedish population study [22]. Similarly, there were no detectable trends in the incidence of dementia or AD from 1975 to 1984 in Rochester, Minnesota [23]. By contrast, Manton et al. found a decline in prevalence of dementia in the United States using data from the National Long Term Care Study from 1982 to 1999 [24]. Therefore, it remains unclear whether the incidence or prevalence of AD, dementia, or cognitive impairment has increased, decreased, or remained stable over time in the United States.
In this article, US trends data are presented from 3 community-based studies and from a fourth study in a national sample. Rocca, Petersen, and Knopman report on trends in the incidence of dementia and AD for Rochester, Minnesota, for the period 1975 through 1994. Hebert and Evans report trends in the incidence of AD from the Chicago Health and Aging Project (CHAP) for the years 1997 through 2008. Hall, Gao, and Unverzagt compare prevalence figures for dementia and AD for 1992 versus 2001 in 2 samples of African-Americans residing in Indianapolis, Indiana. Langa and Larson investigated trends in the prevalence of cognitive impairment for the data collection waves from 1993 and 2002 using national data from the Health and Retirement Study (HRS). In the Discussion section, White provides a synthesis of these findings and gives his perspective on this important topic.
2. Secular trends in the incidence of dementia and AD in Rochester, Minnesota: 1975 through 1994
Incidence rates for dementia and AD from the population of Rochester, Minnesota, were previously reported for 3 different time periods. Data were initially published for the 10 years from 1975 through 1984 [23], then for the 5 years from 1985 through 1989 [25], and most recently, for the 5 years from 1990 through 1994 [26]. In this report, these data were combined over a total of 20 years (1975 through 1994) to investigate secular time trends in the incidence of dementia in a well-defined U.S. population.
The current study was made possible by the medical records-linkage system maintained by the Rochester Epidemiology Project (REP) [27]. Medical care for the populations of Rochester and Olmsted County, Minnesota, is provided largely by the Mayo Clinic at the primary, secondary, and tertiary levels. Additional health care providers in the community participate in the REP, which links essentially all medical information from the local population (the medical records-linkage system). Each provider in the community employs a dossier system (or unit record) whereby all medical information for each individual is accumulated in a single record. Medical diagnoses and surgical interventions from the dossier are routinely abstracted and coded using the hospital version of the International Classification of Diseases, Adapted (H-ICDA) and other coding systems [27,28], and are entered into computerized indexes. Therefore, persons with a given condition can be identified using diagnostic codes.
The indexes of the REP were searched for a list of specific diagnostic codes that might indicate dementia. This list expanded over time from 112 codes for the 1975 through 1984 study [23] to 132 codes for the 1990 through 1994 study [26]. Any subject who received at least 1 dementia code during the study years was considered a potential case. All medical records of each potential case were screened by a specifically trained nurse abstractor and, when applicable, reviewed by a neurologist with special expertise in dementia. The neurologist confirmed the presence of dementia, classified the dementia by type, and determined the year of onset. Because cases of dementia in the general population may remain undetected for a number of years but may ultimately be diagnosed at some point during the natural history of the disease, all medical records of subjects who received 1 of the diagnostic codes of interest during a 6-year period following the last year of the study interval were also reviewed. The use of this extended capture time frame should have assured a more complete case ascertainment [23,25,26].
The diagnostic criteria for dementia were not fully standardized because the REP data were collected as part of routine medical care rather than as part of a research project; however, the ad hoc criteria that were used are similar to those of the Diagnostic and Statistical Manual of Mental Disorders-III-Revised for dementia [29], and to those of the National Institutes of Health for AD [30]. Not all of the subjects affected by dementia had psychometric testing, imaging, or other research tests [23].
The total population living in Olmsted County, Minnesota, was approximately 110,000 at the 1990 US Census (last census included in this study period), was evenly split between men and women (49.0% men), and included approximately 11,000 subjects age 65 years or older. The population was primarily White of European descent. The analyses presented here refer to the incidence of dementia restricted to the city of Rochester rather than the full county [23].
The cases of dementia identified through the medical records-linkage system were used as numerators and US Census data as denominators. However, the census data were corrected by removing prevalent cases of dementia to avoid miscounting the subjects at risk. More details about the calculation of incidence rates were provided elsewhere [23]. Time trends were investigated graphically using age-specific curves for 5-year age classes. Analyses were conducted for dementia and AD separately and included both genders combined because no consistent gender pattern was observed [23]. To increase the stability of the estimates, 3-year moving average incidence rates were graphed. In addition, a series of birth cohort analyses were conducted using the methods described elsewhere [23]. The aims of these re-analyses were mainly descriptive. Since the study covered the target population completely, no sampling was involved, and statistical tests may not be appropriate to interpret the findings [23,31,32]. However, some statisticians argue that despite the descriptive aims of the study and the lack of sampling, statistical testing of the time trends can be done.
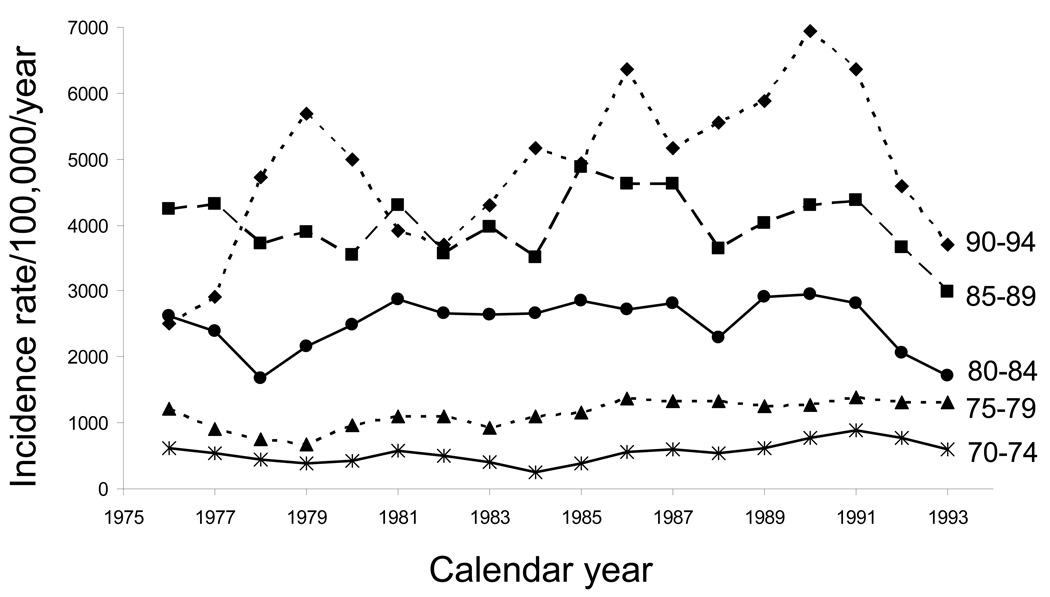
Fig. 1 shows the trend over 20 years for dementia in 5 age classes: 70 to 74, 75 to 79, 80 to 84, 85 to 89, and 90 to 94 years. For all the age classes, the incidence rates showed inconsistent fluctuations (ups and downs) over the 20 years of the study. However, there was some evidence for a declining trend in the second decade of the study, especially for the age groups 80 to 84, 85 to 89, and 90 to 94 years. When Poisson regression was used to test for secular trends, there was no statistically significant calendar year effect for the full 20-year period. However, there was a small but statistically significant decreasing trend in the second decade of the study, 1985 to 1994 [RR, 0.97 per calendar year; 95% confidence interval (CI), 0.95 to 0.99; P = .004]. This finding suggests a 3.0% decline in the incidence of dementia per year or a 30.0% decline over 10 years.
Fig. 1.
Time trends in age-specific incidence rates of dementia in men and women combined from 1975 through 1994 (moving 3-year average incidence rates per 100,000 person-years): Rochester, Minnesota.
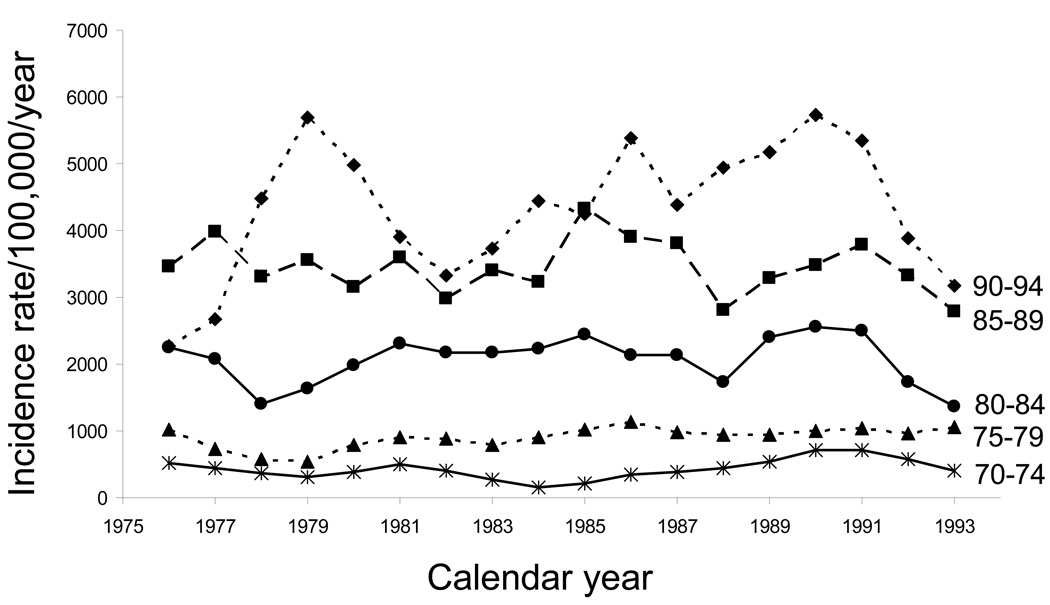
Fig. 2 shows the corresponding time trends of AD for the same 5 age groups over the same 20-year time span. Not surprisingly, the trends for AD were similar to those for all types of dementia combined because AD is the most common type of dementia—about 74.0% to 75.0% of the cases [5]. Poisson regression analyses showed the same patterns of statistical significance as for dementia of all types. Unfortunately, the numbers were too small to investigate the trends for vascular dementia (or for all of the non-AD types of dementia pooled).
Fig. 2.
Time trends in age-specific incidence rates of Alzheimer’s disease in men and women combined from 1975 through 1994 (moving 3-year average incidence rates per 100,000 person-years): Rochester, Minnesota.
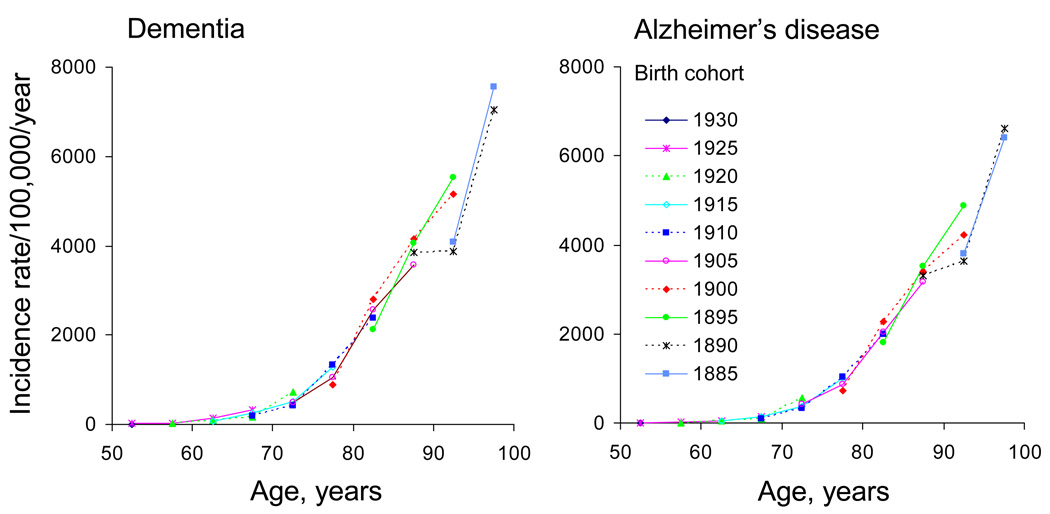
Fig. 3 shows the birth cohort curves for dementia (left panel) and AD (right panel). No obvious birth cohort effects were observed in this population. Different birth cohorts experienced the same incidence of dementia or AD when reaching the same age in different calendar years.
Fig. 3.
Birth cohort analyses of age-specific incidence rates in men and women combined for dementia (left panel) and Alzheimer’s disease (right panel): Rochester, Minnesota. The central year of each birth cohort served as the cohort label. Ten 5-year birth cohorts from 1885 through 1930 were considered.
In conclusion, there were no secular trends in the incidence of dementia when considering the full study period and there were no differences in incidence across the 10 birth cohorts included in the study. However, the data may suggest a declining trend in the second decade of the study, 1985 to 1994. This decline was evident for dementia overall and for AD. The interpretation of these trends remains uncertain.
First, these trends may simply be a chance finding and would not be confirmed if incidence rates for a longer time window were available. Second, the trends may be an artifact caused by some changes in the assignment of diagnostic codes for dementia over time, or in the linkage and storage of data in the REP. However, there were no changes in coding schemes for dementia in the REP between 1985 and 1994. In addition, there were no sizeable changes in patterns of referral to nursing homes inside or outside Olmsted County. Finally, the recognition of dementia by families and by practicing physicians was expected to increase rather than decrease during the period 1985 to 1994 as a result of increased research activity, increased numbers of publications, and increased media attention focused on dementia.
Second, the declining trends may be genuine and may indicate some changes over time in risk factors for dementia. The major mechanisms of dementia can be grouped into 2 broad categories, degenerative and vascular. It has been suggested that both degenerative and vascular mechanisms are involved in the pathogenesis of all cases of dementia and that their clinical separation is artificial [11]. Thus, each patient with dementia falls within a spectrum ranging from pure degenerative dementia to pure vascular dementia, and changes in the incidence of stroke may precede and determine changes in the incidence of dementia. Trends in the incidence of stroke may be responsible for the trends reported here [33]. The average incidence rates of stroke in Rochester, Minnesota, declined by 46.0% between the period 1950 to 1954 and the period 1975 to 1979. The incidence rates stabilized in the 1970s but increased by 17.0% in the period 1980 to 1984 [34]. The incidence rates of stroke remained stable in the period 1985 to 1989 [35]. Trends in the incidence of stroke after 1989 are not available in this community. The relationship between trends in stroke incidence and trends in dementia incidence needs further study. In addition, other trends in risk or protective factors such as education, income, or use of certain medications may also have played a role in the decline.
If the trends in the incidence of dementia for 1985 through 1994 are genuine, they have major implications for research [7,33] and for public health [3]. Therefore, these findings need to be replicated in other populations and should be extended to more recent years (after 1994). The REP offers a unique opportunity to further explore these trends.
3. Short-term trends in the incidence of AD in the Chicago Health and Aging Project (CHAP): 1997 to 2008
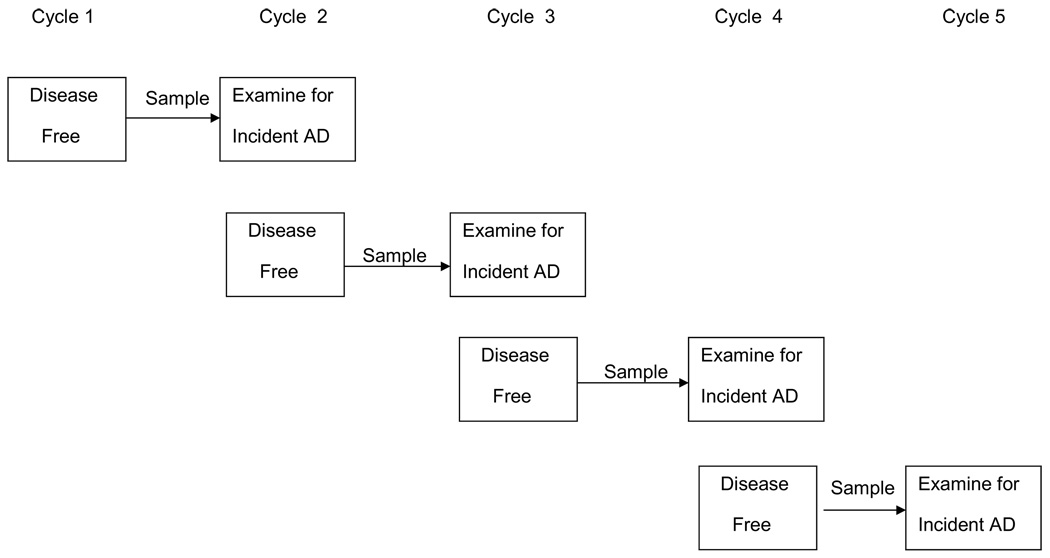
Incidence trends were obtained using data from CHAP [36]. CHAP is a population-based study in Chicago with substantial numbers of both African-American and White participants. The study began in 1993, when all residents age 65 years or older in a defined geographic area were invited to participate. Over time, additional people are invited to participate as they turn 65 years old. In this way, the number of living subjects remains fairly constant (between 6,000 and 7,000) and the full age distribution of the population is maintained. Data are collected in 3-year cycles. Any combination of 2 or more successive cycles is methodologically similar to a standard prospective cohort study (Fig. 4). Individuals are determined to be disease-free at the first of these cycles and a stratified random sample is evaluated for incidence of disease at the second of these cycles, 3 years later [37]. There have been a total of 4 cohort intervals. Evaluations for incident disease have been done approximately continuously since the beginning of the second cycle in 1997, for a total of 11 years. The total number of people who have contributed data to the project increased over time and is presently a little over 10,000.
Fig. 4.
Study design for determining incidence of Alzheimer’s disease in Chicago Health and Aging Project.
These analyses used data from subjects receiving clinical evaluation in the second (first incident) data collection cycle through the fifth cycle from 1997 to 2008. A total of 1,695 subjects were evaluated over this period and 360 developed incident AD. The mean age of the sample was 80.0 years (SD, 5.8). The mean education level was 12.9 years (SD, 3.5) which is similar to the community population and to the US average. Most subjects were women (61.6%) because women have a longer average survival. African-Americans represented 52.5% of the sample.
The date of evaluation was used as a predictor in logistic regression analyses of the probability of incident AD to identify time trends. A logistic model was used rather than a survival model because the window for development of incident disease, the 3 years between data collection cycles, was fairly constant across subjects and without systematic variation over the course of the study. The model adjusted for age, gender, education, race, and interval from the visit when subjects were designated disease-free to the visit when the disease was first diagnosed. The analysis was weighted to reflect the full population from which the samples were drawn and used jackknife variance to correct for the stratified design. Table 1 shows the results of the analysis. If there is a trend toward increased or decreased diagnosis of AD, the calendar year in which the diagnosis was made should appear as a significant variable. The result was null with an odds ratio (OR) of 0.97 and a 95% CI of 0.90 to 1.04 (P = .41). Thus, there was no relation of calendar year of evaluation to disease incidence, suggesting no change in incidence over time.
Table 1.
Results of the logistic model predicting probability of incident Alzheimer's disease: Chicago Health and Aging Project*
| Predictor | OR | 95% CI | P value |
| Calendar year | 0.97 | 0.90–1.04 | .41 |
| Age at evaluation (years) | 1.12 | 1.08–1.16 | <.001 |
| Gender, Men | 1.23 | 0.83–1.82 | .29 |
| Race/ethnicity, African-Americans | 1.77 | 1.19–2.63 | .005 |
| Education (years) | 0.94 | 0.89–1.00 | .07 |
| Interval for disease development† | 0.91 | 0.76–1.09 | .29 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
[36].
Time in years from the visit when last found to be disease-free to the visit when first diagnosed with Alzheimer's disease.
This study had both strengths and limitations. The strengths were that the sample was population-based and biracial. The clinical evaluations for incidence of AD were structured and uniform throughout the study. The analytic methods made it possible to directly examine the change in disease risk over time. The weaknesses of this study limit the generalizability of the findings. The population was only 1 community and the time of observation was limited to 11 years. The data collection cycles themselves were approximately 3 years in duration, so cases with onset occurring over an atypically lengthy period or cases with death shortly after onset would be less likely to be identified. Perhaps the strongest limitation is that small, difficult-to-detect, changes in risk of AD over time could have a substantial impact if they were to continue over a long period. Even a large study over more than a decade such as this one cannot convincingly exclude the possibility of such small changes.
4. Prevalence of dementia and AD in African-Americans in Indianapolis, Indiana: 1992 versus 2001
The Indianapolis-Ibadan Dementia Project was designed to compare morbidity levels and potential risk factors for dementing disorders in population-based samples of African-Americans in Indianapolis, Indiana, and Yoruba in Ibadan, Nigeria. This project, initiated in 1992, provided a unique opportunity to compare prevalence estimates over a decade in community dwelling elderly African-Americans. The population-based sample which was originally recruited in 1992 was enriched in 2001 to replace original participants who had died or were otherwise lost to follow-up. The aim in this section is to report a comparison of the prevalence of dementia and AD for the 1992 and 2001 cohorts, and to report also on differences in the demographic and medical profiles of the cohorts.
In 1992, interviewers went to randomly sampled addresses to enroll self-identified African-Americans age ≥ 65 years; of 2,582 eligible, 2,212 enrolled (9.6% refused, 4.7% were too sick) [38]. In 2001, Medicare lists were used for African-Americans age ≥ 70 years; of 4,260 eligible, 1,892 (44.0%) enrolled, 1,999 (47.0%) refused; the remainder were lost for other reasons. Informed consent was obtained from study participants before each interview. The study was approved by the Institutional Review Board at Indiana University School of Medicine.
The main study followed a 2-stage study design. In the first stage, all participants had an in-home interview. In the second stage, there was a full diagnostic clinical assessment in a subgroup of participants selected from the larger cohort on the basis of performance on the screening interview (good, intermediate, poor). Subjects were selected from all performance groups.
The screening interview relied on an instrument known as the Community Screening Interview for Dementia (CSID). It was specially developed for comparative epidemiological studies of age-associated dementias in different populations. The CSID has good sensitivity and specificity for discriminating dementia from no dementia in community-dwelling populations. Inter-rater reliability and 2-week test re-test reliability are also good [39]. The instrument includes a standardized interview with a close relative about cognitive function and activities of daily living. The instrument also collects information about medical history, alcohol and tobacco use, and current medications (recorded by inspecting containers).
Clinical assessments were conducted in a home visit by a physician and psychometrician. The assessment included the following: (1) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological test battery using normative values by education level, developed for elderly African-Americans [40]; (2) standardized health examination and assessment of function; (3) semi-structured informant interview based upon the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) adapted for American populations [41]; (4) request for medical records including neuroimaging. Diagnostic categories were dementia, cognitive impairment not dementia (CIND), or normal. Diagnosis was made in a consensus conference of clinicians reviewing all clinical data. Clinicians were kept blind regarding CSID scores. Standard diagnostic criteria were used [29,30,42,43]. Blood samples were collected in 10 ml (EDTA) Vacutainer tubes. The samples were centrifuged and red blood cells, buffy coat, and plasma were separated. DNA was extracted from the buffy coat using standard protocols and APOE genotype was obtained via Hhal digestion of amplified products [44]. A separate informed consent was obtained prior to blood collection.
T-tests and chi-square tests were used to compare the cohorts on continuous and categorical variables, respectively. They were also used to compare CSID poor performance group subjects with and without clinical assessment from each cohort. The age-specific prevalence estimates of dementia and AD were calculated using smoothing via logistic regression in which the probability of dementia or AD was modeled as a function of age, gender, and CSID performance group, and predicted probability from the model was used in the estimates [45]. Weighted logistic regression was used to account for different probabilities of selection into the sample for clinical assessment in different performance groups. Standard errors for the age-specific prevalence estimates were approximated using variance-covariance derived from the weighted logistic model. The 95% CIs for the prevalence estimates were constructed on the basis of asymptotic normality. 1990 US Census data for African-Americans in Indianapolis were used for direct age standardization of the prevalence figures. The 1992 prevalence estimates were compared to the 2001 prevalence figures using weighted logistic models with an indicator variable for cohorts.
Table 2 shows the demographic characteristics, medical history, medication use, and APOE ε4 status for the 1992 cohort compared to the 2001 cohort. Table 3 shows the age-specific prevalence of dementia and possible or probable AD for each cohort. Results of the weighted logistic models comparing the prevalence of dementia and AD for the 2 cohorts showed no significant differences (dementia, P = .35; AD, P = .26). For the 2001 cohort, comparisons were made for those who enrolled and those who did not, using information from the Medicare lists. Those who were not enrolled were significantly older (mean age, 79.1 years; SD, 7.4) compared to those who did enroll (mean age, 76.8 years; SD, 5.6; P < .0001). A higher proportion of women enrolled (64.8% versus 59.6%, P = .0006). For the clinical assessment phase, the study participants in the CSID poor performance group who were not clinically assessed in 1992 (n = 88) and 2001 (n = 47) did not differ significantly from those who were assessed, in regard to gender, age, and informant scores. The cognitive scores for those seen compared to those not seen were similar in 1992. However, the cognitive scores were slightly lower for the “not seen” group in 2001 (P = .018).
Table 2.
Comparisons of demographics, medical history, and APOE genotype between the 1992 and 2001 cohorts: African-American sample, Indianapolis, Indiana
| Variables | 1992 (n = 1,500) | 2001 (n = 1,892) | P value |
|---|---|---|---|
| Mean age (SD) | 77.4 (6.0) | 76.8 (5.6) | .004 |
| Mean years of education (SD) | 9.3 (3.2) | 11.3 (2.7) | <.0001 |
| Female, % | 65.3 | 64.8 | .72 |
| Rural childhood residency, % | 34.2 | 24.8 | <.0001 |
| Ever used alcohol regularly, % | 30.6 | 36.1 | .001 |
| Ever smoked, % | 59.5 | 54.6 | .004 |
| Self or Informant-reported history of: | |||
| Cancer, % | 11.9 | 16.7 | .0001 |
| Parkinson’s disease, % | 1.1 | 1.0 | .61 |
| Heart attack, % | 15.6 | 14.0 | .20 |
| Hypertension, % | 64.7 | 75.2 | <.0001 |
| Diabetes, % | 24.4 | 29.3 | .002 |
| Stroke, % | 12.3 | 15.8 | .003 |
| Head injury, % | 8.7 | 8.9 | .84 |
| Depression, % | 6.9 | 11.1 | <.0001 |
| Medication usage | |||
| Antihypertensive, % | 44.2 | 76.0 | <.0001 |
| Antidiabetic, % | 14.4 | 21.6 | <.0001 |
| Statins, % | 1.6 | 24.1 | <.0001 |
| Genetic testing* | |||
| Has ApoE ε4 allele, % | 39.9 | 36.2 | .16 |
Genetic testing was available for 456 subjects in 1992 and 1,133 subjects in 2001.
Table 3.
Age-specific prevalence of dementia and Alzheimer’s disease, and overall standardized prevalence: African-American sample, Indianapolis, Indiana
| Age group (years) | 1992 |
2001 |
||||||
|---|---|---|---|---|---|---|---|---|
| With dementia |
With AD |
With dementia |
With AD |
|||||
| Prevalence (%) |
95% CI | Prevalence (%) |
95% CI | Prevalence (%) |
95% CI | Prevalence (%) |
95% CI | |
| 70 to 74 | 2.5 | 1.6–3.4 | 1.7 | 0.9–2.4 | 1.9 | 0.9–2.9 | 1.3 | 0.4–2.2 |
| 75 to 79 | 4.9 | 3.5–6.2 | 3.5 | 2.3–4.8 | 4.7 | 2.2–7.2 | 4.0 | 1.6–6.4 |
| 80 to 85 | 9.7 | 7.3–12.1 | 7.8 | 5.5–10.1 | 10.3 | 3.6–16.9 | 9.4 | 2.4–16.4 |
| 85+ | 17.5 | 12.8–22.2 | 15.8 | 11.0–20.6 | 23.2 | 4.7–41.6 | 22.5 | 4.6–40.4 |
| Overall standardized prevalence* |
6.8 | 5.8–7.7 | 5.5 | 4.5–6.4 | 7.5 | 4.3–10.6 | 6.8 | 3.7–9.9 |
Abbreviations: 95% CI, 95% confidence interval; AD, Alzheimer’s disease.
Direct standardization by age for subjects age ≥70 years using 1990 US Census data for African-Americans in Indianapolis, Indiana.
There were no differences in the prevalence of dementia or AD for African-Americans living in Indianapolis in 1992 versus 2001. The 2001 cohort had higher levels of hypertension, diabetes, and stroke, conditions associated with risk for dementia and AD, but they also had higher levels of treatment. These differences are consistent with national trends for African-Americans over this time period [46]. The use of statins dramatically increased over this time (from 1.0% in 1992 to 24.0% in 2001). There were 2 conflicting trends with regard to cardiovascular risk factors; incidence rates went up but treatment levels also increased. The APOE ε4 allele was associated with risk for AD in this population; it occurred in about the same frequency in the 2 cohorts and would not be expected to differentially impact the prevalence. Severity ratings of the diagnosed cases were compared and no significant differences were found. There was an increase in the number of nursing homes in Indianapolis over the duration of the study; however, the percentage of African-Americans residing in nursing homes remained constant at 6.0% at both time periods [47,48]. Despite the higher frequency of risk factors and current treatments, there were not significant differences in prevalence between the 2 cohorts.
Limitations of the study include the change in recruitment strategies between 1992 and 2001, which resulted in higher refusal levels in 2001. In 1992, interviewers went door-to-door to randomly selected addresses. By contrast, the study area was greatly expanded to enrich the sample in 2001, and potential participants were initially contacted by mail and telephone, a much less personal approach. The effect of the increase in refusals on prevalence estimates is uncertain. Those who refused to participate were significantly older than those who enrolled, which could result in missing cases, leading to an underestimation of prevalence. Although there was a higher proportion of women in the enrolled group, being a woman was not associated with higher risk [49]. Data on medical illnesses were based upon self report or informant report rather than by direct examination. The individuals who agreed to participate in 2001 had slightly higher educational attainment than those of the 1992 cohort, and education may reduce the risk of dementia. This difference would possibly contribute to a lowering of the prevalence in 2001. If individuals with dementia were surviving longer in the community, this would lead to increased prevalence, whereas higher frequencies of institutionalization in nursing homes would lead to an underestimation. However, the percentages of African-Americans living in nursing homes were the same in 1992 and 2001. Differential mortality for participants with dementia would lead to a decrease in prevalence estimates, but there is no evidence for changes in mortality risk in these cohorts [50].
In conclusion, there were no differences in the prevalence of dementia and AD between 1992 and 2001, despite significant demographic, medical history, and medical treatment differences in these 2 population-based cohorts of African-American elderly.
5. The prevalence of cognitive impairment among older adults in the Health and Retirement Study (HRS): 1993 versus 2002
A large nationally representative study of older Americans was used to identify individuals with ‘cognitive impairment consistent with dementia’ (CI-D). Data were obtained from the 1993 and 2002 waves of the HRS, a biennial, longitudinal, nationally representative survey of US adults [51,52]. The main analytic goal was to identify 2 similar nationally representative cohorts of older individuals (age 70 years or older) in 1993 and 2002, characterize their cognitive function using the same cognitive tests in each year, and then follow each cohort for 2 years to determine mortality for individuals with: 1) normal cognitive function; and 2) CI-D. The CI-D category was further subdivided into mild CI-D and moderate/severe CI-D.
The HRS assesses cognitive function with a 35-point scale that includes: an immediate and delayed 10-noun free recall test to measure memory; a serial seven subtraction test to measure working memory; a counting backwards test to measure speed of mental processing; an object naming test to measure knowledge and language; and recall of the date, the president, and the vice-president to measure orientation. For self-respondents, the presence and severity of CI-D were defined using this 35-point cognitive scale. For those represented by a proxy (about 10.0% in each cohort), the proxy’s assessment of the respondent’s memory and judgment were used to categorize the level of cognitive function.
The definitions and cut-points for these categories were based on prior studies with the HRS data [53], as well as the methods used for the Aging, Demographics, and Memory Study (ADAMS), a supplemental study of dementia in the HRS [54]. The validity of these categories is supported by the clear trends in functional limitations with which they are associated; the mean number of limitations in instrumental activities of daily living (IADLs; preparing meals, grocery shopping, making phone calls, taking medications, managing money) in the normal, mild CI-D, and moderate/severe CI-D categories had an average of 0.4, 1.1, and 2.5 IADL limitations, respectively (P < .001). More detail on the HRS self-report and proxy cognitive measures is available at the HRS web site [55].
The following sociodemographic measures were included in the analyses as independent variables: age (70 to 79 years, 80 to 89 years, ≥ 90 years), race (White, African-American, Other), gender, education (<12 years; 12 years; 13 to 15 years, and ≥ 16 years), potential caregiver network (a spouse and/or living children) and net worth (tertiles; expressed in 1993 dollars). The self-reported chronic medical conditions considered were stroke, diabetes, heart disease, hypertension, lung disease, cancer, psychiatric problems, smoking status, and obesity [self-reported height and weight resulting in a body mass index (BMI) ≥ 30].
Data from 1993 and 2002 were pooled and logistic regression models with a dichotomous dependent variable indicating whether an individual had CI-D (mild, moderate/severe) were estimated. A linear trend variable that took the value of 0 in 1993 and 1 in 2002 was included in the logistic regression models. An OR less than 1 for this trend variable would indicate a decrease in the prevalence of CI-D between 1993 and 2002. Seven separate logistic regression models with different sets of independent variables (e.g., demographic variables, education, cardiovascular risks, and other chronic conditions) were estimated in order to determine which variables were most significantly associated with change in the prevalence of CI-D between 1993 and 2002. All results were adjusted for the complex sampling design of the HRS using appropriate population weights [52]. Statistical analyses were performed with STATA (Release 8.0; Stata Corp, College Station, TX) and SUDAAN (Release 9.0; Research Triangle Institute, Research Triangle Park, NC).
Table 4 shows the characteristics of the 1993 and 2002 study cohorts. Compared to the 1993 cohort, the 2002 cohort was slightly older (mean of 77.8 versus 77.5 years), had significantly more years of education, and had higher net worth (in constant 1993 dollars). Individuals with less than a high school diploma (12 years of education) comprised 42.0% of the sample in 1993, but only 31.0% in 2002. On average, individuals in the 2002 cohort had almost 1 more year of education compared to those in the 1993 cohort (mean of 11.8 versus 11.0 years).
Table 4.
Characteristics of the 1993 and 2002 study cohorts from the Health and Retirement Study
| Variable | 1993 (n = 7,406) | 2002 (n = 7,104) | P value* |
|---|---|---|---|
| Age (years) | .08 | ||
| 70 to 79 | 4,860 (67.0) | 4,494 (64.6) | |
| 80 to 89 | 2,248 (29.1) | 2,258 (31.2) | |
| ≥ 90 | 298 (3.9) | 352 (4.3) | |
| Mean ± SE | 77.5 ± 0.1 | 77.8 ± 0.9 | .02 |
| Race/ethnicity | .004 | ||
| White | 6,237 (90.2) | 6,096 (89.4) | |
| African-American | 1,014 (8.0) | 793 (7.7) | |
| Other | 154 (1.8) | 207 (2.9) | |
| Gender | .1X | ||
| Men | 2,886 (40.0) | 2,990 (40.8) | |
| Women | 4,520 (60.0) | 4,114 (59.2) | |
| Education (years) | <.001 | ||
| <12 | 3,337 (42.0) | 2,350 (31.2) | |
| 12 | 2,151 (30.6) | 2,370 (34.1) | |
| 13 to 15 | 1,055 (14.8) | 1,194 (17.2) | |
| ≥ 16 | 863 (12.7) | 1,190 (17.4) | |
| Mean ± SE | 11.0 ± 0.09 | 11.8 ± 0.10 | <.001 |
| Net worth (1993 dollars) | <.001 | ||
| ≤ 43,500 | 2,668 (32.5) | 1,968 (26.8) | |
| 43,500 to 167,100 | 2,667 (36.8) | 2,202 (30.7) | |
| >167,100 | 2,071 (30.6) | 2,934 (42.5) | |
| Mean ± SE | 179,000 ± 8,400 | 284,000 ± 10,900 | <.001 |
| Potential caregiver network | |||
| Spouse present | 3,625 (50.4) | 3,745 (50.1) | .9X |
| Living child | 6,433 (87.1) | 6,484 (90.4) | <.001 |
| Number of ADLs† impaired | .7X | ||
| 0 | 5,160 (70.8) | 4,989 (70.9) | |
| 1 to 3 | 1,751 (23.0) | 1,676 (23.3) | |
| 4 to 6 | 495 (6.2) | 439 (5.8) | |
| Mean ± SE | .67 ± 0.02 | .66 ± 0.02 | .7X |
| Number of IADLs‡ impaired | <.001 | ||
| 0 | 5,112 (70.3) | 5,631 (80.0) | |
| 1 to 3 | 1,955 (25.5) | 1,141 (15.9) | |
| 4 to 5 | 339 (4.2) | 329 (4.2) | |
| Mean ± SE | .56 ± .02 | .44 ± .02 | <.001 |
| Chronic Conditions | |||
| Stroke | 785 (10.5) | 774 (10.7) | .7X |
| Diabetes | 987 (12.4) | 1,310 (17.8) | <.001 |
| Heart disease | 2,339 (32.0) | 2,448 (34.1) | .005 |
| Hypertension | 3,694 (49.1) | 4,228 (59.6) | <.001 |
| Lung disease | 842 (11.9) | 768 (11.0) | .1X |
| Cancer | 1,014 (14.0) | 1,287 (18.4) | <.001 |
| Psychiatric problem | 805 (10.8) | 942 (13.2) | <.001 |
| BMI | <.001 | ||
| <18.5 | 295 (4.0) | 243 (3.4) | |
| 18.5 to 24.9 | 3,320 (46.6) | 2,858 (41.1) | |
| 25.0 to 29.9 | 2,637 (35.7) | 2,653 (37.7) | |
| ≥ 30.0 | 1,050 (13.7) | 1,261 (17.8) | |
| Smoking Status | <.001 | ||
| Never | 3,508 (47.8) | 3,128 (44.6) | |
| Former | 3,113 (42.5) | 3,381 (47.6) | |
| Current | 729 (9.7) | 541 (7.7) | |
| Respondent Type | .7X | ||
| Self | 6,621 (89.7) | 6,295 (89.9) | |
| Proxy | 785 (10.3) | 809 (10.1) |
Abbreviations: SE, standard error; ADL, activities of daily living; IADL, instrumental activities of daily living; BMI, body mass index.
Values in parentheses are weighted percentages derived using the HRS respondent population weights to adjust for the complex sampling design of the HRS.
P value for chi-square or t-test for a significant difference in proportion or mean between years.
ADLs indicates Activities of Daily Living (eating, transferring, toileting, dressing, bathing, and walking across a room).
IADLs indicates Instrumental Activities of Daily Living (preparing meals, grocery shopping, making phone calls, taking medications, managing money).
In the unadjusted analysis, there was a significant decrease in the prevalence of CI-D between 1993 and 2002, from 12.2% in 1993 to 8.7% in 2002 (P < .001). Table 5 reports the results of 7 different logistic regression models with the presence of CI-D (mild, moderate, or severe) as the outcome variable, using pooled 1993 and 2002 data. The trend variable in the first row of the table represents the odds of CI-D in 2002 compared to 1993. Model 1 shows the statistically significant decline (OR, 0.68) in unadjusted CI-D prevalence noted above. When adjusting for age and gender differences between the 2 cohorts (Model 2), the trend toward decreased CI-D prevalence was slightly larger (the trend OR drops from 0.68 to 0.65), due to the older age of the 2002 cohort and the strong association of older age with increased odds of CI-D. Higher levels of education (Model 3) and net worth (Model 4) were associated with significantly lower odds of CI-D, and accounted for about 43.0% (15.0 percentage points) of the decrease in CI-D prevalence between 1993 and 2002 (i.e., the trend OR increased from 0.65 to 0.80 after adding education and net worth to the model).
Table 5.
Odds ratios for presence of cognitive impairment in 1993 and 2002 (n = 14,476): Health and Retirement Study*
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| Trend (2002 vs. 1993) | 0.68 (0.60–0.77) | 0.65 (0.58–0.73) | 0.76 (0.67–0.86) | 0.80 (0.70–0.91) | 0.77 (0.67–0.87) | 0.74 (0.64–0.86) | 0.72 (0.62–0.83) |
| Age (years) | |||||||
| 70 to 79 | Reference | Reference | Reference | Reference | Reference | Reference | |
| 80 to 89 | 2.38 (2.08–2.73) | 2.21 (1.93–2.52) | 2.11 (1.84–2.41) | 2.30 (1.98–2.67) | 2.27 (1.98–2.61) | 2.34 (2.07–2.72) | |
| ≥90 | 6.82 (5.67–8.21) | 5.56 (4.57–6.75) | 5.00 (4.11–6.08) | 5.86 (4.71–7.29) | 5.63 (4.51–7.04) | 6.01 (4.84–7.46) | |
| Men vs. women | 0.83 (0.72–0.95) | 0.83 (0.72–0.96) | 0.72 (0.62–0.82) | 0.77 (0.66–0.89) | 0.79 (0.66–0.94) | 0.76 (0.64–0.90) | |
| Education (years) | |||||||
| <12 | Reference | Reference | Reference | Reference | Reference | ||
| 12 | 0.32 (0.28–0.38) | 0.40 (0.35–0.47) | 0.44 (0.38–0.51) | 0.44 (0.38–0.51) | 0.45 (0.39–0.52) | ||
| 13 to 15 | 0.24 (0.19–0.29) | 0.32 (0.26–0.40) | 0.35 (0.28–0.43) | 0.35 (0.28–0.44) | 0.36 (0.29–0.45) | ||
| ≥16 | 0.19 (0.15–0.24) | 0.29 (0.22–0.37) | 0.30 (0.23–0.39) | 0.30 (0.23–0.39) | 0.30 (0.23–0.39) | ||
| Net worth (1993 dollars) | |||||||
| ≤43,500 | Reference | Reference | Reference | Reference | |||
| 43,500 to 167,100 | 0.50 (0.43–0.57) | 0.55 (0.47–0.63) | 0.58 (0.50–0.66) | 0.59 (0.51–0.68) | |||
| >167,100 | 0.34 (0.28–0.41) | 0.38 (0.33–0.46) | 0.42 (0.35–0.51) | 0.44 (0.36–0.53) | |||
| Race/ethnicity | |||||||
| White | Reference | Reference | Reference | ||||
| African-American | 2.38 (1.97–2.86) | 2.52 (2.11–3.02) | 2.61 (2.19–3.12) | ||||
| Other | 2.35 (1.70–3.26) | 2.60 (1.88–3.61) | 2.60 (1.85–3.67) | ||||
| Caregiver network | |||||||
| Spouse present | 1.17 (1.01–1.36) | 1.19 (1.03–1.38) | 1.19 (1.03–1.37) | ||||
| Living child | 1.02 (0.82–1.28) | 1.01 (0.81–1.25) | 1.02 (0.83–1.26) | ||||
| Cardiovascular Risks | |||||||
| Stroke | 2.86 (2.49–3.29) | 2.86 (2.48–3.29) | |||||
| Diabetes | 1.08 (0.92–1.26) | 1.07 (0.91–1.25) | |||||
| Hypertension | 0.84 (0.73–0.96) | 0.82 (0.71–0.94) | |||||
| Obesity | 0.90 (0.73–1.11) | 0.90 (0.73–1.11) | |||||
| Heart disease | 0.94 (0.81–1.10) | 0.92 (0.79–1.07) | |||||
| Smoking status | |||||||
| Never | Reference | Reference | |||||
| Former | 0.99 (0.83–1.18) | 0.98 (0.83–1.13) | |||||
| Current | 1.14 (0.85–1.54) | 1.12 (0.83–1.52) | |||||
| Other chronic conditions | |||||||
| Lung disease | 0.97 (0.80–1.17) | ||||||
| Cancer | 0.92 (0.77–1.12) | ||||||
| Psychiatric problem | 1.86 (1.62–2.14) |
Adjusted odds ratios derived using a logistic regression model with pooled 1993 (n = 7,393) and 2002 (n = 7,083) data with cognitive impairment (mild, moderate, or severe) as the dependent variable. Values greater than 1 indicate increased odds of cognitive impairment. 95% confidence intervals are in parentheses.
In a large nationally representative survey of older Americans, the prevalence of CI-D decreased from 12.2% to 8.7% between 1993 and 2002, representing an absolute decrease of 3.5 percentage points, and a relative decrease of nearly 30.0%. The decline in the prevalence of CI-D suggests that, overall, the combined impact of recent trends in medical, lifestyle, demographic, and social factors has been positive for the cognitive health of older Americans. Although the prevalence of some cardiovascular risk factors that are also associated with a higher risk of dementia increased significantly, other factors showed trends that favored a reduced prevalence of CI-D. Most importantly, individuals who were age 70 years or older in 2002 had significantly higher levels of education, on average, than those who were age 70 years or older in 1993. The trend analyses suggest that increasing levels of education and net worth among older Americans explained about 40.0% of the observed relative decrease in CI-D prevalence between 1993 and 2002.
The relationship of temporal changes in various aspects of vascular risk to trends in CI-D is complex and will be important to track in the coming decades. Better control of hypertension and smoking along with more effective drugs to control lipid risk would be expected to delay onset of CI-D, whereas increasing prevalence of diabetes and obesity would be expected to have an opposite effect. In addition, the effects of the increase in diabetes prevalence from 1993 to 2002 on CI-D may take more time to become apparent in population studies like the HRS. This is an important area for future research, given the growing obesity epidemic and the associated rise in diabetes.
One important threat to the validity of this study is the non-random attrition from the HRS cohort. Specifically, if those with cognitive impairment are more likely to drop out of the study than those with normal cognitive function, the prevalence of cognitive impairment may decline over time as an artifact. To further examine the potential impact of differential loss to follow-up on the results, the baseline cognitive status of those who were lost to follow-up between the 1993 and 2002 waves of the study was examined (i.e., those who were not known to be dead and who did not provide either a self-interview or proxy interview in 2002). Of the 7,406 individuals included in the baseline 1993 survey, 453 (6.1%) were lost to follow-up in 2002. Among this group, 6.3% of those who had normal cognitive function in 1993 were lost, 4.6% of those with mild CI-D in 1993 were lost, and 3.4% of those with moderate/severe CI-D were lost. These results suggested that differential loss to follow-up was not an important reason for the lower prevalence of CI-D found in 2002, because the overall loss to follow-up rate was low (93.9% of individuals were accounted for), and those with poorer cognitive function at baseline were actually somewhat less likely to be lost to follow-up by the 2002 wave.
The growth in the elderly population in the coming decades increases the public health importance of better understanding trends in cognitive health and whether a societal investment in building and maintaining cognitive reserve through formal education in childhood, as well as continued cognitive stimulation during work and leisure in adulthood, may help limit the future burden of dementia, especially among the oldest-old. A more detailed account of the work discussed in this section was published earlier [56].
6. Discussion
The 4 time trend studies reported here represent complementary efforts to detect recent trends in the prevalence and incidence of AD, dementia, and cognitive impairment. The impetus for these analyses includes their possible utility to understand determinants and risk factors for these endpoints and to better anticipate the quantity and type of social and health-care needs of America’s older citizens in upcoming years. In particular, these analyses explored the notion that recent improvements in cardiovascular and cerebrovascular disease risks might be reflected in changes in dementia rates.
Heart disease and stroke mortality rates have declined dramatically over the past 4 to 5 decades. By contrast, prevalence levels of obesity, diabetes, and hypertension have increased in recent years in the general population, from childhood through adult life. Mortality improvements are generally attributed to reductions in large artery atherosclerosis associated with reductions in cigarette smoking, better control of serum lipid levels, more effective recognition and treatment of diabetes and hypertension, and more aggressive public health approaches to nutritional and other life style factors. The widespread use of aspirin or other medications has undoubtedly facilitated these trends as well. Depending on the balance between these divergent influences, the result could be either a reduction or an increase in dementia prevalence and incidence rates.
During the past 5 years there has been a growing recognition that many cases of dementia in late life, including those that receive a clinical diagnosis of AD, involve a combination of 2 or more independent pathological processes [57]. The most common combinations involve the Alzheimer process in concert with ischemic cerebrovascular disease and/or cortical Lewy bodies [58]. Autopsy studies have demonstrated that when the defining lesions of 2 or more of these independent processes occur together, the probability that the person had been impaired or demented is additive (i.e., the overall probability reflects the added probabilities of each lesion alone) [59]. Dementing illnesses attributable to mixed pathologies become progressively more common with advancing age, as would be expected based on the strong associations of all 3 of these processes with aging. A recent report that 17.0% of dementia cases in the Honolulu-Asia Aging Study were attributable to untreated or ineffectively treated midlife hypertension [60] supports the idea that improvements in rates of heart disease and stroke and their risk factors will lead to reduced rates of dementia. A demonstrable trend toward lower dementia rates in recent years would provide considerable support for the idea that public health interventions directed at reducing hypertension, smoking, and other risk factors for stroke and heart disease, and at improving general health, may yield the supplementary benefit of preventing some cases of cognitive impairment and dementia.
Three of the four reports presented here were from established community-based epidemiologic studies of dementia, and all employed appropriate clinical and research diagnostic criteria for dementia and AD. The Mayo Clinic report utilized data from the Rochester, Minnesota, population, analyzing rates for dementia diagnoses over a period of 20 years. CHAP employed approximately 10 years of data for samples of mixed ethnicity from a defined community within Chicago, Illinois. The third report examined data from 2 cycles of a project examining rates of dementia in African-American residents of Indianapolis, Indiana, over an interval of about 10 years. A major strength of all 3 studies is their excellent epidemiologic designs, the expertise of the research teams, and the sophistication of analyses applied to their collected data. An inescapable weakness is that the time interval across which rates were compared is relatively short. If dementia rates, like heart and stroke mortality rates, have been gradually falling over the past 30 to 40 years, 10 or even 20 years may not provide enough time for that change to be statistically recognizable. Further, during the time periods examined there have been changes in many social and cultural factors likely to influence endpoint assessments, including a falling enthusiasm for study participation, and the medical or general acceptability of a diagnosis of cognitive impairment. While the Mayo Clinic investigators describe a “blip” of lower rates of dementia in the second period (1985 to 1994), none of the 3 reports provides statistically significant evidence of a clear trend in rates of dementia or AD.
The fourth report presents an analysis of data from 2 waves of a nationally representative survey, the HRS. An algorithmic endpoint based on neuropsychological test scores was used to define cognitive impairment, rather than a criterion-based clinical or research diagnosis of dementia. A significant improvement in the prevalence of this measure was observed between 2 waves of data collection separated by an interval of about 10 years.
Results from the HRS analysis provide quite strong support for falling rates of cognitive impairment in late life in the United States. Conversely, the findings reported from the other 3 studies do not support a definite change in recent decades. Given the current understanding of the likely importance of ischemic cerebrovascular disease and allied risk factors contributing to dementia and aging-related cognitive decline, it seems reasonable to remain cautiously optimistic while continuing to work toward a full understanding of all factors determining the occurrence of dementia.
Acknowledgments
This combined effort was supported by National Institute on Aging grants R01AG034676, U01AG06786, R01AG011101, R03AG029652, R01AG009956, U01AG009740, R01AG015911, R01AG019805, R01AG027010, K08AG019180, U01AG006781, U01AG017155, U01AG019349. Also supporting the project were the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program at the Mayo Clinic, Rochester, Minnesota, and the Interfaculty Program for Health Systems Improvement at Harvard University, Cambridge, Massachusetts.
Rocca, Petersen, and Knopman wish to acknowledge the contribution to the Mayo analyses by Ruth H. Cha, MS, and Brandon R. Grossardt, MS.
Hall, Gao, and Unverzagt thank the elderly Indianapolis, Indiana, participants for their continued involvement with the Indianapolis-Ibadan Dementia Project. They also thank Millicent Pettaway for nursing supervision and data collection, and Jenna York for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement for authors
The authors have no conflicts to disclose. The sponsors had neither a role in the analysis or interpretation of these data, nor in the content of the paper. Appropriate approval procedures were used concerning human subjects.
References
- 1.Kinsella K, Wan H U.S. Census Bureau: International Population Reports, P95/09-1, An Aging World: 2008. Washington, DC: U.S. Government Printing Office; US Census Bureau. 2009
- 2.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord. 2006;21:175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA. Dementia, Parkinson's disease, and stroke in Europe: a commentary. Neurology. 2000;54:S38–S40. [PubMed] [Google Scholar]
- 5.Fratiglioni L, Rocca WA. Epidemiology of Dementia. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2nd ed. Volume 6. Amsterdam: Elsevier; 2001. pp. 193–215. [Google Scholar]
- 6.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 8.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 9.Jedrziewski MK, Lee VM, Trojanowski JQ. Lowering the risk of Alzheimer's disease: evidence-based practices emerge from new research. Alzheimers Dement. 2005;1:152–160. doi: 10.1016/j.jalz.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Grodstein F. Cardiovascular risk factors and cognitive function. Alzheimers Dement. 2007;3:S16–S22. doi: 10.1016/j.jalz.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: How to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortality and life expectancy - mortality by underlying and multiple cause, ages 18+: US, 1981–2007 (Source: NVSS) [ http://www.cdc.gov/nchs/hdi.htm]
- 13.Cutler DM, McClellan M. Is technological change in medicine worth it? Health Aff (Millwood) 2001;20:11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- 14.Health conditions - diabetes, ages 20+, US, 1988–2006 (Source: NHANES) [ http://www.cdc.gov/nchs/hdi.htm]
- 15.Risk factors and disease prevention - hypertension, ages 20+: US, 1988 – 2008 (Source: NHANES) [ http://www.cdc.gov/nchs/hdi.htm]
- 16.Older Americans … key indicators of well-being. [ http://www.agingstats.gov]
- 17.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 18.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57:P163–P172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 19.Flynn JR. The mean IQ of Americans: massive gains 1932 to 1978. Psychol Bull. 1984;101:171–191. [Google Scholar]
- 20.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 22.Rorsman B, Hagnell O, Lanke J. Prevalence and incidence of senile and multi-infarct dementia in the Lundby Study: a comparison between the time periods 1947–1957 and 1957–1972. Neuropsychobiology. 1986;15:122–129. doi: 10.1159/000118254. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- 24.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U.S. elderly population. Adv Gerontol. 2005;16:30–37. [PubMed] [Google Scholar]
- 25.Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59:1589–1593. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol. 2006;63:218–221. doi: 10.1001/archneur.63.2.218. [DOI] [PubMed] [Google Scholar]
- 27.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 28.Commission on Professional and Hospital Activities, National Center for Health Statistics. H-ICDA, Hospital Adaptation of ICDA. 2nd ed. Ann Arbor, MI: 1973. [Google Scholar]
- 29.American Psychiatric Association, American Psychiatric Association Work Group to Revise DSM-III. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DW, Mantel N. On epidemiologic surveys. Am J Epidemiol. 1983;118:613–619. doi: 10.1093/oxfordjournals.aje.a113671. [DOI] [PubMed] [Google Scholar]
- 32.Deming WE. Boundaries of Statistical Inference. In: Smith H, Johnson NL, editors. New Developments in Survey Sampling. New York: Wiley-Interscience; 1969. pp. 652–670. [Google Scholar]
- 33.Gorelick PB, Erkinjuntti T, Hofman A, Rocca WA, Skoog I, Winblad B. Prevention of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13:S131–S139. [PubMed] [Google Scholar]
- 34.Broderick JP, Phillips SJ, Whisnant JP, O'Fallon WM, Bergstralh EJ. Incidence rates of stroke in the eighties: the end of the decline in stroke? Stroke. 1989;20:577–582. doi: 10.1161/01.str.20.5.577. [DOI] [PubMed] [Google Scholar]
- 35.Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 36.Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) J Alzheimers Dis. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 38.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 39.Hall KS, Ogunniyi AO, Hendrie HC, Osuntokun BO, Hui SL, Musick BS, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument, Indianapolis, U.S.A., and Ibadan, Nigeria. Int J Meth Psychiatr Dis. 1996;6:129–142. [Google Scholar]
- 40.Unverzagt FW, Hall KS, Torke AM, Rediger JD, Mercado N, Gureje O, et al. Effects of age, education, and gender on CERAD neuropsychological test performance in an African American sample. Clin Neuropsychol. 1996;10:180–190. [Google Scholar]
- 41.Hendrie HC, Hall KS, Brittain HM, Austrom MG, Farlow M, Parker J, et al. The CAMDEX: a standardized instrument for the diagnosis of mental disorder in the elderly: a replication with a US sample. J Am Geriatr Soc. 1988;36:402–408. doi: 10.1111/j.1532-5415.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Geneva: World Health Organization; 10th revision. 1992
- 43.What is the CDR? [ http://alzheimer.wustl.edu/cdr/AboutCDR/aboutcdr.htm]
- 44.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 45.Beckett LA, Scherr PA, Evans DA. Population prevalence estimates from complex samples. J Clin Epidemiol. 1992;45:393–402. doi: 10.1016/0895-4356(92)90040-t. [DOI] [PubMed] [Google Scholar]
- 46.Health care use and expenditures - personal health care expenditures of Medicare beneficiaries by chronic conditions and type of service, ages 65+: US, 1992–2005 (Source: MCBS) [ http://www.cdc.gov/nchs/hdi.htm]
- 47.Marion County Health Department II: Annual Reports by Nursing Homes. 1989
- 48.Census 2000 Summary File 1 (SF 1) 100-Percent Data, Detailed Tables, Marion County, Indiana. Sex by age (Black or African-American Alone) and Group Quarters Population by Age by Group Quarters Type (Black or African-American Alone) [ http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=DEC&_submenuId=&_lang=en&_ts=]
- 49.Hall K, Gureje O, Gao S, Ogunniyi A, Hui SL, Baiyewu O, et al. Risk factors and Alzheimer's disease: a comparative study of two communities. Aust N Z J Psychiatry. 1998;32:698–706. doi: 10.3109/00048679809113126. [DOI] [PubMed] [Google Scholar]
- 50.Lane KA, Gao S, Hui SL, Murrell JR, Hall KS, Hendrie HC. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5:383–390. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 52.Health and Retirement Study: a longitudinal study of health, retirement, and aging. [ http://hrsonline.isr.umich.edu/]
- 53.Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 55.Wallace RB, Herzog AR, Weir DR, Ofstedal MB, Langa KM, Fisher GG, et al. Ann Arbor, MI: Survey Research Center, University of Michigan; Documentation of Cognitive Functioning Measures in the Health and Retirement Study. 2005 http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf.
- 56.Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 59.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 60.Launer LJ, Hughes T, Yu B, Masaki K, Petrovitch H, Ross GW, et al. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension. 2010;55:1352–1359. doi: 10.1161/HYPERTENSIONAHA.109.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]