Abstract
Aptamers are single stranded DNA or RNA molecules that have been selected using in vitro techniques to bind target molecules with high affinity and selectivity, rivaling antibodies in many ways. In order to use aptamers in research and clinical applications, a thorough understanding of aptamer-target binding is necessary. In this article, we review methods for assessing aptamer-protein binding using separation based techniques such as dialysis, ultrafiltration, gel and capillary electrophoresis, and HPLC; as well as mixture based techniques such as fluorescence intensity and anisotropy, UV-Vis absorption and circular dichroism, surface plasmon resonance, and isothermal titration calorimetry. For each method the principle, range of application and important features, such as sample consumption, experimental time and complexity, are summarized and compared.
Introduction
Nucleic acid aptamers are RNA or single stranded DNA molecules that bind a broad range of targets with high specificity and selectivity. Aptamers are generated through an in vitro combinatorial method referred to as SELEX (Systematic Evolution of Ligands by EXponential enrichment). Since first introduced independently by Tuerk et al. and Ellington et al. in 1990 [1, 2], nucleic acids aptamers have been developed for hundreds of targets [3–5]. Dissociation constants of aptamer-target complexes typically range from the low micromolar to high picomolar levels [3]. Compared to important roles that spontaneous nucleic acid protein interactions play in vivo, such as gene transcription and protein expression, aptamers generated in vitro have a broader range of functions that rival antibodies in analytical applications, clinical diagnoses and therapeutics. The first RNA aptamer based drug, Macugen (Pegaptanib sodium) has been approved by the US FDA in December 2004 and is currently being applied to patients with age-related macular degeneration [6, 7]. Understanding the interactions between nucleic acid aptamers and their targets clarifies binding mechanisms and helps improve rational design of aptamer structures. This demands comprehensive and detailed studies of molecular recognition properties [8], mapping of aptamer and target binding regions [9], kinetic studies of induced conformational changes of aptamers and/or targets upon binding [10], binding stoichiometry and measurement of equilibrium constants. In this article, major analytical techniques developed to measure binding constants are summarized (see Table 1). Although aptamers have been developed for complex targets, such as organelles and cells [11–13], we have chosen to focus this review on aptamers with a single molecular target since this covers the vast majority of aptamer applications.
Table 1.
Comparison of different techniques used to measure aptamer-protein binding
| Technique | Sample consumption | Experiment time and complexity | Kd limit | Equipment |
|---|---|---|---|---|
| Dialysis | ~ 100 µL* | ~ 48 h | 10−8 M [131] | Microequilibrium dialyzer with fluorometer detection [131] |
| Nitrocellulose filter binding | 200 to 500 µL [132, 133]* | ~ 5 min separation. Radioactive label and scintillation count are needed | 10−11M to 10−12M [134, 135] | filters and centrifuge, or membranes and vacuum apparatus, and scintillation counter |
| Gel electrophoresis | 10 µL to 50 µL* | ~ 3 h separation. Gel casting, radioactive labeling, autoradiography, gel cutting and counting are needed | 10−13 M [136, 137] | GE power supply and autoradiography. |
| CE | ~10 µL* in zonal separation | ~ 10 min per sample injection | 10−9 M [138] | Automated CE system with LIF detector |
| HPLC | 100 µL to 18 mL in FA, dependent on column type*[48] | 10 to 30 min per sample injection | 10−6 M [139] | Automated HPLC system with UV detector |
| Fluorescence Intensity | ~150 µL [140] 15 to 25µL* |
~ 10 min per sample, waiting is needed after each titration ~ 10 min | 10−10 M [142] | Spectrofluorometer and cuvette, Microplate reader |
| Fluorescence Anisotropy/Polarization | ~150 µL [140, 141] 15 to 25 µL* | ~ 10 min per sample, waiting is needed after each titration ~ 10 min | 10−9 M [143] | Spectrofluorometer with polarizers and quartz cuvette, Microplate reader |
| UV-Vis absorption | ~ 2.5 mL [102] | ~10 min per sample, waiting is needed after each titration | 10−6 M [102] | Spectrophotometer, quartz cuvette |
| Surface Plasmon Resonance | 10 to 20 µL* | ~ 20 min per sample, aptamer immobilization step is needed | 10−12 M [144] | Biacore SPR instrument and sensor chip |
| Isothermal Titration Calorimetry | ~1.5 mL in sample cell; 200 to 500 µL for total injection volume [120] | 1.5 to 3 hours | 10−8 M to 10−9 M [102] | ITC calorimeter |
Volume is for each sample. 8 to 25 samples are usually required to fit a binding isotherm and obtain a Kd value.
Binding equilibrium and isotherms
A simple binding equilibrium with a 1:1 stoichiometry is described by:
| (1) |
in which A is the aptamer, T is the target, and C is the aptamer-target complex. The equilibrium can be described using either a dissociation constant Kd (eq. 2), or association constant Ka in (eq. 3).
| (2) |
| (3) |
The most common strategy for measuring K is to titrate a constant concentration of one ligand with an increasing concentration of the other. For example, if the initial aptamer concentration remains constant, then the fraction of bound aptamer (fa) is given by:
| (4) |
Eq. 4 takes the general form of a rectangular hyperbola in the first quadrant with one asymptote of fa equaling 1. Kd can be estimated directly from this binding curve using a nonlinear regression analysis. Although many equations have been introduced in various techniques all are essentially variant forms of eq. 4. Similarly eq. 4 is the basis of many forms of graphic plots used to estimate K. A semi logarithmic plot of fa against log [T] is able to present data over three to six orders of magnitude of target concentration and Kd is easily estimated from the point where the curve crosses the fa = 0.5 threshold. Linear forms of the rectangular hyperbola, such as the double reciprocal plot (eq. 5), y reciprocal plot (eq. 6), and x reciprocal plot (eq. 7) are also useful for estimating Kd using linear regressions.
| (5) |
| (6) |
| (7) |
Although eqs. 5–7 are mathematically equivalent, each equation transforms error differently, affecting Kd as well as the confidence in the estimate. Detailed discussions can be found in Kenneth Connors’ book [14] and other articles [15, 16]. Generally, direct analysis of the rectangular hyperbola, the semilogarithmic plot, and the x reciprocal plot (also known as the Scatchard plot) are used most frequently. There are advantages and disadvantages to each approach and it is recommended to perform data analysis using at least two methods to compare the obtained Kd values. It is also recommended to collect data across the binding fraction range of 0.2 to 0.8 [16, 17]. Plots made without data points in this range often give rise to large errors in Kd estimates [18].
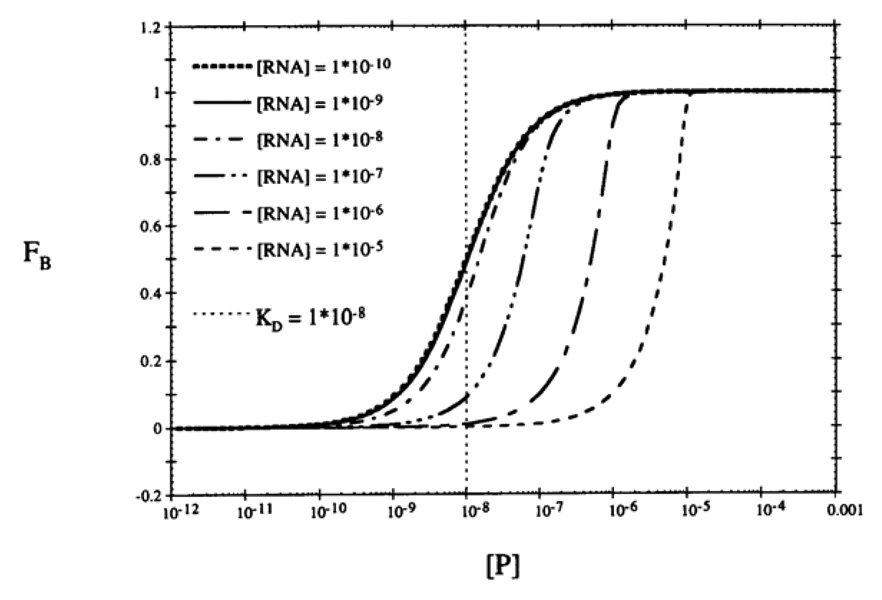
Using eq. 4 directly assumes that the concentration of aptamer is significantly smaller than Kd so that the free target concentration [T] at equilibrium doesn’t change significantly from the total target concentration [T]t upon binding. Figure 1 illustrates simulated plots for an RNA aptamer (Kd = 10−8 M) titrated with a broad concentration range of protein [19]. The binding curves are not affected by RNA concentration when below the value of Kd (i.e. [RNA] = 10−10 M or 10−9 M), and the Kd can be estimated as the protein concentration at 0.5 FB. When the RNA concentration approach Kd (10−8 M), the curves shift and Kd can no longer be estimated directly from the plot since the abscissa is no longer representative of free protein concentration. Here the complex concentration should be calculated by inserting [C]/[A]t into fa, and [T]t-[C] into [T] in equation 4:
| (8) |
Clearly eq. 8 is much more complicated but the sensitivity of some techniques or the extremely low Kd of some aptamers require using concentrations comparable to or higher than Kd.
Figure 1.
Simulated semilogarithmic binding plots of an RNA-protein interaction. In each plot, [RNA] is fixed, a 1:1 binding stoichiometry is assumed and Kd=10−8 M. Figure is reprinted from ref [19] with permission.
In some cases determining the equilibrium concentration of the protein target is made difficult due to complications such as denaturing during purification of recombinant protein, surface adsorption during solution preparation, or degradation/denaturation during storage. Using eqs 4 or 8 in any of these scenarios underestimates binding affinity. If protein loss is significant a fixed concentration of protein, which does not need to be known exactly if <Kd, should be titrated with increasing concentrations of aptamer [20]. Then Kd and total active target concentration [T]ta could be determined via nonlinear regression of equation 9 or linear regression of Scatchard plot as shown in equation 10 where fp is the fraction of target protein bound.
| (9) |
| (10) |
Separation based techniques
Dialysis
Dialysis, ultracentrifugation, and ultrafiltration all separate free aptamer from aptamer-protein complexes based on size differences. In a dialysis experiment, two compartments are separated by a semi-permeable membrane. At the beginning of an experiment, one compartment contains the mixture of aptamer and target, while the other only contains buffer. If the aptamer is smaller than the target and the corresponding complex, only the aptamer can cross the membrane to the other compartment. Note that this method is only applicable in cases where the target is significantly larger than the aptamer. After equilibrium is achieved, free aptamer concentrations at different target concentrations are determined in the second compartment. This technique, although technically simple, suffers from several experimental complications. The chemical potential of two compartments at equilibrium are balanced when the activity of the analytes are equal, which may be significantly different than the analyte concentrations [14]. The net charge of the protein target is balanced by H+ or OH− counterions, giving rise to a pH gradient and differing ligand concentrations across the membrane [21, 22]. The impermeability of the target and complex gives rise to an osmotic pressure gradient, resulting in solvent flow across the membrane and errors in solvent volumes [23]. Other issues encountered in dialysis measurements include long incubation time [24], large volume requirements and nonspecific adsorption [25].
Ultrafiltration
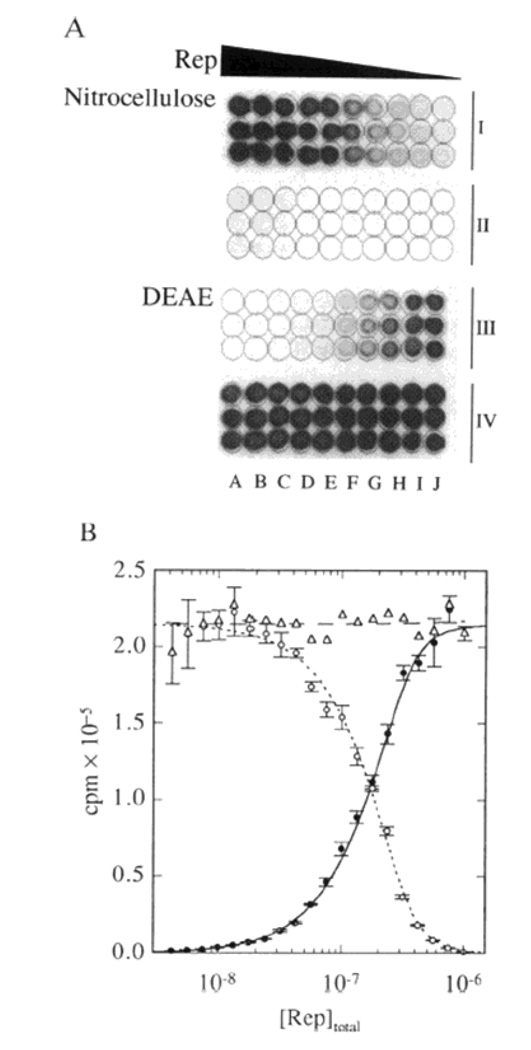
Ultrafiltration based techniques are similar to dialysis in that only free aptamer is able to pass through a filter membrane. In ultrafiltration the solution containing the aptamer and protein target is driven through the membrane using pressure, vacuum or centrifugal force. Aptamer bound to the protein target is retained by the membrane allowing the equilibrium distribution to be assessed across a range of protein target concentrations. The most widely used membrane material is nitrocellulose, which possesses long pores that are nucleic acid permeable but retain proteins as small as 2 kDa [26] by hydrophobic adsorption. Membranes are available in several traditional formats but “dot blot” apparatus are most commonly used due to the minimal material required (see Figure 2). 32P-labeled aptamers are incubated with the protein target at various concentrations pulled through the membrane using vacuum. [27][28] Free protein and the aptamer-protein complex are retained on membrane. The bound fraction is quantified in the membrane using a phosphor imager, Alternatively, free aptamer can be quantified in the filtrate using a scintillation counter.
Figure 2.
Dot Blot analysis of an interaction between HP duplex DNA (0.1 µM) and Rep protein. Nitrocellulose retained the DNA-protein complex while DEAE retained unbound DNA. Wells from left to right (A–J) contained progressively lower concentrations of Rep. Each group of three rows, designated I–IV, contains triplicate sets of data. Rows II and IV are continuations of rows I and III, respectively. (B) Quantitative analysis of the data from A shows the inverse relationship between the radioactive DNA retained on the nitrocellulose (e) and the DEAE (o) filters. Reprinted with permission from [28]
Ultrafiltration based techniques are commonly used due to their speed, sensitivity and low cost. Radioactive labeling allows aptamer concentrations as low as 10−12 M to be used simplifying measurement of Kd’ sin the low pM range. [27] Elimination of the equilibration time of dialysis shortens experiments from days to minutes. There are a number of drawbacks to consider though. In some cases incomplete retention of the aptamer-protein complex by the nitrocellulose membrane has been observed, giving rise to an over estimate of Kd. Incomplete retention could be due to competitive binding between aptamer and membrane for the same binding site on the protein [29]; or the aptamer interfering with interactions between an unstructured hydrophobic region on the protein and the membrane [30]. Another concern is nonspecific adsorption of nucleic acid to the nitrocellulose membrane, which requires control experiments in the absence of protein to be performed and signals to be subtracted to calculate the binding fraction. This is a particular concern for aptamers that have been isolated using nitrocellulose based selections. Protein concentration should be lower than 10−5 M to prevent saturation of binding sites on the nitrocellulose membrane [30]. Difficulties have also been encountered for aptamers capable binding more than one protein molecule [31]. While some have proposed that ultracentrifugation techniques are not an equilibrium method [30], others claim that as long as the concentration ratio of target over complex remains constant, from Equation 2, the filtration is an equilibrium process [32]. Radioactive labeling introduces additional safety and regulatory concerns; however, alternative detection methods with non-radioactive labels have also been developed [33, 34].
Gel Electrophoresis
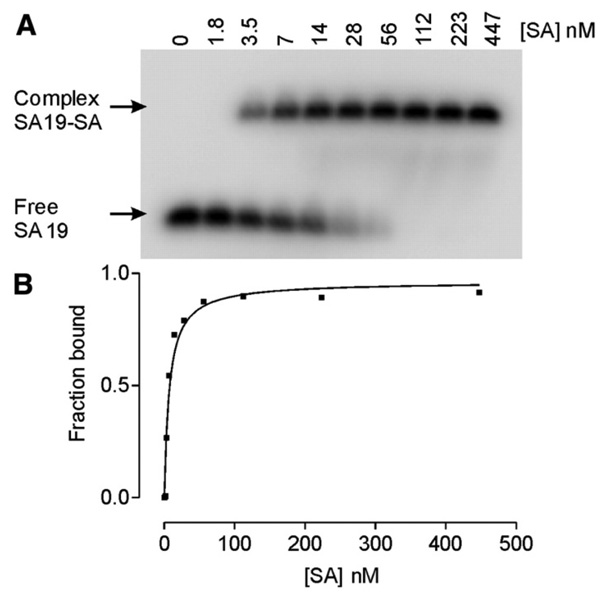
Nucleic acid aptamers are polyanions with a characteristic, strongly negative electrophoretic mobility. Protein targets generally possess lower charge and a lower mobility. The aptamer-protein complex possesses an intermediate electrophoretic mobility between that of the aptamer and target, making separation of the complex from the free aptamer straightforward. After first introduced by Fried and Crothers, and Garner and Revzin, gel electrophoresis has been widely used in studies of nucleic acid-protein binding equilibrium and kinetics [35, 36]. A series of samples are loaded onto a non-denaturing polyacrylamide or agarose gel with a fixed concentration of one component of the equilibrium (i.e. aptamer or protein) and increasing concentrations of the other component. Upon applying a uniform electric field, unbound aptamer moves fast, followed by the complex and finally the free protein. Quantification of the aptamer or proteinis achieved using UV absorbance [36], 32P-labeling of nucleic acids [35], Coomasie blue R-250 staining of protein [35], two-color fluorescent staining of both target and ligand [37–39], or blotting methods [40, 41]. Shown in Figure 3A is a native polyacrylamide gel pattern of samples with 3 nM aptamer and increasing concentrations of streptavidin [42]. The aptamers in free and complexed forms were quantified by radioactive labeling and phosphor imaging to obtain the nonlinear binding curve shown in Figure 3B.
Figure 3.
(A) The gel pattern of a mobility shift assay with 3 nM aptamer and 0–447 nM Streptavidin. Gel pattern was obtained using phosphor autoradiography and a phosphor imager; (B) Nonlinear binding curve was obtained from the above gel pattern with Kd = ~7.0 nM. Figures are reprinted from ref [42] with permission.
Gel electrophoresis separations take from 30 min to several hours. It is therefore necessary to consider association and dissociation rates of the aptamer-protein complex in order to properly interpret observed gel patterns and make valid estimates of equilibrium constants. For a strong aptamer-protein interaction, when the dissociation rate constant is small and the complex half-life is significantly longer than the separation time scale, the free DNA ligand and complex will give rise to distinct bands on the gel. Otherwise, if the complex half-life is comparable or smaller than the separation time, smearing of the bands is expected, if the complex is observed at all. However, observed dissociation is usually much slower than predicted based on solution measurements and distinct bands are obtained [35, 43]. The cross linked structure of the gel confines the dissociated ligands providing an increased opportunity for reassociation before the aptamer and protein can diffuse a significant distance [35, 43–46]. This stabilizing effect in combination with the high resolution of gel electrophoresis separations based on differing size and mobility makes studying even complicated, multiple stoichiometry equilibria possible [35]. Although this method is popular due to its high sensitivity, low cost and various detection methods, it is time consuming in terms of gel casting, separation, and visualization.
Capillary Electrophoresis
Capillary electrophoresis (CE) separates analytes in free solution based on their size and charge. The 20 to 100 µm inner diameter of the separation capillary not only reduces sample volumes but also Joule heating to improve separation speed and efficiency [47]. Many techniques have been developed to study protein interactions with nucleic acids [48–53]. Various approaches have been demonstrated including free zone, frontal analysis (FA), Hummel-Dreyer (HD), affinity CE (ACE), vacancy peak (VP), and vacancy affinity CE (VACE) [54–58]. Many of these approaches are analogous, and actually derived from, similar techniques employed in HPLC analyses of protein-nucleic acid interactions. [49, 59] The following section will focus on zonal CE techniques since these are most commonly applied to aptamer binding.
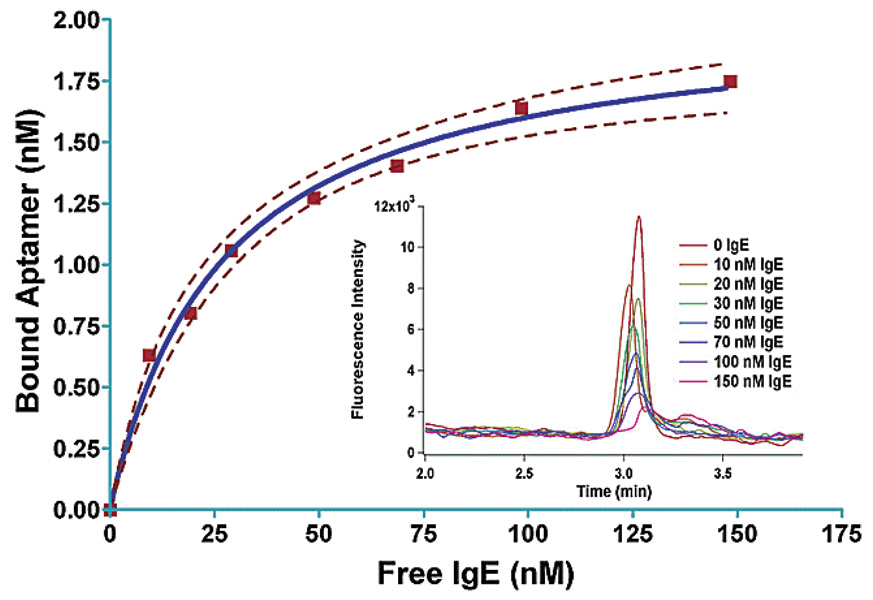
The dissociation rate of aptamer-protein complexes is often slower than the 5–20 minute separation times typical of CE. As dicussed above, the protein and aptamer-protein complex generally have slower mobilities than the unbound aptamer giving rise to distinct peaks. If a fluorescently labeled aptamer is titrated with increasing concentrations of protein, two peaks representing the complex and unbound aptamer are observed in the electropherogram. The bound fraction can be calculated from the decrease in the intensity of the free ligand peak as increasing amounts of protein are added. This technique has been used to characterize aptamers selected via CE-SELEX, a process in which only nucleic acid sequences that stay bound to the target for approximately the same separation time would be collected to evolve aptamers [60–62]. Figure 4 shows an overlay of electropherograms in which the FAM labeled free aptamer peak decreases with increasing human IgE concentration [60].
Figure 4.
Binding curve measured using CE for an aptamer selected to bind human IgE. Dashed lines represent 95% confidence of the fit curve. The figure inset shows fluorescence intensities of free aptamer peaks at different human IgE concentrations in CE electropherograms. Figure is reprinted from ref [60] with permission.
If the aptamer-protein complex has a life time on a time scale similar to that of the separation time, observed peaks will be distorted as the complex dissociates on the column during the separation. While this may be seen as detrimental to the measurement, the distortion of the peaks yields additional kinetic information regarding the complex in addition to the dissociation constant [63]. To avoid assessing areas of unresolved peaks, the first statistical moment of the aptamer signal across the entire electropherogram could be used to obtain dissociation constant as well [64, 65].
High Performance Liquid Chromatography (HPLC)
Chromatography separates analytes based on different interactions with the stationary phase. Zone separations of the free aptamer, protein target and aptamer-protein complex can be used to assess the equilibrium distribution of these components and estimate Kd in a manner similar to the electrophoresis separations described above. Separation mechanisms are generally not as straight forward as in electrophoresis but a number of options are available that separate analytes based on size including size exclusion chromatography (SEC) and internal surface reversed phase chromatography (ISRP) [66, 67]. As in the other separations described above the relationship between dissociation rate and separation time must be considered to properly interpret chromatograms of equilibrium mixtures.
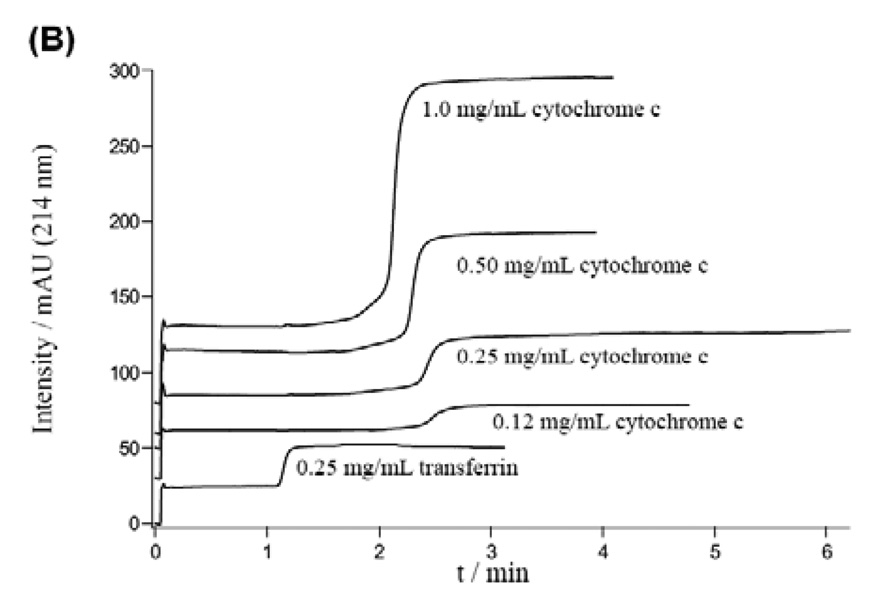
Frontal analysis (FA) can be applied using HPLC columns with immobilized aptamers to measure binding constants as well [68–75]. By carefully controlling the immobilization process, aptamer affinity for the protein target remained unchanged from that observed in solution [68, 76]. These immobilized stationary phases can be prepared in capillary monolithic columns, decreasing sample volumes dramatically [74]. Unlike traditional FA, methods utilizing immobilized columns require a fast association rate between the aptamer and its target. Samples with differing concentrations of protein are continuously applied to the column. As shown in Figure 5, when the target concentration is low, it takes longer time to saturate the aptamers immobilized on the stationary phase, resulting a longer retention and a gentle slope before reaching maximum signal [74]. With higher protein concentrations the front elutes more quickly with a sharper slope. Equation 11 can be used to estimate Kd:
| (11) |
where [P]o is the protein concentration, T is the retention time, To is the dead time, and Lt is the overall number of active aptamer binding sites immobilized on the column [67, 74], which may be different than the total amount of immobilized aptamer and can also be determined from the linear regression. In the above equation, we assume that all the retention is caused by the aptamers. If the monolithic column itself retains the protein and the protein elutes out later than the dead time To, Kd obtained from equation 11 is overestimated and correction of retention time is needed by performing control experiments.”
Figure 5.
Frontal analysis chromatograms obtained using a column immobilized with an aptamer for cytochrome c. Reprinted from [73] with permission.
Mixture based techniques
Fluorescence intensity
Fluorescence techniques have several advantages. Fluorescence signal is measured while the aptamer and protein remain at their equilibrium concentrations. This not only avoids concerns regarding association or dissociation rates, but also allows Kd to be measured under various buffer conditions, which may be limited by certain separation techniques [77]. For example, increasing the ionic strength of the buffer may affect the binding of the complex to a nitrocellulose membrane or increase Joule heat in affinity CE. Fluorescence based methods are generally much faster than separation based methods. There are hundreds of fluorescent probes available with differing lifetimes and excitation/emission wavelengths. Labeling procedures are well characterized, especially for nucleic acids [78–81].
It is not uncommon for the fluorescence of a labeled aptamer to change upon binding its protein target. If binding does induce a change in aptamer fluoresence, this signal can be used directly to estimate binding affinity. The microenvironment of the dye in the aptamer-protein complex could be dramatically different from that in free solution, resulting in a change in fluorescence quantum yield. Many dyes are sensitive to changes in buffer polarity. For example, the quantum yield of fluoresce in increases in less polar environments [82, 83]. Quenching due to energy transfer to the binding partner is also possible [84]. Another possible consequence of binding involves a conformation change and formation of a donor-acceptor complex at the ground state, resulting in a shift in the fluorescent emission profile [85]. As a result, the potential for either fluorescence enhancement or quenching is possible depending on the specific system under study [86–89]. By subtracting fluorescence signals from free ligand and background one can obtain intensity changes and calculate binding fractions. Practically, extra care should be taken if the buffer composition of the protein solution being added is different than that of the aptamer, since even small changes in pH or addition of additives such as glycerol or surfactant could induce a change in fluorescence intensity, generating an artificial “binding curve” as increasing amounts of protein solution is added. A derivative method employs aptamer beacons which incorporate both a fluorescent molecule and a quencher in close proximity in native status [90]. Binding to the target induces a conformation change in the aptamer changing the distance between the fluorescent dye and quencher, giving rise to fluorescent signal. This method requires preknowledge of the aptamer structure and is only applicable in cases where a suitable conformation change is observed. Similarly, if the quencher is another dye whose excitation spectrum overlaps the emission spectrum of the fluorescent dye, then a change in Förster resonance energy transfer (FRET) could be used to monitor binding process [91–93].
Fluorescence Anisotropy/Polarization
When polarized light illuminates a sample, only fluorescent molecules with dipoles in the same orientation as the incoming photons are excited. If the dye rotates slowly in comparison to the emission kinetics, the polarity of the incoming photon is retained in the fluorescence photon. The intensity of fluorescence signal with polarization parallel and perpendicular to that of the excitation source can be compared to assess polarization of the signal. Equation 12 describes how a fluorescent molecules’ lifetime and rotational diffusion affect polarization [94]:
| (12) |
where P is the observed polarization, Po is the limiting polarization when there is no rotation, τ is the fluorescence lifetime, R is the universal gas constant, T is the absolute temperature, η is the buffer viscosity, V is the molar volume of the dye, and 3ηV/RT is Debye rotational relaxation time, which is directly proportional to V. When a fluorescently labeled aptamer binds its protein target the molar volume increases resulting in less rotation and an increase in polarization. Since aptamers are usually smaller than their protein targets, they are usually labeled with dye molecules to achieve the largest change in polarization upon binding.
The definitions of anisotropy and polarization are given in equations 13 and 14 [81]:
| (13) |
| (14) |
Where I∥ represents emission intensity parallel to the incident light and I⊥ represents emission intensity perpendicular to the incident light. Anisotropy and polarization have similar physical meaning and are mathematically interchangeable.
Experimentally, it is important to choose a dye with a fluorescence lifetime that matches its rate of rotation. If the lifetime is too short, polarization is high for both the free ligand and the aptamer-protein complex, making observation of a change difficult. If the lifetime is too long, even the large complex could depolarize and have similar polarization as the free ligand. It is recommended that fluoresce in with a relatively fast lifetime of 4 n sec be used for aptamer-protein complexes smaller than 100 kDa [95, 96]. Long linkers between the dye and aptamer should be avoided since the flexibility of this attachment adds rotational freedom, which may not be significantly affected by binding the protein target. The G factor is an instrument parameter that measures the ratio of parallel and perpendicular signals recorded for non-polarized light. Ideally G should be 1 but polarization and anisotropy should be corrected when it is not [97]. Fluorescence enhancement or quenching upon binding further complicates measurements since these directly affect the fluorescence lifetime of the dye. For example, fluorescence enhancement is the result of an increase in quantum yield and fluorescence lifetime. From equation 12, polarization of the complex will be less than that predicted in the absence of fluorescence change. This increase gives an overestimate of the amount of complex and the corresponding bound fraction, leading to an overestimate of Kd. To correct for this error, the maximal fluorescence enhancement factor Qm is used to correct the apparent bound fraction Fa and give the accurate bound fraction fa: [85]
| (15) |
| (16) |
| (17) |
where Im and Io are the fluorescence intensities of the complex and free aptamer and P, Pf and Pb are polarizations of the sample, free aptamer and complex, respectively.
UV-Vis absorption
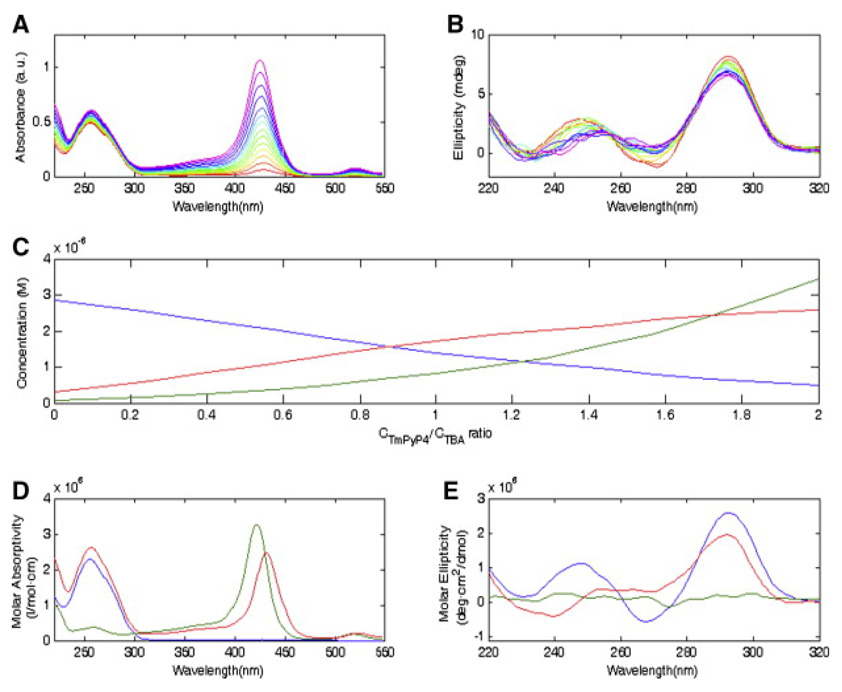
UV-Vis absorption is a powerful and widely used method in studying aptamer-target interactions due to its simplicity, low instrument cost and label free detection. It is well known that DNA has a maximum absorption at 260 nm and almost no absorption in a wide range above 300 nm [98]. If the target is a protein, the absorption in the near UV range (250 nm to 300 nm) due to aromatic residues is much weaker than that of nucleic acids; while the absorption in the far UV range (< 250 nm) is comparable with that of DNA [99, 100]. Binding constants can be estimated if there is an intensity change in the maximum absorption of either the aptamer or its protein target; or if another absorption peak appears in the near visible region due to the presence of metal ions or an extended π-electron system [101]. Figure 6A shows absorption spectra of mixtures of a thrombin-binding aptamer(TBA) and the target porphyrin 5,10,15,20-tetrakis-(N-methyl-4-pyridyl)-21, 23H-porphyrin tetratosylate (TmPyP4) at different ratios while keeping aptamer concentration constant [102]. When the TmPyP4/DNA concentration ratio is low, the band observed at 430 nm is due to the complex. When the ratio increases this band shifts to 422 nm, representing a characteristic absorption from free TmPyP4. The resolved DNA, TmPyP4 and complex spectra are shown in Figure 6D. By subtracting the spectra obtained from the same TmPyP4 concentrations without DNA one can obtain the complex spectra and calculate binding fractions.
Figure 6.
(A) UV-Vis absorption spectra of aptamer-TmPyP4 at a fixed aptamer concentration and increasing TmPyP4/aptamer concentration ratios. (B) CD spectra under the same aptamer/target ratio as in (A). (C) Resolved concentration profiles. (D) Pure resolved UV-Vis absorption spectra of aptamer, TmPyP4, and complex. (E) Pure resolved CD spectra of aptamer, TmPyP4, and complex. Blue, TBA; green, TmPyP4; red, complex. Figures are reprinted from ref [102] with permission.
Circular Dichroism (CD)
Circular Dichroism (CD) is a derivative UV-Vis absorption method that has proven useful in the study of biomolecular secondary structure. Due to the presence of asymmetric carbons in amino acid residues and sugars, proteins and nucleic acids adsorb left and right circularly polarized light differently [103]. This difference can be expressed as an absorption difference (ΔA), differential molar extinction coefficient (Δε), or degree of ellipticity (θ). The three are interchangeable as shown in eq. 18:
| (18) |
in which C is sample concentration and l is the path length [101]. Only well-defined secondary structures give rise to unique CD spectra since free rotations in denaturing molecules cancel each other out. Proteins with α-helices and (anti) parallel β-sheets give distinctive spectra under 250 nm [104, 105] while CD features from DNA structures are predominantally observed above 250 nm [106, 107]. It is therefore possible to calculate binding fraction from signals above 250 nm when titrating DNA with increasing concentration of protein, while at the same time observing protein structural changes using the signal below 250 nm. This technique has even been applied in cases where the protein target has no CD signal at all, such as TmPyP4 shown in Figure 6E. The complex CD spectrum indicates the G-quadruplex structure of the aptamer although the absence of negative peak at 270 nm suggests that formation of this structure is incomplete. Although the signal change is not significant upon binding due to the low molar ellipticity of DNA, which requires at least 10−5 M samples, this technique rivals others in terms of information on stoichiometry and conformational change.
Surface Plasmon Resonance (SPR)
SPR is a phenomenon that occurs on a metal film when the incident light is polarized parallel to the plane of incidence. The evanescent electromagnetic field generated by electron-plasmon oscillation penetrates the metal film into the sample solution near the film. When the light source and metal film are fixed, the angle change of the reflected light is only related to the refractive index in the sample solution near the film. It is found that the resonance signal is related linearly to the immobilized analyte across a broad concentration range [108, 109]. Aptamers are usually chosen to be immobilized on the metal surface because protein targets are usually larger and induce larger changes in refractive index upon binding [110–112]. As shown in Figure 7, when IgE is injected and flows over the immobilized aptamer, binding changes the surface refractive index and the resonance signal increases; after the binding process reaches the steady state a denaturing buffer is introduced at 900s and dissociation occurs [113]. Both the association and dissociation processes are exponential and from the curves association and dissociation rate constants could be calculated, thus dissociation constants could be further calculated [103]. Meanwhile, the steady state signal at different protein concentrations can also be used to calculate binding fractions and obtain Kd.
Figure 7.
SPR sensorgrams of aptamer-IgE interaction at different IgE concentrations. Plots were obtained by subtracting sensorgrams of aptamer IgG at the same concentrations. Figure is reprinted from ref [113] with permission.
There are many advantages of using this technique including label free detection, obtaining both thermodynamic and kinetic information within 20 min, and two approaches for determining Kd [108, 109]. However, caution should be employed to ensure that mass transfer of the protein from the bulk solution in the flow cell to the aptamers on the surface of the SPR chip is not a limiting factor, which would slow the observed association rate and give rise to an underestimate of Kd [114–116]. This can be conveniently checked by evaluating the association rate obtained at different flow rates. Faster mass transfer can be achieved by applying higher flow rate, using a thinner flow cell, or lowering aptamer surface density. Also, immobilization methods should be optimized and Kd estimates should be assessed by complementary techniques to confirm that aptamer affinity remains unchanged when immobilized onto the surface.
Isothermal Titration Calorimetry (ITC)
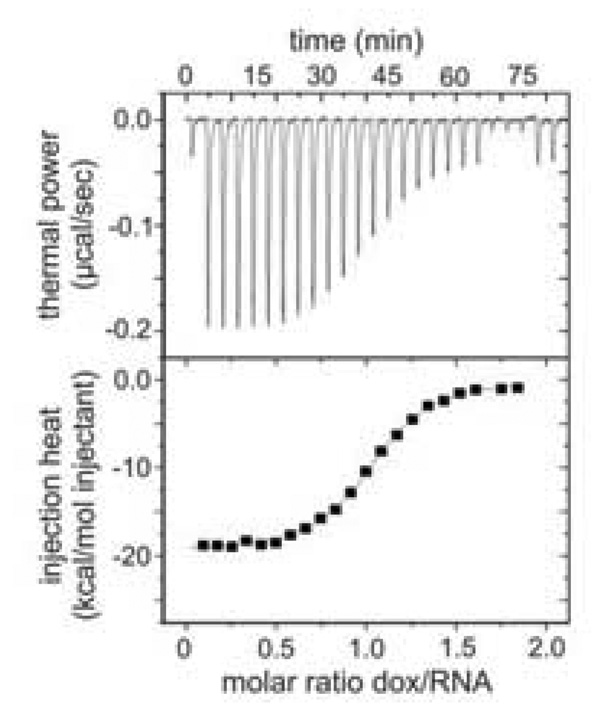
ITC is an excellent alternative to the separation and spectroscopy based methods described above due to its accuracy and simultaneous determination of Kd, stoichiometry and thermodynamic parameters (ΔG, ΔH, and ΔS) [117–119]. ITC relies on the fact that formation of the aptamer-protein complex is an exothermic process that releases heat. A sample cell containing a constant volume of aptamer or protein is titrated with a series of additions of its ligand. Heat is released as increasing amounts of complex are formed. Signal is measured as the amount of electric power needed to keep the temperature in the sample cell the same as the temperature in a control cell containing the same volume of buffer. As binding reaches saturation, less complex, and consequently heat, is generated with every addition. The upper plot in Figure 8 is a raw ITC thermalgram of 2.6 µM RNA aptamer in a volume of 1.44 ml titrated with 36 µM of its target doxycycline in 10 µL injections [120]. The signals are then integrated to give heat and plotted against target/ligand molar ratio in the lower plot.
Figure 8.
Raw ITC data (upper) and thermalgram (lower) of titrating an RNA aptamer with doxycycline. Figures are reprinted from ref [120] with permission.
Data analysis in ITC is more tedious than other techniques reviewed here. The injected heat must be associated with the bound fraction of the sample in the cell and the molar enthalpy of the reaction at a certain temperature. The free titrant concentration in equilibrium needs to be calculated via a quadratic equation. Removing an injection volume out of the cell before each injection to keep the sample volume constant makes the calculation of the afore mentioned two even more complicated (for detailed information, see ref [121]). Practically, any other factors that could trigger a heat release or production upon injection rather than binding, such as bubble formation or sample/titrant dilution heat, should be avoided or corrected.
Although high picomolar Kd have been reported using ITC, measurements in the 10−8 to 10−9 M−1 range are more common. Although ITC can work in the sample concentration range of 10<[sample]/Kd<100 [122], any concentration lower than high nanomolar is not sufficient to generate a significant injection heat signal and the thermalgram is too shallow to be meaningful. If a high nanomolar sample concentration is chosen with a low picomolar Kd binding system, the titration curve is so steep that few points are on the slope, giving rise to significant error in Kd.
Conclusion and Outlook
The sheer number of techniques that have been applied to measuring aptamer-protein binding speaks both to the importance and difficulty of these measurements. It should be emphasized that no one technique is perfect for all cases. Careful consideration of sensitivity, anticipated Kd range, association and dissociation kinetics and sample environment must be taken. Many techniques require structural changes upon binding that are difficult to predict before measurements are made. Techniques that require post selection labeling or immobilization may dramatically affect binding or in other cases could have little effect. Practical considerations such as amount of sample material, instrument cost/availability and analysis time are important as well. Considering all of these factors and the complexity of these measurements we strongly recommend employing at least two complementary techniques to confirm the validity of Kd measurements for aptamer-protein complexes.
The exponential development in detection and fabrication technologiesis making Kd measurements faster while reducing sample volumes. Hybrid technologies that combine multiple techniques for measuring binding are also becoming available. For example, a wider range of detectors are being integrated in separation instruments, especially in CE. Online laser induced fluorescence polarization (LIFP) has been used to monitor both mobility shift and polarization during a separation allowing Kd to be determined simultaneously by complementary techniques [123–125]. Two beam fluorescence cross-correlation spectroscopy can resolve bound aptamer from unbound based on the difference in mobility with improved sensitivity and no need of separation [126]. A CE-UV area imager that measures signal at two points can simultaneously measure binding constant and analytes’ hydrodynamic radius [127]. Microchip CE devices with flow gated injection shortens separations time to as little as 10 seconds, simplifying measurements of aptamers with faster dissociation rates [128, 129]. Micro free flow electrophoresis (μFFE) can be used to monitor the bound and free aptamer across a wide protein concentration range using gradient pumping, generating over 300 data points across the binding curve in as little as five minutes [89]. PDMS microfluidic chips with multiple channels and SPR imaging are able to test multiple aptamers’ affinity toward one target at the same time [130]. The emergence of a new generation of detectors, microfluidic devices and hyphenated techniques have been and will continue to improve aptamer-protein binding measurements with regard to faster speed, lower sample consumption, better sensitivity and higher throughput.
Acknowledgements
The authors grateful acknowledge funding from the National Institutes of Health (R01GM063533).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tuerk C, Gold L. Science. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 2.Ellington AD, Szostak JW. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 3.Tombelli S, Minunni A, Mascini A. Biosensors & Bioelectronics. 2005;20:2424. doi: 10.1016/j.bios.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Pendergrast PS, Marsh HN, Grate D, Healy Judith M, Stanton M. Journal of biomolecular techniques : JBT. 2005;16:224. [PMC free article] [PubMed] [Google Scholar]
- 5.Mosing RK, Bowser MT. J. Sep. Sci. 2007;30:1420. doi: 10.1002/jssc.200600483. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MAA, Keating GM. Drugs. 2005;65:1571. doi: 10.2165/00003495-200565110-00010. [DOI] [PubMed] [Google Scholar]
- 7.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Nature Reviews Drug Discovery. 2006;5:123. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal SS, Mayo MW, Bruno JG, Bronk BV, Batt CA, Chambers JP. Biosensors & Bioelectronics. 2000;15:549. doi: 10.1016/s0956-5663(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 9.Gopinath SCB. Analytica Chimica Acta. 2009;636:117. doi: 10.1016/j.aca.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Patel DJ, Suri AK, Jiang F, Jiang LC, Fan P, Kumar RA, Nonin S. Journal of Molecular Biology. 1997;272:645. doi: 10.1006/jmbi.1997.1281. [DOI] [PubMed] [Google Scholar]
- 11.Herr JK, Smith JE, Medley CD, Shangguan D, Tan W. Anal. Chem. 2006;78:2918. doi: 10.1021/ac052015r. [DOI] [PubMed] [Google Scholar]
- 12.Shangguan D, Li Y, Tang ZW, Cao ZHC, Chen HW, Mallikaratchy P, Sefah K, Yang CYJ, Tan WH. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11838. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamah SM, Healy JM, Cload ST. Accounts of Chemical Research. 2008;41:130. doi: 10.1021/ar700142z. [DOI] [PubMed] [Google Scholar]
- 14.Connors KA. Binding Constants. New York: John Wiley; 1987. [Google Scholar]
- 15.Harrison F, Katti SK. Chemometrics and Intelligent Laboratory Systems. 1990;9:249. [Google Scholar]
- 16.Larsson A. J. Immunol. Methods. 1997;206:135. doi: 10.1016/s0022-1759(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 17.Deranleau DA. J. Amer. Chem. Soc. 1969;91:4044. [Google Scholar]
- 18.Weber G, Anderson SR. Biochemistry. 1965;4:1942. [Google Scholar]
- 19.Hall KB, Kranz JK. Methods in Molecular Biology. Vol. 118. Totowa, New Jersey: 1999. p. 105. [DOI] [PubMed] [Google Scholar]
- 20.Setzer DR. Methods in Molecular Biology. Vol. 118. Totowa, New Jersey: 1999. p. 115. [DOI] [PubMed] [Google Scholar]
- 21.Mapleson WW. Journal of Pharmacological Methods. 1987;17:231. doi: 10.1016/0160-5402(87)90053-2. [DOI] [PubMed] [Google Scholar]
- 22.Tanford C. Physical Chemistry of Macromolecules. New York: Wiley; 1961. [Google Scholar]
- 23.Huang J. Journal of Pharmaceutical Sciences. 1983;72:1368. doi: 10.1002/jps.2600721137. [DOI] [PubMed] [Google Scholar]
- 24.Bowers WF, Fulton S, Thompson J. Clinical Pharmacokinetics. 1984;9:49. doi: 10.2165/00003088-198400091-00007. [DOI] [PubMed] [Google Scholar]
- 25.Henricsson S. Journal of Pharmacy and Pharmacology. 1987;39:384. doi: 10.1111/j.2042-7158.1987.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 26.Ryan PC, Lu M, Draper DE. Journal of Molecular Biology. 1991;221:1257. doi: 10.1016/0022-2836(91)90932-v. [DOI] [PubMed] [Google Scholar]
- 27.Carey J, Cameron V, Dehaseth PL, Uhlenbeck OC. Biochemistry. 1983;22:2601. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- 28.Wong I, Lohman TM. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5428. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oehler S, Alex R, Barker A. Analytical Biochemistry. 1999;268:330. doi: 10.1006/abio.1998.3056. [DOI] [PubMed] [Google Scholar]
- 30.Hall KK, J . RNA-Protein Interaction Protocols: Nitrocellulose filter binding for determination of dissociation constants. Humana Press; 1999. [DOI] [PubMed] [Google Scholar]
- 31.Stockley P. DNA-Protein interactions Principles and Protocols: Filter-Binding Assays. Humana Press; 2009. [Google Scholar]
- 32.Sophianopoulos JA, Durham SJ, Sophianopoulos AJ, Ragsdale HL, Cropper WP. Archives of Biochemistry and Biophysics. 1978;187:132. doi: 10.1016/0003-9861(78)90015-2. [DOI] [PubMed] [Google Scholar]
- 33.Christopoulos TK, Diamandis EP, Wilson G. Nucleic Acids Research. 1991;19:6015. doi: 10.1093/nar/19.21.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czerwinski JD, Hovan SC, Mascotti DP. Analytical Biochemistry. 2005;336:300. doi: 10.1016/j.ab.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Fried M, Crothers DM. Nucleic Acids Research. 1981;9:6505. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garner MM, Revzin A. Nucleic Acids Research. 1981;9:3047. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing D, Agnew J, Patton WF, Hendrickson J, Beechem JM. Proteomics. 2003;3:1172. doi: 10.1002/pmic.200300438. [DOI] [PubMed] [Google Scholar]
- 38.Jing D, Beechem JM, Patton WF. Electrophoresis. 2004;25:2439. doi: 10.1002/elps.200405994. [DOI] [PubMed] [Google Scholar]
- 39.Shcherbakov D, Piendl W. Electrophoresis. 2007;28:749. doi: 10.1002/elps.200600241. [DOI] [PubMed] [Google Scholar]
- 40.Westermeier R. Protein Purification. New York: John Wiley & sons; 1998. [Google Scholar]
- 41.Chen G, Kelly C, Chen H, Leahy A, Bouchier-Hayes D. Journal of Surgical Research. 2001;95:79. doi: 10.1006/jsre.2000.5896. [DOI] [PubMed] [Google Scholar]
- 42.Tahiri-Alaoui A, Frigotto L, Manville N, Ibrahim J, Romby P, James W. Nucleic Acids Research. 2002;30 doi: 10.1093/nar/30.10.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerstle JT, Fried MG. Electrophoresis. 1993;14:725. doi: 10.1002/elps.11501401115. [DOI] [PubMed] [Google Scholar]
- 44.Fried MG. Electrophoresis. 1989;10:366. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- 45.Crothers DM. Nature. 1987;325:464. [Google Scholar]
- 46.Cann JR. Analytical Biochemistry. 1996;237:1. doi: 10.1006/abio.1996.0193. [DOI] [PubMed] [Google Scholar]
- 47.Landers JP. Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques: Introduction to Capillary Electrophoresis. New York: Taylor & Francis Group; 2008. [Google Scholar]
- 48.Oravcova J, Bohs B, Lindner W. Journal of Chromatography B-Biomedical Applications. 1996;677:1. doi: 10.1016/0378-4347(95)00425-4. [DOI] [PubMed] [Google Scholar]
- 49.Busch MHA, Carels LB, Boelens HFM, Kraak JC, Poppe H. Journal of Chromatography A. 1997;777:311. doi: 10.1016/s0021-9673(97)00369-5. [DOI] [PubMed] [Google Scholar]
- 50.Heegaard NHH, Nilsson S, Guzman NA. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 1998;715:29. doi: 10.1016/s0378-4347(98)00258-8. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka Y, Terabe S. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2002;768:81. doi: 10.1016/s0378-4347(01)00488-1. [DOI] [PubMed] [Google Scholar]
- 52.He XY, Ding YS, Li DZ, Lin BC. Electrophoresis. 2004;25:697. doi: 10.1002/elps.200305727. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Weber SG. Trac-Trends in Analytical Chemistry. 2008;27:738. doi: 10.1016/j.trac.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erim FB, Kraak JC. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 1998;710:205. doi: 10.1016/s0378-4347(98)00127-3. [DOI] [PubMed] [Google Scholar]
- 55.Mammen M, Gomez FA, Whitesides GM. Analytical Chemistry. 1995;67:3526. doi: 10.1021/ac00115a023. [DOI] [PubMed] [Google Scholar]
- 56.Sebille B, Thuaud N, Tillement JP. Journal of Chromatography. 1979;180:103. doi: 10.1016/s0021-9673(00)80178-8. [DOI] [PubMed] [Google Scholar]
- 57.Busch MHA, Boelens HFM, Kraak JC, Poppe H, Meekel AAP, Resmini M. Journal of Chromatography A. 1996;744:195. doi: 10.1016/0021-9673(96)00275-0. [DOI] [PubMed] [Google Scholar]
- 58.Busch MHA, Boelens HFM, Kraak JC, Poppe H. Journal of Chromatography A. 1997;775:313. doi: 10.1016/s0021-9673(97)00369-5. [DOI] [PubMed] [Google Scholar]
- 59.Sebille B, Zini R, Madjar CV, Thuaud N, Tillement JP. Journal of Chromatography-Biomedical Applications. 1990;531:51. doi: 10.1016/s0378-4347(00)82280-x. [DOI] [PubMed] [Google Scholar]
- 60.Mendonsa SD, Bowser MT. Journal of the American Chemical Society. 2004;126:20. doi: 10.1021/ja037832s. [DOI] [PubMed] [Google Scholar]
- 61.Mendonsa SD, Bowser MT. Analytical Chemistry. 2004;76:5387. doi: 10.1021/ac049857v. [DOI] [PubMed] [Google Scholar]
- 62.Mendonsa SD, Bowser MT. Journal of the American Chemical Society. 2005;127:9382. doi: 10.1021/ja052406n. [DOI] [PubMed] [Google Scholar]
- 63.Krylov SN. Electrophoresis. 2007;28:69. doi: 10.1002/elps.200600577. [DOI] [PubMed] [Google Scholar]
- 64.Shimura K, Uchiyama N, Enomoto M, Matsumoto H, Kasai K. Analytical Chemistry. 2005;77:564. doi: 10.1021/ac049132r. [DOI] [PubMed] [Google Scholar]
- 65.Shimura K, Waki T, Okada M, Toda T, Kimoto I, Kasai K. Electrophoresis. 2006;27:1886. doi: 10.1002/elps.200500239. [DOI] [PubMed] [Google Scholar]
- 66.Hagestam IH, Pinkerton TC. Analytical Chemistry. 1985;57:1757. [Google Scholar]
- 67.Hage DS, Tweed SA. Journal of Chromatography B. 1997;699:499. doi: 10.1016/s0378-4347(97)00178-3. [DOI] [PubMed] [Google Scholar]
- 68.Deng Q, German I, Buchanan D, Kennedy RT. Analytical Chemistry. 2001;73:5415. doi: 10.1021/ac0105437. [DOI] [PubMed] [Google Scholar]
- 69.Deng Q, Watson CJ, Kennedy RT. Journal of Chromatography A. 2003;1005:123. doi: 10.1016/s0021-9673(03)00812-4. [DOI] [PubMed] [Google Scholar]
- 70.Michaud M, Jourdan E, Villet A, Ravel A, Grosset C, Peyrin E. Journal of the American Chemical Society. 2003;125:8672. doi: 10.1021/ja034483t. [DOI] [PubMed] [Google Scholar]
- 71.Kotia RB, Li LJ, Mcgown LB. Analytical Chemistry. 2000;72:827. doi: 10.1021/ac991112f. [DOI] [PubMed] [Google Scholar]
- 72.Clark SL, Remcho VT. Analytical Chemistry. 2003;75:5692. doi: 10.1021/ac030156s. [DOI] [PubMed] [Google Scholar]
- 73.Connor AC, McGown LB. Journal of Chromatography A. 2006;1111:115. doi: 10.1016/j.chroma.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Q, Li XF, Le XC. Analytical Chemistry. 2008;80:3915. doi: 10.1021/ac702567x. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Q, Li XF, Shao YH, Le XC. Analytical Chemistry. 2008;80:7586. doi: 10.1021/ac801206s. [DOI] [PubMed] [Google Scholar]
- 76.Rupcich N, Nutiu R, Li Y, Brennan JD. Angewandte Chemie, International Edition. 2006;45:3295. doi: 10.1002/anie.200504576. [DOI] [PubMed] [Google Scholar]
- 77.Anderson BJ, Larkin C, Guja K, Schildbach JF. Fluorescence Spectroscopy. 2008:253. doi: 10.1016/S0076-6879(08)03412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heyduk T, Lee JC. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1744. doi: 10.1073/pnas.87.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haralambidis J, Duncan L, Angus K, Tregear GW. Nucleic Acids Research. 1990;18:493. doi: 10.1093/nar/18.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen DJ, Benkovic SJ. Biochemistry. 1989;28:9586. doi: 10.1021/bi00451a006. [DOI] [PubMed] [Google Scholar]
- 81.Jameson DM, Sawyer WH. Biochemical Spectroscopy. 1995:283. [Google Scholar]
- 82.Nag A, Bhattacharyya K. Journal of Photochemistry and Photobiology a-Chemistry. 1989;47:97. [Google Scholar]
- 83.Nakayama K, Endo M, Fujitsuka M, Majima T. Journal of Physical Chemistry B. 2006;110:21311. doi: 10.1021/jp064031d. [DOI] [PubMed] [Google Scholar]
- 84.Livak KJ, Flood SJA, Marmaro J, Giusti W, Deetz K. Pcr-Methods and Applications. 1995;4:357. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 85.Wei AP, Herron JN. Analytical Chemistry. 1993;65:3372. doi: 10.1021/ac00071a007. [DOI] [PubMed] [Google Scholar]
- 86.Drees BL, Rye HS, Glazer AN, Nelson HCM. Journal of Biological Chemistry. 1996;271:32168. doi: 10.1074/jbc.271.50.32168. [DOI] [PubMed] [Google Scholar]
- 87.McAfee JG, Edmondson SP, Zegar I, Shriver JW. Biochemistry. 1996;35:4034. doi: 10.1021/bi952555q. [DOI] [PubMed] [Google Scholar]
- 88.Rachofsky EL, Osman R, Ross JBA. Biochemistry. 2001;40:946. doi: 10.1021/bi001664o. [DOI] [PubMed] [Google Scholar]
- 89.Turgeon RT, Fonslow BR, Jing M, Bowser MT. Analytical Chemistry. 2010;82:3636. doi: 10.1021/ac902877v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan WH, Wang KM, Drake TJ. Current Opinion in Chemical Biology. 2004;8:547. doi: 10.1016/j.cbpa.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 91.Lee W, Obubuafo A, Lee YI, Davis LM, Soper SA. Journal of Fluorescence. 2010;20:203. doi: 10.1007/s10895-009-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Endoh T, Funabashi H, Mie M, Kobatake E. Analytical Chemistry. 2005;77:4308. doi: 10.1021/ac048491j. [DOI] [PubMed] [Google Scholar]
- 93.Juskowiak B. Analytica Chimica Acta. 2006;568:171. doi: 10.1016/j.aca.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 94.Perrin F. Journal de Physique et le Radium. 1926;7:390. [Google Scholar]
- 95.Chen RF, Scott CH. Analytical Letters Part a-Chemical Analysis. 1985;18:393. [Google Scholar]
- 96.Heyduk T, Ma YX, Tang H, Ebright RH. Rna Polymerase and Associated Factors. 1996;(Pt B):495. [Google Scholar]
- 97.Lakowicz JR, Gryczynski I, Gryczynski Z, Dattelbaum JD. Analytical Biochemistry. 1999;267:397. doi: 10.1006/abio.1998.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basu S. Biopolymers. 1967;5:876. doi: 10.1002/bip.1967.360050910. [DOI] [PubMed] [Google Scholar]
- 99.Donovan JW. Phys. Principles Tech. Protein Chem. 1969:101. [Google Scholar]
- 100.Strickland EH, Horwitz J, Kay E, Shannon LM, Wilchek M, Billups C. Biochemistry. 1971;10:2631. doi: 10.1021/bi00789a033. [DOI] [PubMed] [Google Scholar]
- 101.Nienhaus GU, editor. Methods Mol. Biol. Vol. 305. Totowa, NJ, U. S.: 2005. Protein-ligand interactions: Methods and applications. 2005. [DOI] [PubMed] [Google Scholar]
- 102.del Toro M, Gargallo R, Eritja R, Jaumot J. Analytical Biochemistry. 2008;379:8. doi: 10.1016/j.ab.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 103.Moss T, Leblanc B, editors. Methods Mol. Biol. Third Edition. Vol. 543. Totowa, NJ, U. S.: 2009. DNA-Protein Interactions: Principles and Protocols. 2009. [Google Scholar]
- 104.Johnson WC., Jr Annual Review of Biophysics and Biophysical Chemistry. 1988;17:145. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- 105.Johnson WC., Jr Methods of Biochemical Analysis. 1985;31:61. doi: 10.1002/9780470110522.ch2. [DOI] [PubMed] [Google Scholar]
- 106.Johnson BB, Dahl KS, Tinoco I, Jr, Ivanov VI, Zhurkin VB. Biochemistry. 1981;20:73. doi: 10.1021/bi00504a013. [DOI] [PubMed] [Google Scholar]
- 107.Gray DM, Hung S-H, Johnson KH. Methods Enzymol. 1995;246:19. doi: 10.1016/0076-6879(95)46005-5. [DOI] [PubMed] [Google Scholar]
- 108.Fagerstam LG, Frostell-Karlsson A, Karlsson R, Persson B, Ronnberg I. J Chromatogr. 1992;597:397. doi: 10.1016/0021-9673(92)80137-j. [DOI] [PubMed] [Google Scholar]
- 109.Jonsson U, Fagerstam L, Ivarsson B, Johnsson B, Karlsson R, Lundh K, Lofas S, Persson B, Roos H, Ronnberg I, et al. Biotechniques. 1991;11:620. [PubMed] [Google Scholar]
- 110.Di Primo C, Lebars I. Anal. Biochem. 2007;368:148. doi: 10.1016/j.ab.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 111.Balamurugan S, Obubuafo A, Soper SA, McCarley RL, Spivak DA. Langmuir. 2006;22:6446. doi: 10.1021/la060222w. [DOI] [PubMed] [Google Scholar]
- 112.Tang Q, Su X, Loh KP. J. Colloid Interface Sci. 2007;315:99. doi: 10.1016/j.jcis.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 113.Wang J, Lv R, Xu J, Xu D, Chen H. Anal. Bioanal. Chem. 2008;390:1059. doi: 10.1007/s00216-007-1697-x. [DOI] [PubMed] [Google Scholar]
- 114.Schuck P. Biophys. J. 1996;70:1230. doi: 10.1016/S0006-3495(96)79681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schuck P, Minton AP. Trends in Biochemical Sciences. 1996;21:458. doi: 10.1016/s0968-0004(96)20025-8. [DOI] [PubMed] [Google Scholar]
- 116.Schuck P, Minton AP. Anal. Biochem. 1996;240:262. doi: 10.1006/abio.1996.0356. [DOI] [PubMed] [Google Scholar]
- 117.Freire E, Mayorga OL, Straume M. Anal. Chem. 1990;62:950A. [Google Scholar]
- 118.Doyle ML. Current Opinion in Biotechnology. 1997;8:31. doi: 10.1016/s0958-1669(97)80154-1. [DOI] [PubMed] [Google Scholar]
- 119.Holdgate GA. Biotechniques. 2001;31:164. [PubMed] [Google Scholar]
- 120.Mueller M, Weigand JE, Weichenrieder O, Suess B. Nucleic Acids Res. 2006;34:2607. doi: 10.1093/nar/gkl347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lewis EA, Murphy KP. Methods in Molecular Biology. Vol. 305. Totowa, NJ, United States: 2005. p. 11. [DOI] [PubMed] [Google Scholar]
- 122.Crane-Robinson C, Dragan AI, Read CM. Methods in Molecular Biology. Vol. 543. Totowa, NJ, United States: 2009. p. 625. [DOI] [PubMed] [Google Scholar]
- 123.Le XC, Wang QH, Lam MT. Electrophoresis. 2002;23:903. doi: 10.1002/1522-2683(200203)23:6<903::AID-ELPS903>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 124.Wan Q-H, Le XC. Anal. Chem. 1999;71:4183. doi: 10.1021/ac9902796. [DOI] [PubMed] [Google Scholar]
- 125.Wan Q-H, Le XC. Anal. Chem. 2000;72:5583. doi: 10.1021/ac000318+. [DOI] [PubMed] [Google Scholar]
- 126.LeCaptain DJ, Van Orden A. Analytical Chemistry. 2002;74:1171. doi: 10.1021/ac015647w. [DOI] [PubMed] [Google Scholar]
- 127.Ostergaard J, Jensen H. Analytical Chemistry. 2009;81:8644. doi: 10.1021/ac901419x. [DOI] [PubMed] [Google Scholar]
- 128.Gong M, Nikcevic I, Wehmeyer KR, Limbach PA, Heineman WR. Electrophoresis. 2008;29:1415. doi: 10.1002/elps.200700777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong MJ, Wehmeyer KR, Limbach PA, Heineman WR. Electrophoresis. 2007;28:837. doi: 10.1002/elps.200600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang ZZ, Wilkop T, Xu DK, Dong Y, Ma GY, Cheng Q. Analytical and Bioanalytical Chemistry. 2007;389:819. doi: 10.1007/s00216-007-1510-x. [DOI] [PubMed] [Google Scholar]
- 131.Cruz-Aguado JA, Penner G. Journal of Agricultural and Food Chemistry. 2008;56:10456. doi: 10.1021/jf801957h. [DOI] [PubMed] [Google Scholar]
- 132.Cary PD, Kneale GG. Methods in Molecular Biology. Vol. 543. Totowa, NJ, United States: 2009. p. 613. [DOI] [PubMed] [Google Scholar]
- 133.Romaniuk PJ. Journal of Biological Chemistry. 1990;265:17593. [PubMed] [Google Scholar]
- 134.Bock C, Coleman M, Collins B, Davis J, Foulds G, Gold L, Greef C, Heil J, Heilig JS, Hicke B, Hurst MN, Husar GM, Miller D, Ostroff R, Petach H, Schneider D, Vant-Hull B, Waugh S, Weiss A, Wilcox SK, Zichi D. Proteomics. 2004;4:609. doi: 10.1002/pmic.200300631. [DOI] [PubMed] [Google Scholar]
- 135.Kensch O, Connolly BA, Steinhoff HJ, McGregor A, Goody RS, Restle T. Journal of Biological Chemistry. 2000;275:18271. doi: 10.1074/jbc.M001309200. [DOI] [PubMed] [Google Scholar]
- 136.Gaillard C, Strauss F. BMC molecular biology. 2000;1:1. doi: 10.1186/1471-2199-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaouen S, De Koning L, Gaillard C, Muselikova-Polanska E, Stros M, Strauss F. J. Mol. Biol. 2005;353:822. doi: 10.1016/j.jmb.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 138.Drabovich AP, Berezovski M, Okhonin V, Krylov SN. Anal. Chem. 2006;78:3171. doi: 10.1021/ac060144h. [DOI] [PubMed] [Google Scholar]
- 139.Deng Q, German I, Buchanan D, Kennedy RT. Anal. Chem. 2001;73:5415. doi: 10.1021/ac0105437. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Lee HJ, Corn RM. Nucleic Acids Research. 2006;34:6416. doi: 10.1093/nar/gkl738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Potty ASR, Kourentzi K, Fang H, Jackson GW, Zhang X, Legge GB, Willson RC. Biopolymers. 2008;91:145. doi: 10.1002/bip.21097. [DOI] [PubMed] [Google Scholar]
- 142.Chang CC, Wu JY, Chien CW, Wu WS, Liu H, Kang CC, Yu LJ, Chang TC. Analytical Chemistry. 2003;75:6177. doi: 10.1021/ac034789i. [DOI] [PubMed] [Google Scholar]
- 143.Gokulrangan G, Unruh JR, Holub DF, Ingram B, Johnson CK, Wilson GS. Analytical Chemistry. 2005;77:1963. doi: 10.1021/ac0483926. [DOI] [PubMed] [Google Scholar]
- 144.Tsuji S, Tanaka T, Hirabayashi N, Kato S, Akitomi J, Egashira H, Waga I, Ohtsu T. Biochemical and Biophysical Research Communications. 2009;386:227. doi: 10.1016/j.bbrc.2009.06.014. [DOI] [PubMed] [Google Scholar]