Abstract
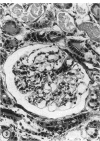
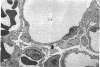
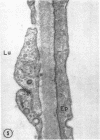
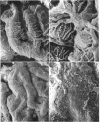
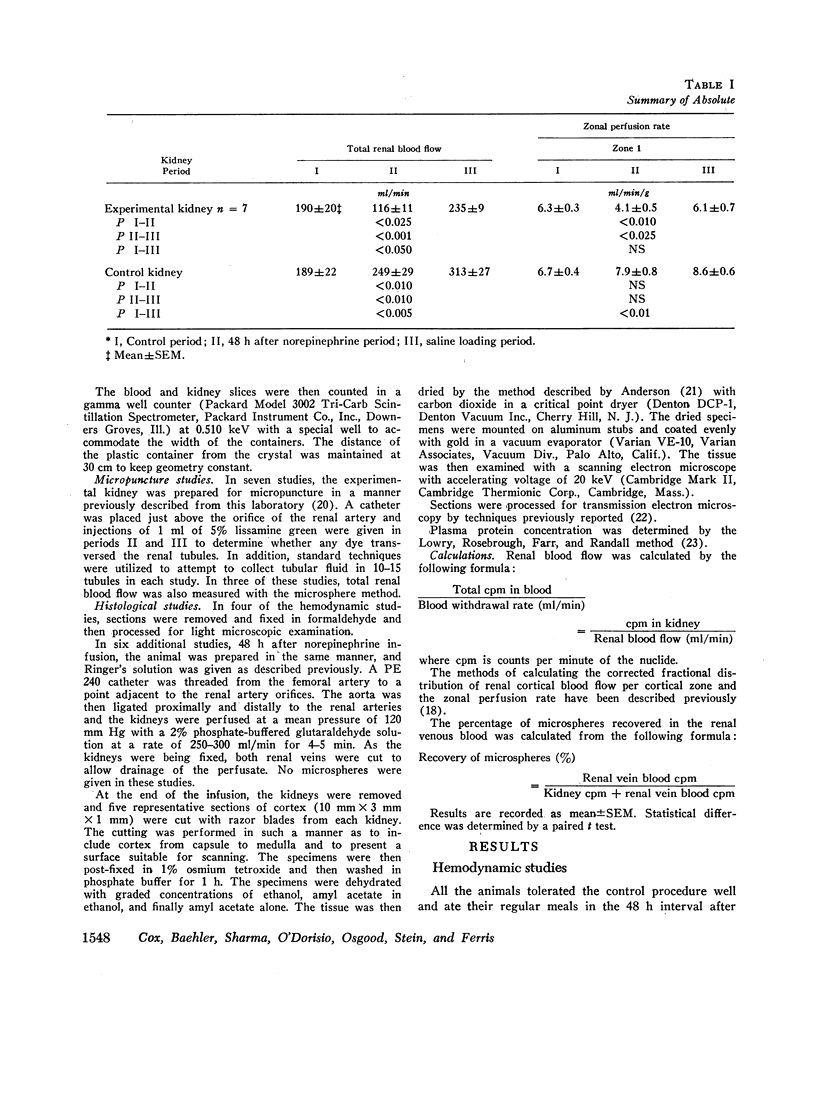
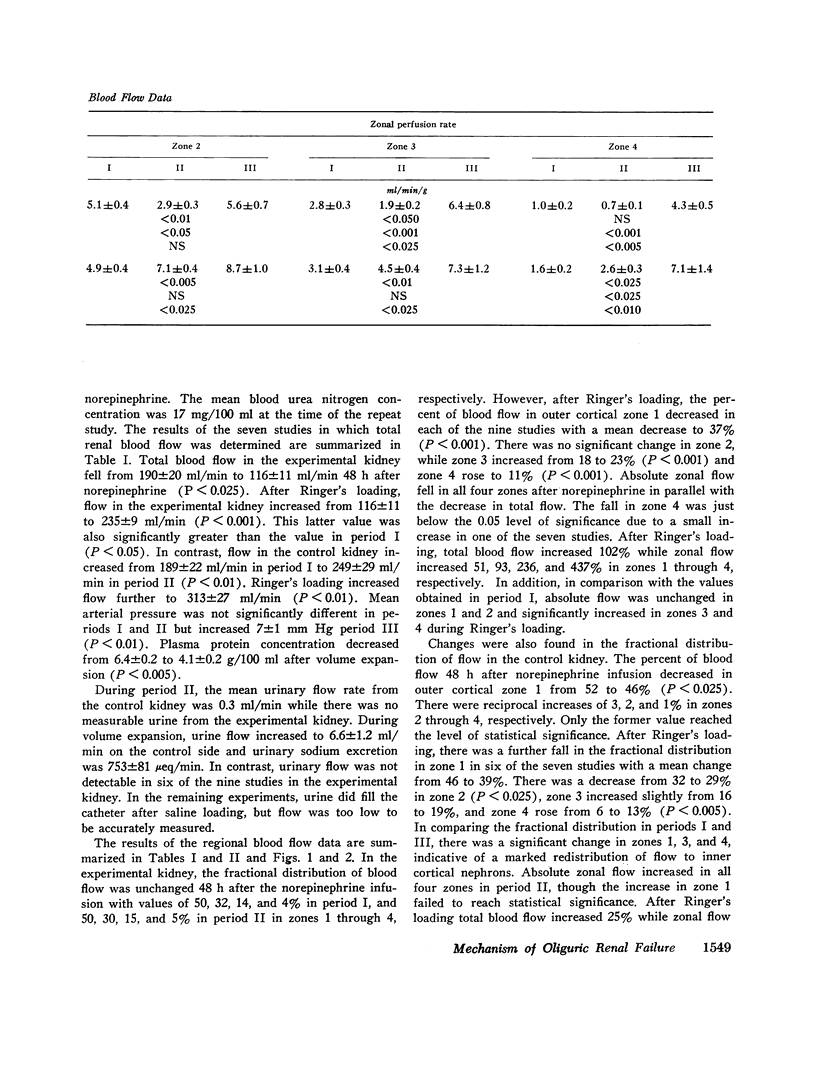
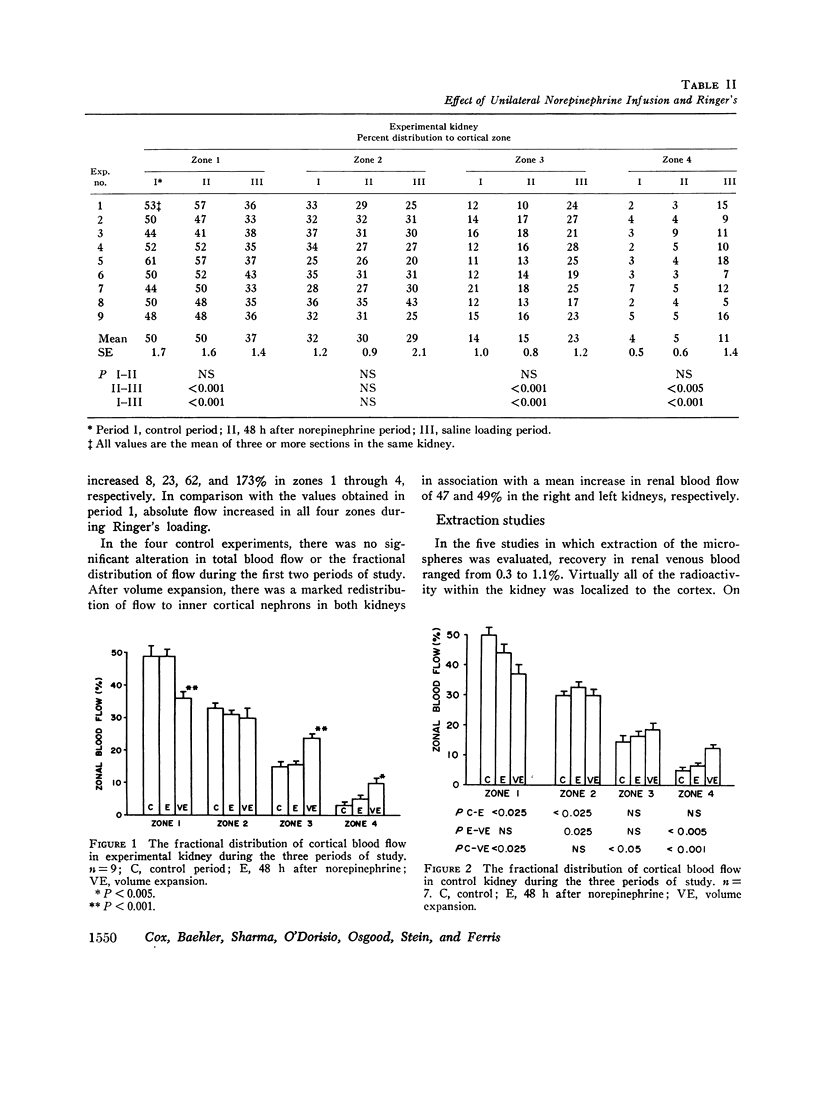
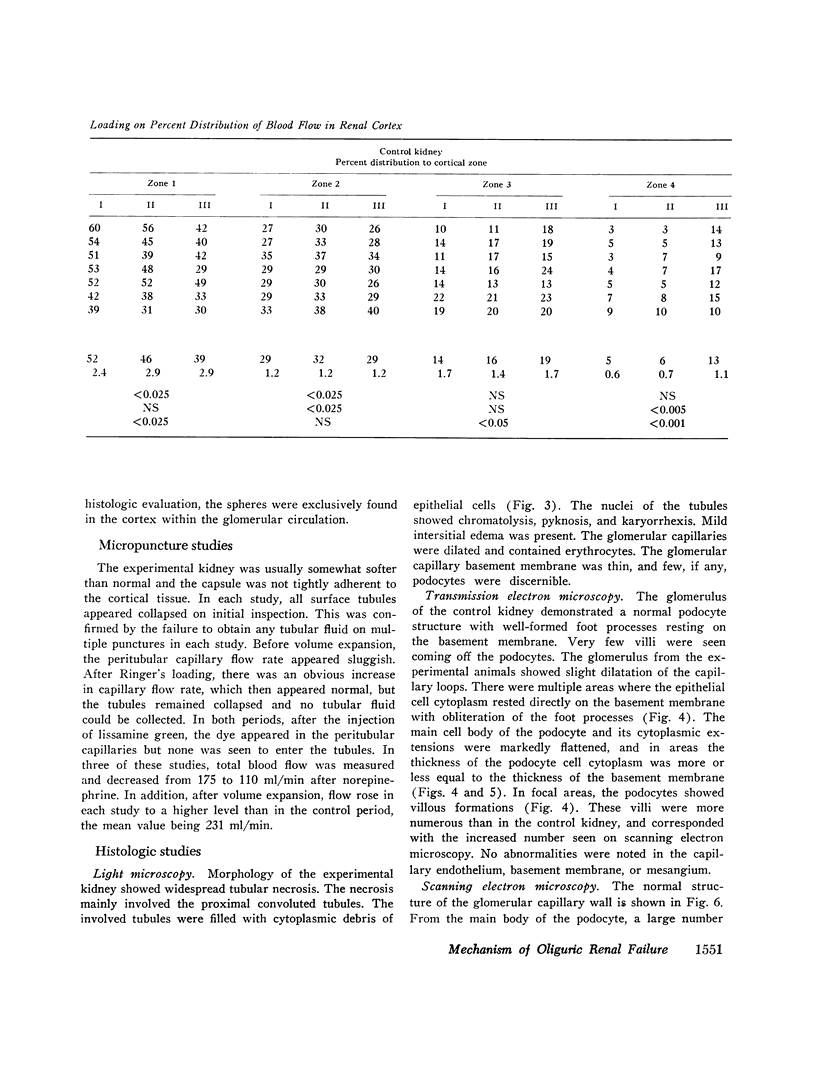
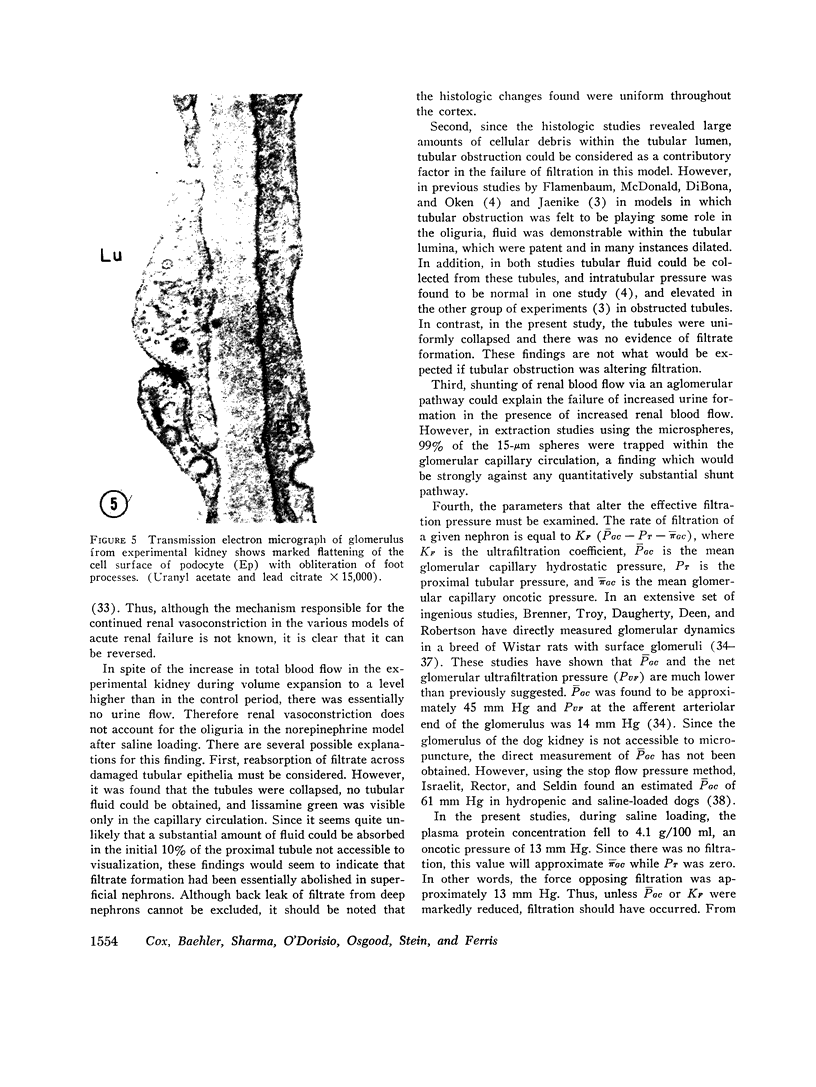
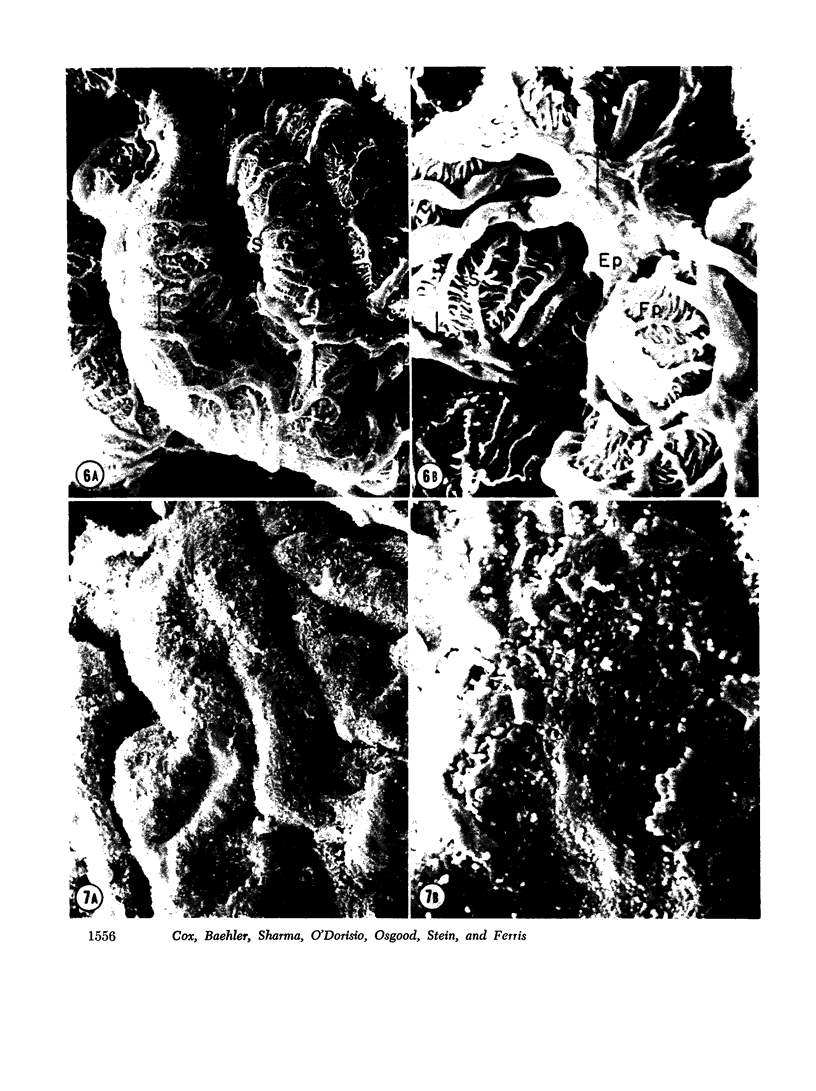
To further evaluate the mechanism of the oliguria of acute renal failure, a model was utilized in which intense and prolonged vasoconstriction produced the unilateral cessation of urine flow. The radioactive microsphere method was used to measure total and regional blood flow before and after the intrarenal infusion of norepinephrine, 0.75 μg/kg/min, for 2 h in the dog. In the control kidney, renal blood flow increased 32% 48 h after norepinephrine in association with a fall in the fractional distribution of flow to the outer cortex. In the experimental kidney, total renal blood flow fell from 190 ml/min before norepinephrine to 116 ml/min at 48 h (P < 0.025) with a uniform reduction in cortical blood flow. After the administration of 10% body wt Ringer's solution, there was a marked redistribution of flow to inner cortical nephrons in both the control and experimental kidney. In addition, there was a marked increase in total blood flow in both kidneys. On the experimental side, flow rose to 235 ml/min, a value greater than in either the control period (P < 0.05) or at 48 h after norepinephrine (P < 0.001). However, in spite of this marked increase in blood flow, there was essentially no urine flow from the experimental kidney. In separate studies, the animals were prepared for micropuncture. In all studies, the surface tubules were collapsed, and there was no evidence of tubular obstruction or leakage of filtrate. Over 99% of the 15-μM spheres were extracted in one pass through the experimental kidney. An analysis of the forces affecting filtration suggested that an alteration in the ultrafiltration coefficient may be responsible, at least in part, for the anuria in this model. In this regard, transmission and scanning electron microscopy revealed a marked abnormality in the epithelial structure of the glomerulus. It is suggested that a decrease in glomerular capillary permeability may be present in this model of acute renal failure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970 Nov;23(5):489–496. [PubMed] [Google Scholar]
- Ayer G., Grandchamp A., Wyler T., Truniger B. Intrarenal hemodynamics in glycerol-induced myohemoglobinuric acute renal failure in the rat. Circ Res. 1971 Aug;29(2):128–135. doi: 10.1161/01.res.29.2.128. [DOI] [PubMed] [Google Scholar]
- BRUN C., CRONE C., DAVIDSEN H. G., FABRICIUS J., HANSEN A. T., LASSEN N. A., MUNCK O. Renal blood flow in anuric human subject determined by use of radioactive Krypton 85. Proc Soc Exp Biol Med. 1955 Aug;89(4):687–690. doi: 10.3181/00379727-89-21917. [DOI] [PubMed] [Google Scholar]
- Bank N., Mutz B. F., Aynedjian H. S. The role of "leakage" of tubular fluid in anuria due to mercury poisoning. J Clin Invest. 1967 May;46(5):695–704. doi: 10.1172/JCI105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Katz M. A., Rector F. C., Jr, Seldin D. W. Measurement of intrarenal blood flow. II. Effect of saline diuresis in the dog. Am J Physiol. 1971 Jun;220(6):1914–1920. doi: 10.1152/ajplegacy.1971.220.6.1914. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971 Aug;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedru M. F., Baethke R., Oken D. E. Renal cortical blood flow and glomerular filtration in myohemoglobinuric acute renal failure. Kidney Int. 1972 Apr;1(4):232–239. doi: 10.1038/ki.1972.33. [DOI] [PubMed] [Google Scholar]
- DALGAARD O. Z. An electron microscopic study on glomeruli in renal biopsies taken from human shock kidney. Lab Invest. 1960 May-Jun;9:364–366. [PubMed] [Google Scholar]
- DEODHAR S. D., CUPPAGE F. E., GABLEMAN E. STUDIES ON THE MECHANISM OF EXPERIMENTAL PROTEINURIA INDUCED BY RENIN. J Exp Med. 1964 Oct 1;120:677–690. doi: 10.1084/jem.120.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech R. J., Hoffman J. I., Noble M. I., Saunders K. B., Henson J. R., Subijanto S. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969 Nov;25(5):581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- FLANIGAN W. J., OKEN D. E. RENAL MICROPUNCTURE STUDY OF THE DEVELOPMENT OF ANURIA IN THE RAT WITH MERCURY-INDUCED ACUTE RENAL FAILURE. J Clin Invest. 1965 Mar;44:449–457. doi: 10.1172/JCI105158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamenbaum W., McDonald F. D., DiBona G. F., Oken D. E. Micropuncture study of renal tubular factors in low dose mercury poisoning. Nephron. 1971;8(3):221–234. doi: 10.1159/000179923. [DOI] [PubMed] [Google Scholar]
- GOMORI P., MUNKACSI S., NAGY Z., TAKACS L., KALLAY K. Ischaemia and arteriovenous anastomoses of the kidney in shock, haemorrhage, dehydration and arterial hypoxia in dogs. Acta Med Acad Sci Hung. 1962;18:119–125. [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968 Apr;47(4):774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg N. K., Epstein M., Rosen S. M., Basch R. I., Oken D. E., Merrill J. P. Acute oliguric renal failure in man: evidence for preferential renal cortical ischemia. Medicine (Baltimore) 1968 Nov;47(6):455–474. doi: 10.1097/00005792-196811000-00001. [DOI] [PubMed] [Google Scholar]
- Hollenberg N. K., Sandor T., Conroy M., Adams D. F., Solomon H. S., Abrams H. L., Merrill J. P. Xenon transit through the oliguric human kidney: analysis by maximum likelihood. Kidney Int. 1973 Mar;3(3):177–185. doi: 10.1038/ki.1973.26. [DOI] [PubMed] [Google Scholar]
- Hornych H., Beaufils M., Richet G. The effect of exogenous angiotensin on superficial and deep glomeruli in the rat kidney. Kidney Int. 1972 Dec;2(6):336–343. doi: 10.1038/ki.1972.117. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. Micropuncture study of methemoglobin-induced acute renal failure in the rat. J Lab Clin Med. 1969 Mar;73(3):459–468. [PubMed] [Google Scholar]
- Jaenike J. R. The renal lesion associated with hemoglobinemia: a study of the pathogenesis of the excretory defect in the rat. J Clin Invest. 1967 Mar;46(3):378–387. doi: 10.1172/JCI105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R., Hollenberg N. K., Busch G. J., Abrams H. L. Prolonged unilateral acute renal failure induced by intra-arterial norepinephrine infusion in the dog. Invest Radiol. 1972 May-Jun;7(3):164–173. doi: 10.1097/00004424-197205000-00006. [DOI] [PubMed] [Google Scholar]
- Ladefoged J., Winkler K. Hemodynamics in acute renal failure. The effect of hypotension induced by dihydralazine on renal blood flow, mean circulation time for plasma, and renal vascular volume in patients with acute oliguric renal failure. Scand J Clin Lab Invest. 1970 Aug;26(1):83–87. doi: 10.3109/00365517009049218. [DOI] [PubMed] [Google Scholar]
- McNay J. L., Abe Y. Redistribution of cortical blood flow during renal vasodilatation in dogs. Circ Res. 1970 Dec;27(6):1023–1032. doi: 10.1161/01.res.27.6.1023. [DOI] [PubMed] [Google Scholar]
- McNay J. L., Miyazaki M. Regional increases in mass and flow during compensatory renal hypertrophy. Am J Physiol. 1973 Jan;224(1):219–222. doi: 10.1152/ajplegacy.1973.224.1.219. [DOI] [PubMed] [Google Scholar]
- O'Dorisio T. M., Stein J. H., Osgood R. W., Ferris T. F. Absence of aglomerular blood flow during renal vasodilatation and hemorrhage in the dog. Proc Soc Exp Biol Med. 1973 Jul;143(3):612–615. doi: 10.3181/00379727-143-37377. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Arce M. L., Wilson D. R. Glycerol-induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J Clin Invest. 1966 May;45(5):724–735. doi: 10.1172/JCI105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector J. B., Stein J. H., Bay W. H., Osgood R. W., Ferris T. F. Effect of hemorrhage and vasopressor agents on distribution of renal blood flow. Am J Physiol. 1972 May;222(5):1125–1131. doi: 10.1152/ajplegacy.1972.222.5.1125. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Rous S. N., Wakim K. G. Kidney function before, during and after compensatory hypertrophy. J Urol. 1967 Jul;98(1):30–35. doi: 10.1016/S0022-5347(17)62817-9. [DOI] [PubMed] [Google Scholar]
- SELLERS A. L., SMITH S., 3rd, MARMORSTON J., GOODMAN H. C. Studies on the mechanism of experimental proteinuria. J Exp Med. 1952 Dec;96(6):643–652. doi: 10.1084/jem.96.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. M., Rosensweig J., Chatterjee S., Moore S., De Champlain M. L. Platelets in hyperacute rejection of heterotopic cardiac allografts in presensitized dogs. An experimental study. Am J Pathol. 1973 Feb;70(2):155–174. [PMC free article] [PubMed] [Google Scholar]
- Stein J. H., Ferris T. F., Huprich J. E., Smith T. C., Osgood R. W. Effect of renal vasodilatation on the distribution of cortical blood flow in the kidney of the dog. J Clin Invest. 1971 Jul;50(7):1429–1438. doi: 10.1172/JCI106626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. H., Osgood R. W., Ferris T. F. Effect of volume expansion on distribution of glomerular filtrate and renal cortical blood flow in the dog. Am J Physiol. 1972 Oct;223(4):984–990. doi: 10.1152/ajplegacy.1972.223.4.984. [DOI] [PubMed] [Google Scholar]
- Steinhausen M., Eisenbach G. M., Helmstädter V. Concentration of Lissamine green in proximal tubules of antidiuretic and mercury poisoned rats and the permeability of these tubules. Pflugers Arch. 1969;311(1):1–15. doi: 10.1007/BF00588058. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Mostofi F. K. Electron microscopic studies of acute tubular necrosis. Early changes in the glomeruli of rat kidney after subcutaneous injection of glycerin. Lab Invest. 1970 Jul;23(1):8–14. [PubMed] [Google Scholar]