Abstract
Recent data show that regulatory cells with transforming growth factor (TGF)-β1-dependent activity are able to restore self-tolerance in overtly diabetic non-obese diabetic (NOD) mice. Thus, TGF-β1 seems to have a relevant role in protection from autoimmune diabetes. Our aim was to investigate the possible significance of serum TGF-β1 measurement in the natural history of diabetes in NOD mice, as well as in children positive for at least one islet-related antibody. Serum TGF-β1 (both total and active) was measured by enzyme-linked immunosorbent assay at monthly intervals in 26 NOD mice during the spontaneous development of diabetes and, on a yearly basis, in nine siblings of patients with type 1 diabetes (T1D) with a follow-up of 4 years. Diabetes appeared between the 12th week of age and the end of the study period (36 weeks) in 17 mice. TGF-β1 serum level variations occurred in the prediabetic period in both NOD mice and humans and diabetes diagnosis followed a continuing reduction of active TGF-β1 (aTGF-β1) serum levels. In mice, aTGF-β1 serum levels measured at 4 weeks of age correlated positively with severity of insulitis, and negatively with percentage of insulin-positive cells. Our findings suggest that in NOD mice serum TGF-β1 levels during the natural history of the diabetes reflect the course of islet inflammation. The measurement of aTGF-β1 in islet-related antibody-positive subjects may provide insights into the natural history of prediabetic phase of T1D.
Keywords: insulitis, NOD mice, prediabetes, TGF-β1, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by the presence of islet-related autoantibodies (IrAbs) and insulitis with progressive loss of beta cells that leads ultimately to overt diabetes [1]. The most recent evidence suggests that impaired regulation of self-reactive immune responses may be the result of abnormal activities of a population of regulatory T cells (Tregs), distinguished by their expression of CD25 molecules and forkhead box P3 (FoxP3) [2]. Tregs are dependent for their action upon the presence of transforming growth factor β1 (TGF-β1), which also regulates their expansion [3,4].
The non-obese diabetic (NOD) mouse develops a spontaneous form of autoimmune diabetes that mimics many features of the human disease [5]. In this model, evidence for the presence of regulatory cells comes from transfer experiments of selected lymphocyte populations which demonstrated the ability of the transferred cells to modulate the onset of diabetes [6,7], and TGF-β1 in both the soluble and membrane-bound form seems to play a relevant role in protection from autoimmune diabetes [8–10].
TGF-β1 is secreted as an inert precursor molecule and is converted to its biologically active form extracellularly [11]. This activation may be mediated by macrophages in the inflammatory sites [12]. Both active TGF-β1 (aTGF-β1) and inactive TGF-β1 are present in the blood [13]. In the present study we measured serum TGF-β1 levels, both total (aTGF-β1 plus inactive TGF-β1) and active alone, at monthly intervals in a group of NOD mice observed during the spontaneous development of diabetes. We also measured serum TGF-β1 levels (both total and active) in nine siblings of patients with T1D who were positive for at least one IrAb (high-risk children). TGF-β1 is well conserved between the species [11]. Therefore, the same enzyme-linked immunosorbent assay (ELISA) can be used for the measurement performed on both the human and mouse serum samples, making comparison between the species more valuable. Our aim was to investigate the possible significance of serum TGF-β1 measurement in the natural history of T1D.
Methods
Mice
Four-week-old female NOD mice were obtained from Charles River Laboratories (Calco, Milan, Italy) and housed under specific pathogen-free conditions in the animal facility at the Istituto Superiore di Sanità in Rome. Animals had free access to water and food. All studies were approved by the Animal Care and Use Committee of the Istituto Superiore di Sanità and by the Italian Ministry of Health. One group of animals (n = 26) was observed up to 36 weeks of age. During the study period, mice were monitored for diabetes onset by measuring blood glucose twice weekly. Sera were collected at entry to the study (at 4 weeks of age) and subsequently at monthly intervals until diagnosis of diabetes or up to the 36th week of age in mice remaining euglycaemic. Sera were stored at −80°C until assayed for the measurement of serum TGF-β1 levels. In a second group, 4-week-old female NOD mice (n = 10) were monitored for 2 months as described above and killed at 12 weeks of age. Pancreases were removed for evaluation of insulitis and beta cell content.
An additional group of 10 female C57BL/6 mice (Harlan Laboratories, S. Pietro al Natisone, Udine, Italy) was also evaluated as non-T1D-prone mice. In these mice sera were collected at monthly intervals at 12, 16 and 20 weeks of age.
Diagnosis of diabetes
Non-fasting whole blood glucose was measured in all animals twice a week using a glucometer (Medisense; Abbott Laboratories, Abbott Park, IL, USA) and reagent strips. In non-diabetic NOD mice, non-fasting blood glucose ranges from 3 to 8 mmol/l. Diabetes was defined as two consecutive readings above 12 mmol/l.
Histology and immunohistochemistry
The pancreases from killed mice were removed and snap-frozen in liquid nitrogen. Sections of 4 µm were cut 40 µm apart throughout the gland and stained with haematoxylin and eosin (H&E; Merck, Whitehouse Station, NJ, USA). Insulitis was scored according to the insulitis score, as described previously [14]. At least 30 islets per pancreas were analysed by two independent observers. Insulin staining and histomorphometric analysis were performed on 4-µm cryostatic pancreatic sections, as described previously [14].
Subjects
Nine siblings of patients with T1D, positive for at least one IrAb (high-risk children) were enrolled into the study and followed-up regularly. They were part of a family study, which included about 220 families. Their sera were collected on a yearly basis for 4 years and stored at the Pediatric Department of the Gaslini Research Institute in Genova and at the Policlinico S. Matteo in Pavia, where the nine children were followed. Serum samples were collected in the morning from fasted children according to a standardized procedure. Five (two boys and three girls) of these nine high-risk children developed T1D within the first 4 years of follow-up, while the remaining four did not. For each patient, sex, age, body mass index (BMI) and type and time of appearance of IrAbs are given in Table 1. Diabetes was diagnosed according to World Health Organization/American Diabetes Association criteria [15].
Table 1.
Distribution and type of islet-related autoantibodies (IrAbs) in the nine siblings of patients with type 1 diabetes (T1D)
| Sex | Age† | BMI† | GADA | IA–2A | GADA & IAA | GADA & IA–2A | GADA & IAA & IA–2A | |
|---|---|---|---|---|---|---|---|---|
| Diabetes progressor siblings (sibling) | ||||||||
| 1 | Male | 13 | 20 | + | (+) | |||
| 2 | Female | 13 | 16 | (+) | + | |||
| 3 | Female | 6 | 15·5 | + | ||||
| 4 | Female | 5 | 17·1 | + | ||||
| 5 | Male | 16 | 15·3 | + | ||||
| Diabetes-free siblings (sibling) | ||||||||
| 6 | Male | 5 | 16 | + | [+] | |||
| 7 | Male | 14 | 16 | + | ||||
| 8 | Female | 5 | 17 | + | ||||
| 9 | Male | 9 | 16 | + | ||||
At the first observation. ( ) Positivity acquired 1 year after entry into the study; [] positivity acquired 2 years after entry into the study. BMI: body mass index; GADA: glutamic acid decarboxylase autoantibodies; IAA: insulin autoantibodies.
As an additional control we analysed two serial serum samples, collected at a 6-year interval, from 12 IrAb-negative children [five boys and seven girls; age at first sample collection: 11 ± 3 years; mean ± standard deviation (s.d.)] who did not develop T1D during the observation period. Children were monitored for 7 years for the eventual development of T1D by data crossing with the Sardinian Eurodiab Register of T1D. The samples were from a sera repository established during a previous study aimed at assessing the prevalence of IrAbs in a cohort of healthy school children, recruited from a population with a high incidence of T1D [16]. Informed consent was obtained from the children's parents authorizing storage of the sera and their possible utilization in other studies aimed at investigating the aetiopathogenesis of T1D.
Islet-related autoantibodies
Glutamic acid decarboxylase autoantibodies (GADA) and IA–2A were measured by radiobinding assay using in vitro-transcribed and translated recombinant human protein, as described previously [17]. Insulin autoantibodies (IAA) were measured using commercial kits (CISBio-international, Bagnol/Ceze, France). Results were considered positive when > 3·0 U/ml for GADA, > 1·0 U/ml for IA-2 and > 10% for IAA.
TGF-β1 – ELISA detection
Total TGF-β1 (tTGF-β1 = circulating active + inactive TGF-β1) and aTGF-β1 (circulating TGF-β1 already activated in vivo) serum concentrations were assessed blind in human and mouse sera in duplicate by ELISA (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. For the detection of tTGF-β1, serum samples were acidified and then neutralized before assay. For the detection of serum aTGF-β1, the activation procedure (acidification) was avoided. The minimum detectable amount of TGF-β1 was less than 7 pg/ml; the variability of the duplicates was less than 7% of the mean value. The interassay variability was < 12% for aTGF-β1 and < 9% for tTGF-β1.
Statistical analysis
Significant differences between groups were determined using parametric tests, non-parametric tests and contingency tables analysis, as appropriate. Values are represented as mean ± standard error of the mean (s.e.m.). All statistical analyses were performed using the Stata Statistical Software release 8·0. For all analyses a P-value < 0·05 was considered significant.
Results
NOD mice studies
TGF-β1 serum levels during diabetes development in NOD mice
Diabetes appeared between the 12th week of age and the end of the study period (36 weeks of age) in 17 of 26 mice. Mean TGF-β1 serum levels (active and total) in the groups of diabetes-free and diabetes progressor mice are shown in Fig. 1. The analysis of serum TGF-β1 in the group of diabetes-free mice (Fig. 1a and b) showed, between the 4th and 8th weeks of age, a significant increase in aTGF-β1 and a significant reduction in tTGF-β1 values at 8 weeks of age when compared to the values at 4 weeks of age. This increase/decrease was transient and followed by non-significant variations of both aTGF-β1 and tTGF-β1 between the 12th and 20th weeks of age. A second significant increase of aTGF-β1, associated this time with a significant increase of tTGF-β1, was seen between the 20th and 28th weeks of age followed by a significant reduction of the values in the following weeks.
Fig. 1.
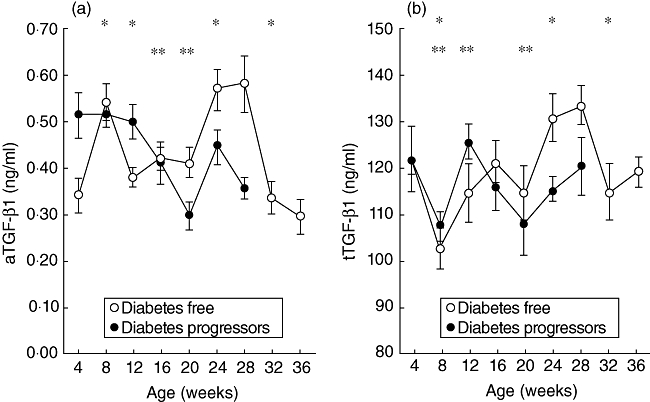
Serum levels of active transforming growth factor (aTGF)-β1 (a) and total TGF (tTGF)-β1 (b) in diabetes-free mice (○n = 9) and diabetes progressor mice (n = 17). Lines represent mean ± standard error of the mean. (a) *(Diabetes-free mice) = P < 0·05; 4 versus 8 weeks of age, 8 versus 12 weeks of age, 20 versus 24 weeks of age and 28 versus 32 weeks of age. **(Diabetes progressor mice) = P < 0·05; 12 versus 16 weeks of age and 12 versus 20 weeks of age. (b) *(Diabetes-free mice) = P < 0·05; 4 versus 8 weeks of age, 20 versus 24 weeks of age and 28 versus 32 weeks of age. **(Diabetes progressor mice) = P < 0·05; 4 versus 8 weeks of age, 8 versus 12 weeks of age and 16 versus 20 weeks of age, by Wilcoxon's signed rank test.
TGF-β1 serum levels in the group of diabetes progressor mice were more heterogeneous than those observed in the diabetes-free group. While tTGFβ1 serum levels in progressors showed a similar variation during the first 8 weeks of observation to that seen in diabetes-free mice (Fig. 1b), the progressor mice showed a greater heterogeneity in the variations of serum levels of aTGF-β1 in the same time-period (see below), so that apparently no significant difference was observable in the aTGF-β1 values in the first 8 weeks of observation (Fig. 1a). However, a significant continuing reduction (two sequential observations) in the values of aTGF-β1 between the 12th and 20th weeks of age was observed, indicating more homogeneous variations of aTGF-β1 values in this group of mice during this period (Fig. 1a). In the same period, tTGF-β1 values also showed a trend towards a reduction, but the variation was only significant at the 20th week of age when compared to the values observed at the 16th week of age.
Comparing the aTGF-β1 values between the two groups of mice (diabetes-free and diabetes progressors) at different time-points (Table 2), it appears that the diabetes progressors show significantly increased values of aTGF-β1 at the 4th week and at the 12th week of age when compared to the values observed in the diabetes-free mice; while at 20 and 28 weeks of age the diabetes progressor mice show a reduction of aTGF-β1 compared to the diabetes-free group. With regard to tTGF-β1 values, the only significant difference between the two groups was observed at 24 weeks of age when the values of tTGF-β1 were significantly lower in diabetes progressors than in diabetes-free mice (mean values: 114 ± 2·60 versus 130 ± 5·17 ng/ml, P = 0·02). Because the aTGFβ1 serum levels appeared to differentiate the diabetes progressor group more effectively from the diabetes-free mice than did the tTGF-β1 variations, the aTGF-β1 serum levels were analysed in greater detail.
Table 2.
Active transforming growth factor (aTGF)-β1 values (ng/ml) in diabetes-free and diabetes progressor mice at different weeks of age
| Age (weeks) | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 |
|---|---|---|---|---|---|---|---|---|---|
| Diabetes-free | |||||||||
| Mean ± s.e.m. | 0·34 ± 0·04 | 0·54 ± 0·04 | 0·38 ± 0·02 | 0·42 ± 0·02 | 0·41 ± 0·03 | 0·57 ± 0·04 | 0·58 ± 0·06 | 0·33 ± 0·03 | 0·29 ± 0·04 |
| n | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Diabetes progressors | |||||||||
| Mean ± s.e.m. | 0·51 ± 0·05 | 0·52 ± 0·03 | 0·50 ± 0·04 | 0·41 ± 0·04 | 0·30 ± 0·03 | 0·45 ± 0·04 | 0·36 ± 0·02 | ||
| n | 17 | 17 | 17 | 16 | 11 | 7 | 3 | ||
| P (Mann–Whitney U-test) | 0·02 | 0·70 | 0·04 | 0·33 | 0·05 | 0·11 | 0·06 | ||
s.e.m.: standard error of the mean.
We performed an additional analysis of the first 8 weeks of observation (from the 4th to the 12th weeks of age) and divided the diabetes progressors into two groups: mice with a decrease or no variation in serum aTGF-β1 (Fig. 2, upper panel, group A) and mice in which an increase in serum aTGF-β1 is observable between the 4th and the 8th weeks of age (Fig. 2, upper panel, group B). The first group of diabetes progressor mice (group A) showed significantly higher values of aTGF-β1 at 4 weeks of age when compared to the values found in diabetes-free mice (0·63 ± 0·08 versus 0·34 ± 0·04 ng/ml, P = 0·002). Group B showed higher values of aTGF-β1 at 12 weeks of age when compared to diabetes-free mice (0·51 ± 0·06 versus 0·38 ± 0·02 ng/ml P = 0·03). Looking at the time of diabetes onset, all the mice but one in group B developed diabetes after the 16th week of age (late onset), while five of eight mice (62·5%) in group A developed diabetes by the 16th week of age (early onset) (P = 0·04; Fig. 2, lower panel).
Fig. 2.
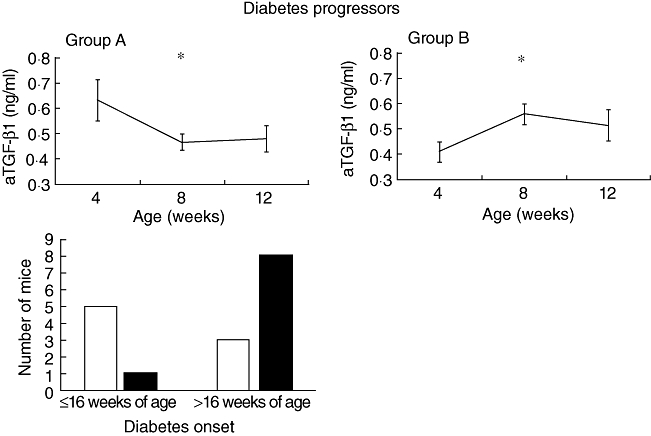
Upper panel: serum active transforming growth factor (aTGF)-β1 during the first 8 weeks of observation in diabetes progressor mice. Group A (n = 8): diabetes progressor mice that show between the 4th and 8th weeks of age reduction or no variation of the aTGF-β1 values. Group B (n = 9): diabetes progressor mice that show an increase of the aTGF-β1 values between the 4th and 8th weeks of age. Lines represent mean ± standard error of the mean. Group A: *P = 0·01, 4 versus 8 weeks of age; group B: *P < 0·01, 4 versus 8 weeks of age by Wilcoxon's signed rank test. Lower panel: distribution of mice belonging to group A (□) and B ( ) according to time of diabetes onset. P = 0·04 by Fisher's exact test.
) according to time of diabetes onset. P = 0·04 by Fisher's exact test.
Association of serum aTGF-β1 levels with grade of insulitis and beta cell loss
We tested the hypothesis that TGF-β1 serum levels could be associated with the inflammatory process in the pancreas. To this end, in a different group of mice (see Methods), we measured serum aTGF-β1 levels and analysed the pancreas for the presence and grade of insulitis and number of insulin-producing β cells in the islets. All the mice were euglycaemic. The grade of insulitis was evaluated in each mouse together with the number of insulin-positive cells, and the results were analysed in relation to aTGF-β1 serum levels at 4, 8 and 12 weeks of age. As shown in Fig. 3, aTGF-β1 serum levels at 4 weeks of age were correlated directly with insulitis (grades 2–3) and correlated inversely with the content of insulin-producing cells in the islets. No correlation was found between aTGF-β1 serum levels at 8 and 12 weeks of age and insulitis or insulin-producing cells. However, when we divided this additional group of mice into two groups, as in Fig. 2, i.e. mice with a decrease or no variation in serum aTGF-β1 (group A) and mice in which an increase in serum aTGF-β1 was observable (group B) between the 4th and the 8th weeks of age, we found that insulin-positive cells represented 39·6 ± 8·7% of the islet area in mice of group A and 75 ± 8·7% in mice of group B (P = 0·02). The mean prevalence of grades 2–3 insulitis in group A was 65·3 ± 15·8% and 38 ± 14% in group B (P = 0·04) (Fig. 3c and d).
Fig. 3.
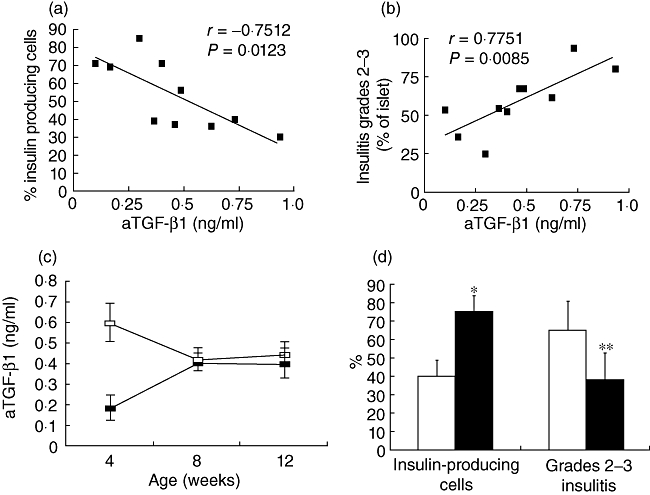
Serum active transforming growth factor (aTGF)-β1 levels correlate in non-obese diabetic (NOD) mice with beta cell loss and insulitis. (a) Correlation between serum aTGF-β1 values at 4 weeks of age and insulin-producing cells evaluated at 12 weeks of age (n = 10); r = −0·7512, P = 0·0123. Insulin staining was performed on 4-µm paraffin-embedded pancreatic sections by incubation with guinea pig anti-porcine insulin primary polyclonal antibody (Dako Corporation, Carpinteria, CA, USA), followed by incubation with peroxidase-conjugated rabbit anti-guinea pig secondary antibody (Dako Corporation). The colour was revealed using the 3,3'-diaminobenzidine revelation system (Vector kit; Vector Laboratory, Burlingame, CA, USA). Histomorphometric analysis of insulin-stained pancreatic sections was carried out with an interactive image analyser (IAS 2000; Delta Sistemi, Rome, Italy) on at least two to three 4-µm sections from each animal. The sections were cut at intervals of ∼40 µm, and the analysis consists in the evaluation of the area of the Langerhans islets occupied by beta-cells (insulin-positive stained cells), in percentage ratio of the total area of the same islets. (b) Correlation between serum aTGF-β1 values at 4 weeks of age and grades 2–3 insulitis evaluated at 12 weeks of age (n = 10); r = 0·7751, P = 0·0085. Sections of 4 µm were cut 40 µm apart throughout the gland and stained with haematoxylin and eosin (H&E; Merck, Whitehouse Station, NJ, USA) for evaluation of the insulitis score using the following scale: 0, intact islet; 1, peri-insulitis; 2, moderate insulitis (< 50% of the islet infiltrated); 3, severe insulitis (≥ 50% of the islet infiltrated). (c) Mean values ± standard error of the mean of aTGF-β1 at the 4th, 8th and 12th weeks of age in the group of mice killed at 12 weeks of age. Mice belonging to group A (□) showed a decrease of aTGF-β1 values between 4 and 8 weeks of age, while mice belonging to group B ( ) showed an increase of aTGF-β1 values. (d) Percentage of insulin-containing cells and insulitis score in groups A (□) and B (
) showed an increase of aTGF-β1 values. (d) Percentage of insulin-containing cells and insulitis score in groups A (□) and B ( ). *P = 0·02 group A versus group B; **P = 0·04 group A versus group B, by Mann–Whitney U-test.
). *P = 0·02 group A versus group B; **P = 0·04 group A versus group B, by Mann–Whitney U-test.
Human studies
We carried out a preliminary investigation on the possibility that aTGF-β1 changes in the prediabetic phase might also occur in humans. For this purpose we analysed 20 serum samples which were positive for at least one IrAb and 40 serum samples negative for IrAb from children monitored for 7 years for the eventual development of T1D by data-crossing with the Sardinian Eurodiab Register of T1D. The samples were from a sera repository established during a previous study aimed at assessing the prevalence of IrAbs in a cohort of healthy school children, recruited from a population with a high incidence of T1D [16]. Full details of this study have been reported elsewhere [18]. None of the 40 IrAbs− children developed diabetes. Among the 20 samples positive for at least one IrAb, 10 were obtained from children who developed T1D at different time-points from the sample collection (1–7 years). While mean active and total TGF-β1 values did not differ between children who did not develop diabetes and those who did, the highest aTGF-β1 values (0·32, 0·33, 0·33 ng/ml) were observed in sera of three children who developed diabetes within 2–3 years from sample collection, suggesting that changes in serum aTGF-β1, related in some way to diabetes onset, might also be present in humans (see Supporting information at end, Fig. S1). Therefore, we measured serum aTGF-β1 in a different cohort of nine children who were siblings of patients with T1D with repeated (yearly) serum collection (see Methods).
Of these nine high-risk siblings, five developed T1D within 4 years of the beginning of follow-up and four did not. Serum aTGF-β1 profiles for all nine children are shown in Fig. 4. In the five children who progressed to T1D, the highest values of aTGF-β1 were found 3 years before clinical onset of the disease, followed by a sustained and progressive decline up to the time of diagnosis (Fig. 4a).
Fig. 4.
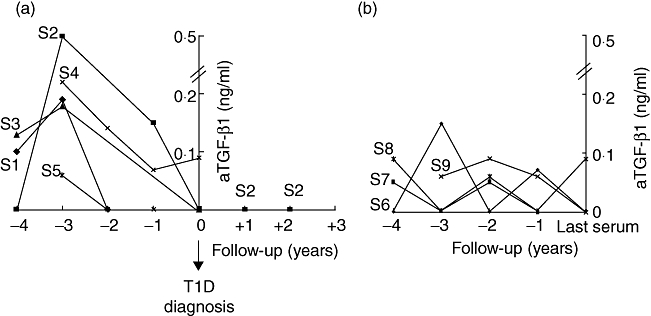
Individual profiles of serum active transforming growth factor (aTGF)-β1 values observed in the five children who developed type 1 diabetes (T1D) during the follow-up period (a) and in the four children who did not (b).
The four high-risk children who did not develop T1D showed multiple mild variations of serum aTGF-β1 during the follow-up period. In this group, only sibling 9 showed a progressive, slight reduction in serum aTGF-β1 levels during the last 2 years of follow-up (Fig. 4b). With regard to individual serum tTGF-β1 profiles, neither group of children showed any substantial modification during the follow-up period (data not shown).
aTGF-β1 values in a non-T1D prone mouse strain and IrAb–/T1D– children
As an additional control we evaluated the serum aTGF-β1 values in a non-autoimmune-prone mouse strain. We performed serial determinations in C57BL/6 mice at 12, 16 and 20 weeks of age, as diabetes progressor mice showed a significant progressive reduction (two sequential observations) in this period in the values of aTGF-β1. As shown in Fig. 5a, during the observation period C57BL/6 mice did not show two consecutive reductions of aTGF-β1 values as found in NOD progressor mice. Comparing the aTGF-β1 values between the groups of NOD mice (diabetes-free and diabetes progressors) and the C57BL/6 control group, it appears that, at the 20th week of age, diabetes progressors show significantly lower values of aTGF-β1 than those observed in the control mice (NOD diabetes progressor mice: 0·30 ± 0·03 versus control mice: 0·51 ± 0·07, mean ± s.e.m.; P = 0·01).
Fig. 5.
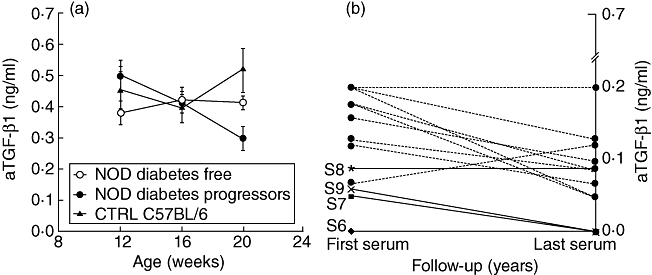
Serum levels of active transforming growth factor (aTGF)-β1 in diabetes-free mice (○n = 9), diabetes progressor mice (•n = 17) and C57BL/6 mice (▴n = 10). Lines represent mean ± standard error of the mean (a). Individual profiles of serum aTGF-β1 values observed in four islet-related autoantibody (IrAb+) siblings who did not develop type 1 diabetes (T1D) during the follow-up period (solid line) and in 12 IrAb–/T1D– children from general population (broken line).
Values observed in IrAb–/T1D– children, although with the limitation of the availability of only two serial serum samples, showed variations similar to those observed in the high-risk IrAb+/T1D− siblings (Fig. 5b).
Discussion
In this study, serum concentrations of the biologically active form of TGF-β1 were measured during the prediabetic phase in a group of female NOD mice as well as in a group of siblings of patients with T1D. A significant relationship between serum aTGF-β1 variations and progression to diabetes was observed in both the animal model and children. Specifically, in the NOD mice, a decrease of aTGF-β1 values in two consecutive observations between the 12th and 20th weeks of age was associated with diabetes onset, while in the same period diabetes-free mice showed relatively stable values, as also observed in the non-diabetes prone strain of C57BL/6 mice. Taken together, these findings demonstrate that aTGF-β1 fluctuations (two consecutive reductions) are associated specifically with the progression to diabetes.
At earlier time-points (4–8 weeks of age) early diabetes progressor mice showed a significant reduction of aTGF-β1 values, while late diabetes progressors and diabetes-free mice showed an increase of the aTGF-β1 values at 8 weeks of age. These observations suggest that serum aTGF-β1 values could be linked to the occurrence and severity of islet inflammation. This hypothesis is supported by the fact that, in the group of mice killed at 12 weeks of age, their aTGF-β1 values at 4 weeks of age correlated directly with the severity of insulitis and inversely with the presence of insulin-producing cells, and that the reduction of aTGF-β values between the 4th and 8th weeks of age was associated with a higher prevalence of grades 2–3 insulitis compared to the mice with an increase of aTGF-β in the same time-period. Thus, serial evaluation of aTGF-β values in the same subject may be helpful in the monitoring of the prediabetic phase, where a time-dependent reduction of the aTGF-β values is associated with destructive insulitis. Furthermore, due to the different progression of insulitis in each mouse, the single aTGF-β values are less informative than the serial values observed in each mouse during the time of observation. Indeed, depending on the progression of insulitis in each mouse, statistically significant differences are found intermittently and the absolute value of aTGF-β might be informative on the course of insulitis only at a very early time-point (4 weeks of age).
The above-described observations suggest that the peaks of serum aTGF-β1 may indicate the presence of waves of inflammation that could be counteracted (successfully or unsuccessfully) by regulatory mechanisms. In this context, relatively stable levels of aTGF-β1 may be a sign of efficient control of the inflammation, while progressive reduction of such values may reflect the shift from controlled to destructive insulitis. According to this hypothesis, in diabetes-free mice the additional late peak of aTGF-β1 observable at 24–28 weeks of age followed by a continuing decrease of the values between the 28th and 36th weeks of age could represent a second wave of islet inflammation that leads eventually to the onset of diabetes. Interpretation of the data collected in NOD mice is supported to a certain extent by the natural history of insulitis in this model. In the NOD mouse, the immune infiltrate of the islets persists for weeks before the onset of overt diabetes, suggesting the presence of immunoregulatory mechanisms (controlled insulitis). In addition, recent data suggest that insulitis may indeed have a relapsing/remitting course as opposed to a continuous course [19,20].
Similarly to NOD mice, variations in serum aTGF-β1 levels can be seen in the prediabetic phase of T1D in high-risk siblings of patients with T1D. Specifically, longitudinal observations demonstrated that, 3 years before overt diabetes, the highest detected values of aTGF-β1 were always followed by a sustained and progressive decreasing trend. Repeated measurements of aTGF-β1 in sera obtained in studies involving newborn offspring of parents with T1D [21], siblings of type 1 diabetic probands [22] and/or genetically at-risk infants from the general population [23] will be necessary to confirm this hypothesis.
In summary, data obtained from the present study suggest that active TGF-β1 serum levels during the natural history of diabetes in NOD mice may represent a biomarker of insulitis. The observations obtained in humans, once confirmed in a larger series of individuals, suggest that it could be a valuable tool for deciphering the natural history of the insulitis process during the silent phase of beta cell loss in subjects at risk for T1D, thus preparing the ground for secondary prevention of the disease.
Acknowledgments
We thank Nazzareno Di Carlo (Department of Infectious, Parasitic, and Immune-Mediated Diseases, Istituto Superiore di Sanità) for animal care and technical assistance. We thank Ms Jean Ringrose for English editing of the manuscript and Mrs Annarosa Monopoli for typing the manuscript.
Disclosure
Nothing to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. (a) Individual values of serum aTGF-β1 in the three groups of Sardinian school children. Bars represent mean. (b) Relationship between serum aTGF-β1 values and time to diabetes onset in the IrAb+ school children who developed T1D. TGF-β1 values were modelled as a fractional polynomial function (degree = 3) of time to diabetes onset in the pre-diabetic individuals from the general population.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750–4. doi: 10.1136/bmj.328.7442.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindley S, Dayan CM, Bishop A, et al. Defective suppressor function in CD4(+) CD25(+) T-cell from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 3.Peng Y, Laouar Y, Li MO, et al. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marie JC, Letterio JJ, Gavin M, et al. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 6.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4 T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–7. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 7.Szanya V, Ermann J, Taylor C, et al. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–5. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 8.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belghith M, Bluestone JA, Barriot S, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 10.Luo X, Yang H, Kim IS, et al. Systemic transforming growth factor-beta1 gene therapy induces Foxp3+ regulatory cells, restores self-tolerance, and facilitates regeneration of beta cell function in overtly diabetic nonobese diabetic mice. Transplantation. 2005;79:1091–6. doi: 10.1097/01.tp.0000161223.54452.a2. [DOI] [PubMed] [Google Scholar]
- 11.Flaumenhaft R, Kojima S, Abe M, et al. Activation of latent transforming growth factor β. Adv Pharmacol. 1993;24:51–76. doi: 10.1016/s1054-3589(08)60933-3. [DOI] [PubMed] [Google Scholar]
- 12.Wallik SC, Figari IS, Morris RE, et al. Immunoregulatory role of transforming growth factor β (TGF-β) in development of killer cells: comparison of active and latent TGF-β1. J Exp Med. 1990;172:1777–84. doi: 10.1084/jem.172.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Frank ME, Jin W, et al. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 14.Calcinaro F, Dionisi S, Marinaro M, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–75. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–8. [PubMed] [Google Scholar]
- 16.Casu A, Pascutto C, Bernardinelli L, et al. Type 1 diabetes among Sardinian children is increasing: the Sardinian diabetes register for children aged 0–14 years (1989–1999) Diabetes Care. 2004;27:1623–9. doi: 10.2337/diacare.27.7.1623. [DOI] [PubMed] [Google Scholar]
- 17.Pastore M, Bazzigaluppi E, Bonfanti R, et al. Two-step islet autoantibody screening for risk assessment of type 1 diabetes in relatives. Diabetes Care. 1998;21:1445–50. doi: 10.2337/diacare.21.9.1445. [DOI] [PubMed] [Google Scholar]
- 18.Locatelli M, Songini M, Bottazzo GF. IDDM Sardinia Project: a study model on the etiopathogenesis of insulin-dependent diabetes mellitus and other autoimmune pathologies. Ann Ist Super Sanità. 1999;35:253–63. [PubMed] [Google Scholar]
- 19.Vukkadapu SS, Belli JM, Ishii K, et al. Dynamic interaction between T cell-mediated β-cell damage and β-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics. 2005;21:201–11. doi: 10.1152/physiolgenomics.00173.2004. [DOI] [PubMed] [Google Scholar]
- 20.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing–remitting disease? Nat Rev Immunol. 2007;7:988–94. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacio E, Hummel M, Walter M, et al. IDDM1 and multiple family history of type 1 diabetes combine to identify neonates at high risk for type 1 diabetes. Diabetes Care. 2004;11:2695–700. doi: 10.2337/diacare.27.11.2695. [DOI] [PubMed] [Google Scholar]
- 22.Gorsuch AN, Spencer KM, Lister J, et al. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet. 1981;2:1363–5. doi: 10.1016/s0140-6736(81)92795-1. [DOI] [PubMed] [Google Scholar]
- 23.Buzzetti R, Galgani A, Petrone A, et al. Genetic prediction of type 1 diabetes in a population with low frequency of HLA risk genotypes and low incidence of the diseases (the DIABFIN study) Diabetes Metab Res Rev. 2004;20:137–43. doi: 10.1002/dmrr.426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


