Abstract
Intravenous immunoglobulin (IVIG) has been used widely to treat immune thrombocytopenic purpura (ITP), but the mechanisms of its action remain unclear. We investigated the affinity for Fcγ receptors (FcγRs) and the thrombocytopenia-ameliorating effect of S-sulfonated gammaglobulin (SGG) and S-alkylated gammaglobulin (AGG), in comparison with unmodified gammaglobulin (GG), in a mouse ITP model. Cleavage of immunoglobulin (Ig)G interchain disulfide bonds by either S-sulfonation or S-alkylation did not decrease the affinity for FcγRIIA (CD32A) and FcγRIIB (CD32B), but did decrease the affinity for FcγRIA (CD64A) and FcγRIIIA (CD16A), presumably because of changes in H-chain configuration. The interchain disulfide bond cleavage decreased the affinity much more for mouse FcγRIV than for mouse FcγRIIB. The ability of AGG to ameliorate ITP was greatly diminished, while SGG, whose disulfide bonds are reconstituted in vivo, was as effective as GG. These results suggest that the interchain disulfide bonds are important for therapeutic effect. It is also suggested that the interaction of IVIG with the inhibitory receptor FcγRIIB is insufficient for effective amelioration of ITP and that, at least in this model, direct binding of IVIG to FcγRIIIA is also required.
Keywords: Fc receptors, idiopathic thrombocytopaenia purpuria/thrombocytopenia, intravenous immunoglobulin therapy, platelets, protein structure/folding
Introduction
Intravenous immunoglobulins (IVIG) are immunoglobulin (Ig) preparations fractionated from pooled plasma of thousands of healthy volunteers. Because the intravenous administration of immunoglobulin (Ig) at high doses sometimes causes anaphylactic adverse reactions, which have generally been ascribed to complement activation by polymeric IgG in the preparations, Igs are treated with polyethylene glycol and/or low pH solution to decrease the amount of polymeric IgG, or modified by S-sulfonation, S-alkylation or with β-propiolactone to reduce complement activation [1].
Imbach et al. [2] found that IVIG therapy was effective in treating immune thrombocytopenic purpura (ITP), an autoimmune disorder characterized by a low platelet count and mucocutaneous bleeding. The antibody-sensitized platelets are eliminated by Fcγ receptor (FcγR)-bearing phagocytes [3]. The mechanisms of IVIG efficacy have been studied extensively, but remain enigmatic. Although some findings suggest that the Fab domain of IgG works in IVIG therapy [4], the Fc fragments from IVIG have been found to be as effective as IVIG itself in clinical ITP therapy [5] and in a mouse ITP model [6], implying an essential role of the interaction of the Fc domain and FcγRs.
There are two functionally different kinds of FcγRs: activating receptors such as FcγRIA (CD64A), FcγRIIA (CD32A) and FcγRIIIA (CD16A), and an inhibitory receptor, FcγRIIB (CD32B) [7]. A simple explanation of the efficacy of IVIG for ITP seems to be blocking of the activating receptors, as autoantibody-bearing platelets do not bind to the activating receptors. It was reported that IgG dimers contained in IVIG, rather than monomers, were effective in the mouse ITP model [8], but another report claimed that large amounts of IgG monomers worked as an antagonist for these receptors [9]. The current understanding of the involvement of those receptors in IVIG efficacy for ITP was reviewed recently by Crow and Lazarus [10], but the mechanisms involved are not known fully.
Samuelsson et al. [6] found that efficacy of IVIG for ITP was abrogated in FcγRIIB-deficient mice and also in mice pretreated with an FcγRIIB-blocking antibody, suggesting a pivotal role of this inhibitory receptor. Furthermore, individual IgG subclasses have different ratios of affinity for activating FcγRIIIA to inhibitory FcγRIIB (A/I ratios), and the IgG subclasses with higher A/I ratios showed more potent cytotoxic activity via FcγRs [11]. In addition, IVIG blocked both the down-regulation of FcγRIIB and the up-regulation of FcγRIIIA by C5a [12]. These findings indicate that the interaction of IVIG with FcγRIIB is essential for its effect on ITP and that IVIG with a lower A/I ratio may be more effective for treatment of ITP.
S-sulfonation [13] or S-alkylation following reduction [14] under mild conditions cleaves the interchain, but not the intrachain, disulfide bonds of IgG, resulting in decreased activity of some effector functions, such as complement activation. Recently, Dall'Acqua et al. [15] showed that recombinant IgG molecules without inter-H chain disulfide bonds had decreased antibody-dependent cellular cytotoxicity. However, the effects of these chemical modifications on the affinity for individual activating and inhibitory receptors and on the efficacy for ITP amelioration have not been investigated.
In the present study, we studied comparatively the affinity for individual FcγRs and the ITP-ameliorating abilities of S-sulfonated GG (SGG), unmodified gammaglobulin (GG) and reduced and S-alkylated GG (AGG). Unexpectedly, cleavage of interchain disulfide bonds did not change the affinity for FcγRIIA and FcγRIIB, although it decreased affinity for FcγRIIIA and FcγRIA. Consequently, the A/I ratios of SGG and AGG were much lower than those of GG. However, AGG showed greatly reduced efficacy in the mouse ITP model, while the efficacy of SGG was equivalent to that of GG. SGG is known to revert to unmodified GG via reconstitution of disulfide bonds in vivo[16] and the affinity of SGG for FcγRIIIA was actually restored by reduction and reoxidation. We also discuss the reason why disulfide bond cleavage resulted in a decrease of affinity for FcγRIIIA, but not FcγRIIs, in terms of the amino acid residues of IgG that are involved in FcγRs-binding.
Materials and methods
Reagents
Gammaglobulin fractions (GG) and S-sulfonated GG (SGG; Venilon™) were kindly provided by the Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan). The contents of IgG1, IgG2, IgG3 and IgG4 in these preparations were 54–58%, 38–43%, 2·2–3·3% and 0·7–0·9%, respectively. There were no significant differences in these contents among the preparations. Polyethylene glycol 4000 (PEG4000) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan) and was added to all solutions of GG, SGG and AGG and their fractions at a final concentration of 0·2% to prevent IgG aggregation.
Biochemical analyses of IVIG preparations
The interchain disulfide bonds between H and L chains were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions, using 10% acrylamide gel. Samples were applied at 5 µg protein/lane and the protein bands were stained with Bio-Safe Coomassie. The compositions of polymers, dimers and monomers in GG, SGG and AGG were analysed by gel filtration high-performance liquid chromatography (HPLC) on a Protein Pak 300SW column (Waters Corporation, Milford, MA, USA) at a flow rate of 1 ml/min in 0·1 M phosphate buffer (pH 6·6) containing 0·14 M NaCl at 25°C. Samples containing 10 µg of protein were injected into the column and the elution profiles were analysed using HPLC ChromNAV (Jasco Corporation, Tokyo, Japan). The contents of polymers and dimers decreased gradually when the preparations were stored in a warm environment, and thus samples were stored at −80°C, kept at 4°C for no longer than a week, and analysed by HPLC just after having been warmed to room temperature.
Fractionation of polymers, dimers and monomers from GG
For small-scale fractionation, 0·8 mg of GG was applied to Protein Pak 300SW at a flow rate of 1 ml/min in 0·1 M phosphate buffer (pH 6·6) containing 0·15 M NaCl, at 25°C. Protein concentrations were determined from the absorbance at 280 nm and components were then analysed by gel filtration HPLC as described above.
For large-scale fractionation, 250 mg of GG was applied to a column (φ2·5 cm × 110 cm) of Sephacryl S-200HR (Sigma-Aldrich Co., St Louis, MO, USA) at a flow rate of 0·2 ml/min in 20 mM phosphate buffer, pH 7·4, containing 0·14 M NaCl and 0·2% PEG4000 [phosphate-buffered saline (PBS)/PEG] to separate the polymer + dimer fraction from monomer. The polymer + dimer fraction used in the mouse ITP model experiments contained 6·3% polymer and 40% dimer, along with monomer, and the monomer fraction was composed of 1·7% dimer and 98% monomer. The starting material, GG, contained 1·3% polymer and 13·5% dimer.
S-sulfonation, S-alkylation and reconstitution of the interchain disulfide bonds of SGG
S-sulfonated GG (SGG) was prepared using the method of Masuho et al. [13], with some modifications. GG 0·5 mg/ml (final concentration), sodium sulfite 270 mM (final concentration; Wako Pure Chemical Industries Ltd) and sodium tetrathionate 70 mM (final concentration; Sigma-Aldrich Co.) were mixed in 50 mM Tris HCl buffer, pH 8·2 containing 0·14 M NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA) and 0·2% PEG4000 (modification buffer). The reaction mixture was incubated at 37°C for 4 h to cleave the interchain disulfide bonds and then dialysed against PBS/PEG. S-alkylated GG (AGG) was prepared as follows. The same batch of GG at the final concentration of 0·5 mg/ml and dithiothreitol at a final concentration of 10 mm were mixed in modification buffer. The reaction mixture was incubated at 37°C for 1 h to reduce the interchain disulfide bonds, and then proteins were allowed to react with iodoacetamide at a final concentration of 50 mM for 30 min at 37°C. The solution was dialysed against PBS/PEG to remove residual reagents. As control, GG was incubated without reagents at 37°C for 2 h.
The disulfide bonds of SGG were reconstituted according to Masuho et al. [17]. 2-Mercaptoethanol at a final concentration of 0·1 M was added to SGG at a final concentration of 5 mg/ml in modification buffer. After reduction at room temperature for 60 min, 2-mercaptoethanol was removed by dialysis against modification buffer. Air oxidation was performed by aerating the outer buffer during dialysis. The samples were then incubated in a rotating tube device at 37°C overnight.
FcγRs-binding assay by ELISA
Recombinant proteins consisting of the extracellular domains of human FcγRs fused with glutathione S-transferase were prepared as described previously [18]. Microtitre plates (Becton-Dickinson Co., Franklin Lakes, NJ, USA) were coated with 4 µg/ml of the FcγR proteins in PBS at 4°C overnight and blocked with 25 mM Tris HCl at pH 7·4 containing 0·14 M NaCl, 0·5% BSA, 2 mM EDTA and 0·05% Tween 20 (ELISA buffer) at 37°C for 2 h. GG, SGG and AGG samples diluted serially in ELISA buffer were dispensed into individual wells of the FcγR-coated plates, which were then incubated at 37°C for 2 h. The plates were washed three times with ELISA buffer, then 0·1 µg/ml horseradish peroxidase (HRP)-conjugated goat anti-human IgG (H + L) affinity-purified F(ab′)2 (Chemicon International, Inc, Temecula, CA, USA) in ELISA buffer was dispensed into the wells and the plates were incubated at 37°C for 1 h. The plates were again washed in the same manner, 0·1 ml of 0·04% o-phenylenediamine dihydrochloride and 0·01% hydrogen peroxide in 0·1 M citrate buffer at pH 5·0 was dispensed into the wells, and the plates were allowed to stand at room temperature for 10 min. Absorbance at 450 nm was measured using a microplate reader Model 550 (Bio-Rad Laboratories, Hercules, CA, USA). The effective concentration (EC50) value was calculated by measuring the IgG concentration that gave 50% of the maximum absorbance in Fcγ receptor-binding ELISA. A/I ratios were calculated by dividing the EC50 for FcγRIIB by the EC50 for FcγRIIIA.
The binding activity for mouse FcγRs was measured using the same methods as those for human FcγRs. Recombinant proteins consisting of the extracellular domains of mouse FcγRs were purchased from R&D systems, Inc. (Minneapolis, MN, USA). As the binding affinity for mouse FcγRs was relatively low, GG and AGG were slightly cross-linked with 2-iminothiolane (Sigma-Aldrich Co.) and N-(8-Maleimidocapryloxy)sulfosuccinimide (Dojindo Laboratories, Tokyo, Japan) [19] and their affinity for mouse FcγRIIB and FcγRIV was assessed by ELISA on the mouse FcγR-coated plates.
Analysis of the three-dimensional structure involved in the interaction between IgG and FcγRs
The three-dimensional structure of the hinge region and Fc domain of human IgG1 (PDB ID; 1E4K) was displayed in the Corey–Pauling–Koltun (CPK) representation using Discovery Studio™ (Accelrys Software Inc., San Diego, CA, USA). The amino acids of IgG involved in the interaction with FcγRIIB and FcγRIII and the cysteines that form inter-H chain disulfide bonds were depicted in different colours, based on reported data [20,21].
Induction and treatment of ITP model mouse
This study was approved by the Animal Experiment Committee of Tokyo University of Science. Male BALB/c mice, 5–6 weeks of age, were purchased from Sankyo Labo Service Corporation (Tokyo, Japan). All mice were kept at the animal facility at Tokyo University of Science, and experiments were conducted while their body weight was in the range of 20–24 g. GG, SGG and AGG at various doses were administered intraperitoneally to the mice, and after 24 h the mice were rendered thrombocytopenic by intraperitoneal administration of 2 µg/mouse of mAb MWReg30 (rat IgG1 antibody against mouse integrin αIIb or CD41; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Blood samples were collected from the tail vein 2 h before and 3, 6 and 24 h after the administration of MWReg30. Approximately 6 µl of blood was diluted with 56 µl of 1% ammonium oxalate and the platelet numbers were counted using an electronic cell counter (Celltac α; Nihon Kohden, Tokyo, Japan). Experiments were performed with groups of five mice, which were administered GG or SGG (five different batches) or AGG (single batch) intraperitoneally. The results shown here are representative of two or three separate experiments. The platelet counts of the five mice in each group were expressed as the mean ± standard deviation (s.d.). Statistical analysis was performed with Student's t-test. The criterion of significance was taken to be P < 0·05.
Results
Binding activity of IVIG for individual FcγRs
IVIG preparations contain non-covalent IgG polymer and dimer, as well as monomer. IVIG preparations containing IgG polymer were reported to be effective in the mouse ITP model, while monomer was not [8]. Thus, GG was separated into polymer, dimer and monomer fractions by gel filtration HPLC. The polymer fraction thus separated was composed of 81% polymer + dimer and 19% monomer. The dimer fraction was composed of 77% polymer + dimer and 23% monomer and the monomer fraction was composed of < 1% polymer + dimer and > 99% monomer (data not shown). The binding activity was examined by ELISA using the extracellular domains of FcγRs (Fig. 1). The polymer fraction was much more active than the monomer fraction, i.e. it showed 140, 160 and 30 times greater affinity for FcγRIIA, FcγRIIB and FcγRIIIA, respectively. The dimer fraction showed 10, three and nine times greater affinity than the monomer fraction for the three receptors, respectively.
Fig. 1.
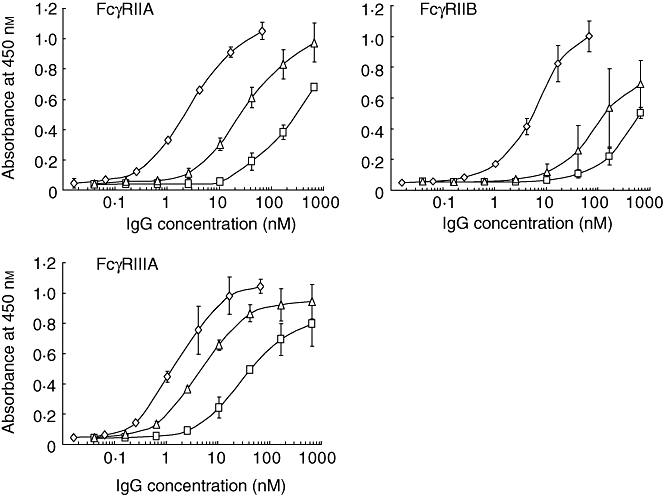
Fcγ receptor (FcγR)-binding activitys of polymer, dimer and monomer fractions from gammaglobulin (GG). Those fractions were separated by gel filtration high-performance liquid chromatography (HPLC) and their binding activity was assessed by enzyme-linked immunosorbent assay (ELISA). The fractions were allowed to react with the extracellular domain of each FcγRIIA, FcγRIIB or FcγRIIIA coated on microtitre plates. The amounts of bound immunoglobulin G (IgG) were measured with horseradish peroxidase (HRP)-conjugated anti-human IgG (H + L) antibodies. Polymer (diamonds), dimer (triangles) and monomer (squares). Those fractions were separated into two independent experiments using two different batches of GG, and ELISA was performed in quadruplicate. The symbols indicate the means ± standard deviation.
The FcγR binding-activity of S-sulfonated GG (SGG) was compared with that of GG by means of FcγR ELISA (Fig. 2). SGG was found to be a little more active than GG in binding to FcγRIIB and as active as GG in binding to FcγRIIA, although it was only 1/40 to 1/50 as active as GG in binding to FcγRIA and FcγRIIIA. GG was composed of 1·3% polymer, 14% dimer and 85% monomer, while SGG was composed of 2·8% polymer, 16% dimer and 81% monomer. The differences between GG and SGG in individual FcγR-binding activity cannot be explained by the differences in composition.
Fig. 2.
Fcγ receptor (FcγR)-binding activity of S-sulfonated gammaglobulin (SGG) preparations. The binding activity of SGG and gammaglobulin (GG) was assessed by means of enzyme-linked immunosorbent assay (ELISA) as described in the legend of Fig. 1. Five different batches of SGG were assessed in triplicate and their individual binding curves are shown. Symbols indicate the means ± standard deviation (s.d.). Three different batches of GG were also assessed; symbols (closed diamonds) indicate the means ± s.d.
Effect of interchain disulfide bond cleavage on FcγR-binding activity
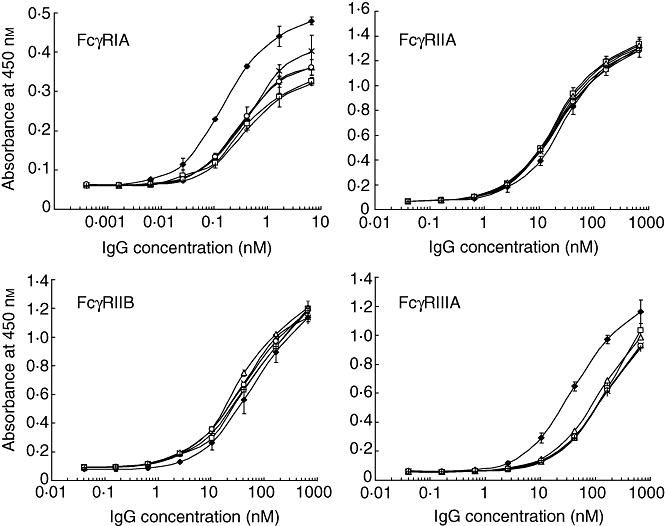
To compare the FcγR binding activity more precisely, SGG and AGG were prepared from the same batch of GG. S-sulfonation or S-alkylation with prior reduction under mild conditions cleaved the interchain disulfide bonds to give H and L chain bands with small amounts of H2L, H2 and HL in SDS-PAGE under non-reducing conditions (Fig. 3a). The polymer and dimer fractions of SGG and AGG showed very similar protein bands in SDS-PAGE, indicating that their interchain disulfide bonds had been cleaved to essentially the same degree (data not shown). There was no significant difference in gel filtration HPLC profiles among GG, SGG and AGG, indicating that their composition of polymers, dimers and monomers is also essentially the same (Fig. 3b).
Fig. 3.
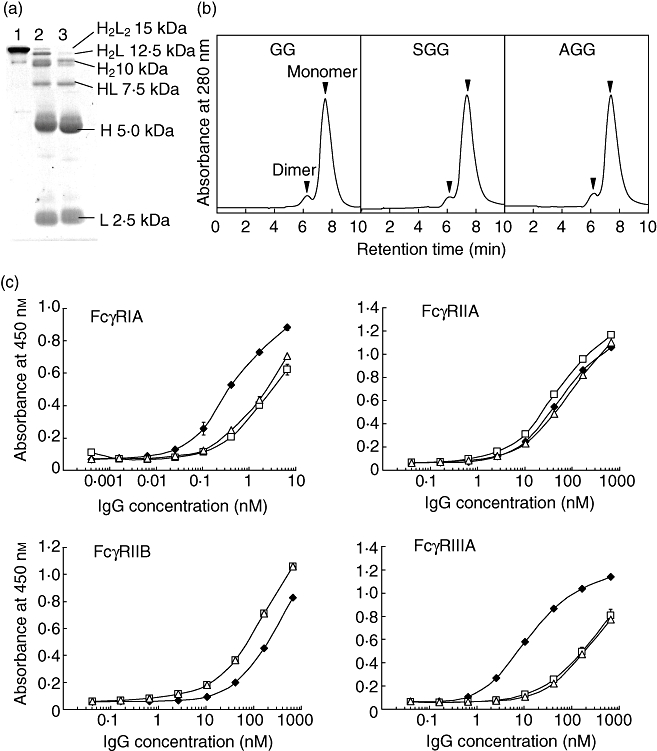
Effect of interchain disulfide bond cleavage on Fcγ receptor (Fcγ)-binding activity. The interchain disulfide bonds of immunoglobulin G (IgG) were cleaved by S-sulfonation or S-alkylation with prior mild reduction, using the same batch of gammaglobulin (GG). (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Lanes 1, 2 and 3 are GG, S-sulfonated gammaglobulin (SGG) and S-alkylated gammaglobulin (AGG), respectively. (b) Profiles of gel filtration high-performance liquid chromatography (HPLC). (c) FcγR-binding activity of SGG (open squares) and AGG (open triangles) compared with GG (closed diamonds). Enzyme-linked immunosorbent assay (ELISA) was performed in triplicate. The symbols indicate the means ± standard deviation.
SGG and AGG showed almost the same binding curves for all four FcγRs (Fig. 3c). Both SGG and AGG were as potent as the parental GG in binding to FcγRIIA and three times more potent than GG in binding to FcγRIIB, whereas their binding activity was decreased to 1/10 and 1/25 for FcγRIA and FcγRIIIA, respectively. The A/I ratios were calculated by dividing the EC50 for FcγRIIB by the EC50 for FcγRIIIA. The A/I ratios of these modified GGs were decreased to 1/70–1/80 compared with that of the parental GG (Table 1). Therefore, cleavage of the interchain disulfide bonds leads to decreased binding activity for FcγRIIIA and a slight enhancement of binding to FcγRIIB.
Table 1.
Comparative binding activities of gammaglobulin (GG), S-sulfonated gammaglobulin (SGG) and S-alkylated gammaglobulin (AGG) for different Fcγ receptors
| EC50 (nM) | |||||
|---|---|---|---|---|---|
| Preparation | FcγRIA | FcγRIIA | FcγRIIB | FcγRIIIA | A/I ratio |
| GG | 0·31 | 60 | 270 | 12 | 23 |
| SGG | 2·8 | 39 | 92 | 300 | 0·31 |
| AGG | 2·2 | 79 | 92 | 320 | 0·29 |
The activity [effective concentration (EC50)] was calculated by measuring the immunoglobulin G (IgG) concentrations that gave 50% of the maximum binding values, based on the results shown in Fig. 3. A/I ratios were calculated by dividing the EC50 for Fcγ receptor IIB (FcγRIIB) by the EC50 for FcγRIIIA.
Why does cleavage of the interchain disulfide bonds decrease the binding activity for FcγRIIIA, but not for FcγRIIs? The amino acids in the Fc fragment involved in binding to FcγRIII were assigned by crystallographic study of the complex of Fc fragment and FcγRIII [22]. FcγRIII is in contact with Leu235, Gly236, Gly237, Ser239 and Asp265 on one H chain and with Leu235, Gly236, Pro329 and Ala330 on the other H chain (Fig. 4a). FcγRIIB, a peptide derived from the Fc domain, Thr256-Pro271, was found to bind to FcγRIIB with an affinity close to that of IgG [20]. Those amino acids were superimposed upon the Fc tertiary structure (Fig. 4b). It appears that a single molecule of FcγRIIB binds to these amino acids on one H chain but not the other H chain. Cleavage of interchain disulfide bonds must change the configuration of the two H chains in the CH2 domain close to the hinge. Thus, it is suggested that the reason for the selective decrease of the affinity for FcγRIIIA by cleavage of the interchain disulfide bonds would be that FcγRIIIA recognizes both H chains, whereas FcγRIIB recognizes only one of them.
Fig. 4.
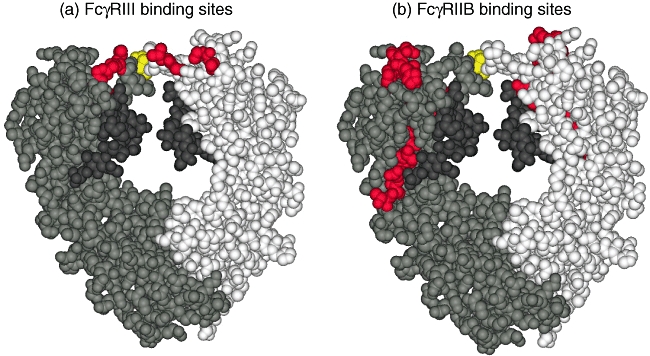
The amino acid residues involved in binding to Fcγ receptor III (FcγRIII) and FcγRIIB. (a) FcγRIII-binding sites. (b) FcγRIIB-binding sites. The three-dimensional structure of Fc fragment is illustrated in the Corey–Pauling–Koltun (CPK) representation using Discovery Studio™ software. The two H chains are drawn in two different colours, grey and white, along with their carbohydrates in dark grey. The amino acid residues involved in binding to FcγRIII and FcγRIIB are depicted in red, and the cysteine residues of H–H interchain disulfide bonds are shown in yellow.
Ability of IVIG to ameliorate thrombocytopenia
The binding activity for mouse FcγRs was measured by the same methods as that for human FcγRs. The mouse orthologues of human FcγRIIB and FcγRIIIA are FcγRIIB and FcγRIV, respectively [11]. GG and AGG were dimerized with chemical linkers because their binding affinity for mouse FcγRs was relatively low. The dimerized GG showed a high affinity for mouse FcγRIV (EC50 14 nM) compared with mouse FcγRIIB (EC50 230 nM), while the dimerized AGG showed EC50 values of 240 nM and 380 nM for FcγRIV and FcγRIIB, respectively (Fig. 5). The A/I ratios were 16 and 1·6 for GG and AGG, respectively. Thus, cleavage of the interchain disulfide bonds resulted in decrease of the A/I ratio for mouse FcγRs, as with the ratio for human FcγRs.
Fig. 5.
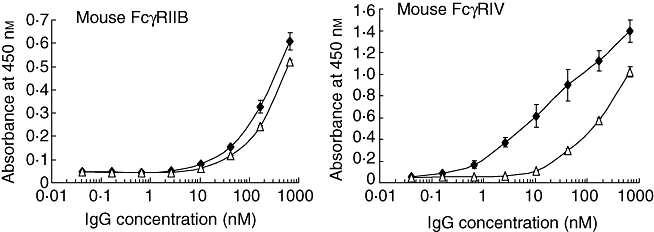
Effect of the interchain disulfide bond cleavage on mouse Fcγ receptor (Fcγ)-binding activity. The activity of dimerized gammaglobulin (GG) (closed diamonds) and dimerized S-alkylated gammaglobulin (AGG) (open triangles) for mouse FcγRIIB and FcγRIV, respectively, was measured by enzyme-linked immunosorbent assay (ELISA) in triplicate and the symbols indicate the means ± standard deviation.
The ability of IVIG to ameliorate thrombocytopenia was assessed in the mouse ITP model. IVIG was administered intraperitoneally to the mice, which were rendered thrombocytopenic 24 h later by intraperitoneal administration of rat IgG1 mAb specific for mouse integrin αIIb (CD41). First, the polymer + dimer fraction and the monomer fraction separated from GG by gel filtration chromatography were compared with respect to amelioration of thrombocytopenia. The polymer + dimer fraction containing 6·3% polymer and 40% dimer ameliorated thrombocytopenia at a dose of 0·2 g/kg, whereas the monomer fraction containing only 1·7% dimer showed no efficacy (Fig. 6a). The parental GG was hardly effective at this dose in ITP. These results are consistent with the binding activity of these fractions for FcγRs (Fig. 1). In addition, the polymer + dimer fraction was five to 10 times more effective than unfractionated GG in the amelioration of thrombocytopenia (Fig. 6b).
Fig. 6.
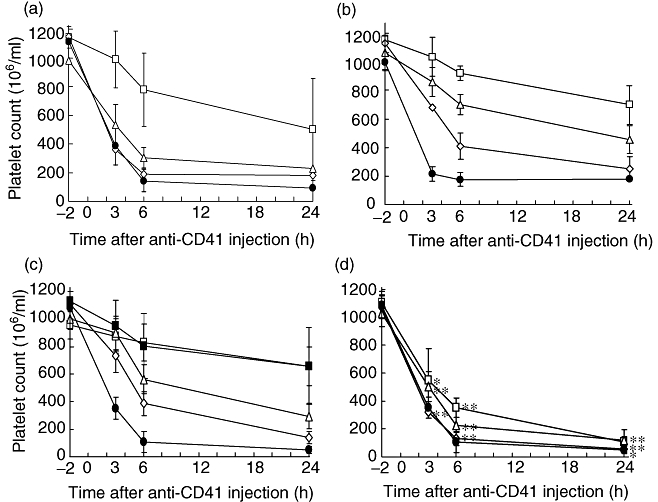
Amelioration of thrombocytopenia with intravenous immunoglobulin (IVIG) preparations. IVIG was administered intraperitoneally to groups of five mice. After 24 h, 2 µg of anti-CD41 monoclonal antibody (mAb) was administered intraperitoneally. Blood samples were obtained 2 h before and 3, 6 and 24 h after administration of anti-CD41 mAb. Platelets were counted by an electronic cell counter. The means ± standard deviation (s.d.) are indicated by symbols with bars. (a) Thrombocytopenia amelioration with GG, polymer + dimer fraction and monomer fraction separated from gammaglobulin (GG). Polymer + dimer fraction (open squares), monomer fraction (open diamonds) or the parental GG (open triangles) was administered intraperitoneally at a dose of 0·2 g/kg. Phosphate-buffered saline (PBS) was used as the control (closed circles). (b) Amelioration of thrombocytopenia with GG at the dose of 2 g/kg (open squares), 1 g/kg (open triangles) or 0·5 g/kg (open diamonds). The control was PBS (closed circles). (c) Amelioration of thrombocytopenia with S-sulfonated gammaglobulin (SGG). The doses are the same as those in (b). In an additional experiment, 6 h before anti-CD41 mAb, 2 g/kg of SGG was administered intraperitoneally and the platelets were counted (closed squares). (d) Amelioration of thrombocytopenia with S-alkylated gammaglobulin (AGG). The doses are the same as those in (b). An asterisk and double asterisk indicate significant differences (P < 0·01 and P < 0·001, respectively) between the platelet counts with S-alkylated gammaglobulin (AGG) and GG. The platelet counts of the five mice in each group were expressed as the mean ± s.d.
Various doses of GG, SGG and AGG were administered 24 h before induction of thrombocytopenia. GG and SGG induced amelioration in a dose-dependent manner (Fig. 6b and c). SGG was as effective as GG at all three doses tested and at all four time-points tested. Further, the platelet counts were higher than those with PBS even at the dose of 0·5 g/kg, with statistical significance (P < 0·001). There was no statistically significant difference (P > 0·01) between the platelet counts in mice treated with SGG and GG. Conversely, AGG was much less effective than either GG or SGG in terms of platelet counts, with statistical significance (P < 0·001 or < 0·01) at all the tested doses and at 3, 6 and 24 h (Fig. 6d). These results suggest that the cleavage of the interchain disulfide bonds greatly decreases not only the binding activity for FcγRIIIA, but also the ability to ameliorate thrombocytopenia.
Restoration of the FcγRIIIA-binding activity of SGG by in vitro reconstitution of the disulfide bonds
In a clinical study of patients with agammaglobulinaemia it was found that the disulfide bonds of SGG were reconstituted [16]. Our finding that the ameliorating ability of SGG was equivalent to that of GG implies that the disulfide bonds are also reconstituted in mice.
To determine whether SGG recovers FcγR-binding activity following reconstitution of the disulfide bonds, three batches of SGG were reduced with 2-mercaptoethanol and then reoxidized by aeration. The reconstituted SGG (R-SGG) showed the H2L2 band with a very small amount of H2L band and without either H or L chain band in SDS-PAGE under non-reducing conditions (data not shown). The FcγR-binding activity of R-SGG was compared with those of SGG and GG. The binding activity for FcγRIIIA was completely recovered (Fig. 7), and the differences in binding to the other three FcγRs between SGG and GG were also abrogated (data not shown). Administration of SGG at 6 h before injection of anti-CD41 mAb ameliorated thrombocytopenia as effectively as administration 24 h before injection, suggesting that the reconstitution of the disulfide bonds occurs within 6 h (Fig. 6c). These results suggest that SGG would recover its binding activity quickly for FcγRIIIA in vivo.
Fig. 7.
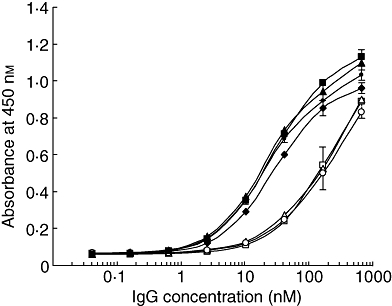
Fcγ receptor IIIA (FcγRIIIA)-binding activity of R-SGG, prepared by in vitro reconstitution of the disulfide bonds of SGG. Three batches of S-sulfonated gammaglobulin (SGG) (open squares, triangles and circles) were reduced separately and re-oxidized to prepare R-SGG (closed squares, triangles and circles). Their FcγRIIIA-binding activity was compared with that of GG (closed diamonds).
Discussion
We determined the binding activity of S-sulfonated GG (SGG) for activating and inhibitory FcγRs compared with that of GG in order to elucidate the mechanism of amelioration of ITP by IVIG, especially in connection with the role of the interchain disulfide bonds in the hinge region of IgG. SGG retained binding activity for both FcγRIIA and FcγRIIB, but showed greatly decreased binding to FcγRIA and FcγRIIIA (Fig. 2). The binding activity would be influenced not only by S-sulfonation, but also by differences in the contents of polymer and dimer in the preparations (Fig. 1). Thus, SGG and mildly reduced and S-alkylated GG (AGG) were prepared from the same batch of GG and compared with the parental GG. SGG and AGG showed the same gel-filtration profiles, confirming that their compositions were the same as that of GG (Fig. 3b). Although the cysteine residues of GG were modified with different residues, -SO3Na for S-sulfonation and -CH2CONH2 for S-alkylation, SGG and AGG showed almost the same binding activity for all four FcγRs. SGG and AGG were found not to show decreased affinity for FcγRIIA and FcγRIIB, but the affinity was decreased to 1/25 for FcγRIIIA and 1/10 for FcγRIA (Fig. 3c). Therefore, the cleavage of interchain disulfide bonds by S-sulfonation or S-alkylation leads to a decrease in the affinity for FcγRIA and FcγRIIIA without any reduction in the affinity for FcγRIIA and FcγRIIB. Consequently, the A/I ratios of SGG and AGG were much lower than those of GG (Table 1).
To understand the different effects of disulfide bond cleavage on the binding activity of IVIG for the different FcγRs, the tertiary structures of the sites that interact with the receptors were analysed by three-dimensional display of the Fc domain. Crystallographic study [22] of the complex of Fc and FcγRIII has revealed that FcγRIII interacts with amino acids of both H chains comprising the N-terminal region of the CH2 domain (Fig. 4a). Conversely, a peptide from the Fc domain of human IgG1, Thr256-Pro271, which bound to FcγRIIB as potently as IgG1 itself [20], was found to be located at a site on the H chain which is distant from the corresponding site of the other H chain (Fig. 4b). Thus, a single molecule of FcγRIIB seems to bind to only one of the two H chains, i.e. FcγRIII recognizes both H chains while FcγRIIB recognizes only one of them. It seems likely that cleavage of the interchain disulfide bonds would change the configuration between the two H chains in the hinge and the CH2 domain [23], and this may be the reason why SGG and AGG retained binding activity for FcγRIIB but showed reduced binding activity for FcγRIIIA.
This explanation is also supported by stoichiometric analyses, indicating that IgG forms a complex with FcγRIII at a molar ratio of 1:1 [24] and a complex with FcγRII at a molar ratio of 1:2 [25]. In this connection, fucose-deficient IgG was much more active than fucosylated IgG in binding to FcγRIII, while fucose-deletion did not affect binding to FcγRII [26]. A comparison of the crystal structure of fucose-deficient IgG with that of the parental IgG revealed only a subtle conformational alteration in a limited region of the Fc domain [27]. It seems likely that modification of the sugar moiety would change the configuration of the two H chains in a similar manner to disulfide bond cleavage.
A mouse ITP model induced with anti-CD41 mAb was employed to evaluate the efficacy of GG, SGG and AGG for ameliorating thrombocytopenia. In connection with the mouse experiments, the binding affinity for mouse FcγRs was assessed by ELISA with the FcγR extracellular domains. The cleavage of interchain disulfide bonds decreased the binding affinity for mouse FcγRIV much more than that for mouse FcγRIIB (Fig. 5) and consequently decreased the A/I ratio, as with that for human FcγRs. In this ITP model, the polymer + dimer fraction apparently ameliorated thrombocytopenia, whereas the monomer fraction was ineffective (Fig. 6a). These results are consistent with previous findings using aged IVIG [8] and immune complexes [28]. SGG in which the disulfide bonds were cleaved reversibly was found to be as effective as GG for amelioration of thrombocytopenia in the mouse ITP model, whereas AGG in which the disulfide bonds were cleaved irreversibly was only one-quarter as effective as GG and SGG (Fig. 6). These results suggest that SGG reverted rapidly to the parental GG via reconstitution of the disulfide bonds. We confirmed that R-SGG, prepared by in vitro reduction and reoxidation, recovered binding activity for FcγRIIIA, as well as the other FcγRs (Fig. 7).
AGG showed a very low ratio of affinity for activating FcγRIIIA to inhibitory FcγRIIB (A/I ratio); its A/I ratio was only 1/80 of that of GG (Table 1). Thus, it was anticipated that AGG would stimulate FcγRIIB more than GG, inducing inhibitory signals, and that AGG would not stimulate FcγRIIIA as much as GG on the receptor-bearing phagocytes, leading to more efficient amelioration than in the case of GG. This view is supported by several reports. For example, the co-clustering of FcγRIIB with FcγRIIA on monocytes induced an inhibitory signal [29]. Mouse IgG subclasses of higher A/I ratio were more cytotoxic [11]. A Fcγ–Fcε fusion protein co-clustered FcγRIIB and FcεRI to suppress the functions of FcεRI [30]. However, the present study revealed that AGG was much less effective than GG in the mouse ITP model. This may indicate that the interaction of polymeric IgG molecules with the inhibitory receptor FcγRIIB is insufficient, and that interaction with FcγRIIIA is necessary for full amelioration of ITP by IVIG.
There are several possible explanations of our result that the direct interaction of IVIG with FcγRIIB is insufficient for ITP amelioration, based on recent work. (i) The simplest explanation would be blocking of the binding of antibody-bearing platelets to FcγRIIIA in the reticuloendothelial system [31]. AGG was 1/25 as active as GG in binding to FcγRIIIA (Table 1). (ii) Boruchov et al. [32] showed that co-stimulation involving not only FcγRIIB, but also FcγRIIIA, was necessary for the inhibitory effect in dendritic cells. Their findings suggest that the interaction of the polymeric IgG molecules in IVIG with not only FcγRIIB, but also FcγRIIIA, would be necessary for ITP amelioration. (iii) The results of adoptive transfer of cells treated with IVIG suggest that IVIG stimulates the ‘initiator’ dendritic cells through FcγRIIIA, but not FcγRIIB, and that the initiator cells affect the secondary ‘effector’ cells, which require FcγRIIB for ITP amelioration [33]. This mechanism does not involve direct binding of IVIG to FcγRIIB. (iv) IVIG has been shown to up-regulate interferon γ receptor 2, suppressing macrophage activation. This mechanism is also dependent upon FcγRIIIA and independent of FcγRIIB [34]. Taken together, our results suggest a significant role of the interactions of IVIG with not only FcγRIIB, but also FcγRIIIA in ITP amelioration.
From the viewpoint of clinical ITP therapy, the results in the mouse ITP model indicate that SGG would be as effective as GG, because R-SGG, which was prepared by reconstituting the disulfide bonds of SGG in vitro, recovered binding activity for FcγRIIIA. Indeed, efficacy of SGG for ITP amelioration has been proved clinically [35]. In contrast to SGG, AGG, in which the interchain disulfide bonds are cleaved irreversibly, showed decreased ability to ameliorate thrombocytopenia in the mouse ITP model, suggesting that AGG would not be suitable for ITP therapy. Nachbaur et al. [36] referred to AGG as one of the available IVIG preparations, but we could not find any literature on ITP therapy with AGG. Lastly, polymer and dimer contained in IVIG were much more active than monomer in terms of FcγR-binding and efficacy in the ITP model, suggesting that IVIG preparations with a higher content of polymeric IgG molecules would be more effective for ITP therapy, although possible adverse effects would need to be considered carefully.
Acknowledgments
We thank both the Chemo-Sero-Therapeutic Research Institute and Teijin Pharma Ltd for their kind supply of GG and SGG. We also thank Dr Shintaro Kamei and Dr Hiroaki Maeda, the Chemo-Sero-Therapeutic Research Institute, and Dr Tsuyoshi Kimura, Teijin Pharma Ltd. for valuable suggestions.
Disclosure
None.
References
- 1.Lemieux R, Bazin R, Neron S. Therapeutic intravenous immunoglobulins. Mol Immunol. 2005;42:839–48. doi: 10.1016/j.molimm.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 2.Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 3.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 4.Basta M, Van Goor F, Luccioli S, et al. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003;9:431–8. doi: 10.1038/nm836. [DOI] [PubMed] [Google Scholar]
- 5.Debré M, Bonnet MC, Fridman WH, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–9. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 7.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–92. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 8.Teeling JL, Jansen-Hendriks T, Kuijpers TW, et al. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: studies in experimental immune thrombocytopenia. Blood. 2001;98:1095–9. doi: 10.1182/blood.v98.4.1095. [DOI] [PubMed] [Google Scholar]
- 9.van Mirre E, Teeling JL, van der Meer JW, Bleeker WK, Hack CE. Monomeric IgG in intravenous Ig preparations is a functional antagonist of FcγRII and FcγRIIIb. J Immunol. 2004;173:332–9. doi: 10.4049/jimmunol.173.1.332. [DOI] [PubMed] [Google Scholar]
- 10.Crow AR, Lazarus AH. The mechanisms of action of intravenous immunoglobulin and polyclonal anti-D immunoglobulin in the amelioration of immune thrombocytopenic purpura: what do we really know? Transfus Med Rev. 2008;22:103–16. doi: 10.1016/j.tmrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:1510–12. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 12.Konrad S, Baumann U, Schmidt RE, Gessner JE. Intravenous immunoglobulin (IVIG)-mediated neutralization of C5a: a direct mechanism of IVIG in the maintenance of a high FcγRIIB to FcγRIII expression ratio on macrophages. Br J Haematol. 2006;134:340–50. doi: 10.1111/j.1365-2141.2006.06185.x. [DOI] [PubMed] [Google Scholar]
- 13.Masuho Y, Tomibe K, Matsuzawa K, Ohtsu A. Development of an intravenous γ-globulin with Fc activities: I. Preparation and characterization of S-sulfonated human γ-globulin. Vox Sang. 1977;32:175–81. doi: 10.1111/j.1423-0410.1977.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber JR, Barrus VA, Siber GR. Decreased protective efficacy of reduced and alkylated human immune serum globulin in experimental infection with Haemophilus influenzae type b. Infect Immun. 1985;47:142–8. doi: 10.1128/iai.47.1.142-148.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall'Acqua WF, Cook KE, Damschroder MM, Woods RM, Wu H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J Immunol. 2006;177:1129–38. doi: 10.4049/jimmunol.177.2.1129. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka T, Abo W, Chiba S, et al. Clinical effect and metabolism of S-sulfonated immunoglobulin in 7 patients with congenital humoral immunodeficiency. Vox Sang. 1979;37:14–20. doi: 10.1111/j.1423-0410.1979.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 17.Masuho Y, Tomibe K, Watanabe T, Fukumoto Y. Development of an intravenous γ-globulin with Fc activities: II. Reconversion of S-sulfonated human γ-globulin into the original γ-globulin. Vox Sang. 1977;32:290–5. doi: 10.1111/j.1423-0410.1977.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagashima H, Tezuka T, Tsuchida W, Maeda H, Kohroki J, Masuho Y. Tandemly repeated Fc domain augments binding avidities of antibodies for Fcγ receptors, resulting in enhanced antibody-dependent cellular cytotoxicity. Mol Immunol. 2008;45:2752–63. doi: 10.1016/j.molimm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Ghetie M-A, Podar EM, Ilgen A, Gordon BE, Uhr JW, Vitetta ES. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci USA. 1997;94:7509–14. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medgyesi D, Uray K, Sallai K, et al. Functional mapping of the FcγRII binding site on human IgG1 by synthetic peptides. Eur J Immunol. 2004;34:1127–35. doi: 10.1002/eji.200324733. [DOI] [PubMed] [Google Scholar]
- 21.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcγ receptor in complex with Fc. J Biol Chem. 2001;276:16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 22.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3·2-Å crystal structure of the human IgG1 Fc fragment-FcγRIII complex. Nature. 2000;406:267–73. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 23.Seegan GW, Smith CA, Schumaker VN. Changes in quaternary structure of IgG upon reduction of the inter heavy-chain disulfide bond. Proc Natl Acad Sci USA. 1979;76:907–11. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghirlando R, Keown MB, Mackay GA, Lewis MS, Unkeless JC, Gould HJ. Stoichiometry and thermodynamics of the interaction between the Fc fragment of human IgG1 and its low-affinity receptor FcγRIII. Biochemistry. 1995;34:13320–7. doi: 10.1021/bi00041a007. [DOI] [PubMed] [Google Scholar]
- 25.Sondermann P, Jacob U, Kutscher C, Frey J. Characterization and crystallization of soluble human Fcγ receptor II (CD32) isoforms produced in insect cells. Biochemistry. 1999;38:8469–77. doi: 10.1021/bi982889q. [DOI] [PubMed] [Google Scholar]
- 26.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 27.Matsumiya S, Yamaguchi Y, Saito J, et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 2007;368:767–79. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Bazin R, Lemieux R, Tremblay T. Reversal of immune thrombocytopenia in mice by cross-linking human immunoglobulin G with a high-affinity monoclonal antibody. Br J Haematol. 2006;135:97–100. doi: 10.1111/j.1365-2141.2006.06245.x. [DOI] [PubMed] [Google Scholar]
- 29.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of FcγRIIb in human monocytic cells. J Biol Chem. 2002;277:5082–9. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 30.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fcγ Fcε functional fusion protein inhibits FcγRI-mediated degranulation. Nat Med. 2002;8:518–21. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. IVIg inhibits reticuloendothelial system function and ameliorates murine passive-immune thrombocytopenia independent of anti-idiotype reactivity. Br J Haematol. 2001;115:679–86. doi: 10.1046/j.1365-2141.2001.03136.x. [DOI] [PubMed] [Google Scholar]
- 32.Boruchov AM, Heller G, Veri M-C, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates ITP via activating Fcγ receptors on dendritic cells. Nat Med. 2006;12:688–92. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- 34.Park-Min KH, Serbina NV, Yang W, et al. FcγRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Uchino H, Yasunaga K, Akatsuka J. A cooperative clinical trial of high-dose immunoglobulin therapy in 177 cases of idiopathic thrombocytopenic purpura. Thromb Haemost. 1984;30:182–5. [PubMed] [Google Scholar]
- 36.Nachbaur D, Herold M, Eibl B, et al. A comparative study of the in vitro immunomodulatory activity of human intact immunoglobulin (7S IVIG), F(ab′)2 fragments (5S IVIG) and Fc fragments. Evidence for post-transcriptional IL-2 modulation. Immunology. 1997;90:212–18. doi: 10.1046/j.1365-2567.1997.d01-2148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


