Abstract
Despite the clinical relevance of anti-factor VIII (FVIII) antibodies (anti-FVIII inhibitors) impairing haemostatic activity of haemophilia A (HA) patients, the immunological mechanisms underlying their production are unknown. Aiming to understand more clearly the immune response in patients with [HAα-FVIII(+)] and without [HAα-FVIII(−)] anti-FVIII inhibitors, we have characterized the cytokine pattern of peripheral blood leucocytes, using an in vitro stimulation of whole blood samples with plasma-derived (pFVIII) or recombinant FVIII (rFVIII). The results highlighted decreased levels of tumour necrosis factor (TNF)-α+ neutrophils with higher interleukin (IL)-5/TNF-α ratio in HAα-FVIII(+). All HA samples displayed decreased levels of IL-10+ monocytes when compared to the blood donor (BD) samples. HAα-FVIII(+) showed lower levels of TNF-α+ monocytes and increased IL-10/TNF-α ratio. Analysis of adaptive immunity revealed increased levels of interferon (IFN)-γ+, TNF-α+ and IL-4+ T-cells, from both CD4+ and CD8+ T cells, in HAα-FVIII(−) when compared to BD. Moreover, increased frequency of IL-10+ B cells and higher levels of α-FVIII IgG1 were observed in HAα-FVIII(−). Basal levels of cytokine+ B-cells, similar to BD, and higher levels of α-FVIII IgG4 are major features in HAα-FVIII(+). The global cytokine profile demonstrated a major anti-inflammatory/regulatory pattern in HAα-FVIII(+), confirmed by the in vitro stimuli with pFVIII or rFVIII. The polarized anti-inflammatory/regulatory immune response in HAα-FVIII(+) and the mixed pattern with a bias towards an inflammatory cytokine profile, modulated by IL-4 in HAα-FVIII(−), may be the key element to drive the development of distinct subclasses of anti-FVIII antibodies. These finding have implications for the design of safe and effective therapeutic protocols to control inhibitors synthesis in HA patients.
Keywords: antibody responses, flow cytometry/FACS, haematology, immune regulation, intracellular cytokine staining
Introduction
Haemophilia A (HA) is an X-linked genetic disease in which a mutation in the F8 gene results in either a lack of circulating functional factor VIII protein or production of a defective FVIII with impaired activity. The treatment of HA patients is based on the infusion of plasma-derived FVIII (pFVIII) or recombinant FVIII (rFVIII). The most prevalent clinical complication observed after repeated FVIII administration in HA patients is the development of anti-FVIII inhibitory antibodies that affect approximately 25% of HA patients and are able to block the FVIII procoagulant activity [1]. Studies from HA experimental models have demonstrated that although anti-FVIII antibodies appeared after four to five injections, FVIII inhibitory antibodies usually appeared after six injections [2]. In humans, the anti-factor FVIII antibodies may comprise distinct immunoglobulin (IgG) subclasses, mainly IgG1 and IgG4, and the anti-FVIII CD4+ T cells recognize a complex repertoire of FVIII epitopes, secreting both T helper type 1 (Th1) and Th2 cytokines, with interleukin (IL)-10 related especially to the anti-FVIII antibody synthesis [3].
Several studies have been focused upon the immune response of HA patients in order to elucidate the mechanism underlying the development of anti-FVIII inhibitors. These studies may contribute to the establishment of alternative immunotherapeutic approaches to prevent or minimize the clinical complication elicited by repeated FVIII exposure. It has been demonstrated that, similar to several other antigen-specific humoral immune responses, the development of anti-FVIII inhibitors seems to be dependent on the activation of FVIII-specific CD4+ T cells, including both pro- and anti-inflammatory/regulatory CD4+ T cells [4–8]. More recently, it has been suggested that discontinuous exposure to the FVIII could impair the activation of T cells, leading to atypical regulatory T cell activity, triggering the synthesis of ‘inhibitors’[3]. Additionally, other studies have described specific cytokine gene polymorphisms, including tumour necrosis factor (TNF)-α and IL-10, as important key factors for FVIII inhibitors development [9–11].
As the anti-FVIII inhibitors are usually life-threatening and difficult to treat, there is an urgent need for improved therapies to reduce the incidence of anti-FVIII inhibitors in HA patients. Despite the clinical relevance of anti-FVIII inhibitory antibodies to the impaired haemostatic activity of patients with HA, the exact immunological mechanisms underlying the production of these are still unknown. In order to understand the underlying immune response to FVIII, we have characterized the cytokine pattern of peripheral blood leucocytes elicited by pFVIII and rFVIII, using a short-term in vitro stimulation of whole blood samples from HA patients with and without FVIII inhibitors. We have examined the differential proinflammatory [interferon (IFN)-γ, TNF-α] and anti-inflammatory/regulatory (IL-4, IL-5 and IL-10) cytokine synthesis by innate (neutrophils and monocytes) and adaptive (CD4+ and CD8+ T cells and B lymphocytes) immunity.
Materials and methods
Study population
This cross-sectional study involved 85 subjects classified into three groups referred as healthy blood donors (BD) (n = 30; mean age = 31·6 ± 12·8 years); HAα-FVIII(−), HA patients without anti-FVIII inhibitors (n = 30; mean age = 27·6 ± 16·6 years); and HAα-FVIII(+), HA patients with anti-FVIII inhibitors (n = 25; mean age = 22·8 ± 15·3 years, mean anti-FVIII inhibitor level = 30·8 UB/ml). All the invited patients attended the Hemominas Foundation, Minas Gerais, Brazil. This study was approved by the ethics committee of the Hemominas Foundation and from National Commission of Ethics in Research, Brazil.
Short-term in vitro culture of whole blood
Heparinized blood samples collected in vacutainer tubes (BD Pharmingen, San Diego, CA, USA) were used for the short-term in vitro cultures as proposed by Peruhype-Magalhães et al. (2006) [12], modified as follows: control cultures (CC) were performed in 14-ml polypropylene tubes (Falcon®; BD Pharmingen, San Jose, CA, USA), using 1·25-ml aliquots of whole blood samples, incubated in the presence of 1·25 ml RPMI-1640 (Sigma, St Louis, MO, USA) for 1 h at 37°C in a 5% CO2 humidified incubator. After the preincubation period, Brefeldin A (BFA) (Sigma) was added at a final concentration of 10 µg/ml and tubes were incubated for 4 h at 37°C in a 5% CO2 humidified incubator. BFA is a used to inhibit the intracellular vesicle trafficking and is important when performing intracellular cytokine studies, as it allows the accumulation of cytokine at the Golgi complex, increasing the sensitivity for further analysis by flow cytometry.
FVIII-stimulated cultures (pFVIII and rFVIII) were, respectively, primed by preincubation of 1·25 ml aliquots of whole blood with 250 µl of plasma-derived FVIII or recombinant FVIII (ProSpec, Rehovot, Israel), diluted previously in sterile water, at a final concentration of 600 ng/ml (0·05 IU/ml for pFVIII and 2·6 IU/ml for rFVIII) for 1 h at 37°C in a 5% CO2 humidified incubator. The physiological concentration of FVIII ranges from 100 to 800 ng/ml (50–150 IU/dl), therefore 600 ng/ml of FVIII used in this study was within the reference range for human plasma [13,14]. The pFVIII- and rFVIII-stimulated tubes were then cultured in the presence of BFA at 10 µg/ml for a further 4 h at 37°C in a 5% CO2 humidified incubator.
Positive control cultures were also performed in order to evaluate the sample viability. For this purpose, 625 µl of aliquots of whole blood samples were incubated in the presence of 580 µl RPMI-1640 plus phorbol 12-myristate 13-acetate (PMA) (Sigma) at 25 ng/ml, ionomycin (Sigma) at 1 mg/ml and BFA at a 10 µg/ml final concentration. Whole blood cultures were incubated for 4 h at 37°C in a 5% CO2 humidified incubator. Blood samples were diluted 1:1 in all cultures performed. Cytokine patterns observed in the positive control cultures, named PMA-stimulated cultures, confirmed the cell antigen-responsiveness capacity for all blood samples, as demonstrated by high levels of IFN-γ+ and TNF-α+ cells observed within total gated lymphocytes (Fig. 1).
Fig. 1.
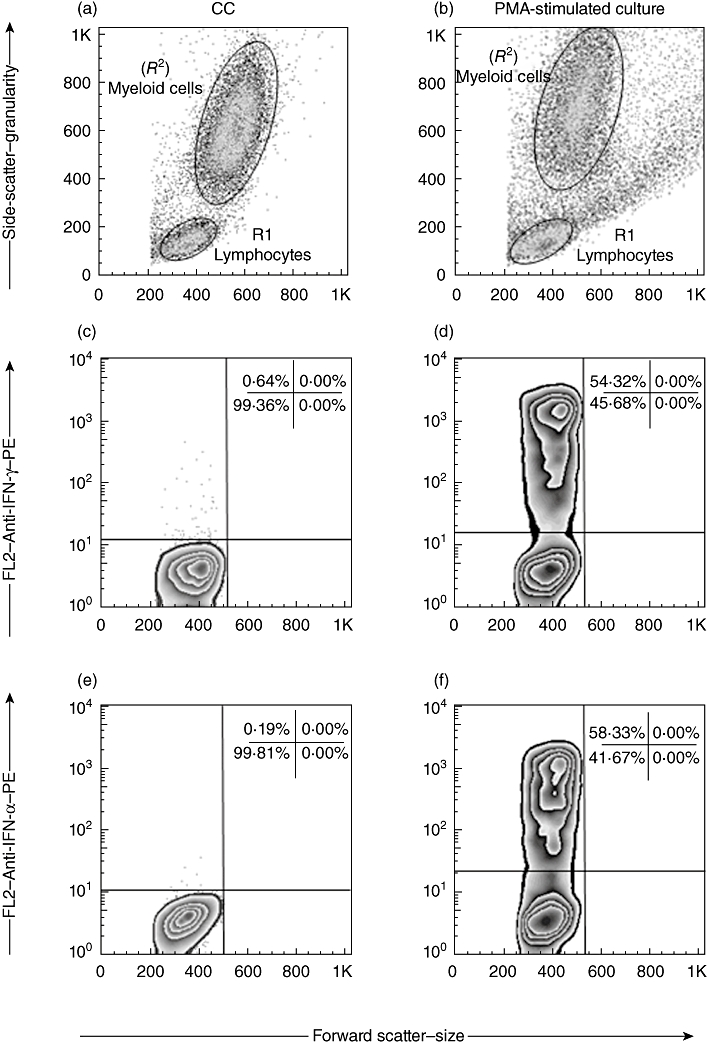
Analysis of cell viability by in vitro phorbol 12-myristate 13-acetate (PMA)-stimulation strategy. Flow cytometry charts were used to first discriminate the lymphocytes (R1) from myeloid cells (R2) in control cultures (a) and PMA-stimulated cultures (b). PMA-activated blast cells can be identified as FSCHigh (500–1k) and SSCHigh (200–600) in (b). Following, the percentage of interferon (IFN)-γ+ cells (c,d) and tumour necrosis factor (TNF)-α+ cells (e,f) within the gated lymphocytes were quantified using the cut-off edge established in the isotopic control staining. The comparative analysis of cytokine+ cells (events above the cut-off line) in the PMA-stimulated cultures and control cultures were used to evaluate cell viability. The percentage of IFN-γ+ and TNF-α+ cells (d,f, respectively) within 50–60% was used as an acceptable parameter of cell viability in all experiments.
Immunophenotyping of cell subsets and intracellular cytokines
After short-term incubation, all cultures (CC, pFVIII and rFVIII) were treated with 2 mM ethylenediamine tetraacetic acid (EDTA solution (Sigma) for 10 min at room temperature and then washed twice with phosphate-buffered saline wash (PBS-W) [PBS 0·5% (w/v) bovine serum albumin (BSA fraction V, ≥96% pure; Sigma) and 0·1% (w/v) sodium azide (Sigma)] by centrifugation at 400 g at 18°C for 7 min. The cells were immunostained in the dark for 30 min at room temperature with TriColor-labelled [TC – phycoerythrin (PE)-cyanin 5 (Cy5)] monoclonal antibodies (mAbs) (Caltag, Burlingame, CA, USA), including anti-CD4 (clone S3.5), CD8 (clone M-L233), CD14 (clone TüK4), CD16 (clone 3G8) or CD19 (clone 4G7) mAbs. After lysing/fixation procedure, the leucocytes were permeabilized by incubation with PBS permeabilization reagent (PBS-P) [PBS-W supplemented with 0·5% (w/v) saponin (Sigma)] for 10 min at room temperature. Fixed/permeabilized cells were then incubated for 30 min in the dark at room temperature in the presence of 20 µl PE-labelled anti-cytokine mAbs (IFN-γ, clone B27; TNF-α, clone MAB11; IL-4, clone MP4-25D2; IL-5, clone TRFK5; and IL-10, JES3-9D7) in the presence of PBS-P, all purchased from e-Bioscience (San Diego, CA, USA). After intracytoplasmic cytokine staining, the leucocytes were washed in PBS-W and fixed with fluorescence activated cell sorter (FACS) FIX solution (1% of paraformaldehyde, 1·02% of sodium cacodilate and 0·66% of NaCl, pH 7·2, all from Sigma) and stored at 4°C for at least 20 min before data acquisition in the flow cytometer.
Flow cytometry acquisition and analysis
After the immunophenotyping procedure, the leucocyte suspensions were run in a FACScalibur® flow cytometer (Becton Dickinson, San Jose, CA, USA) collecting a total 30 000 ungated events/sample. The acquired data were analysed using CellQuest software (Franklin Lakes, NJ, USA). Distinct gating strategies were used to analyse the cytokine-expressing leucocyte subpopulations from the innate (neutrophils and monocytes) and adaptive immunity (T cell subsets and B lymphocytes). Neutrophils were selected as SSCHighCD16High+ cells and monocytes as CD14High+ cells on FL3/anti-CD16-TC or FL3/anti-CD14-TC versus laser side-scatter (SSC) dot plots, respectively. The lymphocyte population was first selected on laser forward-scatter (FSC) versus side-scatter (SSC) dot plots. The number of gated neutrophils, monocytes and lymphocytes analysed ranged from 19 500 to 22 500, 1200 to 2100 and 6000 to 9000, respectively.
Following the initial gate selection, the frequencies of cytokine+ cells were quantified by quadrant statistics applied on FL3/anti-cell surface marker-TC versus FL2/anti-cytokine-PE dot plots, PE-Cy5-labelled antibodies were detected on the FL3 channel, while the PE-labelled antibodies were detected on the FL2 channel. Distinct tubes were used to evaluate the percentage of cytokine+ cells for each T lymphocyte subset (CD4+ and CD8+) and B cells (CD19+). Data were expressed as percentage of cytokine+ cells within gated neutrophils, monocytes and total lymphocytes. The results were assembled further to calculate the global cytokine profile as proposed by Vitelli-Avelar et al. (2008) [15]. Briefly, the median percentage for each cytokine+ cell population was calculated by taking the values obtained for each population studied [HAα-FVIII(+), HAα-FVIII(−) and BD]. Following the definition of the cut-off edge between low and high cytokine producers for each cell population, a multi-colour diagram was created to categorize individual samples as low cytokine producers, high proinflammatory cytokine producers for IFN-γ and TNF-α+ cells and high anti-inflammatory/regulatory cytokine producers for IL-4, IL-5 and IL-10+ cells. After this first assembly the samples corresponding to mixed, high proinflammatory or high anti-inflammatory/regulatory cytokine producers were identified in the multi-colour diagram.
Assessment of FVIII-specific IgG1 and IgG4
The IgG1 and IgG4 reaction to purified pFVIII was performed as described previously [16–18]. Briefly, enzyme-linked immunosorbent assay (ELISA) plates (Nunc-Immuno Maxisorp; Nunc A/S, Roskilde, Denmark) were coated with 100 µl of 1 IU/ml (12·6 µg/ml) plasma-derived FVIII (pFVIII, HemofilM; Baxter Healthcare Corporation, Deerfield, IL, USA) overnight at 4°C in PBS, as described by Bril et al. (2006) [16]. Plates were then washed with PBS-T [PBS plus Tween-20 0·1% (v/v)] and blocked for 1 h at 37°C with PBS, bovine serum albumin (BSA) 1% (w/v) (BSA fraction V, ≥96% pure; Sigma). Different dilutions of plasma samples were incubated for 2 h at 37°C using PBS, BSA 0·1% (w/v). The anti-FVIII binding was detected using a peroxidase-labelled mouse anti-human IgG1 (clone 8c/6–39) and IgG4 (clone HP-6025) (Sigma) diluted (1:1000 and 1:6000, respectively) in PBS, BSA 1% (w/v) for 1 h at 37°C. Following incubation and wash procedures with PBS, Tween-20 0·05% (v/v), 100 µl of substrate solution [4 mg of 1,2-diaminobenzene (OPD), 1·2 µl of H2O2 30% (v/v) in 10 ml of 27 mM citrate, 50 mM Na2HPO4 buffer, pH5·0; Sigma) was added to each well and plates incubated for 20 min at room temperature. Following incubation, the enzymatic reaction was stopped by adding 50 µl of 4 N H2SO4 solution to each well. The optical density (OD) was determined using a filter of 492 nm.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 software package (San Diego, CA, USA). As all data files assume a non-Gaussian distribution, statistical comparisons were carried out using the non-parametric Kruskal–Wallis test followed by Dunn's multiple comparison test to evaluate the cytokine profiles among HAα-FVIII(−), HAα-FVIII(−) and BD. The comparative analyses between control culture and stimulated culture were performed by Wilcoxon's matched-pairs test. The frequency of high and low cytokine producers was compared by contingency table analysis by χ2 test. The differences were considered significant when P-values were <0·05.
Results
Decreased levels of TNF-α+ neutrophils and higher IL-5/TNF-α ratio is the hallmark of HAα-FVIII(+)
Analysis of cytokine+ neutrophils in the control cultures showed a significant lower frequency of TNF-α+ cells in the HAα-FVIII(+) group compared to the HAα-FVIII(−) group (Fig. 2a). After in vitro FVIII stimuli (pFVIII and rFVIII), the HAα-FVIII(+) group showed a significant lower frequency of TNF-α+ cells compared to both the BD and HAα-FVIII(−) groups. However, upon in vitro FVIII stimuli, only the HAα-FVIII(−) group showed a significant reduction in the percentage of TNF-α+ neutrophils compared to the respective control culture. No differences in the percentage of IL-5+ neutrophils were observed in the control cultures. After in vitro FVIII stimuli (pFVIII and rFVIII), the HAα-FVIII(+) group presented a significant increase in the percentage of IL-5+ neutrophils compared to the respective control culture, leading to significant differences compared to both the BD and HAα-FVIII(−) groups. Analysis of the IL-5/TNF-α ratio revealed higher values in the HAα-FVIII(+) group in all experimental conditions analysed compared to the HAα-FVIII(−) group and in the pFVIII-stimulated cultures compared to both the BD and HAα-FVIII(−) groups (Fig. 2a).
Fig. 2.
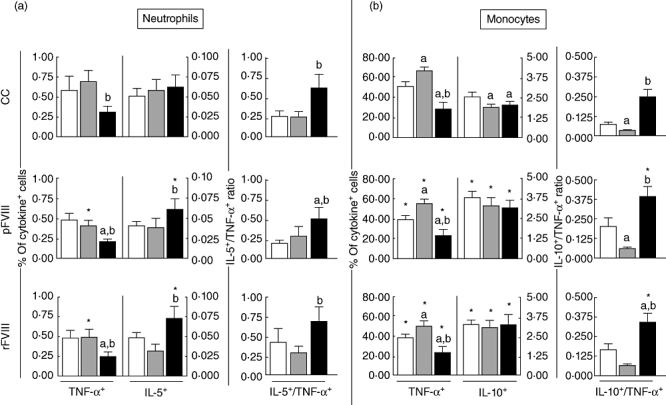
Analysis of intracytoplasmatic cytokine profile of (a) neutrophils and (b) monocytes from peripheral venous blood of blood donor (BD) (white bars), haemophilia A factor VIII [HAα-FVIII(−)] (light grey bars) and HAα-FVIII(+) (dark grey bars) groups. Data from control (CC) and FVIII-stimulated cultures (pFVIII and rFVIII). Statistical significance at P < 0·05 are represented by letters ‘a’, ‘b’ and ‘c’ for comparisons with BD, HAα-FVIII(−) and HAα-FVIII(+) groups, respectively. Significant differences between culture types in the same group were marked with ‘*’. The number of gated neutrophils and monocytes analysed ranged from 19 500–22 500 and 1200–2100, respectively.
Despite both HAα-FVIII(−) and HAα-FVIII(+) displaying decreased levels of IL-10+ monocytes, lower levels of TNF-α+ monocytes observed in HAα-FVIII(+) lead to increased IL-10/TNF-α ratio
Analysis of the cytokine + monocytes revealed lower levels of TNF-α+ cells in the HAα-FVIII(+) group compared to the BD and HAα-FVIII(−) groups in all experimental conditions tested (Fig. 2b). Although, after in vitro stimulation with FVIII, all groups presented significantly lower levels of TNF-α+ monocytes, the distinct profile between the HAα-FVIII(+), BD and HAα-FVIII(−) groups was still observed. Analysis of the IL-10+ monocytes in the control cultures showed lower levels in both the HAα-FVIII(+) and HAα-FVIII(−) groups compared to BD. After FVIII stimulation, all tested groups presented significant increase in the levels of IL-10+ monocytes compared to the respective control cultures. Analysis of the IL-10/TNF-α ratio in all groups showed a dichotomic pattern between the HAα-FVIII(−)and HAα-FVIII(+) group, with higher levels observed in the HAα-FVIII(+) group. Upon in vitro stimulation with FVIII a general increase in the IL-10/TNF-α ratio in monocytes was observed, but was more marked in the BD and HAα-FVIII(+) groups (Fig. 2b).
Increased levels of IFN-γ+, TNF-α+ and IL-4+ T cells, both CD4+ and CD8+, are observed selectively in HAα-FVIII(−)
Analysis of the cytokine+ lymphocytes showed increased levels of IFN-γ+, TNF-α+ and IL-4+ cells in CD4+ T cell subsets in the HAα-FVIII(−) group, independent of the experimental condition tested (Fig. 3a). A similar profile was observed for CD8+ T cells. However, in vitro stimulation with FVIII has a selective impact in HAα-FVIII(−), triggering increased frequency of TNF-α+ and IL-4+ CD8+ T cells. No differences were observed oin the levels of IL-10+ T cells (Fig. 3b).
Fig. 3.
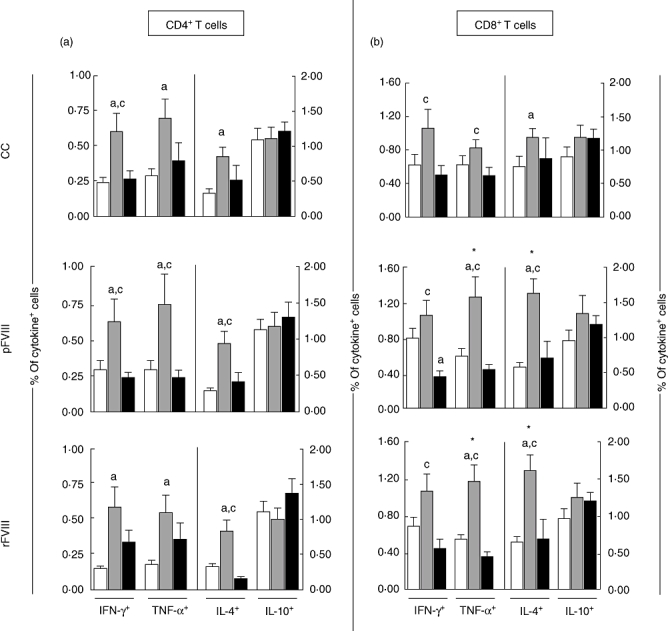
Cytokine profile of (a) CD4+ and (b) CD8+ T cells from peripheral venous blood of blood donors (BD) (white bars), haemophilia A factor VIII [HAα-FVIII(−)] (light grey bars) and HAα-FVIII(+) (dark grey bars) groups. Data from control (CC) and FVIII-stimulated cultures (pFVIII and rFVIII). Statistical significance at P < 0·05 are represented by letters ‘a’, ‘b’ and ‘c’ for comparisons with BD, HAα-FVIII(−) and HAα-FVIII(+) groups, respectively. Significant differences between culture types in the same group were marked with ‘*’. The range of cells represented was derived from 6000–9000 gated events, giving a mean of 7500 lymphocytes. For CD4+ T cells a range of 2700–4050 events were analysed. Similarly for CD8+ T cells, approximately 1920–2880 events were analysed.
Increased frequency of IL-10+ B cells and higher levels of a-FVIII IgG1 are observed in HAα-FVIII(−), whereas basal levels of cytokine+ B cells and higher levels of α-FVIII IgG4 are the major features of HAα-FVIII(+)
The cytokine profile of B cells revealed significantly higher IL-10+ cells in the HAα-FVIII(−) group compared to BD in all experimental conditions tested (Fig. 4a). No significant differences in the frequency of TNF-α+ B cells were observed between the studied groups (Fig. 4a). Analysis of the anti-FVIII antibodies demonstrated significant higher levels of FVIII-specific IgG1 antibodies in the HAα-FVIII(−) group, whereas higher levels of IgG4 anti-FVIII antibodies were observed in the HAα-FVIII(+) group (Fig. 4b).
Fig. 4.
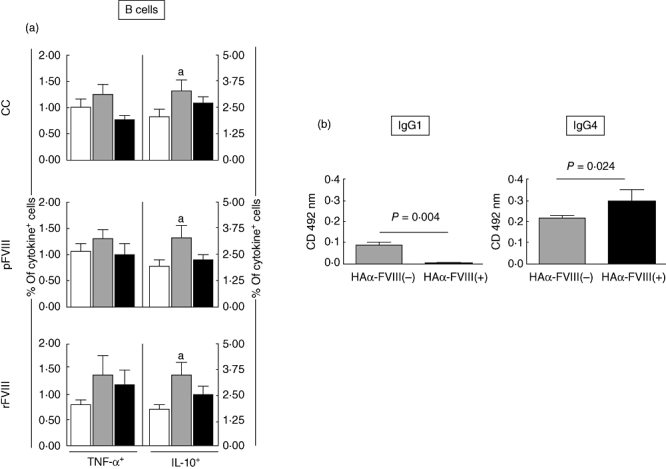
(a) Percentage of tumour necrosis factor (TNF)-α+ and interleukin (IL)-10+ B cells from peripheral venous blood of blood donors (BD) (white bars), haemophilia A factor VIII [HAα-FVIII(−)] (light grey bars) and HAα-FVIII(+) (dark grey bars) groups. Data from control (CC) and FVIII-stimulated cultures (pFVIII and rFVIII). Statistical significance at P < 0·05 is represented by letter ‘a’ for comparisons with BD group. (b) Enzyme-linked immunosorbent assay detection of anti-pFVIII IgG1 and IgG4 subtypes in plasma samples of HAα-FVIII(−) (light grey bars) and HAα-FVIII(+) (dark grey bars) groups. The range of cells represented was derived from 6000–9000 gated events, giving a mean of 7500 lymphocytes. For CD19+ B cells a range of 720–1080 events were analysed.
The global cytokine profile of peripheral blood leucocytes demonstrate a predominance of anti-inflammatory/regulatory pattern in HAα-FVIII(+)
Analysis of the cytokine profiles revealed that most individuals in the BD group had a mixed cytokine pattern. In contrast, the HAα-FVIII(+) group displayed a predominant expression of anti-inflamatory/regulatory cytokines. This profile was statistically distinct compared with that observed for the BD and HAα-FVIII(−) groups. Only patient no. 17 in the HAα-FVIII(+) group presented a non-polarized cytokine profile. The HAα-FVIII(−) group showed a mixed pattern, with a bias towards an inflammatory cytokine profile, modulated by IL-4 (Figs 5 and 6).
Fig. 5.
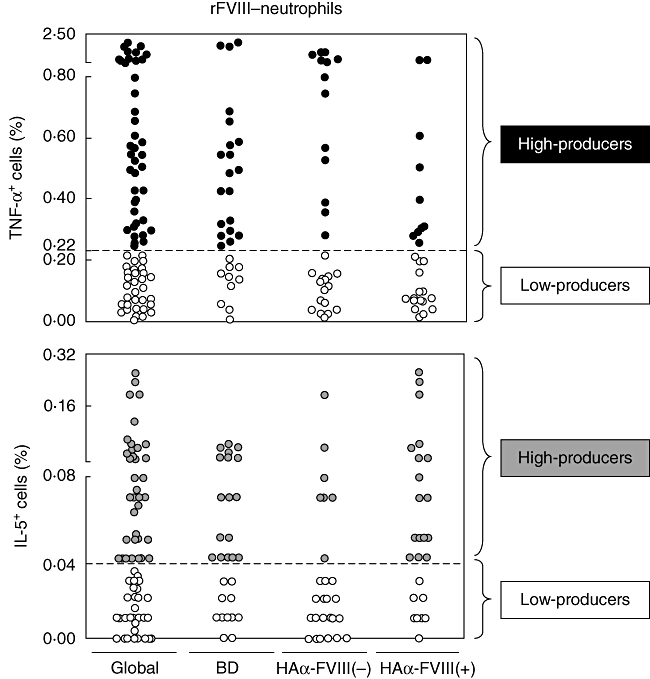
Representative scattergraphs of tumour necrosis factor (TNF)-α+ and interleukin (IL)-5+ neutrophils employed to establish the concept of low cytokine producers (white), high proinflammatory (black) or anti-inflammatory/regulatory (grey) cytokine producers. Low cytokine-producers were defined for values of cytokine+ cells lower than the global median, whereas high cytokine producers were defined for values of cytokine+ cells higher or equal the global median cut-off.
Fig. 6.
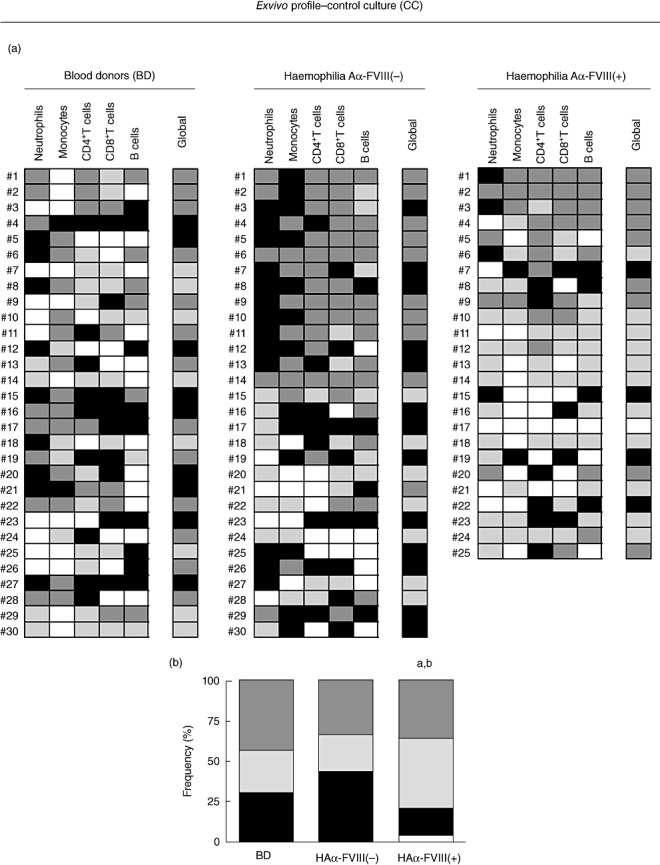
(a) Control culture cytokine profile represented by colour diagram: low-cytokine producers ( ); high proinflammatory cytokine producers (
); high proinflammatory cytokine producers ( ); high anti-inflammatory/regulatory cytokine producers (
); high anti-inflammatory/regulatory cytokine producers ( ); and mixed cytokines producers (
); and mixed cytokines producers ( ). Global cytokine profile was determined by the combination of individual cells patterns. (b) Overall cytokine patterns representing the frequency of pro- and anti-inflammatory/regulatory and mixed cytokine profiles of blood donors (BD(, haemophilia A factor VIII [HAα-FVIII(−)] and HAα-FVIII(+) groups. Statistical significance at P < 0·05 are represented by letters ‘a’ and ‘b’ for comparison with BD and HAα-FVIII(−) groups, respectively.
). Global cytokine profile was determined by the combination of individual cells patterns. (b) Overall cytokine patterns representing the frequency of pro- and anti-inflammatory/regulatory and mixed cytokine profiles of blood donors (BD(, haemophilia A factor VIII [HAα-FVIII(−)] and HAα-FVIII(+) groups. Statistical significance at P < 0·05 are represented by letters ‘a’ and ‘b’ for comparison with BD and HAα-FVIII(−) groups, respectively.
In vitro stimuli with pFVIII or rFVIII confirm further the shift of the global cytokine profile of HAα-FVIII(+) towards an anti-inflammatory/regulatory pattern
After in vitro stimulation with pFVIII and rFVIII, despite minor changes the global cytokine profiles still remained distinct between the studied groups. The BD group sustained a mixed cytokine profile, whereas the anti-inflammatory/regulatory cytokine profile was maintained in the HAα-FVIII(+) group. The in vitro stimulation with pFVIII led to a slight change in the global cytokine pattern in the HAα-FVIII(−) group. However, the global profile in this group still displayed a higher inflammatory profile compared to HAα-FVIII(+) (Fig. 7).
Fig. 7.
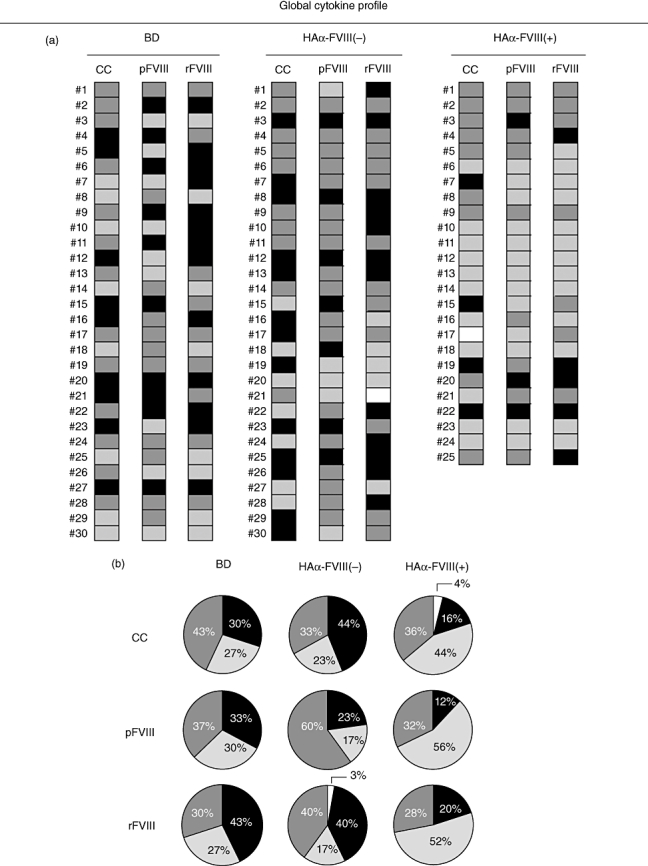
(a) Global cytokine profile determined by the combination of individual cells patterns of blood donors (BD), haemophilia A factor VIII [HAα-FVIII(−)] and HAα-FVIII(+) groups in control and stimulated (pFVIII and rFVIII) cultures. (b) Overall cytokine patterns representing the frequency of pro- and anti-inflammatory/regulatory and mixed cytokine profiles of BD, HAα-FVIII(−) and HAα-FVIII(+) groups in control and stimulated (pFVIII and rFVIII) cultures.
Discussion
Treatment with FVIII infusions may induce the development of anti-FVIII inhibitors in approximately 25% of HA patients [19]. In the past three decades, advances in clinical immunology research have improved understanding of the mechanism underlying the development of anti-FVIII inhibitors that affect the FVIII therapy negatively. Several factors, including those related to the therapeutic scheme as well as those associated with the patient immune response [4–11], seems to affect this phenomenon. Several key points have been evaluated, including the impact of FVIII gene mutation on immune response pathways [20,21], the polymorphisms of HLA genes and also cytokine genes [9–11,22,23] as relevant predictive factors of differential cell activation and cytokine synthesis during the development of anti-FVIII inhibitors in HA patients. Aiming to focus further on the role of the immune system for establishment/maintenance of the FVIII inhibitor profile, in this study we have characterized the cytokine profile of peripheral blood leucocytes from HA patients with and without anti-FVIII inhibitors, prior to and after in vitro stimulation with FVIII.
Our data highlighted a shift towards an anti-inflammatory/regulatory immune response in the innate immunity cells from HAα-FVIII(+) patients, as demonstrated by an increased IL-5/TNF-α ratio in neutrophils and IL-10/TNF-α ratio in monocytes. Despite that the FVIII stimuli in vitro was able to trigger a general increase of IL-10+ monocytes, the predominance of an anti-inflammatory/regulatory global cytokine profile was sustained exclusively in the HAα-FVIII(+) group. These findings emphasize further that the anti-inflammatory/regulatory-predominant cytokine profile in the innate immunity cells is related somehow to the development/maintenance of FVIII inhibitors. Our data also demonstrated that the defined anti-inflammatory/regulatory global cytokine profile observed for the HAα-FVIII(+) group was still observed, even after in vitro stimulation with pFVIII or rFVIII. Conversely, analysis of the innate immunity cells from the HAα-FVIII(–) group showed levels of cytokines in neutrophils compared to the BD group with a dominant proinflammatory cytokine profile. These patients presented increased levels of TNF-α+ monocytes that lead to a lower IL-10/TNF-α ratio, consistent with the predominance of a proinflammatory immune profile. These data are consistent with the association of haplotypes that define intermediate synthesis of IL-10 with the inhibitor's development [11].
Analysis of the adaptive immune compartment demonstrated that the HAα-FVIII(+) group showed basal levels of cytokines+ cells within their T and B lymphocytes, comparable with those observed in the BD reference group. This microenvironment deficient of T cell cytokines and the presence of IL-5 and IL-10 derived, respectively, from neutrophils and monocytes may favour the activation of B cells towards the synthesis of IgG4. We consider that the deficient co-stimulation promoted by CD4+ T cells during the B cell activation play a relevant role driving the immune response towards the synthesis of T independent antibody response, especially in the enriched monocyte-derived IL-10 microenvironment observed in the HAα-FVIII(+) group. It has been demonstrated that there is a direct implication of IL-10 in humoral responses, particularly in compartments where the T–B cell interplay determines the subsequent immune response, increasing IgG4 secretion [24]. The paradigm of B lymphocyte activation consists of a two-signal model that has been proposed by Bretcher and Cohn (1970) [25]. This proposal has developed from the premise that recognition of antigen alone is insufficient to stimulate naive B cells, as this could potentially induce autoreactive responses, whereas the cognate T–B cell interaction would lead to a full B cell response. Recent evidence has suggested that T cell cytokine-deficient B cell activation is part of the humoral immune response, and therefore the antigen alone, or antigen plus signals provided by cells other than T cells, can provide all the necessary signals to induce a B cell response.
Moreover, analysis of the adaptive immune compartment revealed that the HAα-FVIII(−) group presented a typical proinflammatory modulated cytokine profile typified by increased levels of IFN-γ+ and TNF-α+ T cells along with enhanced levels of IL-4+ T cells, both CD4+ and CD8+ subsets. Our data also demonstrated that this proinflammatory modulated cytokine profile was preserved after in vitro stimuli with FVIII, as evidenced by increased synthesis of TNF-α and IL-4+ by CD8+ T cells, observed selectively in the HAα-FVIII(−) group. According to our findings, recent study demonstrated that all T cell clones from one patient without FVIII inhibitors displayed along with the ability to produce IL-4, a predominance of a proinflammatory immune response characterized by enhanced levels of IFN-γ[26]. Further analysis of our data demonstrated that a typical IL-10-modulated immune profile in B cells and higher levels of α-FVIII IgG1 with no apparent inhibitory functions are observed in HAα-FVIII(−). Previous studies have suggested that the secretion of IgG1 antibodies is dependent upon B cells synthesizing IL-10 [27]. Analysis of the global cytokine profile revealed further that peripheral blood leucocytes from the HAα-FVIII(−) group presented a mixed cytokine pattern in the presence of in vitro pFVIII stimuli, with a predominant proinflammatory immune response in the presence of rFVIII, observed similarly in the BD reference group, contrasting with the predominance of an anti-inflammatory/regulatory pattern in the HAα-FVIII(+) group. These results are in agreement with the data obtained from the single cell analysis. Based on these findings, we hypothesize that the proinflammatory modulated immune response determined by increased IFN-γ and TNF-α produced by T cells, controlled by increased levels of IL-4+ T cells and IL-10+ B cells, may favour the synthesis of anti-FVIII IgG1, avoiding the development of anti-FVIII IgG4 inhibitors. It is possible that HAα-FVIII(−) patients control the development of anti-FVIII inhibitory IgG4 antibodies using a dual pathway by: (i) modulating the proinflammatory immune response by the recruitment of IL-4+ CD4+ and CD8+ T cells and (ii) up-regulating the production of IL-10 by B cells favouring the synthesis of anti-FVIII IgG1 antibodies in a T cell-dependent fashion. The production of IgG1 mediated by proinflammatory T cell-derived cytokines stimulation is also well established [28].
Based on these findings, we hypothesize that the anti-inflammatory/regulatory-predominant microenvironment determined by neutrophils and monocytes, along with the basal levels of cytokines produced by T cells and B lymphocytes, may favour the synthesis of anti-FVIII IgG4 with inhibitory activity. Previously, reports have suggested the relevance of an anti-inflammatory/regulatory immune response, with the involvement of CD4+ T cells to drive the synthesis of anti-FVIII IgG4 inhibitors [3,7]. In fact, it has been suggested that a proinflammatory cytokine pattern may be important in initiating the anti-FVIII immune response and the shift towards an anti-inflammatory/regulatory cytokine profile in CD4+ T cells may represent the striking point to the development of a strong inhibitor production [3]. Our data suggested that the maintenance of anti-FVIII production in our patients, despite being related to an anti-inflammatory/regulatory cytokine pattern, did not count with a relevant T cell response. It is possible that differences in the methodological approach used in our study, i.e. the use of whole blood samples and autologous plasma in the cultures to reproduce the in vivo microenvironment, revealed these phenomena not previously reported when working with isolated PBMC, CD4+ T cell clones and absence of autologous plasma.
According to our hypothesis, during the initial FVIII infusions there is probably a T cell-dependent proinflammatory guided response that leads to anti-FVIII IgG1 non-inhibitory antibody. After repeated exposures to intravenous FVIII, with higher frequencies in severely affected patients, the shift towards an anti-inflammatory/regulatory T cell cytokine-deficient pattern may occur, favouring the class-switch to IgG4 anti-FVIII inhibitors. It is possible that the establishment of immunotherapeutic protocols should be implemented early after the beginning of FVIII treatment to maintain the T cell-dependent immunity and avoid the shift towards a T cell anti-inflammatory/regulatory cytokine-deficient response that favours the production of anti-FVIII inhibitors and at the same time improve the life quality and treatment success of HA patients. An important issue to consider in future investigations is the kinetic conversion of proinflammatory towards an anti-inflammatory/regulatory immune response against FVIII during the treatment of HA patients. It is possible that the maintenance of a T cell-mediated immune response with a proinflammatory modulated profile may be the key to avoid the development of anti-FVIII inhibitory IgG4 immune responses. In this context, it is also relevant to define the role of regulatory T cells (Tregs) and Bregs cells in the control of the anti-FVIII immune response in patients who do not develop FVIII inhibitors.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG – BPD-00054/10) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) financial support. O.A.M.F. and A.T.C. are thankful to Conselho Nacional de Desenvolvimento Cinetífico e Tecnológico (CNPq) for the research fellowship (PQ) and C.V.R. acknowledges the FAPEMIG for the BIP fellowship programme.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339:594–8. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 2.Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95:1324–9. [PubMed] [Google Scholar]
- 3.Hu G, Guo D, Key NS, Conti-Fine BM. Cytokine production by CD4+ T cells specific for coagulation factor VIII in healthy subjects and haemophilia A patients. Thromb Haemost. 2007;97:788–94. [PubMed] [Google Scholar]
- 4.Singer ST, Addiego JE, Reason DC, Lucas AH. T lymphocyte proliferative responses induced by recombinant factor VIII in hemophilia A patients with inhibitors. Thromb Haemost. 1996;76:17–22. [PubMed] [Google Scholar]
- 5.Reding MT, Wu H, Krampf M, et al. CD4+ T cell response to factor VIII in hemophilia A, acquired hemophilia, and healthy subjects. Thromb Haemost. 1999;82:509–15. [PubMed] [Google Scholar]
- 6.Reding MT, Wu H, Krampf M, et al. Sensitization of CD4+ T cells to coagulation factor VIII: response in congenital and acquired hemophilia patients and in healthy subjects. Thromb Haemost. 2000;84:643–52. [PubMed] [Google Scholar]
- 7.Reding MT, Lei S, Lei H, et al. Distribution of Th1- and Th2-induced anti-factor VIII IgG subclasses in congenital and acquired hemophilia patients. Thromb Haemost. 2002;88:568–75. [PubMed] [Google Scholar]
- 8.Hu G-L, Okita DK, Diethelm-Okita BM, Conti-Fine BM. Recognition of coagulation factor VIII by CD4+ T cells of healthy humans. J Thromb Haemost. 2003;1:2159–66. doi: 10.1046/j.1538-7836.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- 9.Astermark J, Oldenburg J, Carlson J, et al. Polymorphisms in the TNFA gene and the risk of inhibitor development in patients with hemophilia A. Blood. 2006;108:3739–45. doi: 10.1182/blood-2006-05-024711. [DOI] [PubMed] [Google Scholar]
- 10.Astermark J, Oldenburg J, Pavlova A, Berntorp E, Lefvert AK, MIBS Study Group Polymorphisms in the IL10 but not in the IL1beta and IL4 genes are associated with inhibitor development in patients with hemophilia A. Blood. 2006;107:3167–72. doi: 10.1182/blood-2005-09-3918. [DOI] [PubMed] [Google Scholar]
- 11.Chaves D, Belisário A, Castro G, Santoro M, Rodrigues C. Analysis of cytokine genes polymorphism as markers for inhibitor development in haemophilia A. Int J Immunogenet. 2010;37:79–82. doi: 10.1111/j.1744-313X.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- 12.Peruhype-Magalhães V, Martins-Filho OA, Prata A, et al. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin Exp Immunol. 2006;146:124–32. doi: 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balagué C, Zhou J, Dai Y, et al. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood. 2000;95:820–8. [PubMed] [Google Scholar]
- 14.Provan D, Singer CRJ, Baglin T, Lilleyman SJ. Oxford handbook of clinical haematology. 3nd edn. New York: Oxford University Press; 2009. [Google Scholar]
- 15.Vitelli-Avelar DM, Sathler-Avelar R, Teixeira-Carvalho A, et al. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol. 2008;68:516–25. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 16.Bril WS, Van Helden PM, Hausl C, et al. Tolerance to factor VIII in a transgenic mouse expressing human factor VIII cDNA carrying an Arg(593) to Cys substitution. Thromb Haemost. 2006;95:341–7. doi: 10.1160/TH05-08-0559. [DOI] [PubMed] [Google Scholar]
- 17.Van Helden PM, Van Den Berg HM, Gouw SC, et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. 2008;142:644–52. doi: 10.1111/j.1365-2141.2008.07232.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaves DG, Velloso-Rodrigues C, Moreau V, et al. Reactivity profile of anti-factor VIII antibodies with designed synthetic peptides mimicking epitopes of the C2 and a1 domains. Br J Haematol. 2008;141:708–15. doi: 10.1111/j.1365-2141.2008.07043.x. [DOI] [PubMed] [Google Scholar]
- 19.Reding MT. Immunological aspects of inhibitor development. Haemophilia. 2006;12:30–5. doi: 10.1111/j.1365-2516.2006.01363.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee CA, Lillicrap D, Astermark J. Inhibitor development in hemophiliacs: the roles of genetic versus environmental factors. Semin Thromb Hemost. 2006;32:10–14. doi: 10.1055/s-2006-946909. [DOI] [PubMed] [Google Scholar]
- 21.Boekhorst J, Lari GR, D'Orion R, et al. Factor VIII genotype and inhibitor development in patients with haemophilia A: highest risk in patients with splice site mutations. Haemophilia. 2008;14:729–35. doi: 10.1111/j.1365-2516.2008.01694.x. [DOI] [PubMed] [Google Scholar]
- 22.Hay CR, Ollier W, Pepper L, et al. HLA class II profile: a weak determinant of factor VIII inhibitor development in severe haemophilia A. Thromb Haemost. 1997;77:234–7. UKHCDO Inhibitor Working Party. [PubMed] [Google Scholar]
- 23.Oldenburg J, Picard JK, Schwaab R, Brackmann HH, Tuddenham EG, Simpson E. HLA genotype of patients with severe haemophilia A due to intron 22 inversion with and without inhibitors of factor VIII. Thromb Haemost. 1997;77:238–42. [PubMed] [Google Scholar]
- 24.Satoguina JS, Weyand E, Larbi J, Hoerauf AT. Regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–26. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 25.Bretcher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1040–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 26.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114:1423–8. doi: 10.1182/blood-2009-01-200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brière F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (slgD) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–62. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbas A, Murphy K, Sher A. Functional diversity of helper T cell lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]


