Abstract
Dendritic cells (DC) and regulatory T cells (Tregs) are vital to the development of transplant tolerance. Curcumin is a novel biological agent extracted from Curcuma longa (turmeric), with anti-inflammatory and anti-oxidant activity mediated via nuclear factor (NF)-κB inhibition. We investigated the immunomodulatory effects of curcumin on human monocyte-derived and murine DC. Human monocyte-derived DC (hu-Mo-DC) were generated in the presence (CurcDC) or absence (matDC) of 25 µM curcumin, and matured using lipopolysaccharide (1 µg/ml). DC phenotype and allostimulatory capacity was assessed. CD11c+ DC were isolated from C57BL/6 mice, pretreated with curcumin and injected into BALB/c mice, followed by evaluation of in vivo T cell populations and alloproliferative response. Curcumin induced DC differentiation towards maturation-arrest. CurcDC demonstrated minimal CD83 expression (<2%), down-regulation of CD80 and CD86 (50% and 30%, respectively) and reduction (10%) in both major histocompatibility complex (MHC) class II and CD40 expression compared to matDC. CurcDC also displayed decreased RelB and interleukin (IL)-12 mRNA and protein expression. Functionally, CurcDC allostimulatory capacity was decreased by up to 60% (P < 0·001) and intracellular interferon (IFN-γ) expression in the responding T cell population were reduced by 50% (P < 0·05). T cell hyporesponsiveness was due to generation of CD4+CD25hiCD127loforkhead box P3 (FoxP3)+ Tregs that exerted suppressive functions on naïve syngeneic T cells, although the effect was not antigen-specific. In mice, in vivo infusion of allogeneic CurcDC promoted development of FoxP3+ Tregs and reduced subsequent alloproliferative capacity. Curcumin arrests maturation of DC and induces a tolerogenic phenotype that subsequently promotes functional FoxP3+ Tregsin vitro and in vivo.
Keywords: curcumin, dendritic cells, maturation arrest, regulatory T cells
Introduction
Dendritic cells (DC) are a heterogeneous population of professional antigen-presenting cells (APC) that potently initiate primary immune responses and possess the ability to regulate both innate and adaptive immunity [1,2]. DC are critical to the maintenance of central and peripheral tolerance [3] and modify adaptive T cell proliferation essential to the immunological response to infection, inflammation and alloimmunity [4]. DC capacity to regulate T cell responses reflects provision of critical signals mediated via the co-stimulation molecule B7 [5,6] and tumour necrosis factor (TNF) family members [7,8], in addition to cytokines [9], whose expression is altered by the microenvironment sensed through DC surface receptors, including Toll-like receptors (TLR) [10] and CD40 [7].
DC subsets and immature or maturation-resistant DC are more likely to promote tolerance [11,12], although phenotypically mature DC (myeloid and plasmacytoid) demonstrate tolerogenic potential [13–16]. The ratio of resting/immature to activated/mature DC may also determine tolerance induction [17–19], as may a T helper type 2 (Th2)-polarized helper–T cell response [20,21]. Tolerogenic DC display phenotypic maturation and but lack functional maturation, and are characterized by high major histocompatibility complex (MHC) expression, low co-stimulatory molecule expression, an inability to generate biologically active interleukin (IL)-12 and high IL-10 production [11,12,22]. The tolerizing capabilities of DC have been linked to the induction or expansion of regulatory T cells (Tregs) [23]. Tregs predominate in the CD4+CD25hi T cell fraction and the transcription factor forkhead winged helix protein-3 (FoxP3) [24] is accepted widely as the most specific marker for Tregs, although in humans it may be expressed transiently by conventional (effector) T cells. Additional markers, such as CD62L [25], an absence of CD127 [26] and intracellular cytotoxic T lymphocyte antigen (CTLA)-4 [27,28] have been used to define Tregs.
The production of in vitro-propagated maturation-arrested DC is a promising method for inducing tolerance. A variety of immunosuppressive drugs have shown this capacity through nuclear factor (NF)-κB inhibition, including Bay 11-7082 [29], corticosteroids [30], cyclosporin [31], mycophenolate mofetil [32], rapamycin [33], deoxyspergualin (DSG) [34] and vitamin D3[35]. Antigen-exposed DC lacking the RelB subunit of NF-κB also have the capacity to suppress previously primed immune responses after transfer to primed recipients, indicating that RelB is required for functional DC maturation [29]. Curcumin, an extract of Curcuma longa (turmeric), has a long history of medicinal use. More recently, anti-oxidant [36,37], anti-inflammatory [38], anti-microbial [39–41] and anti-proliferative [42] properties have been identified. Its pleiotropic activity arises from suppression of NF-κB activity via inhibition of I kappa B kinase (IKK)-α phosphorylation [43] and prevention of nuclear translocation of NF-κBp65 subunit [44]. We demonstrate in this study that curcumin, through its inhibitory effect on NF-κB, directs DC differentiation towards a tolerogenic phenotype that expands FoxP3+ Tregsin vitro and in vivo.
Methods
Generation of human monocyte-derived DC (hu-Mo-DC)
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats from healthy donors (South Australian Red Cross Blood Service) using Ficoll-Hypaque (Amersham Biosciences, Brown Deer, WI, USA) density gradient centrifugation, as described previously [45]. PBMC were cultured for 7 days in complete medium (CM): RPMI with 10% v/v heat-inactivated fetal bovine serum (Sigma Aldrich, St Louis, MO, USA), 2 mM l-glutamine (MultiCel; Trace Scientific, Noble Park, Victoria, Australia), penicillin–streptomycin 50 U/ml (MultiCel; Trace Scientific), supplemented with 800 U/ml human recombinant granulocyte colony-stimulating factor (rhGM-CSF; Sandoz Australia, Sydney, Australia) and 400 U/ml rhIL-4 (eBiosciences, San Diego, CA, USA). DC were treated with 25 µM curcumin (Sigma Aldrich) and matured using lipopolysaccharide 1 µg/ml (LPS; R&D Systems, Minneapolis, MN, USA).
Phenotypic analysis of hu-Mo-DC or T cells
DC were stained with the following fluorochrome-conjugated anti-human monoclonal antibodies (mAb): CD14 (MY-4), CD209 (DCN46), CD80 (L307·4), CD86 (FUN1), CD83 (HB15e), human leucocyte antigen D-related (HLA-DR) (G46-6) from BD Biosciences (San Jose, CA, USA), CD40 (mAB89; Immunotech, Marseille, France) and immunoglobulin-like transcript 2 (ILT2) (HP-F1; Beckman Coulter, Hialeah, FL, USA). Unconjugated ILT4 (42D1, Santa Cruz Biotechnology, Santa Cruz, CA, USA), PDL1 and PDL2 (M1H1 and M1H18, respectively; R&D Systems) mAb were detected with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Ig)G1 secondary antibody (Southern Biotech, Birmingham, AL, USA). T cells were stained for expression of CD3 (UCHT1), CD4 (OKT4), CD25 (BC96), IFN-γ (45.B3), CD127 (eBioRDR5), IL-17 (eBio64DEC17), CD62L (DREG-56) and FoxP3 (PCH101, all eBioscience, San Diego, CA, USA). T cells were stained immediately following primary mixed lymphocyte reaction (MLR) to assess intracellular IFN-γ, stimulated with staphylococcal enterotoxin B (SEB; 1 µg/ml, Sigma Aldrich) for 24 h to detect IL-17, or rested for 48 h prior to staining for FoxP3 (to eliminate transient FoxP3 expression by conventional T cells). Briefly, cells were suspended in phosphate-buffered saline (PBS) [with 1% w/v HI fetal bovine serum (FBS) and 0·1% w/v sodium azide] and Fc-receptor binding was inhibited by incubation with 1% rabbit serum (Sigma Aldrich). Cells were incubated at 4°C for 20 min with mAb, fixed with fluorescence activated cell sorter (FACS) lysing solution (BD Biosciences) or Fix/Perm solution (eBioscience) for intracellular staining. For unconjugated antibodies, secondary antibody was added at 4°C for 30 min. Appropriately conjugated, isotype-matched IgG antibodies were used as negative controls. Flow cytometry was performed using FACSCanto (Becton Dickinson, San Jose, CA, USA) and analysed using FACS diva version 6·1·1 (BD Pharmingen, San Diego, CA, USA).
MLR
Primary MLR
γ-irradiated (30 gray) DC were washed extensively, and used as stimulators of allogeneic T cells enriched by passage of monocyte-depleted PBMC through a nylon-wool column (Boehringer Mannheim Biochemica, Indianapolis, IN, USA). Where indicated, fluorescence-activated sorted CD4+ T cells from monocyte-depleted PBMC were used.
Secondary MLR
Five days after co-culture, T cells were isolated using anti-CD3 immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells (1 × 104/well) were cultured with naive syngeneic T cells at various ratios (1:1–1:20) or restimulated in the presence of irradiated mature DC (1 × 104/well, from the same or third-party donor).
All cells were cultured in CM in quintuplicate wells in a 96-well round-bottomed plate at 37°C in 5%CO2 for 5 days. In the final 16–18 h of incubation 1 µCi of [3H]-thymidine (Amersham Biosciences) was added. Cells were harvested onto glass-fibre filters (Wallac Oy, Turku, Finland) and counted in a Microbeta® Counter (Tomtec, Hamden, CT, USA). Results are expressed as mean counts per minute (cpm) ± standard deviation (s.d.).
Immunofluorescence for NF-κBp50
DC were stained for NF-κBp50 as described previously [46]. Briefly, cells were adhered to Lab-Tek® chamber slides (Nunc Nalge International, Rochester, NY, USA), incubated with NF-κBp50 (clone H119; Santa Cruz Biotechnology) and washed twice with PBS. Secondary antibody (FITC goat anti-rabbit IgG; Santa Cruz Biotechnology) was added for 30 min, and 4′,6-diamindino-2-phenylindole (DAPI; Molecular Probes) for 5 min. Slides were washed three times in PBS, mounted with fluorescent mounting medium (Dako, Glostrup, Denmark) and imaged on an ApoTome microscope (Zeiss, Oberkochen, Germany).
Real-time PCR
Total RNA was extracted using Qiagen RNeasy® Mini Kits (Qiagen, Hilden, Germany) as per manufacturer's instructions and quantitated using the Experion® RNA Stdsens Analysis Kit (Bio-Rad Laboratories, Hercules, CA, USA). One microgram of RNA was reverse-transcribed and PCR amplification was performed using in a Rotorgene 2000 real-time cycler (Corbett Research, Mortlake, Australia). Reactions were performed using AmpliTaq Gold® PCR Master Mix (Applied Biosystems, Foster City, CA, USA), SYBR Green (Adelab, Adelaide, Australia), designed primers (Geneworks, Adelaide) and cDNA of template, standard, or non-template control. Results were and normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT1), and analysed with Rotor-gene version 5·0 (Corbett Research).
Enzyme-linked immunosorbent assay (ELISA)
IL-12p70 and IL-10 concentrations were quantified in 24-h supernatants from DC (1 × 106/ml) cultured with rhIFN-γ (20 ng/ml; Prospec-Tany, Rehovot, Israel) and rhCD40L (5 µg/ml; R&D) using the ELISA Ready-SET-GO! Kit (eBiosciences) according to the manufacturer's instructions.
Animals
Male 8–12-week-old C57BL/6 (H2Kb), BALB/c (H2Kd) and C3H (H2Kk) mice were purchased from and maintained under pathogen-free conditions in the Institute of Medical and Veterinary Science Animal Facility (IMVS, Adelaide, Australia). The University of Adelaide and IMVS animal ethics committees approved all experimental protocols.
Isolation of murine DC and assessment of DC localization
CD11c+ DC were purified from spleen and lymph nodes of C57BL/6 mice using immunomagnetic beads (Miltenyi Biotec) and cultured with or without curcumin (25 µM) overnight. Cells (1 × 106) were washed extensively and resuspended in PBS for intravenous injection into BALB/c mice. DC were labelled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiI, 2 µg/ml; Molecular Probes, Eugene, OR, USA) to assess systemic distribution. Seven days after injection, liver, spleen, kidney, mesenteric and inguinal lymph nodes were isolated, embedded in octreotide (OCT) and snap-frozen. Seven µm cryosections were fixed in ice-cold acetone (Sigma Aldrich) for 5 min and dried at room temperature. Sections were stained at room temperature with anti-CD11c (clone N418; Biolegend, San Diego, CA, USA), anti-DEC205 (clone NLDC-145; Miltenyi) and DAPI, washed in PBS, mounted and imaged.
Isolation of splenocytes and proliferation assay
Mononuclear cells (MNC) from spleen were purified using density gradient separation and Tregs were identified by flow cytometric staining for CD4 (clone L3T4), CD25 (clone PC61·5) and FoxP3 (clone FJK-16s, all eBioscience), as described above. MNC were added to γ-irradiated MNC from C57BL/6 (same donor) or C3H (H2Kk, third-party) in a 1:1 ratio (1 × 105/well) in a 5-day MLR. Radioisotope incorporation and flow cytometric assessment of the responding T cell population was determined as described above.
Statistical analysis
Data are expressed as mean + s.d. Statistical comparison between groups was performed using the Student's t-test (parametric variables) using stata version 11·0 (Stata Corporation, College Station, TX, USA), with P < 0·05 deemed as significant.
Results
Curcumin modifies the expression of DC positive co-stimulatory and negative regulatory molecules
Hu-Mo-DC were generated from PBMC in 7-day cultures with GM-CSF and IL-4. Treatment with curcumin did not affect hu-Mo-DC development, as demonstrated by the loss of CD14 (<10% expression) and high levels of DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) expression (>90%). Pretreatment of DC with curcumin (CurcDC) prior to incubation with LPS inhibited up-regulation of cell-surface expression of positive co-stimulatory molecules: CD80, CD86, MHC class II, CD40 and CD83 (Fig. 1a). CurcDC phenotype was similar to immDC. Curcumin (and subsequent LPS exposure) also down-regulated expression of the negative regulatory molecules PD-L1 and PD-L2 by 30% and 75%, respectively (compared to matDC), although expression of ILT2 and ILT4 was not affected significantly (Fig. 1b). These data indicate that preincubation of hu-Mo-DC with curcumin prior to stimulation with LPS reduces the capacity of DC to express cell surface co-stimulatory, maturation and regulatory molecules that are normally induced by a maturation stimulus.
Fig. 1.
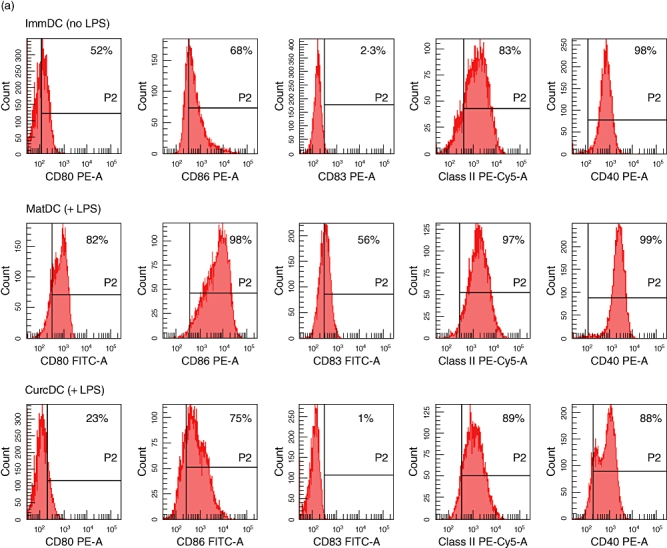
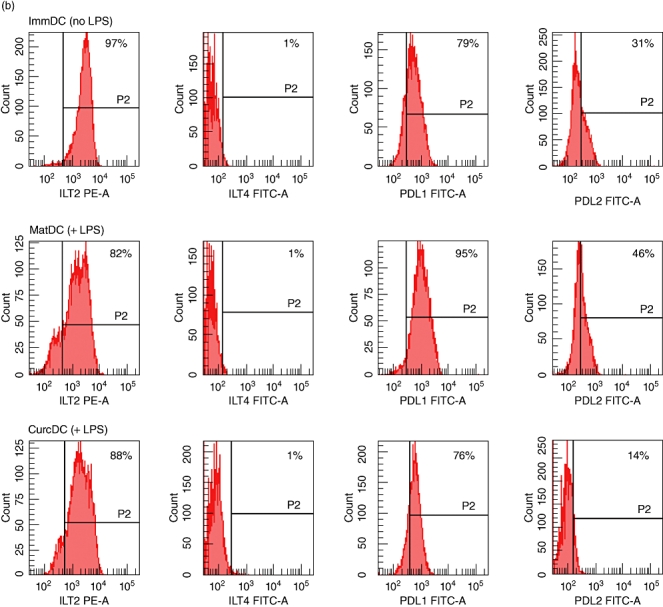
Curcumin alters positive and negative cell surface molecule expression on human monocyte-derived dendritic cells (hu-Mo-DC). Hu-Mo-DC were generated as described in Materials and methods, exposed to curcumin (or complete medium) and matured with lipopolysaccharide (LPS) (where applicable) for 24 h. Flow cytometry was used to compare the phenotypic profile of immature DC (immDC, no LPS), mature DC (matDC, exposed to LPS) and curcumin DC (CurcDC) (exposed to LPS). Data are shown gated to the DC population by forward-/side-scatter and the line (P2) indicates isotype control. (a) CurcDC failed to up-regulate positive co-stimulatory molecules when exposed subsequently to LPS, particularly CD83. (b) Curcumin inhibited expression of negative regulatory molecules PDL1 and PDL2 compared to both matDC and immDC. Immunoglobulin-like transcript 2 (ILT2) and ILT4 expression remained unchanged. These figures are from an individual experiment representative of four performed.
CurcDC are maturation-arrested
RelB-deficient DC promote immune suppression [29]; consequently, we next examined NF-κB activity after DC exposure to curcumin in vitro. CurcDC were incubated overnight with LPS to promote NF-κB signalling. Reverse transcription–polymerase chain reaction (RT–PCR) results demonstrated significantly reduced expression of RelB, compared to LPS-treated controls (mat DC, Fig. 2a), consistent with the theory that curcumin arrests DC maturation in response to activation signals [38,44,46,47]. Confocal microscopy confirmed significant cytoplasmic fluorescence and nuclear translocation of NF-κBp50 in matDC (Fig. 2b). However, CurcDC demonstrated little cytoplasmic and nuclear NF-κB-p50 binding (Fig. 2c).
Fig. 2.
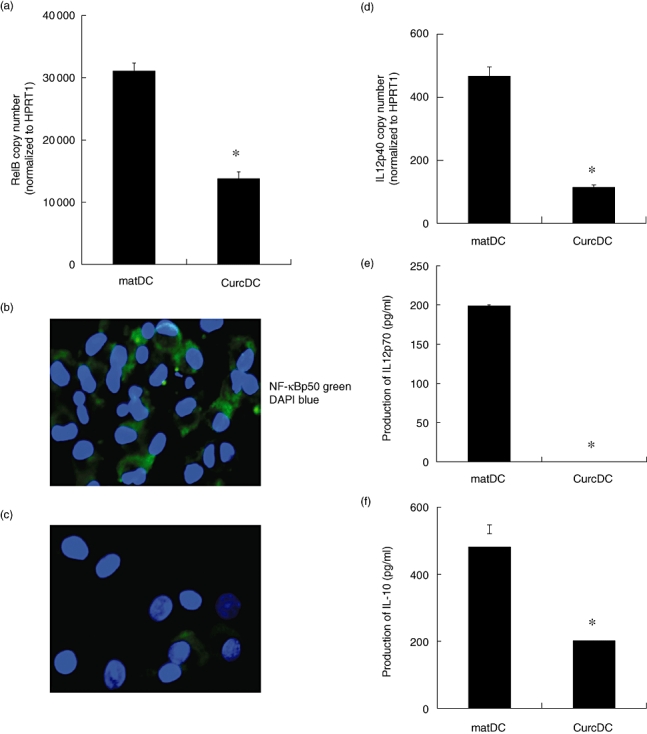
Curcumin generates maturation-arrested dendritic cells (DC). Mature DC (matDC) and curcumin DC (CurcDC) were washed extensively and exposed to additional CD40L and interferon (IFN)-γ to mimic inflammatory conditions in vivo, as might occur following exposure to alloantigen. (a) Compared to matDC, CurcDC demonstrated down-regulated RelB mRNA expression (decreased by 56%, *P < 0·001) consistent with a maturation-arrested state. (b,c) MatDC (b) or CurcDC (c) were fixed and analysed by confocal microscopy. Immunofluorescent staining for nuclear factor (NF)-κBp50 subunit demonstrated failure of up-regulation and nuclear translocation in CurcDC despite a robust maturation stimulus. Cell nuclei [4′6-diamino-2-phenylindole (DAPI)] are coloured blue and NF-kBp50 is coloured green. (d,e) CurcDC demonstrated lower mRNA expression of interleukin (IL)-12p40 (decreased by 63%, *P < 0·001) compared to matDC (d), and failed to produce detectable quantities of IL-12p70 (e). (f) CurcDC were capable of producing significant IL-10, albeit in reduced quantities compared to matDC (*P < 0·001). Reverse transcription–polymerase chain reaction results are mean ± standard deviation of quadruplicate measurements, normalized to a housekeeping gene (HPRT1), and are representative of six independent experiments. Cytokine protein production is from triplicate wells assessed by enzyme-linked immunosorbent assay, and representative of four independent experiments.
NF-κB transcriptional activity is required for expression of cytokines, including IL-12 [48] and IL-10 [49], and we next compared cytokine mRNA expression and secretion in DC in the presence or absence of curcumin, following stimulation with IFN-γ and CD40L. Maturation with LPS alone failed to demonstrate sufficient IL-12p70 production in either DC population. IL-12p40 mRNA expression was down-regulated in CurcDC compared to controls (matDC, Fig. 2d). Concurrent measurement of supernatant cytokine production (by ELISA) demonstrated negligible production of biologically active IL-12 by CurcDC (Fig. 2e). CurcDC were still able to produce IL-10 (Fig. 2f). These data are consistent with the observed suppression of NF-κB nuclear activity by curcumin.
CurcDC inhibit T cell responsiveness in a primary MLR
Allogeneic T cells were stimulated with CurcDC and compared to proliferation with both matDC and immDC in a DC-MLR. Compared to matDC, CurcDC potently inhibited allogeneic T cell proliferation at all stimulator–responder ratios, at a level similar to immDC (Fig. 3a).
Fig. 3.
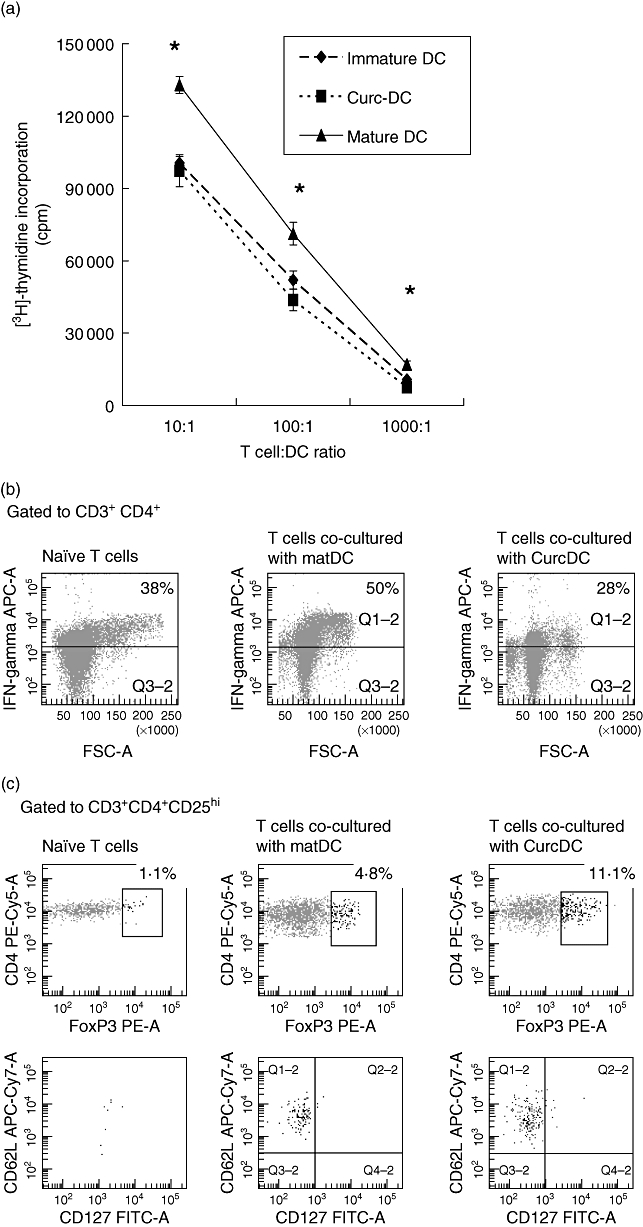
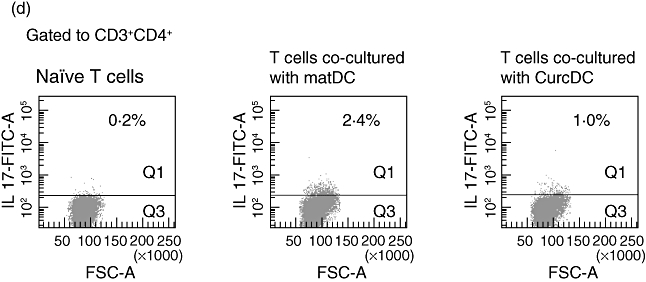
Curcumin dendritic cells (CurcDC) demonstrate impaired allostimulatory capacity mediated by CD4+CD25hiforkhead box P3 (FoxP3)+ regulatory T cells. (a) T cells were co-cultured with immature DC (immDC) mature DC (matDC) or CurcDC at differing T cell : DC ratios for 5 days and proliferation was determined using [3H]-thymidine incorporation added in the final 18 h of culture. Compared to matDC, CurcDC showed lower T cell allostimulatory capacity at all T cell : DC ratios, comparable to immDC. Results shown are mean ± standard error of the mean (*P < 0·01 matDC versus CurcDC and matDC versus immDC). Results are from an individual experiment and representative of four independent experiments performed. T cells co-cultured in a primary mixed lymphocyte reaction (MLR) were then isolated using CD3+ magnetic beads and analysed by flow cytometry. (b) T cells co-cultured with CurcDC demonstrated a 50% reduction in intracellular interferon (IFN)-γ expression compared to matDC. (c) T cell hyporesponsiveness was mediated by the generation of CD4+CD25hiFoxP3+ T cells. This population was also CD127loCD62L+, further confirming a regulatory phenotype. (d) Co-culture with CurcDC [and subsequent stimulation with staphylococcal enterotoxin B (SEB)] failed to induce T helper type 17 (Th17) cells; (b–d) demonstrate results from one of five independent experiments.
T cell hyporesponsiveness following co-culture with CurcDC is associated with lower IFN-γ expression and induction of CD4+CD25hiFoxP3+ Tregs
The responding T cell populations from the primary MLR to assess whether CurcDC induced FoxP3+ Tregs as a mechanism of reduced alloproliferative capacity. We compared CurcDC with matDC only, mimicking the effect of allogeneic DC in vivo. T cells were separated from the stimulating DC population using CD3+ immunomagnetic beads (95% purity, data not shown). The proportion of T cells expressing IFN-γ was reduced in response to CurcDC (27% versus 50%, Fig. 3b), in keeping with previous reports of a deviation away from Th1 helper–T cell generation [50]. The proportion of Tregs was increased significantly following co-culture with CurcDC compared to matDC (11·1% versus 4·8%, Fig. 3c). This population was CD127lo and CD62L+ (Fig. 3c), confirming a Treg phenotype. In addition, there was a concurrent decrease in intracellular IL-17 expression following stimulation with SEB (1·0% versus 2·1%, Fig. 3d).
T cells primed with CurcDC exert suppressive function consistent with Tregs
To address whether T cells from the primary MLR exerted a subsequent tolerogenic function, T cells were restimulated with DC from the same donor or third party. T cells primed with CurcDC were less responsive to subsequent allogeneic stimulation (Fig. 4a), and flow cytometric analysis demonstrated a concurrent expansion in the CD4+CD25hiFoxP3+CD127lo Treg population, although this effect was not specific for the primary donor antigen (Fig. 4b). Consistent with this tolerogenic pattern, CD4+ T cells primed with CurcDC exerted regulatory functions on naive syngeneic T cells (Fig. 4c). T cell proliferative responses were not altered by duration of the secondary MLR, with comparisons made at 2 and 5 days (data not shown). These results indicate that CurcDC induce FoxP3+ Tregs capable of promoting regulatory function on naive T cells.
Fig. 4.
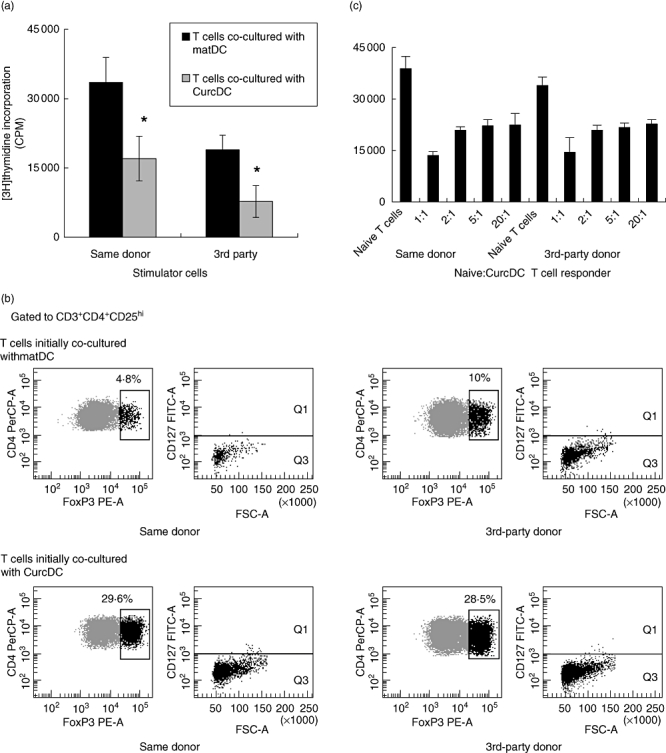
Curcumin dendritic cells (CurcDC) are tolerogenic. (a) T cells were co-cultured initially with CurcDC or mature DC (matDC), isolated from the stimulating cell population using CD3+ magnetic beads, and restimulated in a 5-day mixed leucocyte reaction (MLR) (*P < 0·01). Stimulating DC were harvested from the same donor or third party and subsequent T cell hyporesponsiveness was not limited to the primary donor antigen. (b) Compared to matDC, restimulation of T cells co-cultured initially with CurcDC significantly expanded forkhead box P3 (FoxP3)+ regulatory T cells that were CD127lo. (c) Fluorescence activated sorted CD4+ T cells were allostimulated for 5 days in the presence of CurcDC, purified using CD3+ immunomagnetic beads and co-cultured with naive syngeneic T cells at ratios of 1:1 to 20:1. Suppression of naive T cell proliferation confirmed regulatory function. These figures are representative of three independent experiments.
Allogeneic murine CD11+ DC migrate systemically
To address in vivo applicability of our findings, splenic CD11c+ DC from C57BL/6 mice were isolated using immunomagnetic bead separation (80% purity, data not shown). Previous studies in mice have demonstrated that exposure to 25 µM curcumin is sufficient to inhibit immunostimulatory function [44,47], therefore DC (1 × 106) were treated overnight with (CurcDC) or without curcumin (immDC) at this concentration and injected into BALB/c mice. Control (naive) mice received PBS alone. DC were fluorescent-labelled to assess systemic distribution after 7 days. Confocal microscopy demonstrated widespread presence of DiI+ DC in liver, spleen and kidney, in addition to mesenteric and inguinal lymph nodes (data not shown). No such staining was observed in organs of PBS-injected mice (data not shown). Immunofluorescent staining and confocal microscopy of spleen demonstrated co-localization of DiI-labelled DC, CD11c and Dec205 (Fig. 5a), confirming DC migration and phenotype in vivo.
Fig. 5.
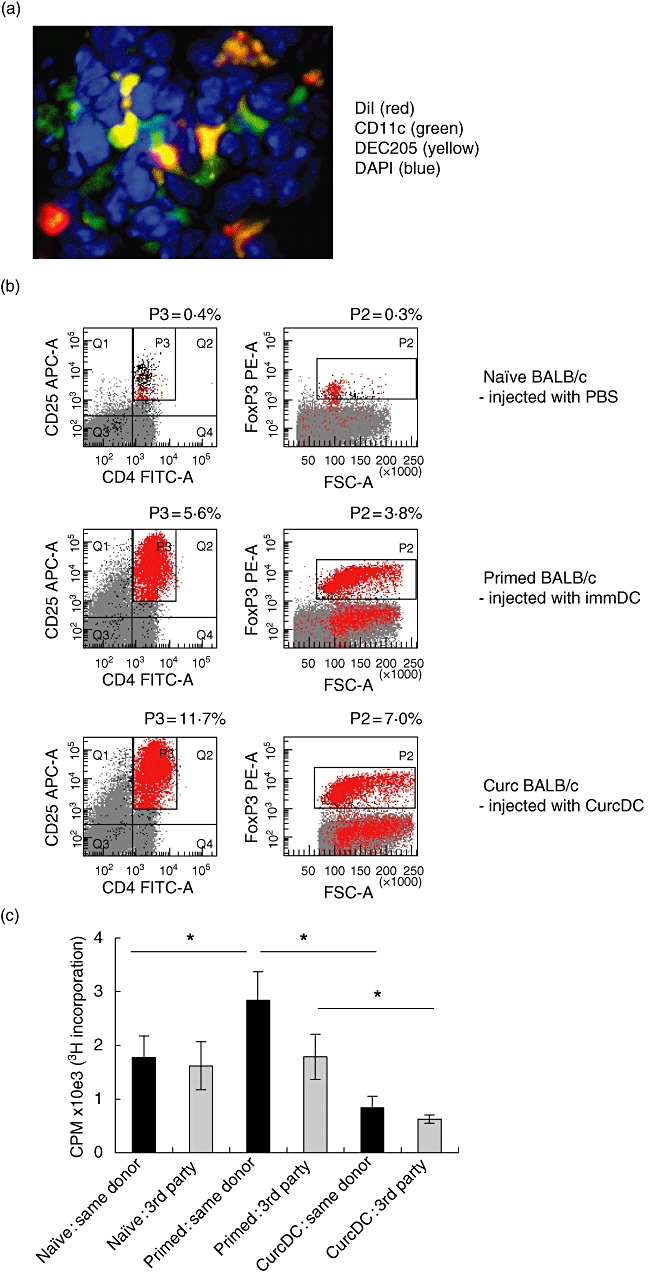
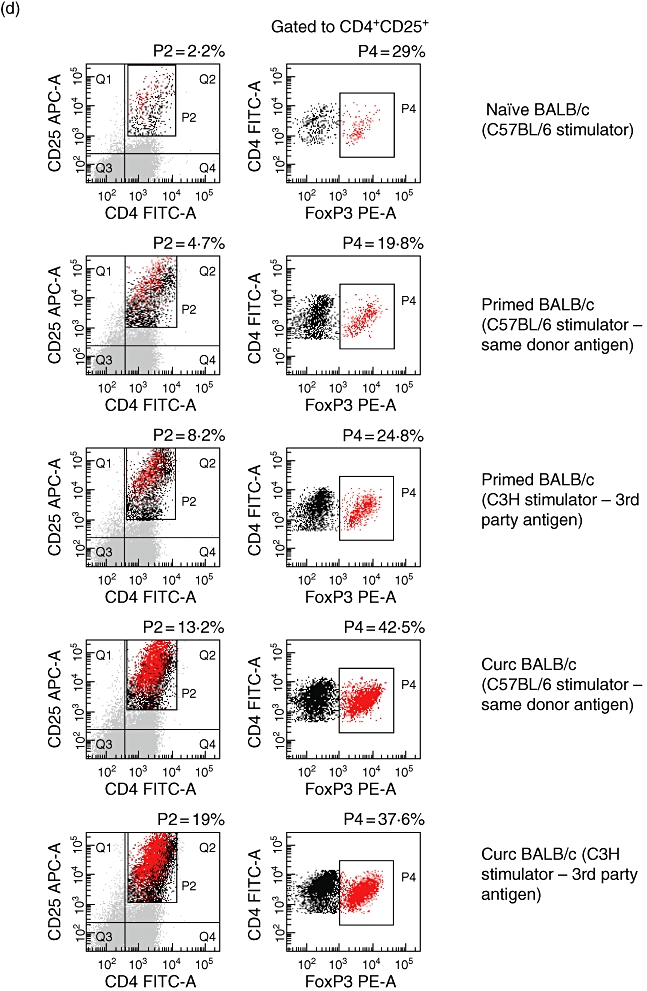
Murine curcumin dendritic cells (CurcDC) distribute systemically, promote forkhead box P3 (FoxP3)+ regulatory T cell expansion in vivo and impair subsequent alloproliferative responses in a non-alloantigen-specific manner. Splenic CD11c+ DC from C57BL/6 (H-2b) mice were isolated using immunomagnetic beads, stained with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiI) and injected intravenously into BALB/c (H-2d) mice to assess systemic distribution. Seven days after injection, organs were removed, snap-frozen, stained and analysed by confocal microscopy (original magnification, 40×). (a) Spleen from BALB/c mice injected with DiI-labelled DC demonstrated red fluorescent cells (DiI+ DC) with co-localization of staining for CD11c (green) and DEC205 (yellow). (b) CD11c+ C57BL/6 DC were incubated with 25 µM (CurcDC) or complete medium (immDC) overnight, washed and injected into BALB/c mice. Splenocytes were isolated after 7 days and flow cytometry performed to assess FoxP3 expression. Infusion of immDC or CurcDC both demonstrated an increase in the CD4+CD25hiFoxP3+ population compared to naive mice [injected with phosphate-buffered saline (PBS)]. (c) Splenocytes were restimulated ex vivo for 5 days with primary donor (C57BL/6) or third-party donor (C3H) antigen (irradiated mononuclear cells). Infusion of immDC led to an antigen-specific primed immune response. However, CurcDC impaired alloproliferation non-specifically compared to both naive (PBS-injected) and immDC-injected mice (*P < 0·01). Results are expressed as mean ± standard deviation of quintuplicate measurements from one experiment, and are representative of four independent experiments. (d) The responding CD4+ T cell population in the secondary mixed lymphocyte reaction was analysed, demonstrating expansion of the CD4+CD25hiFoxP3 following infusion of both immDC and CurcDC, although the increase was substantially greater in the latter. Results are representative of two separate experiments.
Allogeneic CurcDC induce CD4+CD25hiFoxP3+ Tregsin vivo and immune hyporesponsiveness in vitro
Analysis of splenocytes in BALB/c mice injected with PBS, immDC or CurcDC demonstrated marked expansion of the CD4+CD25+FoxP3+ population in the latter two groups (0·3% versus 3·8% versus 7·0%, Fig. 5b). Restimulation of splenocytes with primary donor (B6) or third-party antigen (C3H) showed suppression of the alloproliferative response in mice injected with CurcDC compared to both naive and immDC-injected mice (Fig. 5c), an effect that was not specific for the primary donor antigen.
Flow cytometric analysis of the responding cell population in the secondary MLR demonstrated expansion of CD4+CD25hiFoxP3+ T cells (Fig. 5d), most marked in mice injected with CurcDC.
Discussion
In this study, we demonstrated that hu-Mo-DC propagated under standard conditions and subsequently exposed to curcumin show impaired ability to undergo phenotypic and functional maturation following exposure to LPS, a potent TLR agonist. CurcDC were maturation-arrested, as evidenced by (i) down-regulated positive co-stimulatory molecule expression, particularly CD83 (Fig. 1a); (ii) low mRNA expression of the transcription factor RelB (Fig. 2a) and failure to up-regulate NF-κBp50 (Fig. 2c); and (iii) negligible production of IL-12p70 following stimulation with LPS and/or CD40L and IFN-γ (Fig. 2e). The most significant features of CurcDC were reduced RelB expression/NF-κB nuclear translocation and the inability to produce bioactive IL-12 following exposure to CD40L and IFN-γ. These stimuli mimic the conditions likely to be encountered by allogeneic DC when introduced in vivo[51,52]; ligation of CD40 on unmodified DC by CD40L (CD154) induces DC maturation and abrogates tolerogenic properties [53]. The resistance of CurcDC to maturation was observed following withdrawal of curcumin, indicating that it is a characteristic of the generated DC.
Several additional features of CurcDC were identified: negative regulators of DC phenotype were affected differentially. PDl-1 (and PD-L2) expression was reduced, although still detectable to allow engagement of the T cell ligand PD-1. ILT2 and ILT4 expression was unaffected. These cell surface molecules are members of the immunoglobulin gene superfamily that signal via immunotyrosine-based inhibitory motifs to inhibit cellular responses. They bind a broad range of HLAs, although demonstrate higher-affinity interactions with HLA-G than with classical MHC I [54]. ILT2, in particular, may be modulating the primary T cell response by decreasing T cell receptor (TCR)-ζ phosphorylation required for ZAP70 complex formation, thus altering actin cytoskeleton formation essential for T cell activation [55]. The tolerogenic characteristics of CurcDC also reflect a lower ratio of positive co-stimulatory to inhibitory signals.
Tolerogenic DC induce immune tolerance through several pathways, including clonal T cell depletion or exhaustion, anergy, deviation of Th differentiation or generation of Tregs[20,23]. We report for the first time the phenotypic profile of proliferating T cells in response to curcumin-treated hu-Mo-DC, and demonstrate that inhibition of T cell alloproliferative capacity correlates with expansion of the CD4+CD25hiFoxP3+ Tregpopulation. CurcDC failed to produce IL-12p70 essential for polarization of a Th1 response; expression of IFN-γ in the responding T cells was also down-regulated, in keeping with development of a Th2-deviated microenvironment reported previously [50]. IFN-γ is critical to the generation of FoxP3+ Tregs[56–58]; its reduced presence, in conjunction with decreased TCR co-stimulation due to reduced CD80, CD86 and CD40 expression on CurcDC, increased the propensity for FoxP3+ Treggeneration.
CurcDC-primed T cells demonstrate reduced alloproliferative capacity following restimulation with alloantigen (Fig. 4a), with concomitant expansion of CD4+CD25hiCD127loFoxP3+ Tregs (Fig. 4b) capable of regulating naive syngeneic T cell proliferation (Fig. 4c). This is consistent with previous reports that co-stimulation is required for induction and expansion of FoxP3+ Tregs, which is mediated by the production of IDO from DC [59,60]. However, the regulatory effect was not alloantigen-specific, in keeping with previous studies [27,61,62]. Multiple CD4+ Tregs subsets have been identified, including FoxP3+ Tregs, CD4+CD25–FoxP3– IL-10 producing Tr1 cells [63,64], and transforming growth factor-beta (TGF-β+) Th3 cells [65]. The use of CD3+ purified T cells did not exclude the generation of other regulatory subsets which are not exclusive to CD4+ enriched T cells: CD8+[15,66], CD4–CD8–[67], gammadelta [68] and natural killer (NK) T cells [69] have also been implicated.
An additional finding was the decreased production of Th17 CD4+ helper T cells. Th17 cells express the IL-23 receptor [70], transcription factors retinoic acid-related orphan receptor (ROR)γt [71,72] and RORα (16), and produce IL-22 and IL-17 [73]. They also share a developmental pathway with FoxP3+ Tregs, requiring the addition of IL-6 in the presence of TGF-β[74]. Thus, the cytokine microenvironment and anti-inflammatory effects induced by curcumin in vitro, in addition to the up-regulation of FoxP3+ Tregs, may have altered Th17 CD4+ T cell lineage commitment.
Murine bone-marrow-derived DC exposed to curcumin have been shown previously to generate FoxP3+ Tregsin vitro[47]. The stability and maturation-resistance of CurcDC under inflammatory conditions is crucial to their successful application as tolerogenic agents in vivo that down-regulate anti-donor T cell responses. DC that are resistant to maturation offer a considerable advantage over conventional ‘immature’ DC, as demonstrated by our in vivo results. The latter undergo maturation in vivo, sensitizing the recipient, and limiting their effectiveness in promoting regulatory T cells. We have demonstrated that infusion of allogeneic DC induced FoxP3+ Tregs generation in vivo (Fig. 5b), although only CurcDC reduced subsequent alloproliferative responses (Fig. 5c) with significant expansion of the FoxP3+ compartment (Fig. 5d).
In conclusion, we report that exogenous curcumin deviates hu-Mo-DC towards tolerogenic DC, capable of generating FoxP3+ Tregs. The advantage of curcumin is its low cytotoxicity and potency as an NF-κB inhibitor, in addition to antioxidant and anti-inflammatory properties. Thus, our present observations provide a new way of generating tolerogenic DC ex vivo as a therapeutic tool, with potential applications in vivo.
Disclosure
All authors (NMR, SK, PTC) have no financial disclosures nor conflict of interest.
References
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392:245–51. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman R, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morelli A, Thomson A. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Coinhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald R, Freeman G, Sharpe A. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 7.Quezada S, Jarvinen L, Lind E, Noelle R. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima Y, Tanaka Y, Tozawa Y, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed] [Google Scholar]
- 9.Moser M, Murphy K. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Kaisho K, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 11.Barratt-Boyes S, Thomson A. Dendritic cells: tools and targets for transplant tolerance. Am J Transplant. 2005;5:2807–13. doi: 10.1111/j.1600-6143.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 12.Lutz M, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–49. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 13.Albert M, Jegasthesan M, Darnell R. Dendritic cell maturation is required for cross-tolerization of CD8+T cells. Nat Immunol. 2001;2:1010–17. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 14.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 15.Gilliet M, Liu Y. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moseman E, Liang X, Dawson A, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 17.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk A. Induction of interleukin-10 producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garza K, Chan S, Suri R, et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–8. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonuleit H, Schmitt E, Steinbrink K, Enk A. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 20.Coates P, Thomson A. Dendritic cells, tolerance induction and transplant outcome. Am J Transplant. 2002;2:299–307. doi: 10.1034/j.1600-6143.2002.20403.x. [DOI] [PubMed] [Google Scholar]
- 21.Arpinati M, Green C, Heimfeld S, Heuser J, Anasetti C. Granulocyte–colony stimulating factor mobilises T helper 2-inducing dendritic cells. Blood. 2000;95:2484–90. [PubMed] [Google Scholar]
- 22.Hackstein H, Thomson A. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Immunol. 2004;4:4–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 23.Wood K, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot J, Gavin M, Rudensky A. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 25.Kang S, Tang Q, Bluestone J. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Putman A, Xu-Yu A. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin E, O'Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interluekin-10. Immunity. 2003;18:155–67. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 30.Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–81. [PubMed] [Google Scholar]
- 31.Lee J, Ganster R, Geller D, Burckart G, Thomson A, Lu L. Cyclosporine A inhibits the expression of costimulation molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68:1255–63. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 32.Lagaraine C, Hoarau C, Chabot V, Velge-Roussel F, Labranchu Y. Mycophenolic acid-treated human dendritic cells have a mature migratory phenotype and inhibit allogeneic responses via direct and indirect pathways. Int Immunol. 2005;17:351–63. doi: 10.1093/intimm/dxh215. [DOI] [PubMed] [Google Scholar]
- 33.Hackstein H, Taner T, Zahorchak AF, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–63. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Bernier S, Ichim T, et al. LF15-0195 generates tolerogenic dendritic cells by suppression of NF-kappaB signalling through inhibition of IKK activity. J Leukoc Biol. 2003;74:438–47. doi: 10.1189/jlb.1102582. [DOI] [PubMed] [Google Scholar]
- 35.Penna G, Adorini L. 1-alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 36.Balasubramanyam M, Koteswari A, Kumar R, Monickaraj S, Maheswari J, Mohan V. Curcumin-induced inhibition of cellular reactive oxygen species generation: novel therapeutic implications. J Biosci. 2003;28:715–21. doi: 10.1007/BF02708432. [DOI] [PubMed] [Google Scholar]
- 37.Ruby A, Kuttan G, Babu K, Rajasekharan K, Kuttan R. Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett. 2003;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 38.Yadav V, Mishra K, Singh D, Mehrotra S, Singh V. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–97. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 39.Apisariyakul A, Vanittanakom N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae) J Ethnopharmacol. 1995;49:163–9. doi: 10.1016/0378-8741(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 40.Mazumder A, Raghavan K, Weinstein J, Kohn K, Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995;49:1165–70. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- 41.Negi P, Jayaprakasha G, Jagan Mohan Rao L, Sakariah K. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agric Food Chem. 1999;47:4297–300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 42.Garg A, Buchholz T, Aggarwal B. Chemosensitization and radiosensitization of tumours by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Aggarwal B. Activation of transcription factor NF-kB is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 44.Kim G, Kim K, Lee S, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kB as potential targets. J Immunol. 2005;174:8116–24. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 45.Lim W, Kireta S, Russ G, Coates P. Human plasmacytoid dendritic cells regulate immune responses to Epstein–Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood. 2007;109:1043–50. doi: 10.1182/blood-2005-12-024802. [DOI] [PubMed] [Google Scholar]
- 46.Capini C, Jaturanpinyo M, Chang H-I, et al. Antigen-specific suppression of inflammatory arthritis using liposomes. J Immunol. 2009;182:3556–65. doi: 10.4049/jimmunol.0802972. [DOI] [PubMed] [Google Scholar]
- 47.Cong Y, Wang L, Konrad A, Schoeb T, Elson C. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39:3134–46. doi: 10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- 48.Grumont R, Hochrein H, O'Keeffe M, et al. C-Rel regulates interleukin 12 P70 expression in CD8+ dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194:1021–32. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Garra A, Murphy K. From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce T(H)1 development. Nat Immunol. 2009;10:929–32. doi: 10.1038/ni0909-929. [DOI] [PubMed] [Google Scholar]
- 50.Shirley S, Monpetit A, Lockey R, Mohapatra S. Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun. 2008;374:431–6. doi: 10.1016/j.bbrc.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caux C, Massacrier C, Vanbervliet B, et al. Activation of dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morelli A, Zahorchak A, Larregina A, et al. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–23. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 53.Grohnmann U, Fallarino F, Silla S, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166:277–83. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 54.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514–21. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 56.Feng G, Gao W, Strom T, et al. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of TH17 responses and generation of Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:2521–7. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng G, Wood K, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+FoxP3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–89. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+CD25– T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–41. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang J, Huddleston S, Fraser J, Khoruts A. De novo induction of antigen-specific CD4+CD25+FoxP3 regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–9. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 60.Munn D, Sharma M, Mellor A. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 61.Eljaafari A, Li Y-P, Miossec P. IFNγ, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009;183:2932–45. doi: 10.4049/jimmunol.0804352. [DOI] [PubMed] [Google Scholar]
- 62.Thornton A, Shevach E. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 63.Roncarolo M, Bacchetta R, Boridgnon C, Narula S, Levings M. Type 1 regulatory T cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 64.Roncarolo M, Gregori S, Battaglia M, Bacchetta R, Fleischauer K, Levings M. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 65.Weiner H. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhou J, Carr R, Liwski R, Stadnyk A, Lee G. Oral exposure to alloantigens generates intragraft CD8+ regulatory T cells. J Immunol. 2001;167:107–13. doi: 10.4049/jimmunol.167.1.107. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Yang L, Young K, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–9. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 68.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 69.Seino K-I. Requirements for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577–81. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aggarwal S, Ghilardi N, Xie M, de Sauvage F, Gurney A. Interleukin-23 promotes a distinct CD4 T cell activation state characterised by the production of interluekin-17. J Biol Chem. 2003;278:1910–14. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 71.Ivanov I, McKenzie B, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 72.Yang X, Nurieva R, Martinez G, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγt. Immunity. 2008;28:647–59. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langrish C, Chen Y, Blumenschein W, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]


