Abstract
There is emerging interest in the application of mesenchymal stem cells (MSC) for the prevention and treatment of autoimmune diseases, graft-versus-host disease and allograft rejection. It is, however, unknown how inflammatory conditions affect phenotype and function of MSC. Adipose tissue-derived mesenchymal stem cells (ASC) were cultured with alloactivated peripheral blood mononuclear cells (PBMC) (mixed lymphocyte reaction: MLR), with proinflammatory cytokines [interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-6] or under control conditions, and their full genome expression and function examined. Proinflammatory cytokines mainly increased indoleamine-2,3-dioxygenase expression, whereas ASC cultured with MLR showed increased expression of COX-2, involved in prostaglandin E2 production. Both conditions had a stimulatory, but differential, effect on the expression of proinflammatory cytokines and chemokines, while the expression of fibrotic factors was decreased only in response to proinflammatory cytokines. Functional analysis demonstrated that inflammatory conditions affected morphology and proliferation of ASC, while their differentiation capacity and production of trophic factors was unaffected. The immunosuppressive capacity of ASC was enhanced strongly under inflammatory conditions. In conclusion, ASC showed enhanced immunosuppressive capacity under inflammatory conditions, while their differentiation capacity was preserved. Therefore, in vitro preconditioning provides ASC with improved properties for immediate clinical immune therapy.
Keywords: cytokines, gene array, immunosuppression, inflammation, mesenchymal stem cell, microarray, mixed lymphocyte reactions
Introduction
Mesenchymal stem cells (MSC) are found in a variety of tissues, including bone marrow, skin and adipose tissue [1–3] and can be expanded easily in vitro. MSC are thought to have tissue regenerative properties, in the first place via their multi-lineage differentiation capacity [2] and, perhaps more importantly, via the secretion of trophic factors that may activate local progenitor cells [4]. In addition, MSC have potent immunomodulatory capacity. They inhibit the proliferation of T cells [5,6] and inhibit dendritic cell maturation [7,8]. These properties make MSC promising for a diversity of clinical applications; for example, for the prevention and treatment of autoimmune diseases and bone marrow rejection. Recently, interest has developed in the use of MSC in solid organ transplantation [9,10]. These conditions are associated with an inflammatory milieu. A restraint for therapeutic use of MSC may, however, be the limited understanding of their characterization and biology under various inflammatory conditions [10–13]. It has been reported that the immunosuppressive effects of ASC are mediated via soluble factors, and enhanced further if direct cell–cell contact between ASC and immune cells was allowed [14]. Different studies have attributed the immunosuppressive effect of MSC to different immunosuppressive factors. These include indoleamine 2,3-dioxygenase (IDO) [15–17], prostaglandin E2[18], transforming growth factor (TGF)-β and hepatocyte growth factor (HGF) [5], HLA-G [19], nitric oxide [20], interleukin (IL)-10 [21] and haem oxygenase [22]. In addition, there is evidence that cell–membrane interactions between MSC and immune cells via the adhesion molecules intercellular adhesion molecule (ICAM)-1 or vascular cell adhesion molecule (VCAM)-1 play a crucial role in the immunomodulatory capacity of MSC [14,23]. Thus, the immunomodulatory capacity of MSC is a multi-factorial process. The activity of these processes may depend upon local immunological conditions. It has been demonstrated that in the absence of inflammation, MSC can stimulate lymphocyte survival and proliferation [24]. Under inflammatory conditions a high production of cytokines, such as interferon (IFN)-γ, tumour necrosis factor (TNF)-α and IL-6, are largely produced and MSC may respond to these factors by changing their immunomodulatory function [25–27]. Exposure of MSC to IFN-γ has been reported to up-regulate the expression of IDO, TGF-β and HGF [25,28] and it was demonstrated recently that IFN-γ-activated MSC are more effective for the treatment of graft-versus-host disease [29]. Effective application of MSC in organ transplantation may require potent and immediate immunosuppressive effects. In vitro activation of MSC could therefore be beneficial for clinical effectiveness of MSC in organ transplantation.
In the present study, we investigated whether different inflammatory conditions affected the gene expression, phenotype and function of adipose tissue-derived mesenchymal stem cells (ASC). ASC were cultured with alloactivated peripheral blood mononuclear cells (PBMC) (mixed lymphocyte reaction, MLR) or with a cocktail of proinflammatory cytokines containing IFN-γ, TNF-α and IL-6, while their functions and full genome expression were examined.
Materials and methods
Isolation and culture of ASC
ASC were isolated and expanded from perirenal adipose tissue of four living healthy kidney donors, as described previously [30,31]. These donors (three males, one female, mean age 46 ± 7 years) were approved to donate their kidney after routine screening. They did not use immunosuppressive medication.
In brief, perirenal fat was minced and digested with 0·5 mg/ml collagenase type IV (Invitrogen, Paisley, UK) in RPMI-1640 (Invitrogen) for 30 min at 37°C. The cells were then washed, transferred to culture flasks and kept in minimum essential medium (MEM)-α medium (Invitrogen) with 100 U/ml penicillin and 100 µg/ml streptomycin (p/s; Invitrogen) and 15% fetal calf serum (FCS) (Biowhittaker, Verviers, Belgium) at 37°C, 5% CO2 and 95% humidity. After 3 days, non-adherent cells were removed and adherent cells continued in culture. Cultures were refreshed with ASC-culture medium twice a week. At 90% confluence, adherent cells were removed from culture flasks by incubation in 0·05% trypsin-ethylenediamine tetraacetic acid (EDTA) at 37°C and cells were used for experiments or frozen at −150°C until use. ASC were used for experiments at between passages 2–5.
To confirm whether the perirenal fat-derived cells were indeed ASC, they were characterized by flow cytometry, differentiated in osteogenic and adipogenic lineages and added to MLR to test their immunosuppressive capacity, as described previously [30,31]. For independent experiments, ASC were used from different ASC donors.
Exposure of ASC to inflammatory conditions
ASC were seeded at 10 000 cells/cm2 and cultured under two inflammatory conditions for 7 days. The first condition consisted of alloactivated PBMC at a ratio of 10:1, in which the PBMC were separated from ASC by a 0·4 µm pore size transwell membrane (Greiner Bio-one, Essen, Germany). The second condition consisted of a proinflammatory cytokine cocktail containing 50 ng/ml IFN-γ (U-Cytech, Utrecht, the Netherlands), 20 ng/ml TNF-α (PeproTech, London, UK) and 10 ng/ml IL-6 (PeproTech).
Measurement of ASC diameter and proliferation
Adherent cells were removed from culture flasks by incubation in 0·05% trypsin-EDTA at 37°C and cells put into cell-counting chambers (Bürker–Türk chamber; Brand, Wertheim, Germany). Cells were photographed microscopically (Axiovert 200M; Carl Zeiss, Munich, Germany) at 40× high-performance field (HPF) Ph2. Cell diameters were measured using AxioVision software (version 4·7.1) (Carl Zeiss).
Proliferation of ASC cultured under the previously described conditions was determined by counting the living cells manually using cell-counting chambers. To avoid contamination of PBMC in ASC-MLR co-cultures, transwell-membrane inserts were used (Greiner Bio-one, Alphen a/d Rijn, the Netherlands).
Flow cytometric characterization of ASC
Adherent cells were removed from culture flasks by incubation in 0·05% trypsin-EDTA at 37°C and then washed twice with fluorescence activated cell sorter (FACS)Flow (BD Biosciences, San Jose, CA, USA). Next, cell suspensions were incubated with antibodies against CD86-fluorescein isothiocyanate (FITC), CD166-phycoerythrin (PE), human leucocyte antigen D-related (HLA-DR)-allophycocyanin (APC)-cyanin 7 (Cy7) (all from BD Biosciences), CD40-PE, CD80-PE, HLA-avidin–biotin complex (ABC)-PE (all from Serotec, Oxford, UK), CD90-APC and CD105-FITC (all from R&D Systems, Abingdon, UK) at room temperature (RT) protected from light for 30 min. After two washes with FACSFLOW, flow cytometric analysis was performed using an eight-colour FACSCANTO-II with FACSDIVA Software (BD Biosciences) and FlowJo Software (Tree Star Inc., Palo Alto, CA, USA).
Differentiation capacity of ASC
Osteogenic differentiation was induced by culturing confluent ASC cultures in α-MEM supplemented with 1% p/s, 15% heat-inactivated fetal bovine serum (FBS), 5 mmβ-glycerophosphate (Sigma-Aldrich, Munich, Germany), 50 µg/ml l-ascorbic acid-phosphate (Sigma-Aldrich) and 10 nm dexamethasone (Sigma-Aldrich) for 21 days. Cells were then washed with phosphate-buffered saline (PBS) and fixed in cold 4% paraformaldehyde for 5 min at room temperature. After two washes with H2O, cells were incubated in 1% silver nitrate in H2O at room temperature on a light box until blackening occurred. The cells were then washed three times with H2O, incubated in 2·5% sodium thiosulphate in H2O for 5 min at room temperature, washed twice with H2O and photographed.
Adipogenic differentiation was induced by culturing confluent ASC cultures in α-MEM supplemented with 1% p/s, 15% heat-inactivated FBS, 50 µg/ml l-ascorbic acid-phosphate (Sigma-Aldrich), 500 µm 3-isobutyl-1-methylxanthine (IBMX; Fluka, Buchs, Switzerland), 60 µm indomethacin (Fluka) and 10 nm dexamethasone (Sigma-Aldrich) for 21 days. Cells were then fixed in 60% isopropanol for 1 min, and incubated in filtered 0·3% oil red O (Sigma-Aldrich) solution in 60% isopropanol for 10 min to stain lipid droplets. After several washes with PBS the cells were photographed.
Isolation of PBMC
PBMC were isolated from buffy coats of healthy volunteers using Ficoll-Paque™ Plus (GE Healthcare, Uppsala, Sweden) separation and stored at −135°C until use.
MLR
The immunosuppressive capacity of pretreated ASC was tested in MLR. In MLR, 5 × 104 responder PBMC were stimulated by 5 × 104γ-irradiated (40 Gy) allogeneic PBMC in RPMI-1640 + 10% HI-FBS in round-bottomed 96-well plates (Nunc, Roskilde, Denmark). ASC were added at the beginning (day 0) or at the end (day 6) of the 7-day MLR to responder cells at a 1:5 ratio. On day 7, proliferation was measured following incorporation of [3H]-thymidine (0·5 µCi/well) during a 16-h incubation using a β-plate reader. To determine the proliferation capacity of the PBMC, 5 × 104 cells were stimulated with 1 µg/ml PHA for 3 days and [3H]-thymidine incorporation was measured.
To determine the importance of IDO in the immunosuppressive effect of the ASC pretreated under the different conditions, ASC were added to MLR, as described above, with addition of the IDO1-inhibitor 1-methyl-L-tryptophan (1-MT) (Sigma-Aldrich). 1-MT was prepared by dissolving in 1 m hydrochloric acid and diluted in RPMI-1640 + 10% heat-inactivated FBS. Finally, the pH of the solution was neutralized by adding 1 m sodium hydroxide. The solution was filtered before use.
Isolation of RNA and gene expression analysis
ASC of four healthy donors were seeded at passage four at 10 000 cells/ cm2. The cells were cultured for 7 days under control conditions or with alloactivated PBMC (separated by a transwell membrane), or in the presence of the proinflammatory cytokine cocktail. ASC were then harvested by trypsinization and RNA isolated using MINI columns (Qiagen, Valencia, CA, USA). The RNA quality and quantity was assessed using the RNA 6000 Nano kit on a 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA). Samples with RNA integrity numbers of >8 were selected; 100 ng of total RNA was used to prepare sense-strand biotinylated DNA according to the manufacturer's ‘Whole transcript sense target labeling’ protocol (Affymetrix, Santa Clara, CA, USA). Hybridization to Affymetrix Human Gene 1·0 ST arrays (764 885 probe sets, representing 28 869 annotated genes), staining, washing and scanning (Scanner 3000) procedures were performed as described by Affymetrix and performed by the Erasmus MC Center for Biomics. Probe set summarization, array QC and annotations of the probe sets were performed using Affymetrix ‘Gene Expression Consolle’ (Affymetrix). All the different QC metrics analysed met the standards required by Affymetrix and showed an overall comparability of the signal distribution obtained from the different arrays. Principal component analysis was used to assess the underlying structure of the data set and define correlation relationships among samples (Partek Inc., St Louis, MO USA). Probe sets expressed differentially among conditions were identified using the class comparison tool implemented in BRB ArrayTools (National Cancer Institute, Bethesda, MD, USA). Briefly, we identified genes that were expressed differentially among the two classes using a random-variance t-test. The random-variance t-test is an improvement over the standard separate t-test as it permits sharing information among genes about within-class variation without assuming that all genes have the same variance. Genes were considered statistically significant if their P-value was less than 0·0001. A stringent significance threshold was used to limit the number of false positive findings. A ‘per gene’ estimate of the false discovery rates among genes passing the test was also computed. The false discovery rate associated with a row of the table is an estimate of the proportion of the genes with univariate P-values less than or equal to the one in that row that represent false positives. The Benjamini–Hochberg method for false discovery rate control was used for this estimation [32,33]. Genes passing the test threshold were clustered and displayed as a heatmap using Spotfire (Spotfire Inc., Somerville, MA, USA). The change in gene expression of a number of genes (IDO, IL-6, IL-8, CXCL10) as measured by microarray was confirmed by real-time reverse transcription–polymerase chain reaction (RT–PCR). In brief, ASC were precultured under control, MLR (in transwell culture systems) or cytokine conditions and trypsinized at day 7. Total RNA was isolated and cDNA synthesized as described previously [34]. Quantitative gene expression was determined using TaqMan Universal PCR Master Mix and assays-on-demand for IDO (Hs 00158027.m1), IL-6 (Hs 00174131.m1), IL-8 (Hs00174114.m1) and CXCL10 (Hs 00171042.m1) (all Applied Biosystems, Foster City, CA, USA) on a StepOnePlus (Applied Biosystems).
Statistical analysis
Data were analysed using paired t-test or Wilcoxon's signed-rank test depending on the distribution of the data as tested with the Kolmogorov–Smirnov test for normality. Parametric data are expressed as mean ± standard deviation (s.d.), while non-parametric data are expressed as median (interquartile range). Statistical significance was defined as P < 0·05 (two-tailed).
Results
Effect of inflammatory conditions on ASC gene expression
To investigate the effect of inflammatory conditions on ASC gene expression, ASC were cultured with alloactivated PBMC or proinflammatory cytokines and full genome expression analysis carried out by microarray. ASC were cultured for 7 days under control conditions and inflammatory conditions, either with alloactivated PBMC (MLR) separated by a transwell membrane or with a proinflammatory cytokine cocktail containing IFN-γ, TNF-α and IL-6. The gene expression profiles of ASC derived from four different non-pooled donors showed strong clustering within the different treatment groups, as shown in Fig. 1 and Table 1. ASC that were cultured in the presence of MLR for 7 days showed significant up-regulation of 233 genes and down-regulation of 334 genes compared to ASC cultured under control conditions. ASC that were cultured in the presence of proinflammatory cytokines showed significant up-regulation of 635 genes and down-regulation of 296 genes. Hierarchical clustering demonstrated that gene expression changes in response to both inflammatory stimuli only partly overlapped (Fig. 1a,b), indicating that ASC respond in a significantly different manner to alloactivated PBMC then to proinflammatory cytokines. This was evidenced further by the comparison of ASC cultured with MLR with ASC cultured with cytokines, which resulted in the identification of 1080 genes that showed significantly different expression (Fig. 1c). The most significant changes in gene expression are described below. In addition, real-time RT–PCR analysis on four relevant genes (IDO, IL-6, IL-8 and CXCL10) was performed to confirm the data obtained by microarray (data not shown). The pattern of gene expression changes was similar in microarray and RT–PCR analysis. Only the increase in IDO expression in ASC with MLR was a great deal larger in the RT–PCR analysis than in the microarray analysis.
Fig. 1.

Hierarchical clustering of genes up- (red) and down-regulated (green) by adipose tissue-derived mesenchymal stem cells (ASC) after culture under inflammatory conditions compared to average gene expression level of all samples, measured by microarray. ASC were cultured under control conditions, with alloactivated peripheral blood mononuclear cells (PBMC) (mixed lymphocyte reaction: MLR) or with proinflammatory cytokines (n = 4). Microarray data is deposited in Gene Expression Omnibus (GEO) number GSE18662 at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18662. (a) Gene expression profile of ASC cultured with MLR compared to control ASC. ASC cultured with MLR showed partial overlap with ASC cultured with proinflammatory cytokines. (b) Gene expression profile of ASC cultured with proinflammatory cytokines compared to control ASC. ASC cultured with proinflammatory cytokines showed partial overlap with ASC cultured with MLR. (c) Gene expression profile of ASC cultured with MLR compared to ASC cultured with proinflammatory cytokines. ASC cultured with inflammatory conditions showed partial overlap with control ASC.
Table 1.
Gene expression data of adipose tissue-derived mesenchymal stem cells (ASC) cultured under control conditions or with inflammatory conditions
| Genes | Intensity ASC (control) (mean + s.d.) (log2) | Intensity ASC (MLR) (mean + s.d.) (log2) | Intensity ASC (cytokines) (mean + s.d.) (log2) | Fold change intensity ASC (MLR) versus ASC (control) | Fold change intensity ASC (cytokines) versus ASC (control) | Difference ASC (MLR) versus ASC (cytokines) (P < 0·0001) |
|---|---|---|---|---|---|---|
| Immunosuppressive factors | ||||||
| HLA-G | 11·8 ± 0·2 | 12·7 ± 0·2 | 13·5 ± 0·1 | 1·8* | 3·3* | − |
| COX-2 | 8·0 ± 0·4 | 11·3 ± 0·4 | 8·5 ± 0·3 | 10·2 | 1·5 | + |
| IDO | 4·1 ± 0·1 | 5·6 ± 0·7 | 12·8 ± 0·1 | 2·9 | 394·1* | + |
| HGF | 5·2 ± 0·3 | 4·6 ± 0·3 | 7·1 ± 0·3 | 0·7 | 3·7* | + |
| Guanylate binding proteins (GBP) | ||||||
| GBP1 | 9·1 ± 0·4 | 10·9 ± 0·3 | 12·9 ± 0·1 | 3·6* | 14* | + |
| GBP2 | 6·5 ± 0·2 | 8·9 ± 0·5 | 11·0 ± 0·1 | 5·3* | 22·2* | + |
| GBP3 | 8·2 ± 0·3 | 9·0 ± 0·8 | 11·0 ± 0·4 | 1·8 | 7·3* | + |
| GBP4 | 5·1 ± 0·3 | 7·7 ± 0·9 | 11·8 ± 0·1 | 6·1* | 102·0* | + |
| GBP5 | 4·5 ± 0·1 | 6·5 ± 1·1 | 12·7 ± 0·2 | 3·9 | 290·5* | + |
| GBP6 | 4·1 ± 0·1 | 4·6 ± 0·3 | 6·7 ± 0·4 | 1·5 | 6·3* | + |
| GBP7 | 4·9 ± 0·1 | 5·4 ± 0·2 | 5·6 ± 0·2 | 1·4 | 1·7 | − |
| Myxovirus resistance genes | ||||||
| 1 | 6·2 ± 0·5 | 6·8 ± 0·3 | 10·5 ± 0·2 | 1·5 | 19·1* | + |
| 2 | 6·4 ± 0·5 | 6·4 ± 0·1 | 9·7 ± 0·2 | 1·0 | 9·7* | + |
| Proinflammatory factors | ||||||
| IL-1α | 5·8 ± 0·6 | 7·4 ± 0·9 | 4·0 ± 0·5 | 3·1 | 0·3* | + |
| IL-1β | 6·3 ± 0·4 | 9·1 ± 0·7 | 5·4 ± 0·2 | 7·1* | 0·5* | + |
| IL-6 | 11·9 ± 0·2 | 13·1 ± 0·1 | 11·9 ± 0·1 | 2·2* | 1·0 | + |
| IL-8 | 6·6 ± 0·4 | 12·1 ± 0·4 | 8·5 ± 0·4 | 45·6* | 3·7 | + |
| TNF-10 | 4·5 ± 0·4 | 5·0 ± 0·2 | 10·3 ± 0·1 | 1·4 | 53·4* | + |
| TNF-13b | 5·2 ± 0·2 | 5·8 ± 0·2 | 8·7 ± 0·3 | 1·5 | 11·2* | + |
| IL-33 | 6·0 ± 0·4 | 9·3 ± 0·4 | 6·8 ± 0·4 | 10·5* | 1·8 | + |
| Serum amyloid | ||||||
| 1 | 5·6 ± 0·3 | 10·6 ± 0·4 | 6·6 ± 1·1 | 31·3* | 1·9 | + |
| 2 | 5·9 ± 0·3 | 10·2 ± 0·3 | 6·2 ± 0·5 | 20·0* | 1·2 | + |
| HLA class I | ||||||
| A | 12·1 ± 0·1 | 12·9 ± 0·1 | 13·7 ± 0·1 | 1·7* | 3·0* | − |
| B | 12·0 ± 0·2 | 13·0 ± 0·2 | 13·9 ± 0·1 | 2·0* | 3·5* | − |
| C | 12·0 ± 0·1 | 12·7 ± 0·1 | 13·6 ± 0·1 | 1·7* | 3·2* | + |
| E | 10·7 ± 0·1 | 11·8 ± 0·2 | 12·7 ± 0·1 | 2·1* | 3·8* | + |
| F | 6·9 ± 0·1 | 8·0 ± 0·1 | 9·4 ± 0·1 | 2·2* | 5·9* | + |
| G | 11·8 ± 0·2 | 12·7 ± 0·2 | 13·5 ± 0·1 | 1·8* | 3·3* | − |
| HLA class II | ||||||
| DPα1 | 7·4 ± 0·8 | 7·8 ± 1·0 | 12·9 ± 0·3 | 1·3 | 44·9* | + |
| DPβ1 | 6·8 ± 0·4 | 7·0 ± 0·3 | 11·7 ± 0·3 | 1·1 | 29·4* | + |
| DQα1 | 6·0 ± 0·1 | 6·3 ± 0·2 | 12·3 ± 1·7 | 1·2 | 78·9* | + |
| DRα | 5·2 ± 0·1 | 5·3 ± 0·1 | 12·4 ± 0·1 | 1·1 | 143·8* | + |
| DRβ3 | 5·4 ± 0·1 | 5·8 ± 0·2 | 10·8 ± 1·0 | 1·4 | 44·3* | + |
| DMα | 6·9 ± 0·2 | 6·5 ± 0·2 | 10·9 ± 0·1 | 0·8 | 16·0* | + |
| DMβ | 6·5 ± 0·3 | 6·6 ± 0·1 | 10·8 ± 0·2 | 1·1 | 19·4* | + |
| Chemokine ligands (CXCL) | ||||||
| CXCL1 | 7·3 ± 0·4 | 11·4 ± 0·3 | 10·0 ± 0·2 | 17·7* | 6·7* | + |
| CXCL3 | 6·4 ± 0·2 | 7·9 ± 0·4 | 6·4 ± 0·2 | 2·8 | 1·0 | − |
| CXCL5 | 8·3 ± 0·4 | 11·6 ± 0·7 | 8·8 ± 0·2 | 10·0 | 1·4 | + |
| CXCL6 | 6·3 ± 0·5 | 10·7 ± 0·5 | 9·5 ± 0·3 | 21·2* | 9·4* | − |
| CXCL9 | 4·0 ± 0·2 | 4·1 ± 0·1 | 11·7 ± 0·1 | 1·1 | 209·0* | + |
| CXCL10 | 3·7 ± 0·4 | 6·5 ± 4·8 | 12·7 ± 0·2 | 7·0 | 521·9* | + |
| CXCL11 | 4·0 ± 0·3 | 4·8 ± 0·8 | 12·0 ± 0·2 | 1·8 | 250·7* | + |
| CXCL12 | 9·9 ± 0·6 | 8·9 ± 0·4 | 11·1 ± 0·3 | 0·5* | 2·3 | − |
| Chemokine ligands (CCL) | ||||||
| CCL2 | 10·2 ± 0·3 | 12·2 ± 0·2 | 12·4 ± 0·1 | 3·9* | 4·5* | − |
| CCL5 | 6·8 ± 0·6 | 9·6 ± 0·6 | 11·6 ± 0·4 | 7·1* | 27·4* | + |
| CCL7 | 4·9 ± 0·1 | 6·3 ± 0·4 | 9·1 ± 0·7 | 2·5 | 17·2* | + |
| CCL8 | 4·9 ± 0·2 | 5·8 ± 0·6 | 10·2 ± 0·9 | 1·9 | 40·7* | + |
| CCL13 | 6·2 ± 0·4 | 8·8 ± 0·9 | 9·8 ± 0·7 | 5·9* | 12·1* | − |
| CCL20 | 5·0 ± 0·1 | 8·1 ± 0·2 | 5·1 ± 0·4 | 8·1* | 1·0 | + |
| CCL28 | 5·3 ± 0·1 | 6·7 ± 0·3 | 5·9 ± 0·2 | 2·5* | 1·4 | − |
| Collagens | ||||||
| Iα1 | 13·9 ± 0·1 | 13·4 ± 0·1 | 11·1 ± 0·4 | 0·7 | 0·1* | + |
| Iα2 | 13·8 ± 0·1 | 13·5 ± 0·1 | 12·0 ± 0·3 | 0·8 | 0·3* | + |
| IIα1 | 7·6 ± 0·1 | 7·6 ± 0·1 | 6·7 ± 0·1 | 1·0 | 0·5* | + |
| IIIα1 | 13·0 ± 0·1 | 13·4 ± 0·1 | 10·4 ± 0·4 | 1·2 | 0·2* | + |
| IVα1 | 11·4 ± 0·2 | 11·4 ± 0·2 | 8·9 ± 0·3 | 1·0 | 0·2* | + |
| IVα2 | 10·3 ± 0·2 | 10·1 ± 0·3 | 8·9 ± 0·3 | 0·9 | 0·4* | + |
| Vα1 | 10·2 ± 0·1 | 9·4 ± 0·1 | 7·6 ± 0·1 | 0·6 | 0·2* | + |
| Vα2 | 11·5 ± 0·1 | 11·5 ± 0·1 | 9·4 ± 0·3 | 1·0 | 0·2* | + |
| VIα3 | 11·2 ± 0·3 | 10·9 ± 0·2 | 9·8 ± 0·4 | 0·8 | 0·4* | + |
| XIIα1 | 12·4 ± 0·1 | 11·6 ± 0·1 | 9·6 ± 0·3 | 0·6 | 0·1* | + |
| XIVα1 | 7·6 ± 0·4 | 5·8 ± 0·3 | 5·6 ± 0·2 | 0·3 | 0·2* | − |
| XVα1 | 10·6 ± 0·6 | 9·1 ± 0·8 | 8·0 ± 0·7 | 0·4* | 0·2* | − |
| XVIα1 | 8·7 ± 0·1 | 8·1 ± 0·2 | 7·6 ± 0·1 | 0·7 | 0·5* | − |
P < 0·0001.
HGF: hepatocyte growth factor; HLA: human leucocyte antigen; IDO: indoleamine 2,3-dioxygenase; IL: interleukin; MLR: mixed leucocyte reaction; s.d.: standard deviation; TNF: tumour necrosis factor.
Immunosuppressive factors
It is well recognized that multiple factors are involved in the immunosuppressive function of ASC [5,15,18,19]. In our hands, there was no up-regulation of the anti-inflammatory factors IL-10, TGF-β, iNOS or haem oxygenase by ASC after culture with MLR or proinflammatory cytokines. There was minor up-regulation of HGF (fourfold) and HLA-G (threefold) (Fig. 2a). However, IDO expression was 394-fold increased by ASC cultured with the inflammatory proinflammatory cytokines. The increase in IDO expression was significantly smaller in ASC cultured with MLR (threefold). In contrast, ASC cultured with MLR had 10-fold increased levels of COX-2, which may result in increased production of anti-inflammatory prostaglandin E2. Increased COX-2 expression was not seen in ASC cultured with proinflammatory cytokines.
Fig. 2.

Gene expression of adipose tissue-derived mesenchymal stem cells (ASC) cultured under control conditions, with alloactivated peripheral blood mononuclear cells (PBMC) (mixed lymphocyte reaction: MLR) or with proinflammatory cytokines for 7 days. mRNA expression is shown as log2-transformed median fluorescence intensities of four independent samples as measured by microarray. (a) Immunosuppressive factors; (b) guanylate binding proteins; (c) myxovirus resistance genes; (d) proinflammatory factors; (e) serum amyloid; (f) human leucocyte antigen (HLA) class I; (g) HLA class II; (h) chemokine ligands (CXCL); (i) chemokine ligands (CCL); (j) collagens.
Exposure of ASC to the inflammatory conditions resulted in strong induction of genes for guanylate binding proteins (GBP) and myxovirus resistance genes, which have anti-viral and anti-microbial function. The largest increases were observed for GBP5 (291-fold), GBP4 (102-fold), GBP2 (22-fold) and GBP1 (14-fold) in ASC cultured with proinflammatory cytokines (Fig. 2b). In addition, ASC cultured with proinflammatory cytokines strongly up-regulated the expression of myxovirus resistance genes 1 (19-fold) and 2 (10-fold) (Fig. 2c). This increase in expression was not observed in ASC cultured with MLR.
Proinflammatory factors
Although ASC can exert immunosuppressive activity, they also express genes for proinflammatory factors (Fig. 2d). IL-6 was expressed highly under all culture conditions. After exposure of ASC to alloactivated PBMC, we found a 46-fold up-regulation of IL-8, while the expression of IL-1β (sevenfold) and IL-33 (11-fold) also increased. In contrast, culture of ASC with proinflammatory cytokines up-regulated the expression of TNF superfamily (TNFSF) member 10 and member 13B by factors 53 and 11, respectively. ASC did not express IL-2. Serum amyloid A1 and A2, factors produced by the liver in response to inflammatory stimuli, showed strongly increased gene expression after culture of ASC with alloactivated PBMC (31-fold and 20-fold, respectively) (Fig. 2e), while these factors were not up-regulated in ASC cultured with proinflammatory cytokines.
HLA expression
ASC expressed high levels of HLA class I, whereas HLA class II levels were low under control conditions (Fig. 2f,g). In the presence of alloactivated PBMC, HLA class I expression by ASC was increased slightly (twofold) and HLA class II expression did not change significantly. In contrast, ASC cultured with proinflammatory cytokines up-regulated the expression of HLA class I genes up to sixfold and HLA class II up to 144-fold.
Chemokines
Next, the effect of inflammatory conditions on the chemoattractive properties of ASC was examined. Culture of ASC with MLR or proinflammatory cytokines induced differential expression of several chemokines. ASC cultured with MLR increased the expression of the neutrophil, monocyte and eosinophil attractants CXCL1 (18-fold) and CXCL6 (21-fold) (Fig. 2h). ASC cultured with proinflammatory cytokines showed strong increases in the expression of the T lymphocyte attractants CXCL9 (209-fold), CXCL10 (522-fold) and CXCL11 (251-fold), whereas the neutrophil, monocyte and eosinophil attractants CXCL1 and CXCL6 showed weaker increases (sevenfold and ninefold).
Chemokines of the CCL-motive were also induced specifically by ASC depending on the inflammatory stimulus (Fig. 2i). In ASC cultured with MLR the expression of CCL2 (fourfold), CCL5 (sevenfold), CCL13 (sixfold), CCL20 (eightfold) and CCL28 (threefold) was increased significantly compared to control ASC. Culture of ASC with the proinflammatory cytokines strongly increased the expression of CCL2 (fivefold), CCL5 (27-fold), CCL7 (17-fold), CCL8 (41-fold) and CCL13 (12-fold), but had no effect on the lymphocyte attractants CCL20 and CCL28.
Fibrosis
To investigate whether inflammatory conditions stimulate ASC to induce fibrosis, we examined the expression of collagens by ASC. Proinflammatory cytokines reduced significantly the expression of 13 of a total of 45 types of collagens (Fig. 2j). Culture of ASC with MLR reduced expression of collagen type 15α1 only (threefold). ASC may also induce fibrosis via the secretion of factors such as connective tissue growth factor, TGF-β and platelet-derived growth factor that act on other cell types. The expression of these factors by ASC, however, did not change in response to inflammatory conditions. Furthermore, except from small increases in actin α1 (0·2-fold) and actin γ2 (2·0-fold) after culture with MLR, no significant changes in gene expression of cytoskeletal proteins such as actins or intermediate filaments were observed in ASC after exposure to proinflammatory conditions.
Effect of inflammatory conditions on the phenotype of ASC
Next, functional analysis of ASC cultured under inflammatory conditions was performed. ASC cultured under inflammatory conditions showed morphological changes compared to ASC cultured under control conditions (Fig. 3a). ASC cultured under control conditions grew in a monolayer and were distributed equally on the surface of the culture flask, while ASC cultured with alloactivated PBMC clustered in star-shaped formations.
Fig. 3.
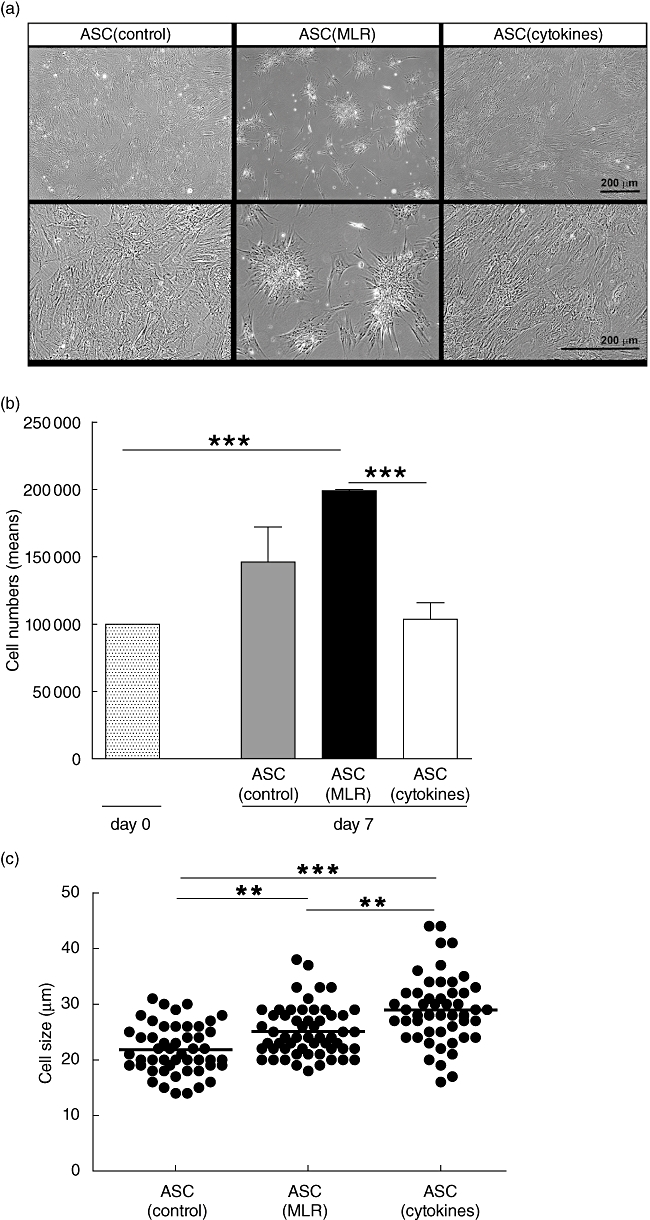

Effect of inflammatory conditions on the phenotype of adipose tissue-derived mesenchymal stem cells (ASC). ASC were cultured under control conditions, with alloactivated peripheral blood mononuclear cells (PBMC) (mixed lymphocyte reaction: MLR) in transwell culture systems or with proinflammatory cytokines. For each independent experiment, ASC were used from different ASC donors. (a) Morphology of ASC. ASC cultured under control conditions and with proinflammatory cytokines formed confluent monolayers, while ASC co-cultured with MLR formed star-like clusters of cells. Representative examples shown of four experiments with four different ASC cultures. (b) Proliferation of ASC. Absolute number of viable ASC was counted after 7 days in culture. Data represent the mean ± standard error of the mean of six experiments with six different ASC cultures. (c) Cell size of ASC. ASC cultured with MLR and proinflammatory cytokines increased significantly in size compared to ASC cultured under control conditions. Approximately 50 individual trypsinized ASC per culture condition were measured. (d) Flow cytometric analysis of ASC. One representative example of each condition is shown of three independent experiments using three different ASC cultures. *P < 0·05; **P < 0·01; ***P < 0·001.
The number of ASC cultured for 7 days with MLR increased compared to control ASC cultures (Fig. 3b). In contrast, the number of ASC treated with proinflammatory cytokines was reduced significantly.
Culture of ASC with MLR or proinflammatory cytokines increased significantly the diameter of ASC (Fig. 3c). ASC cultured under control conditions had a diameter of 21 (interquartile range 19–25) µm. After culture with MLR, ASC had a diameter of 24 (22–28) µm and treatment of ASC with inflammatory cytokines led to an increase in cell diameter to 29 (25–32) µm.
To investigate whether the immunophenotype of ASC changed after culture with inflammatory factors, flow cytometric analysis was performed (Fig. 3d). ASC expressed the characteristic cell surface markers CD90, CD105 and CD166 and the expression of these markers was unaffected by culture of ASC with MLR or proinflammatory cytokines. Levels of HLA class I expression by ASC were independent of inflammatory culture conditions. Control ASC were slightly positive for HLA class II (6%), while culture of ASC with MLR or proinflammatory cytokines resulted in an increase in HLA class II-positive cells of 62% and 86%, respectively. Independently of culture conditions, ASC stained positive for the co-stimulatory molecule CD80 and were weakly positive for CD86. CD40 was not expressed on control or MLR-cultured ASC, but culture of ASC with proinflammatory cytokines induced expression of CD40.
Effect of inflammatory conditions on the differentiation capacity of ASC
ASC, cultured previously for 7 days under inflammatory conditions, were cultured under adipogenic and osteogenic conditions for 3 weeks (Fig. 4). Independent of previous culture conditions, ASC were able to differentiate in adipogenic and osteogenic lineages.
Fig. 4.

Effect of inflammatory conditions on the differentiation capacity of adipose tissue-derived mesenchymal stem cells (ASC). ASC cultured under control conditions, with mixed lymphocyte reaction (MLR) or with proinflammatory cytokines, were continued in culture for 3 weeks under osteogenic or adipogenic conditions. One representative example is shown of four experiments using four different ASC donors. (a) Osteogenic differentiation. Deposition of calcified nodules (brown/black) was visualized by von Kossa staining. (b) Adipogenic differentiation. Lipid droplets (red) were stained with oil-red-O.
Effect of inflammatory conditions on the immunosuppressive capacity of ASC
To examine the effect of inflammatory conditions on the immunosuppressive capacity of ASC, pretreated ASC were added to mitogen (PHA)-stimulated or alloactivated PBMC at different concentrations. At an ASC-PBMC ratio of 1:5, ASC inhibited PHA-stimulated PBMC proliferation significantly after 3 days (Fig. 5a). At this ratio, ASC cultured under control conditions inhibited the PHA-stimulated proliferation by 50 ± 26%, ASC pretreated with MLR by 59 ± 6% and ASC pretreated with proinflammatory cytokines by 84 ± 9%. At lower concentrations (1:20 and 1:50), ASC pretreated with proinflammatory cytokines were still able to inhibit significantly the proliferation of PHA-stimulated PBMC by 36 ± 27% and 20 ± 20%, respectively, whereas ASC cultured under control conditions or with alloactivated PBMC did not show this capacity.
Fig. 5.

Effect of inflammatory conditions on the inhibition of peripheral blood mononuclear cell (PBMC) proliferation by adipose tissue-derived mesenchymal stem cells (ASC). ASC were pretreated under control conditions, with alloactivated PBMC mixed lymphocyte reaction (MLR) or with proinflammatory cytokines conditions and added to mitogen- or alloactivated PBMC. The proliferation in the test conditions is normalized to the proliferation in the control situation (i.e. MLR without addition of ASC, which is set to 100%). (a) Effect of pretreated ASC on the proliferation of phytohaemagglutinin (PHA)-stimulated PBMC. ASC were added to PBMC at various concentrations (1:5, 1:20, 1:50) (n = 8). Proliferation was measured by [3H]-thymidine incorporation on day 3. (b) Effect of pretreated ASC added on day 0 to MLR on the proliferation of alloactivated PBMC (MLR). ASC were added to PBMC at various concentrations (1:5, 1:20, 1:50) (n = 6). Proliferation was measured by [3H]-thymidine incorporation on day 7. (c) Effect of pretreated ASC added on day 6 to MLR. ASC were added to responder PBMC at a 1:5 ratio (n = 11). Proliferation was measured by [3H]-thymidine incorporation on day 7. (d) Effect of IDO1-inhibitor 1-methyl-l-tryptophan (1-MT) on the capacity of pretreated ASC to inhibit the proliferation of PHA-stimulated PBMC (n = 4). ASC were added to responder PBMC at a 1:5 ratio. Proliferation was measured by [3H]-thymidine incorporation on day 3. (e) Effect of IDO1-inhibitor 1-MT on the capacity of pretreated ASC to inhibit the proliferation in MLR (n = 8). ASC were added to responder PBMC at a 1:5 ratio. Proliferation was measured by [3H]-thymidine incorporation on day 7. *P < 0·05; **P < 0·01; ***P < 0·001.
Comparable effects of pretreatment conditions on the immunosuppressive capacity of ASC were observed when pretreated ASC were added to MLR for 7 days (Fig. 5b). At an ASC–PBMC ratio of 1:5, ASC cultured under control conditions inhibited the proliferation of alloactivated PBMC by 44 ± 25%, but this effect disappeared at a 1:20 ratio, and at a ratio of 1:50 they even stimulated the proliferation. ASC cultured previously with MLR inhibited the proliferation by 55 ± 3% (at 1:5 ratio). At lower concentrations (1:20 or 1:50), ASC precultured with MLR had no inhibitory effects. ASC pretreated with MLR, however, did not stimulate the proliferation as observed with control ASC. Pretreatment of ASC with proinflammatory cytokines increased further the immunosuppressive capacity of ASC. At a ratio of 1:5 to responder cells, these pretreated ASC inhibited the proliferation in MLR by 76 ± 18%. Their immunosuppressive effect was still present at lower ratios and the proliferation of alloactivated PBMC was inhibited by 42 ± 35% and 32 ± 27% at a ratio of 1:20 and 1:50, respectively.
To examine whether the anti-proliferative effect of ASC was instant, ASC were added on day 6 of a 7-day MLR at a 1:5 ratio (Fig. 5c). Addition of control and MLR-precultured ASC did not inhibit, but stimulated, the proliferation of responder cells in MLR by 26 ± 21% and 24 ± 19%, respectively. In contrast, ASC pretreated with proinflammatory cytokines inhibited PBMC proliferation by 25 ± 14% during the final day of the 7-day MLR (P < 0·001). Thus, pretreatment with MLR increased the capacity of ASC to inhibit the proliferation of mitogen and alloactivated PBMC. Pretreatment of ASC with proinflammatory cytokines resulted in even stronger and instant immunosuppressive function of ASC.
Because of the striking increase in the expression of IDO by ASC cultured with proinflammatory cytokines, the importance of IDO as a mediator of the enhanced immunosuppressive capacity of ASC was investigated. Pretreated ASC were added to PHA-stimulated PBMC or MLR in the presence or absence of the IDO inhibitor 1-MT. 1-MT had no effect on the capacity of control and MLR pretreated ASC to inhibit the proliferation of PHA and alloactivated PBMC (Fig. 5d,e). However, 1-MT decreased significantly the inhibitory effect of ASC pretreated with proinflammatory cytokines. The percentage inhibition of PHA-stimulated PBMC reduced from 84 ± 8% to 64 ± 17% and the inhibition of MLR from 68 ± 20% to 29 ± 45% after addition of 1-MT. The reduction of the immunosuppressive capacity of proinflammatory cytokine-activated ASC by 1-MT confirms the involvement of IDO in the increased immunosuppressive activity of ASC.
Discussion
In the present study we have demonstrated that inflammatory conditions have an important impact on the phenotype and function of ASC. Stimulation of ASC with MLR was used to study the effect of a range of inflammatory cytokines that are associated with immune responses. Stimulation with the proinflammatory cytokines IFN-γ, TNF-α and IL-6 represents a controlled and reproducible method of immune activation of ASC. Culture of ASC with alloactivated lymphocytes (MLR) or proinflammatory cytokines did not affect their differentiation capacity and production of trophic factors. Both inflammatory conditions, however, affected ASC morphology, proliferation and gene expression of cytokines, chemokines and HLA molecules. These gene expression changes led to increased immunosuppressive capacity of ASC.
Exposure of ASC to MLR or a cocktail of proinflammatory cytokines resulted in a change in ASC morphology and distribution in culture. The typical monolayer distribution of ASC changed to a star-shaped clustered distribution of ASC after culture in an inflammatory milieu. This effect was most striking in cultures of ASC in the presence of MLR. The clustering could be the result of differential expression of cell adhesion molecules. Whereas cadherin and selectin expression was not affected, the expression of a number of integrins changed modestly in ASC in the presence of MLR compared to control ASC and ASC cultured with proinflammatory cytokines. We also observed that ASC cultured with MLR showed a high proliferation rate, while culture with proinflammatory cytokines resulted in ASC with enlarged cell size and dramatically reduced proliferation. These findings indicate that ASC are affected in a different manner by the two inflammatory conditions used.
Inflammatory conditions not only affected the phenotype of ASC, but also the immunosuppressive function of ASC. Culture of ASC with MLR improved the capacity of ASC to inhibit the proliferation of mitogen or alloantigen-stimulated lymphocytes. Culture of ASC with proinflammatory cytokines enhanced the immunosuppressive capacity of ASC even further. In contrast to ASC precultured under control conditions, ASC pretreated with proinflammatory cytokines were able to inhibit lymphocyte proliferation when added at day 6 of a 7-day MLR. This suggests that proinflammatory cytokines activate the immunosuppressive machinery of ASC. This can lead to immediate immunosuppressive activity when ASC are added to an active MLR.
The increased immunosuppressive effect of these ASC was dependent largely upon increased expression of IDO. Blocking IDO reduced the immunosuppressive effect of cytokine-treated ASC to levels found in control ASC, but did not abolish the immunosuppressive capacity completely. This shows that IDO is important for the induced immunosuppressive capacity of ASC treated with cytokines, but less so for the basic immunosuppressive capacity of ASC. As a consequence, other factors must play a role in the immunosuppressive function of ASC, of which several have been reported in the literature, such as HGF, HLA-G and nitric oxide (NO) [5,19,20]. We found high expression of HLA-G, TGF-β1 and COX-2, which have been reported to be involved in the immunosuppressive effect of ASC [5,18,19]. In MLR-cultured ASC we found strong up-regulation of COX-2, which could indicate that prostaglandin E2 is responsible for some of the enhanced immunosuppressive capacity of these cells.
Culture under inflammatory conditions not only changed the expression of anti-inflammatory factors by ASC, but also increased the expression of HLA class I. The expression of HLA class II was increased predominantly by proinflammatory cytokines, whereas culture of ASC with MLR had less effect. Up-regulation of HLA makes ASC potentially more immunogenic. This could have consequences for clinical application of ASC of allogeneic origin.
Inflammatory conditions also increased the expression of proinflammatory factors and chemokines. The type of proinflammatory factors and chemokines produced by ASC depended upon the inflammatory condition. Whereas ASC cultured with MLR showed up-regulation of chemokines for neutrophils, monocytes and macrophages, in particular, culture of ASC with proinflammatory cytokines resulted in the up-regulation of chemokines for T lymphocytes. The relevance of the chemoattraction of the different immune cells by ASC is not clear, but could lead to binding of activated immune cells to ASC [23]. Close contact of activate immune cells and ASC may increase the efficacy of the immunomodulatory function of ASC [20,35]. These results indicate that ASC can exhibit diverse immunomodulatory effects. The local inflammatory milieu is of crucial importance for the balance between the pro- and anti-inflammatory effects of ASC. Furthermore, it determines the mechanisms that ASC employ to execute their immunomodulatory function.
Apart from their immunomodulatory properties, ASC have potential to support tissue regeneration. While this is mediated partially via their differentiation in other cell types [2], there is now increasing evidence that the regenerative effect of ASC is also the result of the production of trophic factors, which stimulate resident progenitor cells [4]. Under inflammatory conditions, ASC maintained the capacity to differentiate in adipogenic and osteogenic lineages. Moreover, inflammatory conditions did not affect the expression of growth factors, such as epidermal growth factor, VEGF and fibroblast growth factors by ASC, and even increased the expression of stem cell factor. Thus, while ASC gain immunosuppressive capacity under inflammatory conditions, their regenerative capacity is preserved.
A suggested undesired property of ASC is their potential transformation into fibrosis [36]. We found that culture of ASC with MLR had no effect on collagen gene expression, while culture of ASC with proinflammatory cytokines induced down-regulation of the expression of multiple collagens. The expression of connective tissue growth factor, TGF-β and platelet-derived growth factor, which can induce epithelial–mesenchymal transition, was not affected by inflammatory conditions. This suggests that inflammatory conditions do not favour the induction of fibrosis by ASC.
The present study demonstrates that the type of inflammatory stimulus affects the response of ASC. In an alloactivated setting, ASC remain functional and even enhance their immunosuppressive function. Their immunosuppressive activity can be enhanced further by culturing ASC with proinflammatory cytokines. This offers the possibility to generate ASC in vitro with strong and instant immunosuppressive capacity. The potential regenerative capacity of ASC is not affected by inflammatory conditions and there is no evidence for an increased risk of fibrosis. Therefore, immune activation of ASC could be of benefit for potential clinical immune therapy with ASC.
Acknowledgments
The authors thank the Department of Surgery of the Erasmus Medical Center Rotterdam for collecting the perirenal adipose tissue of the living kidney donors. We also thank Zeliha Ozgur for technical assistance. Microarray data are deposited in Gene Expression Omnibus (GEO), number GSE18662 at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18662 (free, accessible from 20 October 2010).
Disclosure
The authors have nothing to disclose.
References
- 1.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 5.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 7.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 8.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–73. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 9.Crop M, Baan C, Weimar W, Hoogduijn M. Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int. 2009;22:365–76. doi: 10.1111/j.1432-2277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Dahlke MH, Hoogduijn M, Eggenhofer E, et al. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88:614–19. doi: 10.1097/TP.0b013e3181b4425a. [DOI] [PubMed] [Google Scholar]
- 11.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp. 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 14.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–8. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 16.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 19.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 20.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Wu DQ, Hu YH, et al. In vitro cultivation of islet-like cell clusters from human umbilical cord blood-derived mesenchymal stem cells. Transl Res. 2008;151:293–302. doi: 10.1016/j.trsl.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 23.Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol. 2009;39:3436–46. doi: 10.1002/eji.200939584. [DOI] [PubMed] [Google Scholar]
- 24.Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–60. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 25.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 26.Najar M, Rouas R, Raicevic G, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570–83. doi: 10.1080/14653240903079377. [DOI] [PubMed] [Google Scholar]
- 27.Hemeda H, Jakob M, Ludwig A, Giebel B, Lang S, Brandau S. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 28.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–63. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597–604. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 31.Crop MJ, Baan CC, Korevaar SS, et al. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 32.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–75. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 34.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant. 2009;9:222–30. doi: 10.1111/j.1600-6143.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 35.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 36.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]


