Abstract
Breast milk contains pro- and anti-inflammatory cytokines and chemokines with potential to influence immunological maturation in the child. We have shown previously that country of birth is associated with the cytokine/chemokine profile of breast milk. In this study we have investigated how these differences in breast milk affect the cellular response of cord blood mononuclear cells (CBMCs) and intestinal epithelial cells (IECs, cell line HT-29) to microbial challenge. Ninety-five women were included: 30 from Mali in West Africa, 32 Swedish immigrants and 33 native Swedish women. CBMCs or IECs were stimulated in vitro with breast milk, alone or in combination with lipopolysaccharide (LPS) or peptidoglycan (PGN). Breast milk in general abrogated the LPS-induced down-regulation of surface CD14 and Toll-like receptor (TLR)-4 expression on CB monocytes, while inhibiting the PGN-induced TLR-2 up-regulation. However, breast milk from immigrant women together with LPS induced a lower CBMC release of interleukin (IL)-6 (P = 0·034) and CXCL-8/IL-8 (P = 0·037) compared with breast milk from Swedish women, while breast milk from Swedish women and Mali women tended to increase the response. The same pattern of CXCL-8/IL-8 release could be seen after stimulation of IECs (HT-29). The lower CBMC and IEC (HT-29) responses to microbial compounds by breast milk from immigrant women could be explained by the fact that breast milk from the immigrant group showed a divergent pro- and anti-inflammatory content for CXCL-8/IL-8, transforming growth factor-β1 and soluble CD14, compared to the other two groups of women. This may have implications for maturation of their children's immune responses.
Keywords: breast milk, cord blood, cytokines, intestinal epithelial cells, maternal country of birth
Introduction
A child is born with a sterile intestine but is heavily colonized during the first weeks of life. A regulated recognition of microorganisms by the intestinal epithelial cells (IECs) is crucial for maintaining gut homeostasis. Colonization of the neonatal gut is needed for production of polymeric immunoglobulin A (IgA) [1], and it takes several months before IgA in the gut has reached protective levels. During this time the breast milk will provide the child with IgA, with highest concentrations in colostrum. Also, immune cells, anti-inflammatory [e.g. interleukin (IL)-10, transforming growth factor (TGF-β)] and proinflammatory [e.g. IL-1β, IL-6, CXCL-8/IL-8, tumour necrosis factor (TNF)] factors in the breast milk are important for protection of the newborn from pathogens and regulating the immune response [2]. Indeed, breast feeding protects against necrotizing enterocolitis [3], gastrointestinal infection [4] and respiratory infections [5] in the newborn. In contrast, the role of breast-feeding in allergy prevention remains controversial, and it is debated whether the different immunomodulatory components in human milk differ between mothers [6]. Maternal allergic disease has indeed been shown to influence the immunological content of the milk [7–10].
There is a lower incidence of some immune-mediated diseases in developing than developed countries and immigrants maintain their lower risk [11,12]. However, the offspring of immigrants have an increased immune-mediated disease risk compared to the indigenous population of the developed country [13–16]. This might reflect stimulation resulting from maternal characteristics indicated by breast milk. We demonstrated recently that mothers born in a developing country who migrated to Sweden after age 10 years have increased breast milk levels of TGF-β1, IL-6 and IL-8 compared to the indigenous population of their new country [17].
Although the neonatal immune system is immature compared with adults, it has the ability to mount significant innate immune responses against microorganisms through pattern recognition receptors, of which the Toll-like receptors (TLRs) are the best characterized. TLR-2 signals in response to a wide range of microbial associated molecular patterns, including peptidoglycan (PGN). TLR-4 co-operates with CD14 expressed by monocytes and macrophages in response to lipopolysaccharide (LPS) [18]. Importantly, cells lacking membrane CD14 becomes LPS-responsive in the presence of soluble CD14 (sCD14). sCD14 is also involved in bridging innate and adaptive immune responses through regulating the activation and functions of B cells [19] and activated T cells [20]. Further, breast milk contains high concentrations of sCD14, believed to add to the protection of the digestive tract [21,22].
We have shown previously that immunological factors in breast milk could be influenced by maternal exposures in childhood [17]. The aim of this study was to investigate how breast milk modulates anti-microbial responses by cord blood mononuclear cells (CBMCs) and intestinal epithelial cells (IECs) (HT-29), and whether these effects could be associated with pro- and anti-inflammatory properties of the milk.
Materials and methods
Subjects
Mothers who completed full-term singleton pregnancies with uncomplicated deliveries and with no notable morbidity in mother or child were recruited consecutively during the same period at delivery units in Örebro County Sweden. In Sweden, a total of 33 mothers who were born and resided in Sweden throughout their childhood, and a total of 32 mothers who themselves were born and spent at least the first 10 years of their life in a developing country and subsequently immigrated to Sweden were recruited. Countries of origin were the following (number of women): Somalia (13), Congo (two), Afghanistan (one), Pakistan (one), Togo (two), Burundi (two), Eritrea (three), Morocco (two), Iraq (four) and Burma (one). Mothers who were born and resided permanently in the area around Bamako in Mali in West Africa were also recruited (n = 30). The medical ethics committees of the Uppsala region of Sweden and in Bamako, Mali approved this study. For stimulation experiments with CBMCs and IECs (HT-29), breast milk were selected randomly from the three cohorts [women born and resident in Sweden (n = 11), women born in a developing country but resident in Sweden (n = 10) and women born and resident in Mali (n = 11)].
Breast milk collection
Breast milk samples were collected between 3 and 5 days following delivery. Some 10–15 ml of breast milk was collected in sterile tubes using a manual breast pump. Within 2 h of collection, the sample was centrifuged for 20 min, 900 g at 4°C. The fat layer and cellular compartments were removed and the remaining whey was aliquoted and stored at −80°C.
Cytometric bead array
IL-1β, IL-6, IL-10, IL-12p70, TNF and CXCL-8/IL-8 levels in breast milk were measured using cytometric bead array (CBA) (BD Biosciences, San Jose, CA, USA), according to the manufacturer's instructions and as described previously [17]. The IL-12p70 levels were too low for reliable detection and were not included in further analysis.
Enzyme-linked immunosorbent assay (ELISA) for sCD14, CXCL-8/IL-8, TGF-β1, TGF-β2, sIL-6R, sgp130 and IgA
sCD14, CXCL-8/IL-8, TGF-β1, TGF-β2, sIL-6R, sgp130 and total IgA levels in breast milk and in culture supernatants from IECs (HT-29) were determined using ELISA (sCD14, CXCL-8/IL-8, TGF-β1, TGF-β2, sIL-6R and sgp130 ELISAs from R&D Systems, Abingdon, Oxon, UK and human IgA ELISA quantitation kit from Bethyl Laboratories Inc., Montgomery, TX, USA), according to the manufacturer's instructions. The optical density was determined using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) set at 450 nm.
Collection of cord blood mononuclear cells
Seven cord bloods (CB) were collected at Örebro University Hospital, Sweden from children born to mothers with full-term singleton pregnancies with uncomplicated deliveries. These mothers did not participate in the study. Cord blood samples were aspirated from the umbilical cord vein into heparinized Vacutainer tubes after careful wiping of the cord with alcohol. CBMCs were isolated and cryopreserved as described previously [23].
In vitro activation of cells
CBMCs
Seven CBs from children born to mothers not participating in the study were used repeatedly to minimize variation due to individual differences between the donors. CBMCs were thawed and washed three times in culture medium [RPMI-1640 supplemented with HEPES (20 mm), penicillin (100 U/ml), streptomycin (100 µg/ml), l-glutamine (2 mm); HyClone Laboratories Inc, South Logan, UT, USA] and 10% heat-inactivated fetal calf serum (Gibco, Invitrogen, Auckland, New Zealand). The cells were diluted to a concentration of 1 × 106 cells/ml with culture medium and were plated in 48-well plates (Costar, Cambridge, UK) either with addition of culture medium only, breast milk diluted 1 : 10, LPS (Escherichia coli B, 1 ng/ml; Sigma Aldrich, Stockholm, Sweden), PGN (Staphylococcus aureus, 1 µg/ml; Sigma Aldrich) or breast milk in combination with LPS or PGN. The cells were incubated at 37°C in 6% CO2 atmosphere for 3 h (for analysis of cell surface markers by flow cytometry) and 24 h (for measurement of secreted cytokines and the chemokine CXCL-8/IL-8 in cell culture supernatants). After the incubation time, the cells were subjected to analysis by flow cytometry and the supernatants were collected through centrifugation and stored at −20°C until analysed further. Kinetic and titration studies were performed to determine the optimal time-points and concentrations for the experiments.
IECs
The human colon carcinoma epithelial cell line HT-29 was obtained from American Type Culture Collection. A total of 200 000 HT-29 cells were grown in medium (McCoys 5A; LGC Standards, Teddington, UK) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Invitrogen) in 48-well plates (Costar) for 24 h until reaching 90% confluence. The cells were washed once with culture medium and incubated for 24 h in new media supplemented with either LPS (100 ng/ml; Sigma-Aldrich), breast milk (diluted 1 : 10) or breast milk in combination with LPS. To investigate how breast milk sCD14 affected the response of IECs (HT-29) to LPS, breast milk (diluted 1 : 100) was incubated together with an anti-human CD14 antibody (20 µg/ml, clone MY4, IgG2b; Beckman Coulter, Brea, CA, USA) or an isotype-matched control antibody (20 µg/ml, IgG2b; Dako, Glostrup, Denmark) for 2 h at room temperature before adding the milk together with LPS (100 ng/ml; Sigma-Aldrich) to the cells as described above. After the incubation time, the supernatants were collected through centrifugation and stored at −20°C until analysed further. Kinetic and titration studies were performed to determine the optimal time-points and concentrations for the experiments.
Flow cytometry
CBMCs were preincubated for 10 min with 10% normal mouse serum to block Fc receptors and stained thereafter with the following antibodies: anti-CD14 fluorescein isothiocyanate (FITC) (IgG2b, Leu-M3; BD Biosciences), anti-TLR-2 phycoerythrin (PE) (IgG2a, TL2·1; eBioscience, San Diego, CA, USA), and anti-TLR-4 PE (IgG2a, HTA125; eBioscience), isotype-matched antibodies: IgG2b (BD Biosciences) and IgG2a (eBioscience) and subjected to flow cytometry as described previously [23] using a BD fluorescence activated cell sorter (FACSCalibur) flow cytometer (BD Biosciences) and analysed using CellQuest software (BD Biosciences). The results for the CBMCs are presented as stimulation index of mean fluorescence intensity (MFI) of stimulated/unstimulated monocytes. Further, HT-29 cells were stained with anti-TLR-4 PE (IgG2a, HTA125; eBioscience) to confirm TLR-4 surface expression.
Statistical analyses
The distributions of cytokine levels and receptor expression were skewed, so this skewness was eliminated using the lnskew0 procedure provided by the STATA version 10·0 software package. The distribution for IL-6 release after stimulation of CBMCs did not require transformation. Each of the transformed cytokine levels or transformed receptor expression was the dependent variable in linear regression analysis. Statistical significance was defined as P < 0·05 or 95% confidence intervals (CI) that did not include 0·00. For stimulation experiments with CBMCs corrections were made for variation between cord blood samples using a cluster function.
Results
Cytokines, chemokines and sCD14 in breast milk
In a comparison of 64 breast milk samples from two different groups of women (immigrant women living their first 10 years in a developing country but giving birth in Sweden, and native Swedish women), we have shown previously that immigrant women differ in their breast milk composition compared to women who grew up in Sweden [17]. In this study we have included a group of women from Mali in West Africa (n = 30) and here we show that, similar to the milk from immigrant women (n = 32), milk from Mali women contained higher levels of TGF-β1 than milk from native Swedish women [n = 33, P = 0·049, regression coefficient (B) −0·17, 95% confidence interval (CI), −0·35 to −0·0005; Table 1]. However, the Mali women also had significantly higher levels of sCD14 in breast milk compared with both women immigrating to Sweden (P = 0·001, B –0·38, 95% CI, −0·59 to −0·17) and Swedish women (P = 0·015, B −0·26, 95% CI, −0·47 to −0·05; Table 1).
Table 1.
Levels of cytokines, chemokines and sCD14 in breast milk
| Cytokine/chemokine/sCD14 (pg/ml) | Mali women (n = 30) | Immigrant women† (n = 32) | Swedish women† (n = 33) | P-value |
|---|---|---|---|---|
| IL-1β | 5·14 (1·6–67) | 4·9 (2·7–129) | 5·8 (2·8–46) | n.s. |
| IL-6 | 41 (5·2–462) | 44 (7·0–411) | 29 (5·4–1 097) | n.s. |
| CXCL-8/IL-8 | 249 (33–5 728) | 479 (61–3 219) | 305 (57–6 955) | 0·043‡ |
| IL-10 | 4·9 (2·2–19) | 5·3 (2·4–44) | 5·1 (2·7–35) | n.s. |
| TNF | 4·7 (2·7–172) | 4·5 (1·8–34) | 4·3 (2·6–69) | n.s. |
| TGF-β1 | 1312 (449–2 557) | 1038 (435–1 789) | 974 (502–2 314) | 0·049‡, 0·044‡ |
| TGF-β2 | 3727 (256–14 789) | 3105 (528–13 328) | 3703 (623–16 211) | n.s. |
| sCD14 (µg/ml) | 20·2 (13·1–42·2) | 15·2 (8·1–35·4) | 16·8 (10·3–31) | 0·001§, 0·015‡ |
Previously published results [17].
Statistically significant higher levels compared to Swedish women.
Statistically significant higher levels compared to immigrant women.
IL: interleukin; TNF: tumour necrosis factor; TGF: transforming growth factor; n.s.: non-significant.
Breast milk modulates CD14, TLR-4 and TLR-2 expression on cord blood monocytes following stimulation with microbial compounds
The effect of breast milk on CBMC anti-microbial responses (e.g. LPS and PGN) was first investigated in terms of surface expression of CD14, TLR-4 and TLR-2 on CB monocytes. CBMCs were cultured with breast milk for 3 h with or without the addition of LPS and PGN.
Stimulation of CBMCs with breast milk alone did not alter the CB monocyte expression of CD14, TLR-2 or TLR-4 (data not shown). This was true for breast milk from all three groups of women. LPS induced a decreased monocyte expression of CD14 and TLR-4, while PGN induced an increase in surface TLR-2 expression on monocytes, as expected from previous findings [23,24]. Breast milk in general abrogated the LPS-induced down-regulation of surface CD14 and TLR-4 expression (Fig. 1a–d), while inhibiting the PGN-induced TLR-2 up-regulation (Fig. 1e,f).
Fig. 1.
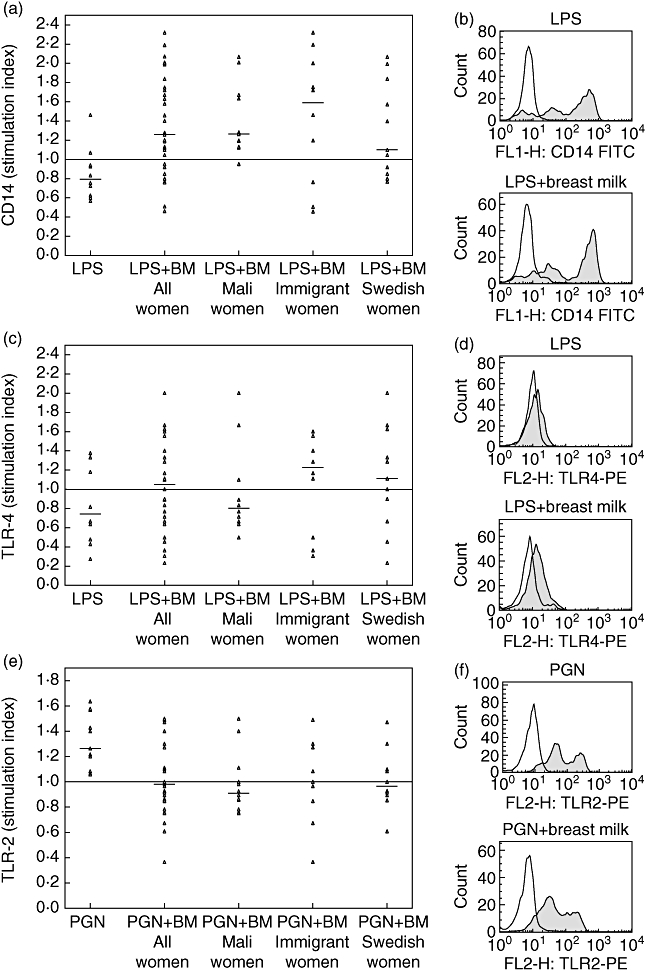
Regulation of monocyte cell surface expression of (a,b) CD14, (c,d) Toll-like receptor (TLR)-4 and (e,f) TLR-2 after stimulation of cord blood mononuclear cells (CBMCs) with lipopolysaccharide (LPS) or peptidoglycan (PGN) alone or in combination with breast milk from Mali women (n = 11), immigrant women (n = 10) or Swedish women (n = 11). Each dot represents the mean fluorescence intensity ratio of receptor expression for stimulated/unstimulated monocytes. (b,d,f) Representative histogram plots displaying receptor expression (shaded) and isotype control (unshaded). BM: breast milk.
Maternal country of birth associates with breast milk influence on IL-6 and CXCL-8/IL-8 CBMC responses to LPS
To investigate whether the observed modulation of CD14 and TLR expression on CB monocytes was reflected in an effector response, we measured the release of IL-6, CXCL-8/IL-8, IL-1β, IL-10 and TNF in culture supernatants of CBMCs stimulated for 24 h with breast milk with or without the addition of LPS or PGN.
Stimulation of CBMCs with breast milk alone induced moderate release of IL-6 and IL-8 with no differences between the three groups (data not shown). In general, breast milk tended to increase the response of CBMCs towards LPS. However, in analogy with the results observed at the cell surface, breast milk from immigrant women together with LPS induced a statistically significant lower release of IL-6 (P = 0·034, B −7·5, 95% CI, −14·2 to −0·79) and CXCL-8/IL-8 (P = 0·037, B −0·04, 95% CI −0·07 to −0·003) compared to breast milk from Swedish women (Fig. 2a,b). The same trend was seen when stimulating CBMCs with breast milk together with PGN, although it did not reach statistical significance (data not shown). Adjustment for IL-6 and CXCL-8/IL-8 in breast milk did not alter the statistically significant results; neither did adjustment for maternal age (data not shown).
Fig. 2.
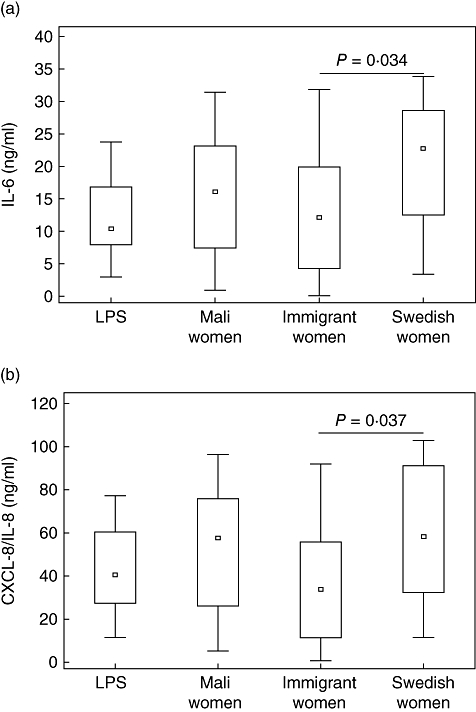
Release of (a) interleukin (IL)-6 and (b) CXCL-8/IL-8 from cord blood mononuclear cells (CBMCs) after stimulation with lipopolysaccharide (LPS) or LPS in combination with breast milk from Mali women (n = 11), immigrant women (n = 10) or Swedish women (n = 11). BM: breast milk.
No significant differences were seen between the groups regarding the breast milk effect on CBMC production of IL-1β, IL-10 or TNF.
Breast milk from immigrant women induces a lower response of IECs (HT-29) to LPS in terms of CXCL-8/IL-8 release compared to women from Mali and Swedish women
Contents of breast milk could potentially aid in the response of the neonate to antigens encountered through the gastrointestinal tract. We therefore investigated the effect of breast milk on IECs (HT-29) either alone or in combination with LPS.
Initially, TLR-4 expression by the HT-29 cells was confirmed by flow cytometry (Fig. 3a). Stimulation with breast milk alone induced low release of CXCL-8/IL-8, only slightly higher than medium only, and no differences were seen between the groups. Stimulation with 100 ng/ml LPS alone induced approximately 20 times higher CXCL-8/IL-8 levels compared to stimulation with breast milk only (data not shown). However, when stimulating IECs (HT-29) with breast milk in combination with LPS significantly increased levels of CXCL-8/IL-8 were released into culture supernatants. Interestingly, stimulation with breast milk from immigrant women together with LPS resulted in lower release of CXCL-8/IL-8 in culture supernatants compared with stimulation with breast milk from Mali women or Swedish women, although not statistically significant (Fig. 3b).
Fig. 3.
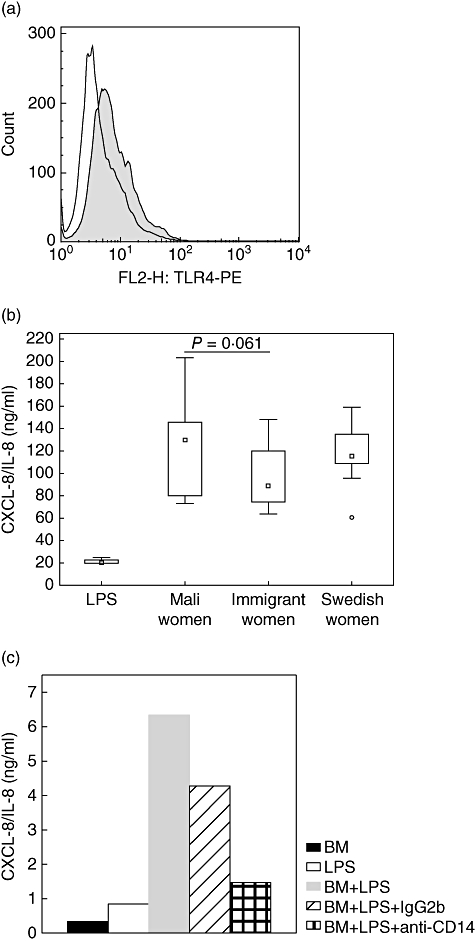
(a) Representative histogram plot displaying Toll-like receptor (TLR)-4 expression on intestinal epithelial cells (IECs) (HT-29) (shaded) and isotype control (unshaded). (b) Release of CXCL-8/IL-8 after stimulation of IECs (HT-29) with lipopolysaccharide (LPS) together with breast milk from Mali women (n = 10), immigrant women (n = 10) or Swedish women (n = 10). (c) Blocking milk sCD14 with an anti-human CD14 antibody (MY4, immunoglobulin G2b, 20 µg/ml), but not an isotype control inhibited the LPS-induced release of CXCL-8/IL-8. Outliers, BM: breast milk.
The response of IECs (HT-29) to LPS stimulation was shown to be dependent on breast milk sCD14. Blocking milk sCD14 with an anti-human CD14 antibody (MY4, IgG2b, 20 µg/ml) inhibited the release of CXCL-8/IL-8 from HT-29 cells after LPS stimulation (Fig. 3c).
IL-6 trans-signalling capacity of breast milk
IL-6 is critical for resolving innate immunity and promoting acquired immunity. A disruption in this transition may predispose to inflammatory disorders and autoimmunity. We therefore investigated potential differences in breast milk IL-6 trans-signalling capacity by measuring sIL-6R and the antagonist to IL-6 trans-signalling sgp130 [25] in breast milk from the three groups of women.
All breast milk samples contained high levels of both sIL-6R and sgp130. However, sgp130 concentrations were more than 10 times higher than the concentration of sIL-6R, which indicates a competitive inhibition of the IL-6/sIL-6R response. Large individual differences both in sIL-6R and sgp130 concentrations were observed, but women from Mali, immigrant women or native Swedish women did not differ from each other (Table 2).
Table 2.
Levels of sIL-6R and sgp130 in breast milk
| Soluble receptors (ng/ml) | Mali women (n = 29) | Immigrant women (n = 31) | Swedish women (n = 31) | P-value |
|---|---|---|---|---|
| sIL-6R | 5·5 (2·3–10·6) | 4·6 (1·8–14·2) | 5·7 (2·2–11·2) | n.s. |
| sgp130 | 65·7 (25·3–323) | 55 (26·8–198) | 67·1 (14–158) | n.s. |
n.s.: non-significant.
Breast milk TGF-β1 and TGF-β2 associates with IgA levels in breast milk
TGF-β1 is involved in the induction of IgA. We examined the relationship of breast milk TGF-β1 and TGF-β2 levels with total IgA levels in breast milk from all women and found a significant association. When subdividing the breast milk samples further according to group, a strong association between TGF-β1 and TGF-β2 and IgA in breast milk from the Mali women could be seen, but not for the immigrant women or the Swedish women (Table 3).
Table 3.
Correlation between total immunoglobulin A (IgA) and transforming growth factor (TGF)-β1 and total IgA and TGF-β2 levels in breast milk
| Associations | n | Coefficient | 95% CI | P-value |
|---|---|---|---|---|
| Total IgA and TGF-β1 | ||||
| All women | 92 | 0·15 | 0·04 to 0·27 | 0·01 |
| Mali women | 29 | 0·45 | 0·17 to 0·74 | 0·003 |
| Immigrant women | 32 | 0·03 | −0·13 to 0·19 | 0·73 |
| Swedish women | 31 | 0·12 | −0·08 to 0·31 | 0·23 |
| Total IgA and TGF-β2 | ||||
| All women | 92 | 0·29 | 0·05 to 0·53 | 0·016 |
| Mali women | 29 | 0·79 | 0·24 to 1·35 | 0·007 |
| Immigrant women | 32 | 0·28 | −0·04 to 0·61 | 0·09 |
| Swedish women | 31 | 0·04 | −0·42 to 0·51 | 0·85 |
The table shows the regression coefficients with 95% confidence intervals calculated using linear regression; bold values indicate statistical significance. CI: confidence interval.
Because both Mali women and immigrant women had increased TGF-β1 levels in breast milk compared with Swedish women, we investigated if there could be differences in breast milk IgA levels between the groups. Although TGF-β and IgA levels were associated, no significant differences in breast milk IgA levels could be observed between the three groups of women (Fig. 4).
Fig. 4.
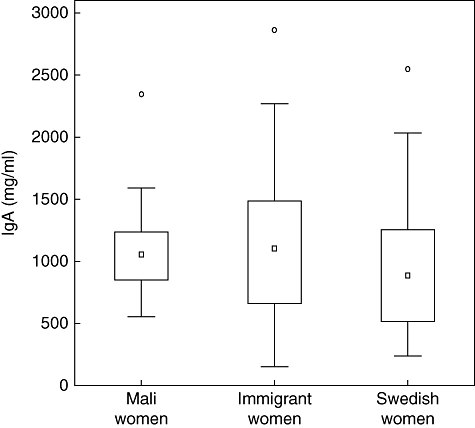
Concentrations of total immunoglobulin A (IgA) in breast milk from Mali women (n = 29), immigrant women (n = 31) and Swedish women (n = 32). Outliers, extreme outliers for IgA not shown. BM: breast milk.
Discussion
This study investigated how inflammatory (e.g. cytokines, the chemokine CXCL-8/IL-8, IL-6 trans-signalling and sCD14) and anti-inflammatory (e.g. IL-10, TGF-β1 and TGF-β2) components of breast milk influence CBMC and IEC (HT-29) responses towards microbial compounds. The women were either living in Mali (high microbial exposure throughout life) or born in a developing country before immigrating to Sweden (high microbial exposure only in early life), and these two groups were compared with samples from native Swedish women (low microbial exposure throughout life).
To assess the modulation of microbial responses by breast milk, stimulation of CBMCs with breast milk in combination with either LPS or PGN was performed (Fig. 1a–f). The addition of breast milk abrogated the LPS-mediated down-regulation of CD14 and TLR4 on CB monocytes, with a more pronounced effect by breast milk from immigrant women. This was also mirrored by a lower release of IL-6 and CXCL-8/IL-8 after stimulation with breast milk from immigrant women together with LPS, compared to stimulation with LPS and breast milk from Swedish women (Fig. 2a,b). Regarding PGN stimulation, breast milk in general tended to inhibit the up-regulation of TLR-2 expression on CB monocytes in response to PGN, which could be a mechanism for down-modulating an immune response to Gram-positive bacteria in the neonatal intestine. Adjustment for IL-6 and CXCL-8/IL-8 in breast milk did not alter the statistical significance of results, indicating that other characteristics beyond IL-6 and CXCL-8/IL-8 in the milk are of importance. Further, children are born with a T helper type 2 (Th2)-biased immune system [26]. However, exposure to pathogenic and non-pathogenic microbes in the environment during early life is believed to drive the maturation of the immune system towards Th1 immunity, thus balancing the overall immune response [27]. Breast milk from immigrant women did not enhance responses to microbial components (LPS, PGN) to the same extent as breast milk from the other two groups of women. This could have consequences for the maturation of their children's own immune responses, rendering them more susceptible to inflammatory disorders such as allergy and other immune-mediated diseases later in life. Indeed, it has been shown that although immigrants moving from a developing country to an industrialized country maintain a low risk for developing immune-mediated diseases, their children have a higher risk of developing diseases such as inflammatory bowel disease (IBD) and allergy compared to the indigenous population of their new country [13,14,16]. The heterogeneity of the immigrants in this study helps to rule out a simple genetic mechanism, and our data support the hypothesis that parental ‘programming’ (e.g. epigenetic, in utero priming or breast feeding) and differential pattern of exposure between parents and the offspring is a probable explanation for this phenomenon.
Recognition of commensal bacteria by TLRs in the intestine is important for gut homeostasis [28]. Under normal conditions, the epithelial cells are stimulated by the commensal bacteria to produce cytokines (IL-6, TNF, TGF-β1), heat-shock proteins and other factors involved in cytoprotection, tissue repair and angiogenesis [29,30]. Cytokines in breast milk may have important functions to protect the neonatal gut during bacterial colonization. When stimulating IECs (HT-29) with LPS in combination with breast milk, we observed a potent enhancement of the CXCL-8/IL-8 response compared to that induced by LPS alone, similar to what has been reported previously by others [21,31]. Breast milk contains large amounts of sCD14 which may enable IECs to respond to microbial stimuli in terms of LPS [21]. Interestingly, IECs (HT-29) showed a less prominent increase in CXCL-8/IL-8 production after stimulation with LPS and breast milk from immigrant women compared to LPS and breast milk from Mali women or Swedish women (Fig. 3b). This is in line with our results with CBMCs, where a lower release of IL-6 and CXCL-8/IL-8 after stimulation with breast milk from immigrant women together with LPS was seen. The lower amounts CXCL-8/IL-8 released by the IECs (HT-29) after stimulation with breast milk from immigrant women together with LPS could be due to a lower sCD14 content of breast milk from these women compared to women from Mali (P = 0·001) and Swedish women (non-significant, Table 1). Indeed, when blocking breast milk sCD14 with an anti-human CD14 antibody, the release of CXCL-8/IL-8 after LPS stimulation was inhibited and the extent of the inhibition was dependent upon the amount of sCD14 in breast milk (Fig. 3c and data not shown). This is in agreement with previously published data [21,31]. Further, a study by Blais et al.[32] reported that only low amounts of sCD14 can be found in faeces of breast-fed infants and that sCD14 is more susceptible to pancreatin digestion than pepsin. This suggests that sCD14 may aid in the protection of the upper digestive tract, while only small amounts of sCD14 will reach the distal gastrointestinal tract to avoid excessive inflammation in this heavily colonized area. High amounts of sCD14 in breast milk might therefore be important to maintain the ability to respond to pathogenic bacteria in the intestine. Also, interaction between sCD14 and B cells have been shown to promote IgG1 but not IgE responses from tonsillar B cells and PBMCs [19], and by interacting directly with activated T cells sCD14 can regulate T cell activation and function negatively [20]. However, we cannot rule out that other milk factors, not investigated in this study, have influenced the response of IECs towards LPS. The less stimulatory effect of breast milk from immigrant women on IEC (HT-29) responses to microbial stimulation could have implication for how the child responds to antigens encountered through the gastrointestinal tract during breast feeding, and might affect the maturation of the child's immune responses.
Further, breast milk contains high levels of sIL-6R which renders cells lacking IL-6R responsive to IL-6. IL-6 is important for the transition from an innate to an acquired immune response, but is also involved in diseases characterized by chronic inflammation [25]. IL-6 exerts its biological functions through signalling via at least one subunit of gp130, together with either its membrane-bound receptor (IL-6R) or its soluble receptor (sIL-6R). Whereas gp130 is expressed ubiquitously, IL-6R expression is limited to certain cell types. However, IL-6-unresponsive cells may be rendered IL-6-responsive via sIL-6R, a mechanism termed IL-6-trans-signalling. The more than 10 times higher concentrations of sgp130 in breast milk compared to sIL-6R, seen in our study, indicate that the IL-6 trans-signalling mediated by breast milk is highly regulated. However, large individual variations were seen between breast milk levels of both sIL-6R and sgp130.
TGF-β is important for class-switching to IgA in human B cells [33] and for induction of oral tolerance [34]. Infants have a limited capacity to produce TGF-β, but will be provided with TGF-β from breast milk for at least 3 months of lactation, which is a longer period than for other milk immune factors [35], and TGF-β in colostrum has been associated with infant IgA production [36]. We observed associations between levels of TGF-β1, TGF-β2 and total IgA levels in breast milk from all women (n = 92, data not shown). Interestingly, when subdividing the women according to group, strong associations between breast milk TGF-β1, TGF-β2 and IgA could be seen only in breast milk from Mali women but not from Swedish or immigrant women (Table 3). The higher breast milk TGF-β1 levels in women from Mali as well as in immigrant women suggests that exposure during childhood, potentially through epigenetic events, may be of importance for TGF-β1 levels in breast milk. However, we cannot rule out the possibility that contemporaneous exposures such as infections, dietary habits and stress could have influenced the results. TGF-β1 levels in breast milk have been associated negatively to early onset of allergic sensitization, probably by promoting low-dose oral tolerance induction and IgA generation in response to dietary antigens [37,38]. No differences in total IgA levels between breast milk from the three groups of women were observed (Fig. 4). However, IgA in breast milk exhibits specificity for an array of common intestinal and respiratory pathogens in the maternal environment, helping to protect both the gut and the upper airways of the child. All breast milk samples contained large quantities of IgA and differences in specificity rather than total amount of IgA might be of greater importance in protection of the newborn child. Indeed, a study from Italy reported increased levels of E. coli-specific secretory IgA in breast milk from immigrant women believed to be part of a persisting immunological secretory memory [39]. Further, although both immigrant and Mali women had significantly higher amounts of TGF-β1 in breast milk a positive association between TGF-β1, TGF-β2 and IgA could be seen only in breast milk from Mali women, indicating a complex regulation of breast milk IgA, potentially by environmental factors.
The sample size in this study is rather small, resulting in limitations in statistical power. However, rather than relying on a single association that may have arisen by chance, it is possible to demonstrate consistency across the findings for several measures. The face validity of these findings is high, as they are in the expected directions. Further, lack of detailed characteristics for the Mali women limits the interpretation, although we have shown previously that immune-mediated diseases and living conditions did not account for any of the differences in breast milk characteristics between immigrant women and Swedish women [17]. There might be heterogeneity in infectious exposures among the Mali women and uncertainty about their health status. However, their uncomplicated pregnancy and delivery strongly indicate good health.
In conclusion, results from this study suggest that immunological factors in breast milk in general have the potential to modulate CBMC and IEC (HT-29) responses to microbial compounds. However, we show clearly that breast milk from different groups of women varies in its pro-and anti-inflammatory content, which is interesting in relation to the debate on breast-feeding and allergy protection. Our results indicate that not only maternal disease (i.e. allergy, which has been shown by others [7–10]) influences breast milk content, but perhaps also maternal environmental factors: not only current exposures, but also exposure during childhood. Breast milk from immigrant women was less efficient in enhancing the response of CBMCs and IECs (HT-29) to microbial compounds, evident both at the cell surface level and in terms of cytokine production. This may have implications for maturation of their children's immune responses, rendering them more susceptible to inflammatory disorders later in life. Further, these findings may help to explain why rapid transition between generations, in terms of exposure to microorganisms, may increase immune-mediated disease risk. Such transition could occur through rapid economic change within countries or through migration between countries.
Acknowledgments
We would like to thank all the women participating in the study, and we are grateful to the staff at Örebro University Hospital, Sweden and in Bamako, Mali, who assisted with sample collection. The work was supported by grants from the Swedish Asthma and Allergy Association's Research Foundation, Örebro University Hospital Research Committee; KI Fonden; Visceral; the Swedish Research Council (grant: 74X-15160-03-2 and 57X-15160-05-02), the Vardal Foundation, the Cancer- and Allergy Foundation, the Swedish Medical Research Society, the Golden Jubilee Memorial Foundation and HRH Crown-Princess Lovisa and Axel Tielman, Hesselman and the Mjölkdroppen and Salén foundations. The collection of the Mali data was supported by grants from BioMalPar, the Mérieux Foundation and MRTC-CVD-Maryland Malaria Training grant of FIC/NIH.
Disclosure
The authors have no conflict of interest.
References
- 1.Suzuki K, Fagarasan S. How host–bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–31. doi: 10.1016/j.it.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Hosea Blewett HJ, Cicalo MC, Holland CD, Field CJ. The immunological components of human milk. Adv Food Nutr Res. 2008;54:45–80. doi: 10.1016/S1043-4526(07)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 4.Monterrosa EC, Frongillo EA, Vasquez-Garibay EM, Romero-Velarde E, Casey LM, Willows ND. Predominant breast-feeding from birth to six months is associated with fewer gastrointestinal infections and increased risk for iron deficiency among infants. J Nutr. 2008;138:1499–504. doi: 10.1093/jn/138.8.1499. [DOI] [PubMed] [Google Scholar]
- 5.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–32. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 6.Duncan JM, Sears MR. Breastfeeding and allergies: time for a change in paradigm? Curr Opin Allergy Clin Immunol. 2008;8:398–405. doi: 10.1097/ACI.0b013e32830d82ed. [DOI] [PubMed] [Google Scholar]
- 7.Böttcher MF, Jenmalm MC, Garofalo RP, Björksten B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:157–62. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Böttcher MF, Jenmalm MC, Björksten B, Garofalo RP. Chemoattractant factors in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:592–7. doi: 10.1203/00006450-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Böttcher MF, Jenmalm MC, Björksten B. Cytokine, chemokine and secretory IgA levels in human milk in relation to atopic disease and IgA production in infants. Pediatr Allergy Immunol. 2003;14:35–41. doi: 10.1034/j.1399-3038.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- 10.Snijders BE, Damoiseaux JG, Penders J, et al. Cytokines and soluble CD14 in breast milk in relation with atopic manifestations in mother and infant (KOALA Study) Clin Exp Allergy. 2006;36:1609–15. doi: 10.1111/j.1365-2222.2006.02613.x. [DOI] [PubMed] [Google Scholar]
- 11.Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16–25. doi: 10.1007/s00535-006-1995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Hertzen LC, Haahtela T. Asthma and atopy – the price of affluence? Allergy. 2004;59:124–37. doi: 10.1046/j.1398-9995.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery SM, Morris DL, Pounder RE, Wakefield AJ. Asian ethnic origin and the risk of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:543–6. doi: 10.1097/00042737-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Kieffer EC, Martin JA, Herman WH. Impact of maternal nativity on the prevalence of diabetes during pregnancy among U.S. ethnic groups. Diabetes Care. 1999;22:729–35. doi: 10.2337/diacare.22.5.729. [DOI] [PubMed] [Google Scholar]
- 15.Hemminki K, Li X. Cancer risks in childhood and adolescence among the offspring of immigrants to Sweden. Br J Cancer. 2002;86:1414–18. doi: 10.1038/sj.bjc.6600227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Amsterdam JG, Bischoff EW, Hady M, Opperhuizen A, Steerenberg PA. The prevalence of allergic sensitisation in immigrant children in the Netherlands. Int Arch Allergy Immunol. 2004;133:248–54. doi: 10.1159/000076831. [DOI] [PubMed] [Google Scholar]
- 17.Amoudruz P, Holmlund U, Schollin J, Sverremark-Ekström E, Montgomery SM. Maternal country of birth and previous pregnancies are associated with breast milk characteristics. Pediatr Allergy Immunol. 2009;20:19–29. doi: 10.1111/j.1399-3038.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias MA, Rey Nores JE, Vita N, et al. Cutting edge: human B cell function is regulated by interaction with soluble CD14: opposite effects on IgG1 and IgE production. J Immunol. 2000;164:3480–6. doi: 10.4049/jimmunol.164.7.3480. [DOI] [PubMed] [Google Scholar]
- 20.Rey Nores JE, Bensussan A, Vita N, et al. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur J Immunol. 1999;29:265–76. doi: 10.1002/(SICI)1521-4141(199901)29:01<265::AID-IMMU265>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Labeta MO, Vidal K, Nores JE, et al. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med. 2000;191:1807–12. doi: 10.1084/jem.191.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal K, Donnet-Hughes A. CD14: a soluble pattern recognition receptor in milk. Adv Exp Med Biol. 2008;606:195–216. doi: 10.1007/978-0-387-74087-4_7. [DOI] [PubMed] [Google Scholar]
- 23.Amoudruz P, Holmlund U, Malmstrom V, et al. Neonatal immune responses to microbial stimuli: is there an influence of maternal allergy? J Allergy Clin Immunol. 2005;115:1304–10. doi: 10.1016/j.jaci.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Amoudruz P, Holmlund U, Saghafian-Hedengren S, Nilsson C, Sverremark-Ekström E. Impaired Toll-like receptor 2 signalling in monocytes from 5-year-old allergic children. Clin Exp Immunol. 2009;155:387–94. doi: 10.1111/j.1365-2249.2008.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 26.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–76. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Zheng M, Klinman DM, Gierynska M, Rouse BT. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci USA. 2002;99:8944–9. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBouder E, Rey-Nores JE, Raby AC, et al. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol. 2006;176:3742–52. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 32.Blais DR, Harrold J, Altosaar I. Killing the messenger in the nick of time: persistence of breast milk sCD14 in the neonatal gastrointestinal tract. Pediatr Res. 2006;59:371–6. doi: 10.1203/01.pdr.0000199907.61549.94. [DOI] [PubMed] [Google Scholar]
- 33.van Vlasselaer P, Punnonen J, de Vries JE. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992;148:2062–7. [PubMed] [Google Scholar]
- 34.Strobel S. Oral tolerance, systemic immunoregulation, and autoimmunity. Ann NY Acad Sci. 2002;958:47–58. doi: 10.1111/j.1749-6632.2002.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 35.Hawkes JS, Bryan DL, James MJ, Gibson RA. Cytokines (IL-1beta, IL-6, TNF-alpha, TGF-beta1, and TGF-beta2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res. 1999;46:194–9. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa J, Sasahara A, Yoshida T, et al. Role of transforming growth factor-beta in breast milk for initiation of IgA production in newborn infants. Early Hum Dev. 2004;77:67–75. doi: 10.1016/j.earlhumdev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Kalliomäki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-beta in breast milk: a potential regulator of atopic disease at an early age. J Allergy Clin Immunol. 1999;104:1251–7. doi: 10.1016/s0091-6749(99)70021-7. [DOI] [PubMed] [Google Scholar]
- 38.Saarinen KM, Vaarala O, Klemetti P, Savilahti E. Transforming growth factor-beta1 in mothers' colostrum and immune responses to cows' milk proteins in infants with cows' milk allergy. J Allergy Clin Immunol. 1999;104:1093–8. doi: 10.1016/s0091-6749(99)70094-1. [DOI] [PubMed] [Google Scholar]
- 39.Ciardelli L, Garofoli F, Avanzini MA, et al. Escherichia coli-specific secretory IgA and cytokines in human milk from mothers of different ethnic groups resident in northern Italy. Int J Immunopathol Pharmacol. 2007;20:335–40. doi: 10.1177/039463200702000213. [DOI] [PubMed] [Google Scholar]


