Abstract
This open-label multi-centre study evaluated a new intravenous immunoglobulin, Gammaplex®, in the treatment of 50 patients with primary immunodeficiency and significant hypogammglobulinaemia. Patients treated previously with other intravenous immunoglobulins received Gammaplex® on their same infusion schedule for 1 year; 22 were on a 21-day and 28 on a 28-day regimen (300–800 mg/kg/infusion). There were no serious, acute bacterial infections, whereas six subjects (12·0%) had at least one such infection in the 6 months before enrolment. Forty subjects (80·0%) had at least one non-serious infection; the median number of infective episodes per subject per year was 3·07. Antibiotics were taken by 38 subjects therapeutically and prophylactically by 16 at some time. Fewer than half (46·0%) missed any time off work or school because of infection or other illness. Trough immunoglobulin (Ig)G levels were above 6·00 g/l in all subjects at all assessments after 15 weeks with two exceptions. Overall, 21·2% of infusions were associated with an adverse event up to 72 h after infusion. The frequency of adverse events increased with infusion rate. Headache was the most common product-related adverse event (7·5% of 703 infusions). In conclusion, Gammaplex® is effective in primary immunodeficiency and is well tolerated.
Keywords: immunodeficiency-primary, intravenous immunoglobulin (IvIg), common variable immunodeficiency disease (CVID) and X-linked agammaglobulinaemia (XLA)
Introduction
Primary immunodeficiency diseases (PID) are a heterogeneous group of conditions associated with intrinsic defects of the immune system. Many are characterized by hypogammaglobulinaemia and/or defective antibody production and increased susceptibility to infection [1].
Since the early 1950s, patients have been given replacement therapy with plasma-derived immunoglobulin (Ig)G. The initial products were given intramuscularly (i.m.), but had limited efficacy because of the relatively small quantities that could be given i.m. In the 1980s, intravenous immunoglobulins (IVIG) became available, and are now the prime treatment for patients with impaired humoral immunity [2–4].
Gammaplex® 5% is a newly developed, highly purified IVIG, manufactured from plasma from healthy US donors. The manufacturing process includes three distinct virus inactivation/reduction steps, namely solvent/detergent, 20 nm virus filtration and terminal low pH incubation.
Gammaplex® 5% is presented as a solution for intravenous (i.v.) administration, containing 5 g human normal immunoglobulin and 5 g d-sorbitol (as a stabilizer) in 100 ml of buffer solution. Sorbitol has a negligible risk of renal toxicity [5] and excellent stability properties, allowing room temperature storage for 2 years. The immunoglobulins present are virtually 100% IgG; the typical IgA content is ∼ 4 µg/ml. Using the European Pharmacopoeial high-performance liquid chromatography (HPLC) size exclusion chromatographic method, the IgG is composed of monomers 91·5%, dimers 7·6%, aggregates (multimers) 0·9% and fragments 0%.
The primary objective of this study was to determine if Gammaplex® was efficacious with respect to the incidence of serious, acute bacterial infections (SABIs), defined as bacterial pneumonia, bacteraemia or sepsis, osteomyelitis/septic arthritis, visceral abscess or bacterial meningitis in individuals with PID [6].
Materials and methods
Study design
This non-comparative study was conducted at seven centres in the United States from February 2006 to November 2007. It was designed in line with the applicable US and European Union (EU) guidelines [6,7] and was approved by the Institutional Review Board of each centre. Informed consent was obtained from subjects aged ≥ 18 years, or from the parent/guardian of children < 18 years. Assent was also obtained if appropriate.
Trough IgG levels were recorded on two infusions of prior product before starting Gammaplex® and before each infusion. Subjects on a 21-day infusion schedule had up to 16 Gammaplex® infusions; subjects on the 28-day schedule had up to 12 infusions. The scheduled duration of treatment was 12 months. Two follow-up visits were made after the last infusion.
The primary efficacy end-point was the incidence of SABIs [6]. Assuming a Poisson distribution and an underlying SABI rate of 0·5/subject/year, 40–50 evaluable subjects would ensure > 80% power to reject a null hypothesis of one or more events/subject/year using a one-sided test at the 0·01 level.
Subject selection
Subjects with PID and hypogammaglobulinemia, aged ≥ 3 years, weight ≥ 27·5 kg (≥ 37 kg for the pharmacokinetic substudy), were recruited. All subjects had been receiving IVIG at a dose that had not changed by ± 50% of the mean dose for at least 3 months before entry, at 300–800 mg/kg/infusion every 21 or 28 days. Trough serum IgG levels at study entry had to be at least 3·00 g/l above those before IVIG therapy and ≥ 6·00 g/l.
Exclusion criteria included: history of severe anaphylaxis to blood products; selective IgA deficiency or a history of antibodies to IgA; alanine transaminase (ALT) or aspartate aminotransferase (AST) > 2·5 times upper limit of normal; history of renal disease; history of deep vein thrombosis or thrombotic complications of IVIG therapy; secondary immunodeficiency, such as chronic lymphocytic leukaemia; receiving long-term, high-dose steroids or receiving immunomodulatory or immunosuppressive drugs.
Treatment
Gammaplex® was administered intravenously via a 15–20 micron filter at 300–800 mg/kg/infusion every 21 or 28 (±4) days, following the subject's previous schedule.
The dose was based on recommended and/or common practices [8–10]. Infusions began at 0·01 ml/kg/min (30 mg/kg/h) for the first 15 min. If tolerated, the rate was advanced every 15 min to 0·02 ml/kg/min (60 mg/kg/h), 0·04 ml/kg/min (120 mg/kg/h), 0·06 ml/kg/min (180 mg/kg/h) and to 0·08 ml/kg/min (240 mg/kg/h) thereafter. If an adverse event of moderate or severe intensity occurred, the infusion rate was reduced until symptoms subsided, and then resumed at a rate tolerated by the subject.
Diary cards
The subject or parent/guardian completed daily diary cards to record adverse events; oral temperature; any presumed infection; physician/emergency room visits; school/work days missed because of infection or illness; and concomitant medication, including antibiotics.
Pharmacokinetics
Trough levels of IgG and IgG subclasses at every infusion and at the first follow-up were determined, using n anti-sera to human IgG subclasses 1–4 (Dade Behring, Deerfield, IL, USA) [11]. Antibodies against specific antigens [tetanus, Streptococcus pneumoniae (subtypes 4, 6B, 9V, 14, 19), Haemophilus influenzae B] were determined before infusions 1, 4, 8 and 12 and for subjects on a 21-day schedule before infusion 16. H. influenzae B and S. pneumoniae antibodies were determined by enzyme immunoassays and microsphere photometry, respectively.
The IgG half-life was determined in a subset of 24 subjects (nine on the 21-day schedule and 15 on the 28-day schedule) using a non-compartmental method (WinNonlin Pharsight Corporation, San Diego, CA, USA).
Blood samples were obtained between infusions 7 and 8 (28-day schedule) or infusions 9 and 10 (21-day schedule); sampling times were immediately pre- and post-infusion, 1 and 24 h after the infusion, and 2, 4, 7, 14, 21 and 28 days later. (The next infusion was delayed for those in the pharmacokinetic substudy on the 21-day schedule.)
Analysis of results
The primary efficacy variable was the estimated SABI rate and was calculated from the total number of observed infections and the total number of subject years on study. Secondary efficacy end-points (i.e. days off work/school, acute care visits or hospitalizations, fever incidence, non-serious infections, antibiotic use, IgG concentrations, safety and tolerability) were summarized descriptively and expressed per subject per year, where applicable.
Results
Study subjects
Fifty subjects were enrolled; 22 were on a 21-day and 28 on a 28-day infusion schedule. The majority (45 subjects, 90·0%) completed the study and returned for both follow-up visits. One subject on a 21-day infusion schedule and two subjects on a 28-day schedule discontinued treatment because of an adverse event (paraesthesia, bronchospasm and pregnancy, respectively); another on a 28-day schedule was lost to follow-up after 10 infusions; a further subject withdrew consent after seven infusions.
All subjects had received prior IVIG therapy, and eight had a documented adverse reaction to their prior IVIG therapy.
The subjects were aged 9–78 years; 92% had a diagnosis of common variable immunodeficiency (Table 1). Twenty-seven had a documented infection within the prior 6 months, with five subjects having had one and one subject having had three SABIs.
Table 1.
Study subjects
| Age (years) | |
| Mean (s.d.) | 44·0 (19·10) |
| Range | 9–78 |
| Male : female | 26 (52·0%):24 (48·0%) |
| Diagnosis | |
| Common variable immunodeficiency | 46 (92·0%) |
| X-linked agammaglobulinaemia | 4 (8·0%) |
| Number with infection in past 6 months | 27 (54·0%) |
| Number of infections in past 6 months | |
| 0 | 23 (46·0%) |
| 1 | 23 (46·0%) |
| 2 | 2 (4·0%) |
| 3 | 2 (4·0%) |
| Number (%) with SABI in past 6 months | 6 (12·0%) |
| Number of SABIs in past 6 months | |
| 0 | 44 (88·0%) |
| 1 | 5 (10·0%) |
| 2 | 0 |
| 3 | 1 (2·0%) |
| Number with ongoing infections | 2 (4·0%) |
SABI: serious, acute bacterial infection; s.d.: standard deviation.
A total of 703 infusions of Gammaplex® were given to the 50 subjects. Only one infusion – the first infusion in one subject – was given below the dose range of 300–800 mg/kg. Deviations from the specified infusion rates occurred in 21 infusions (13 subjects); seven infusions were in the same subject. The mean dose (range) per infusion for those on the 21-day schedule and the 28-day schedule was 469·4 mg/kg (330–693) and 466·2 mg/kg (326–790), respectively.
Infections
There were no SABIs in any subject with an onset between the first infusion of Gammaplex® and the first follow-up (Table 2).
Table 2.
Infections during Gammaplex® therapy
| Number with SABIs | n | 0 |
| Number who recorded an infection during study | n (%) | 40 (80·0%) |
| *Presumed infectious episodes per subject per year | Mean (s.d.) | 3·28 (3·024) |
| Median | 3·07 | |
| Range | 0–14·9 | |
| n | 50 | |
| *Presumed infectious episodes per subject per year | None | 10 (20·0%) |
| > 0–< 3 | 13 (26·0%) | |
| 3–< 5 | 16 (32·0%) | |
| 5–< 10 | 9 (18·0%) | |
| ≥ 10 | 2 (4·0%) | |
| Sinus infections (subjects) | n (%) | 19 (38·0%) |
| Overall upper respiratory tract infections (subjects) | n (%) | 22 (44·0%) |
| Lower respiratory tract infections (subjects) | n (%) | 10 (20·0%) |
| Urinary tract infections (subjects) | n (%) | 6 (12·0%) |
| Gastrointestinal infections (subjects) | n (%) | 9 (18·0%) |
| Other infections (subjects) | n (%) | 19 (38·0%) |
SABIs (serious, acute, bacterial infections), defined as bacterial pneumonia, bacteraemia or sepsis, osteomyelitis/septic arthritis, visceral abscess or bacterial meningitis in accordance with regulatory guidance and definitions [6]. ‘Other’ infections were those recorded for four subjects or fewer each.
If the patient recorded a possible infection on their diary card for any day, an infection was presumed whether or not it was confirmed by a health care professional, so the number of ‘presumed infectious episodes’ is a worst case for real infections. s.d.: standard deviation.
One subject was completing treatment for bacterial pneumonia at the time of the first Gammaplex® infusion.
Forty subjects (80·0%) recorded at least one non-serious infection during the study (Table 2). These presumed infections involved primarily the upper respiratory tract (e.g. sinusitis).
Antibiotics
Thirty-eight subjects (76·0%) received a total of 108 courses of therapeutic systemic antibiotics, and 16 subjects (32·0%) received 33 courses for prophylaxis. Seven subjects were on prophylactic antibiotics throughout the study, and two were on continuous prophylaxis for approximately 6 months.
Fever
There was a median of 0 days of fever per subject per year (maximum 8·9); 32 subjects (64%) had no episode of fever (> 38°C).
Visits to the physician and/or hospital emergency room (ER) and hospitalization
There was a median of 2·14 (mean 5·55) unscheduled physician visits per year (Table 3). Visits to the ER were very uncommon (median 0; mean 0·40). One subject had multiple visits associated with planned lumbar surgery, recurrent wheezing and a coincidental squamous cell carcinoma.
Table 3.
Physician and/or hospital emergency room (ER) visits
| Statistic | Physician visit n (%) | Hospital ER visit n (%) | |
|---|---|---|---|
| Number of visits per year because of medical problem including infection | Mean (s.d.) | 5·55 (8·242) | 0·40 (0·847) |
| Median | 2·14 | 0 | |
| Range | 0–42·1 | 0–4·12 | |
| Number of visits per year because of medical problem including infection | 0 | 11 (22·0%) | 38 (76·0%) |
| > 0–< 1 | 28 (56·0%)* | 1 (2·0%) | |
| > 1–< 2 | 6 (12·0%) | ||
| > 2–< 3 | 4 (8·0%) | ||
| > 3–< 7 | 1 (2·0%) | ||
| 7–< 14 | 5 (10·0%) | 0 | |
| 14–< 21 | 2 (4·0%) | 0 | |
| 21–< 35 | 3 (6·0%) | 0 | |
| 35–< 70 | 1 (2·0%) | 0 |
Number of covers > 0–< 7.
s.d.: standard deviation
Four subjects were hospitalized, three on one occasion each for less than 7 days/year (one subject had planned lumbar surgery, one had a uterine haemorrhage, one had viral gastroenteritis). The fourth subject (on a 28-day schedule) was hospitalized three times; the first admission was for planned disc surgery; the next two admissions were for thrombosis (after infusion 5) and chest pain (infusion 7), each of which was classified as possibly related to Gammaplex®. Risk factors for this subject were anti-phospholipid syndrome and obesity (body mass index: 35·7).
Days off school or work
Most subjects (54·0%) did not miss a day off school/work because of an infection or other medical problem (Fig. 1). The mean ± standard deviation (s.d.) number of missed days (per subject per year) was 8·73 (34·41) with a median of zero missed days.
Fig. 1.
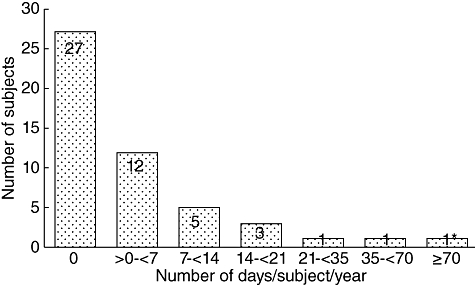
Number of school/work days missed because of infection or illness. *This subject underwent lumbar disc surgery, experienced recurrent wheezing and had a coincidental squamous cell carcinoma (240 days off school/work).
IgG concentrations
In the IgG half-life substudy, the IgG concentration versus time profiles were similar in patients on 21-day and 28-day schedules (Fig. 2). The mean half-life for total IgG was 41·1 days for all 24 subjects analysed, and was comparable for the 21-day and 28-day infusion schedules: 41·6 and 40·8 days, respectively.
Fig. 2.
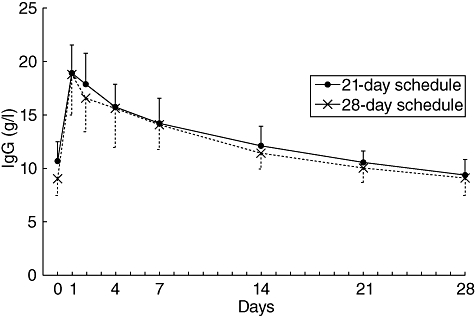
Concentrations of immunoglobulin (Ig)G against time in patients who received mean 467 mg/kg Gammaplex® administered on a 21-day or 28-day schedule. Results shown as mean ± standard deviation; n = 9 for the 21-day schedule and n = 15 for the 28-day schedule.
The median trough total IgG was calculated per visit for subjects on the 21-day infusion schedule and was 9·36–12·40 g/l. The median values per visit for the 28-day infusion schedule were slightly lower: 8·33–11·40 g/l.
Trough IgG levels remained consistently above 6·00 g/l after 15 weeks, with two exceptions in two subjects: 5·89 g/l at visit 12 of the 21-day infusion schedule and 5·25 g/l at visit 6 of the 28-day infusion schedule.
Combining 21- and 28-day schedules, levels of IgG1 (median 5·66–7·28 g/l), IgG2 (median 2·77–3·49 g/l) and IgG3 (median 0·22–0·29 g/l) remained stable during Gammaplex® therapy. Levels of serum IgG4 plateaued at a median of 0·066 g/l at visit 10.
Trough-specific IgG antibody levels did not differ between infusion schedules: median levels of antibodies to H. influenzae B and tetanus were 2·0–2·4 mg/l and 1·9–2·6 IU/ml, respectively. Stable levels were also observed for the S. pneumoniae serotypes.
Safety and tolerability
All 50 subjects experienced one or more adverse events, but only 24 (48·0%) had a possible product-related event.
Of the 703 infusions, 137 (19·5%) were associated with an adverse event (regardless of causality) up to 48 h after an infusion; the upper 95% confidence limits for the proportions were 22·1%: the majority (57%) were not considered product-related by the clinicians. In the next 24 h adverse events were associated with a further 12 infusions, five of which were regarded as product-related.
In total, 237 adverse events were associated with an infusion, giving an overall rate of one event per three infusions. The frequency of subjects with adverse events during infusion increased in association with the rate (and therefore duration) of infusion but 98% of infusions were tolerated at the highest rate.
Discussion
The primary efficacy variable for this study was the annual incidence of SABIs, with FDA guidance regarding a rate of < 1·0 as evidence of efficacy [6]. None of the 50 subjects developed a SABI, although six (12·0%) reported at least one such infection in the previous 6 months, while on another IVIG product. This study provides good evidence for the efficacy of Gammaplex® in PID with annual rates of SABIs substantially lower than the Food and Drug Administration (FDA) guidance requirement [6] and similar to those reported for other recent IVIG products [12–15].
The present study also included several secondary efficacy variables, with the results consistently supporting the conclusion that Gammaplex® is efficacious in PID. Overall, 46% of subjects missed days at school or work with the mean number of days missed being 8·73/subject/year. These results are similar to the 43% and 7·83 days/subject/year reported during treatment with Flebogamma® 5%; very similar results were also observed in these two studies for physician/ER visits [14]. The incidence and type of non-serious infections were also in line with those described for other IVIG products [10,12,13].
Trough IgG levels were > 6·00 g/l after 15 weeks on Gammaplex® in all but two instances and remained fairly stable over time. The median trough total IgG levels before infusion (range 8·33–12·40 g/l) were within the range for subjects without PID, and as shown previously to confer good protection against infection in PID [10].
The mean IgG half-life following Gammaplex® infusion was 41·1 days. A slightly shorter IgG half-life has been reported for some IVIG preparations in subjects with PID: Alyanakian et al. [16] reported a mean IgG half-life of 35·9 days, while Ballow and colleagues [17] compared two products and reported mean half-lives of 34·86 and 33·51 days. However, others have found half-lives closer to those in this study: a mean half-life of 44·6 days was reported for the 28-day schedule of Flebogamma® (although the 21-day schedule was associated with a shorter half-life of 29·9 days), and a mean half-life of 41 days for 21/28 day schedules of Octagam [14,15]. In conclusion, a Gammaplex® dosing schedule based on infusions every 21 or 28 days is appropriate.
The FDA guidance for investigation of IVIG in PID specifies a safety end-point of the proportion of infusions with one or more temporally associated adverse events, the target being an upper one-sided 95% confidence limit of 0·40 (i.e. 40% of infusions). In our study, the upper 95% confidence limits were 22·1% by 48 h and 23·9% by 72 h, well within the specified limit. These adverse events included any infections reported at the time of the infusions, even though infections were also an efficacy end-point. The calculated incidence is thus, arguably, a ‘worst-case scenario’, as the adverse event incidence would be lower if infections were excluded from the calculations. The adverse event profile with Gammaplex® compares well with that reported for other IVIG products [10,12–14].
Overall, there was good compliance with the planned regimen of Gammaplex® dose and incremental infusion rates. The infusions were generally well tolerated: only five of the 50 subjects discontinued the study prematurely, with three subjects withdrawing because of an adverse event (possibly related to Gammaplex® in two cases). One subject was hospitalized because of adverse events possibly related to Gammaplex® (thrombosis and chest pain), and thrombotic events are a known complication of IVIG therapy [18].
In conclusion, this long-term study demonstrated that Gammaplex® was effective in preventing SABIs in patients with PID with significant hypogammaglobulinaemia, provided adequate IgG levels and was well tolerated.
Acknowledgments
The study was supported by Bio Products Laboratory and co-ordinated by INC Research Inc. The authors thank Dr M. Berger for his help and advice, the patients and their carers for their participation in the study and all staff at the study centres for their contributions.
Disclosure
Nothing to disclose.
References
- 1.Wood P, Stanworth S, Burton J, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149:410–23. doi: 10.1111/j.1365-2249.2007.03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liese JG, Wintergerst U, Tympner KD, et al. High- vs. low-dose immunoglobulin therapy in the long-term treatment of X-linked agammaglobulinemia. Am J Dis Child. 1992;146:335–9. doi: 10.1001/archpedi.1992.02160150075025. [DOI] [PubMed] [Google Scholar]
- 3.Roifman CM, Lederman HM, Lavi S, et al. Benefit of intravenous IgG replacement in hypogammaglobulinemic patients with chronic sinopulmonary disease. Am J Med. 1985;79:171–4. doi: 10.1016/0002-9343(85)90006-3. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles C, Siegal FP, Smithwick EM, et al. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann Intern Med. 1984;101:435–9. doi: 10.7326/0003-4819-101-4-435. [DOI] [PubMed] [Google Scholar]
- 5.Chapman SA, Gilkerson KL, Davin TD, et al. Acute renal failure and intravenous immune globulin: occurs with sucrose-stabilized, but not with d-sorbitol-stabilzed, formulation. Ann Pharmacother. 2004;38:2059–67. doi: 10.1345/aph.1E040. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Safety, Efficacy, and Pharmacokinetic Studies to Support Marketing of Immune Globulin Intravenous (Human) as Replacement Therapy for Primary Humoral Immunodeficiency. Draft guidance November 2005 (finalized June 2008)
- 7.Committee for Proprietary Medicinal Products. Note for guidance on the clinical investigation of human normal immunoglobulin for intravenous administration (IVIG) London: 29 June 2000, CPMP/BPWG/388.95 rev. 1.
- 8.Berger M. A history of immune globulin therapy, from the Harvard Crash Program to monoclonal antibodies. Curr Allergy Asthma Rep. 2002;2:368–78. doi: 10.1007/s11882-002-0069-z. [DOI] [PubMed] [Google Scholar]
- 9.Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;1:1075–7. doi: 10.1016/s0140-6736(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 10.Eijkhout HW, van der Meer JWM, Kallenberg GCM, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia: a randomized double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165–74. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ochs HD, Morell A, Skvaril F, et al. Survival of IgG subclasses following administration of intravenous gammaglobulin in patients with primary immunodeficiency diseases. In: Morell A, Nydegger UE, editors. Clinical use of intravenous immunoglobulins. London: Academic Press Inc.; 1986. pp. 77–85. [Google Scholar]
- 12.Church JA, Leibl H, Stein MR, et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IVIG 10%] in patients with primary immunodeficiency. J Clin Immunol. 2006;26:388–95. doi: 10.1007/s10875-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 13.Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of Privigen®, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29:137–44. doi: 10.1007/s10875-008-9231-2. [DOI] [PubMed] [Google Scholar]
- 14.Berger M, Pinciaro PJ, Flebogamma® 5% Investigators Safety, efficacy and pharmacokinetics of Flebogamma® 5% [Immune globulin intravenous (human)] for replacement therapy in primary immunodeficiency diseases. J Clin Immunol. 2004;24:389–96. doi: 10.1023/B:JOCI.0000029108.18995.61. [DOI] [PubMed] [Google Scholar]
- 15.Ochs HD, Pinciaro PJ, Octagam Study Group Octagam® 5%, an intravenous IgG product, is efficacious and well tolerated in subjects with primary immunodeficiency diseases. J Clin Immunol. 2004;24:309–14. doi: 10.1023/B:JOCI.0000025453.23817.3f. [DOI] [PubMed] [Google Scholar]
- 16.Alyanakian MA, Bernatowska E, Scherrmann JM, et al. Pharmacokinetics of total immunoglobulin and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency disorders. Vox Sang. 2003;84:188–92. doi: 10.1046/j.1423-0410.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 17.Ballow M, Berger M, Bonilla FA, et al. Pharmacokinetics and tolerability of a new intravenous immunoglobulin preparation, IGIV-C, 10% (Gamunex™, 10%) Vox Sang. 2003;84:202–10. doi: 10.1046/j.1423-0410.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalakas MC, Clark WM. Strokes, thromboembolic events and IVIg. Rare incidents blemish an excellent safety record. Neurology. 2003;60:1736–37. doi: 10.1212/01.wnl.0000074394.15882.83. [DOI] [PubMed] [Google Scholar]


