Abstract
Pancreas transplantation is an option to achieve better metabolic control and decrease chronic complications in patients with diabetes. Xenotransplantation becomes an important alternative. In this study, we show the clinical outcome of patients with type 1 diabetes transplanted with neonatal pig islets without immunosuppression. In a longitudinal study of 23 patients with type 1 diabetes, who received porcine islets between 2000 and 2004, we registered demographic and clinical characteristics every 3 months and chronic complications evaluation yearly. Porcine C-peptide was measured in urine samples under basal conditions and after stimulation with l-arginine. More than 50% were female, median current age was 20·8 years, median diabetes duration at transplantation 5·5 years, median current diabetes duration 11 years and median time post-transplantation 5·7 years. Their media of glycosylated haemoglobin reduced significantly after the first transplantation. Insulin doses remain with a reduction greater than 33% in more than 50% of the patients. Before transplantation, 14 of the 21 patients presented mild chronic complications and currently only two patients presented these complications. Porcine C-peptide was present in all urine samples under basal conditions and increased post-stimulation with l-arginine. These patients achieved an excellent metabolic control after the first transplantation. This could explain, as well as the remaining function of transplanted cells, the low frequency of chronic complications compared to patients with similar diabetes duration and age.
Keywords: chronic complications, metabolic control, porcine C peptide, type 1 diabetes, xenotransplantation
Introduction
Type 1 diabetes (DM1) is a metabolic disorder caused by the inability to produce insulin due to autoimmune destruction of the pancreatic beta cells [1]. Currently, after the results reported by the Diabetes Chronic Complications Trial (DCCT), most patients receive an intensive therapy of three or more injections of insulin per day or subcutaneous insulin infusion; nevertheless, more than 60% of patients remain with poor metabolic control defined by glycosylated haemoglobin A1c (HbA1c) > 7% [2,3].
The determination of C-peptide in serum or urine is considered the gold standard for transplant function [4]. Patients with pancreatic or islet transplants have restored insulin secretion with a remission of nephropathy [5], normalized hepatic glucose production and enhanced glucose uptake [6], decreased glomerular hyperfiltration and albuminuria [4,7,8]. Adequate insulin therapy does not correct such phenomena [9]. These beneficial effects have been attributed, at least in part, to the presence of C-peptide, a product released during insulin folding and maturing that acts as a biologically active hormone with important physiological effects [10]. Unfortunately, there are no long-term studies about the beneficial effects of this molecule on the remission of chronic diabetic complications.
These data suggest that the development of therapies directed to restoring islet function in DM1 would improve glycaemic control as well as reduce the incidence of chronic complications more effectively than aggressive insulin therapy. As an alternative to insulin, several transplantation and islet regeneration protocols have been established, with differing levels of success [11–17]. We have reported previously a 4-year follow-up of 12 patients who received porcine islets in a subcutaneous device without immunosuppressive therapy [18], and 3-year follow-up of a patient who became insulin-independent for a long period [19]. Half these patients showed significant reductions in their exogenous insulin requirements, and all the patients showed insulin-positive cells in biopsies of their grafts, but only some patients had detectable levels of circulating porcine insulin regardless of reductions in their exogenous insulin requirements [18]. At that time we were not able to detect porcine C peptide in serum.
In this study we report the clinical follow-up of this group of patients and a second group of 11 additional patients treated with the same xenotransplant protocol, but with cell cultures made in Mexico and not in New Zealand, as were the first cell cultures.
Methods
The clinical trial was regulated by the ethics and research committees of the Hospital Infantil de Mexico and the Facultad de Medicina at the Universidad Nacional Autonoma de Mexico, as well as the National Transplant Center and the National Bioethics Committee of the Health Ministry. The consent forms were in agreement with national and international guidelines, including the Helsinki Declaration.
Patients with type 1 diabetes were invited to participate under these criteria inclusion: human C-peptide below 0·5 ng/ml (low function of pancreatic islets), positive antibodies [anti-glutamic acid decarboxylase (GAD) and anti-islet-cell antibodies (ICA)], duration of diabetes for 2 years or longer, absence of severe chronic complications and poor metabolic control (Hb A1c > 7%). Adolescent patients were invited to participate because they have the poorest glycaemic control and would not need immunosuppressive therapy. The first group of 12 patients (group A) received the first cellular infusion in 2000 and the second group of 11 patients (group B) in 2002–03. Donor animals were male 7–10-day-old piglets, bred in New Zealand in a specific pathogen-free environment in accordance with the Association of Laboratory Animal Care. Four collagen-generating devices were implanted subcutaneously in the anterior wall of the patient's abdomen under general anaesthesia. The devices consist of 6 × 0·8-cm surgical grade stainless steel mesh tubes, with a polytetrafluoroethylene (PTFE) rod in its interior. Two months after the implant of the devices the transplant procedure was carried out by exposing the end of each device, removing the PTFE rod and infusing 250 000 islets of Langerhans and 30–100 Sertoli cells per islet into the collagen tube. The median of cellular infusions for each patient was 3, with a total of 68.
Twenty-three patients were entered into the two trials, but two patients elected to withdraw from the study for personal reasons. The remaining 21 patients were evaluated every 3 months, with a complete physical examination and HbA1c. Chronic complications were evaluated yearly.
Retinopathy
Fundoscopy consists of the evaluation of the retina by an ophthalmologist through an ophthalmoscope via a dilated pupil to detect evidence of diabetic retinopathy and the degree of involvement. There are three potential findings in diabetic patients: (i) no signs of diabetic retinopathy; (ii) non-proliferative diabetic retinopathy (microaneurysms, haemorrhage, hard exudates); or (iii) proliferative diabetic retinopathy (newly formed blood vessels and/or fibrous tissue into the vitreous cavity). Fluorangiography is a highly specialized test consisting of an imaging technique used commonly by physicians to obtain real-time moving images of the internal structures of a patient through the use of a fluoroscope. In its simplest form, a fluoroscope consists of an X-ray source and fluorescent screen between which a patient is placed. The presence of microaneurisms, exudates and haemorrhage is evaluated.
Neuropathy
Neuropathy is evaluated through speed of nerve conduction by a specialist in rehabilitation not related to this protocol. This is an electrophysiological test that evaluates the amplitude and velocity of peripheral nerves to assess the degree of diabetic neuropathy. It includes bilateral median sural and peroneal amplitude and nerve conduction velocity on the dominant side, and median motor and sensory amplitudes and nerve conduction velocity on the non-dominant side.
Peripheral neuropathy evaluation by bilateral electrophysiological studies of the peroneal motor nerve were conducted with the XL-Tek NeuroMax8 (Oakville, ON, Canada) device. The limb was stimulated at the ankle, fibular head and popliteal fossa. Response amplitudes, latencies and nerve conduction velocities were collected.
Nephropathy
Nephropathy is evaluated by microalbuminuria (MA). MA is usually the first sign of diabetic nephropathy and is associated with increased cardiovascular morbidity and mortality. It is defined as urinary albumin excretion in a 24-h urine collection equal to 30–300 mg/24 h or urinary albumin excretion rate 20–200 µg/min (timed urine collection) or urinary albumin concentration 20–200 mg/l (spot urine collection). In this study the MA will be considered when albuminuria is 30–300 mg/24 h.
Between late 2006 and early 2007 the patients were asked to collect two 24-h urine samples on two different days; the first sample had their habitual treatment with exogenous insulin; the second sample, as well as receiving their insulin treatment, were stimulated with l-arginine, 2 g every 6 h starting the night prior to the collection and continuing for the next 24 h, in order to determine porcine C-peptide. This was also determined in 10 non-transplanted patients with type 1 diabetes (similar age and diabetes duration) and eight healthy volunteers.
All xenotransplanted patients showed insulin- and glucagon-positive cells in biopsies from their grafts and some of the patients tested showed circulating porcine insulin after intravenous glucose tolerance test (IVGTT) regardless of the level of decrease in exogenous insulin requirements [18]. Anti-pig antibodies (APA) and anti-Gal antibodies were analysed [20] and porcine endogenous retrovirus (PERV) tests were negative [21]
Sample concentration and porcine C-peptide determination
The sample volumes were measured, then filtered through 0·22 µm low-protein-binding filters (Millipore, Billerica, MA, USA). Fifty-ml aliquots were concentrated × 10 through a 1000 Da cut-off filter (Millipore). Porcine C-peptide determinations were carried out using a porcine C-peptide-specific radioimmunometric assay (Linco Research, St Charles, MO, USA), according to the manufacturer's instructions. The possibility of cross-reactivity with human C-peptide using this technique is less than 1%.
Statistical analysis
Data are presented as median and range for abnormal distribution and mean and standard deviation for determinations with a normal distribution. In order to establish significance, data groups were tested by Wilcoxon's test. P-values were significant when P < 0·05. Fisher's exact test was used to analyse the frequency of chronic complications.
Results
Demographic data of the patients included in this study are presented in Table 1.
Table 1.
Demographic characteristics of patients
| Number of patients (n) | 21 (11 F, 10 M) |
| Current age (years) | 20·8 (16·2–23·7) |
| Diabetes duration pre-xenotransplant (years) | 5·5 (3·3–9·7) |
| Current diabetes duration (years) | 11 (7·1–15·9) |
| Time post-xenotransplant (years) | 5·7 (2·6–7·7) |
M: male; F: female.
The HbA1c basal media was 10·8 ± 2·4%, post-device 7·8 ± 2·02%, post-first transplant 6·9 ± 0·91%, post-second transplant 8·2 ± 1·5%, post-third transplant 8·2 ± 1·7% and currently is 9·26 ± 2·37% (Fig. 1), statistically significant until post-second transplant.
Fig. 1.
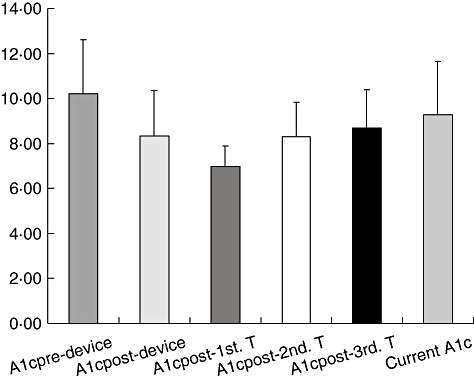
Media of haemoglobin A1c, P = 0·000006, 0·000004, 0·001, 0·1 and 0·27; basal versus post-device just before transplantation, post-first transplant, post-second transplant, post-third transplant and at the moment of porcine C-peptide determination, respectively.
The current media insulin dose for all 21 patients is currently 0·86 ± 0·56 U/kg/day compared to 1·11 ± 0·43 U/kg/day just prior to transplant (Fig. 2), and is statistically significant.
Fig. 2.
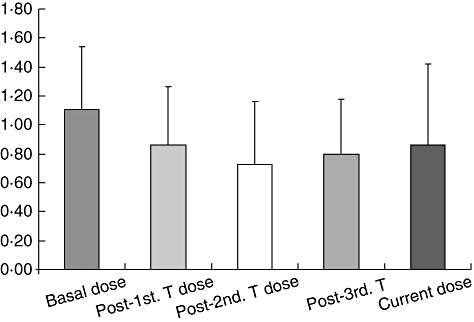
Insulin dose (U/kg/day), P = 0·02, 0·00004, 0·001 and 0·059 for basal versus post-first transplant, second transplant, third transplant and at the time of porcine C peptide determination.
Fourteen of the 21 patients who remained in the study had some evidence of mild chronic complications prior to xenotransplantation, while currently only two patients have this finding (Table 2). One of these patients, a 23-year-old female, was depressed due to a death in the family and subsequently showed poor metabolic control, so we decided to remove her device in 2004. She now has non-proliferative diabetic retinopathy and MA. The second patient, who has retained her xenograft, had MA pretransplant and went into remission for 3 years post-transplant; however, she has recently presented evidence of MA again. The remaining 19 patients continue to be free of chronic complications.
Table 2.
Chronic complications before transplantation and currently
| Chronic complications | Before transplantation | Currently |
|---|---|---|
| Neuropathy* | ||
| Abnormal speed of nervous conduction | 5 (23·8%) | 0 (0%) |
| Retinopathy** | ||
| NPDR | 5 (23·8%) | 1 (4·7%) |
| Nephropathy*** | ||
| Microalbuminuria | 8 (38·1%) | 2 (19%) |
| Number of patients with chronic complications† | 14 (66·7%) | 2 (9·5%) |
P = 0·048 (P < 0·05)
P = 0·184
P = 0·067
P = 0·0003 (P < 0·001).
NPDR: non-proliferative diabetic retinopathy.
All patients were treated intensively with two doses of neutral protamine Hagedorn (NPH) insulin and three or more of ultrarapid insulin. In the first (basal level) urine collections we detected low amounts of porcine C-peptide in all patients (0·62 µg/24 h ± 0·27), which increased significantly after stimulation with l-arginine (24·18 µg/24 h ± 16·82), P < 0·0001 (Fig. 3). In both control groups (type 1 diabetic patients without transplantation and healthy volunteers), urinary porcine C-peptide was undetectable even post-stimulation with l-arginine.
Fig. 3.
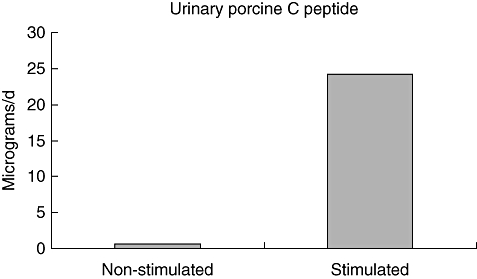
Levels of porcine C peptide in urine from transplanted patients. *P < 0·000004, using paired Student's t-test, comparing non-stimulated and stimulated porcine C-peptide levels.
To determine whether exogenous human insulin has small quantities of C-peptide that could be recognized by anti-porcine C-peptide antibody, we tested NPH, glargine and ultrarapid insulins for the molecule. Traces of C-peptide were detected only in the glargine insulin, which none of our patients was using.
Discussion
It is important to note that there was a significant reduction of HbA1c after the device and a greater reduction after the first transplantation of neonatal pig islets. This could be attributed to the intensive insulin regimen that patients began to use after implantation of the device, but the time between the implanted device and the transplanted porcine islets was 2–11 months; the patients were their own controls because they were receiving continuous intensive insulin treatment, and even then we observed a significant difference between HbA1c post-device and post-first transplantation. Furthermore, the insulin dose reductions were also statistically significant; currently there is a reduction greater than 33% in more than 50% of the patients, while other authors have reported reductions of no greater than 15% [22] or 12 units for a short time [23] when patients begin intensive insulin treatment. One of the patients in the first group remains with 5 units of insulin prebreakfast as a single dose and HbA1c between 7 and 8%.
Conversely, these patients did not develop severe chronic complications despite long-term diabetes duration; only two of 21 patients have mild chronic complications, and in basal conditions 14 of 23 patients presented mild chronic complications. Comparing these data with international studies of patients with similar diabetes duration and age, the frequency of chronic complications in our patient group is low. Olsen et al. [24] studied 339 young Danish patients with DM1; 88% were treated with intensive therapy but only 11% had HbA1c below 8% and they had an unacceptably high prevalence of chronic diabetic complications, such as MA in 9%, retinopathy in 60% and neuropathy in 62%. Donaghue et al. [25] reported retinopathy in 24% of young patients with type 1 DM during a 6-year follow-up. Other longitudinal studies (10 years or more of follow-up) have reported diabetic retinopathy in 84% [26] and 78% of the cases [27]. At this point, our principal limitation was the absence of a follow-up control group of chronic complications using intensive therapy.
These patients produced detectable porcine C-peptide in their urine for several years after transplant. Groth et al. [13,28] reported measurable low levels of porcine C peptide for 6 months following the transplantation of porcine islets in diabetic recipients of kidney allografts receiving immunosuppression. Recently, Elliot et al. [29] reported survival of 9·5 years of porcine islets xenotransplanted in one patient, who reduced his insulin dose by 30% but returned to pretransplant level at week 49; urinary porcine C-peptide was detectable in the first 11 months at similar levels to those found in our patients post-stimulation. Our results suggest prolonged survival of the implanted cells using the device [30,31] by the presence of urinary porcine C-peptide, the important insulin dose reduction, the immunological response and biopsies positive for porcine islets, as well as initial excellent metabolic control. In basal conditions our patients produced small quantities of porcine C-peptide, but this increased after stimulation with l-arginine, although even this increase is below normal levels of human urinary C-peptide (65–262 µg/day). It is possible that blood glucose levels, maintained by exogenous insulin, are not enough to stimulate production of insulin from transplanted islets because their survival has partially finished or they are partially sensitive to blood glucose levels for their subcutaneous location. Another possibility is that there was no functional maturation of the neonatal islets for strict metabolic control [32].
Individual urinary porcine C-peptide levels in patients were not related to the exogenous insulin requirements. In the assay used, porcine C-peptide cross-reacts with human C-peptide at a level of less than 1%. Therefore, the possibility that we are measuring endogenous production or from exogenous commercial insulin is null, and we can conclude that the porcine C-peptide detected comes from the transplanted islets. Evidently, the urine levels of porcine C-peptide are extremely low, but in comparison with the healthy control group and patients with type 1 DM without xenotransplant, the values measured were statistically significant.
Recently, it has been reported that C peptide is a biologically active hormone with various physiological effects [3–7]. Therefore, this suggests that the low frequency of chronic complications found in our patients could be explained by porcine C peptide, despite its relatively modest basal level, although further studies are required to confirm this hypothesis. Another possibility could be the effect of metabolic memory reported by some authors [33], as our patients achieved values of glycated HbA1c around 7% during the first year post-transplantation, just into adolescence, when metabolic control is the poorest.
Barnett et al. published the importance of establishing islet immunoisolation through magnetocapsules [34], but using our device we are achieving immunoprotection as demonstrated by the function of the islets through porcine C-peptide and reduction in insulin requirements in more than 81% of the patients (more than 50% of patients remain with a reduction greater than 33%), and through low levels of antibodies already reported [20]. Perhaps our results could be improved if we use an immunosuppressive regimen [35]
The main limitation in this study was the absence of a control group and the small sample size of patients; therefore, a randomized blind control trial is necessary to confirm these findings and provide accurate evidence of islet function and metabolic benefit.
Conclusion
These patients achieved good glycaemic control after the first transplantation (HbA1c = 6·9%) and important decreases in their insulin doses, which could be explained by the remaining function of transplanted cells. This good metabolic control, the presence of porcine C peptide and probably the metabolic memory effect may explain the low frequency of chronic complications compared to patients with similar diabetes duration and age.
Disclosure
Nothing to declare.
References
- 1.American Diabetes Association. Standards of medical care in diabetes – 2006. Diabetes Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- 2.DCCT/EDIC Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 4.Coppelli A, Giannarelli R, Vistoli F, et al. The beneficial effects of pancreas transplant alone on diabetic nephropathy. Diabetes Care. 2005;28:1366–70. doi: 10.2337/diacare.28.6.1366. [DOI] [PubMed] [Google Scholar]
- 5.Luzi L, Perseghin G, Brendel MD, et al. Metabolic effects of restoring partial beta-cell function after islet allotransplantation in type 1 diabetic patients. Diabetes. 2001;50:277–82. doi: 10.2337/diabetes.50.2.277. [DOI] [PubMed] [Google Scholar]
- 6.Wahren J, Johansson BL, Wallberg-Henriksson H. Does C-peptide have a physiological role? Diabetología. 1994;37(Suppl 2):S99–107. doi: 10.1007/BF00400832. Review. [DOI] [PubMed] [Google Scholar]
- 7.Zhong Z, Kotova O, Davidescu A, et al. C-peptide stimulates Na+, K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci. 2004;61:2782–90. doi: 10.1007/s00018-004-4258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebsomen L, Pitel S, Boubred F, et al. C-peptide replacement improves weight gain and renal function in dibetic rats. Diabetes Metab. 2006;32:223–8. doi: 10.1016/s1262-3636(07)70272-0. [DOI] [PubMed] [Google Scholar]
- 9.Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren T. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabet Med. 2000;17:181–9. doi: 10.1046/j.1464-5491.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- 10.Johansson BL, Sjoberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35:121–8. doi: 10.1007/BF00402543. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen JS, Frandsen M, Parving HH. The effect of intravenous insulin infusion on kidney function in insulin-dependent diabetes mellitus. Diabetologia. 1981;20:199–204. doi: 10.1007/BF00252628. [DOI] [PubMed] [Google Scholar]
- 12.Elliott RB, Escobar L, Tan PL, et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc. 2005;37:3505–8. doi: 10.1016/j.transproceed.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Groth CG, Andersson A, Korsgren O, et al. Transplantation of porcine fetal islet-like cell clusters into eight diabetic patients. Transplant Proc. 1993;25:970. [PubMed] [Google Scholar]
- 14.Toso C, Morel P, Bucher P, et al. Insulin independence after conversion to tacrolimus and sirolimus-based immunosuppression in islet-kidney recipients. Transplantation. 2003;76:1133–4. doi: 10.1097/01.TP.0000090394.71832.E5. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Okitsu T, Iwanaga Y, et al. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet. 2005;365:1642–4. doi: 10.1016/s0140-6736(05)66383-0. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro A, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 17.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 18.Valdes-Gonzalez RA, White DJG, Dorantes LM, et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419–27. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 19.Valdés-González RA, White DJG, Dorantes LM, et al. Three-year follow-up of a type 1 diabetes mellitus patient with an islet xenotransplant. Clin Trans. 2007;21:352–57. doi: 10.1111/j.1399-0012.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 20.Valdes-Gonzalez RA, Dorantes LM, Garibay GN, et al. Unexpected immunoresponse to Gal and APA antigens in diabetic type 1 patients receiving neonatal pig islets after 6 years. J Clin Immunol. 2007;27:266–74. doi: 10.1007/s10875-007-9079-x. [DOI] [PubMed] [Google Scholar]
- 21.Valdes-Gonzalez R, Dorantes LM, Bracho E, Rodriguez-Ventura A, White D. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long term porcine islet xenotransplantation. J Med Virol. 2010;82:331–4. doi: 10.1002/jmv.21655. [DOI] [PubMed] [Google Scholar]
- 22.Frolich-Reiterer EE, Ong KK, Regan F, Salzano G, Acerini CL, Dunger DB. A randomized cross-over trial to identify the optimal use of insulin glargine in prepubertal children using a three-times daily insulin regimen. Diabet Med. 2007;24:1406–11. doi: 10.1111/j.1464-5491.2007.02277.x. [DOI] [PubMed] [Google Scholar]
- 23.Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess. 2004;8:1–171. doi: 10.3310/hta8430. Review. [DOI] [PubMed] [Google Scholar]
- 24.Olsen BS, Johannesen J, Borch-Johnsen AK, et al. Metabolic control and prevalence of microvascular complications in young Danish patients with type 1 diabetes mellitus. Diabet Med. 1999;16:79–85. doi: 10.1046/j.1464-5491.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 25.Donaghue KC, Craig ME, Chan AK, et al. Prevalence of diabetes complications 6 years after diagnosis in an incident cohort of childhood diabetes. Diabet Med. 2005;22:711–18. doi: 10.1111/j.1464-5491.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- 26.De Médeiros-Quenum M, Ndiaye PA, Cissé A, et al. Epidemiological aspects of diabetic retinopathy in Senegal. J Fr Ophtalmol. 2003;26:160–3. [PubMed] [Google Scholar]
- 27.Ben Hamouda H, Messaoud R, Grira S, et al. Prevalence and risk factors of diabetic retinopathy in children and young. J Fr Ophtalmol. 2001;24:367–70. [PubMed] [Google Scholar]
- 28.Groth CG, Tibell A, Wenneberg L, Korsgren O. Xenoislet transplantation: experimental and clinical aspects. J. Mol Med. 1999;77:153–4. doi: 10.1007/s001090050325. [DOI] [PubMed] [Google Scholar]
- 29.Elliot RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9·5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–61. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Valdes R, Cravioto MA, Tenopala J. Biological encapsulation as a new model for preservation of islets of Langerhans. Transplant Proc. 1998;30:481. doi: 10.1016/s0041-1345(97)01365-1. [DOI] [PubMed] [Google Scholar]
- 31.Valdés-González RA, Silva-Torres ML, Ramírez-González B, Terán L, Ormsby CE, Ayala-Sumuano JT. Improved method for isolation of porcine neonatal pancreatic cell clusters. Xenotransplantation. 2005;12:240–44. doi: 10.1111/j.1399-3089.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- 32.Tatsuya K, Tatsuya G. Delayed functional maturation of neonatal porcine islets in recipients under strict glycemic control. Xenotransplantation. 2007;14:333–8. doi: 10.1111/j.1399-3089.2007.00414.x. [DOI] [PubMed] [Google Scholar]
- 33.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnett BP, Arepally A, Karmarkar PV, et al. Magnetic resonance-guided, real time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13:986–91. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 35.Balamurugan A, Nelson E, Ramakrishna B. Efect of various immunosuppressive monotherapies on survival and histopathology of monkey islet xenografts in rats. Xenotransplantation. 2007;14:316–22. doi: 10.1111/j.1399-3089.2007.00409.x. [DOI] [PubMed] [Google Scholar]


