Abstract
This study tested the hypothesis that pregnant female baboons exhibit increased levels of various inflammatory mediators in serum resulting from ligature-induced periodontitis, and that these profiles would relate to periodontal disease severity/extent in the animals. The animals were sampled at baseline (B), mid-pregnancy (MP; two quadrants ligated) and at delivery (D; four quadrants ligated). All baboons developed increased plaque, gingival inflammation and bleeding, pocket depths and attachment loss following placement of the ligatures. By MP, both prostaglandin E2 (PGE2) and bactericidal permeability inducing factor (BPI) were greater than baseline, while increased levels of interleukin (IL)-6 occurred in the experimental animals by the time of delivery. IL-8, MCP-1 and LBP all decreased from baseline through the ligation phase of the study. Stratification of the animals by baseline clinical presentation demonstrated that PGE2, LBP, IL-8 and MCP-1 levels were altered throughout the ligation interval, irrespective of baseline clinical values. IL-6, IL-8 and LBP were significantly lower in the subset of animals that demonstrated the least clinical response to ligation, indicative of progressing periodontal disease. PGE2, macrophage chemotactic protein (MCP)-1, regulated upon activation, normal T cell expressed and secreted (RANTES) and LBP were decreased in the most diseased subset of animals at delivery. Systemic antibody responses to Fusobacterium nucleatum, Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Campylobacter rectus were associated most frequently with variations in inflammatory mediator levels. These results provide a profile of systemic inflammatory mediators during ligature-induced periodontitis in pregnant baboons. The relationship of the oral clinical parameters to systemic inflammatory responses is consistent with a contribution to adverse pregnancy outcomes in a subset of the animals.
Keywords: antibody, baboons, inflammation, periodontitis, pregnancy
Introduction
Historically, adaptive immunity has been the focus of immunological investigations related to infectious diseases, due to the specificity of adaptive immunity and the opportunity to create and evaluate vaccine strategies to individual pathogens. However, during the initial contact with a primary infection, the host protective armamentarium is focused upon inflammation and innate immunity. Fundamentally, the innate immune system prevents entry of microorganisms into tissues or, once they have gained entry, eliminates them prior to the occurrence of disease. Thus, the immune system is an interactive network of cellular and molecular processes that are responsible for recognizing and eradicating pathogens and other noxious molecules. The acute phase response (APR) represents an early and highly complex reaction to remove noxious challenge and restore homeostasis. This process is accomplished by substantial increases in the plasma levels of acute phase proteins that can modulate immune cell function and neutralize the noxious components challenging the systemic circulation [1,2]. C-reactive protein (CRP) is a classic member of this family and one of the soluble pathogen-associated molecular pattern (PAMP) recognition receptors. Lipopolysaccharide binding protein (LBP) and bactericidal permeability inducing factor (BPI) are additional acute phase proteins with direct actions providing anti-bacterial resistance in innate immunity. In addition, systemic cytokine/chemokine responses can be identified in patients with periodontitis [3–5]. Interleukin (IL)-1β, tumour necrosis factor (TNF)-α and IL-6 are principal pro-inflammatory cytokines with pleotropic biological activities on immune and non-immune cells, as well as in osteogenic pathways). IL-8 (CXCL8) is the major neutrophil chemokine, while macrophage chemotactic protein (MCP)-1 (CCL2), a major chemoattractant and maturation signal for macrophages, and regulated upon activation, normal T cell expressed and secreted (RANTES; CCL5) is a member of the IL-8 superfamily of cytokines. It is a selective attractant for memory T lymphocytes and monocytes. These chemokines have all been detected in the serum of patients with microbial infections [6–10], including periodontitis [11–14]. However, chronic stimulation of these biomolecules generally represents dysregulated responses, and is associated frequently with systemic disease sequelae [15–21].
In some cases, particularly with polymicrobial infections at mucosal surfaces, innate immune mechanisms may function exceptionally well to manage surface colonization by commensal opportunistic pathogens and maintain homeostasis [22–25]. Nevertheless, with respect to a number of chronic inflammatory diseases, the interaction between the challenge (e.g. bacteria) and the inflammatory and innate immune response can result in collateral damage of the local tissues. Adverse pregnancy outcomes provide a potential example of these ramifications of a dysregulated host response. Ascending vaginal infections trigger the local production of various inflammatory mediators and matrix metalloproteinases (MMP), resulting in amnionitis that impact placental functions negatively and lead potentially to fetal infection [26–32]. Reports described relationships between the presence of inflammatory mediators in amniotic fluid and uterine contractions and/or birth in humans and non-human primates. Proinflammatory cytokines/chemokines, immunomodulatory and immunosuppressive cytokines and prostanoids [e.g. prostaglandin E2 (PGE2)] are produced by the amniotic and decidual membranes and can be found in fetal circulation and amniotic fluid, often associated with premature delivery. Expanding literature supports that the levels of many of these cytokines/chemokines in serum are also reflective of, and potentially contribute to, the risk for premature rupture of membranes (PROM) with preterm labour and delivery [26,32–35]. Consequently, relationships between serum and local cytokine levels and their association with adverse pregnancy outcomes are possible.
Periodontitis is a chronic oral infection with polymicrobial biofilms triggering a localized immunoinflammatory lesion. The chronic inflammation undermines the integrity of the epithelium and initiates connective tissue destruction, with an eventual outcome of bone resorption and potential for exfoliation of teeth [13,36–38]. The balance of inflammation, innate immunity and adaptive immunity interfacing with the complex commensal biofilms, controlling pathogens that emerge in the biofilms, minimizing local collateral tissue damage from chronic inflammation and down-regulating systemic responses to the infections remain ill-defined. The commensal opportunistic pathogens provoke both a localized and systemic response during the disease [39–41], with systemic inflammatory responses being generally low in individuals with a healthy periodontium or in subjects with reversible gingival inflammation (i.e. gingivitis) and increasing in periodontitis patients [40,42]. Thus, an interaction between the systemic responses to periodontitis and the changes that occur during pregnancy could be predicted to increase the risk of adverse pregnancy outcomes [43–45].
The objectives of this study were to document profiles of various systemic inflammatory mediators in female baboons during their pregnancy resulting from ligature-induced periodontitis. The targeted mediators would be those that could contribute to adverse pregnancy outcomes and might be predictive of the biological risk linking periodontal disease with these events. These data should contribute to the development of a pathway that explores the contribution of oral infection and systemic host responses to birth outcomes using a non-human primate model.
Materials and methods
Animal husbandry, experimental design and clinical measures
An experimental cohort of 288 Papio anubis (168 experimental; 120 controls) were examined in this study. Inclusion in the study is dependent upon the following criteria: (i) dams must have a minimum of 20 teeth; (ii) be in good general health based upon an examination by the veterinarian; (iii) range in age from 6–13 years; and (iv) have produced previous offspring. Mothers were excluded if they demonstrated systemic illness that required veterinary treatment during the course of the project that would adversely impact the pregnancy outcome (i.e. infection) and/or administration of antibiotic and/or anti-inflammatory therapy, which could confound the onset and severity of periodontitis. Loss of body weight ≥15% also excluded the baboon from further participation in this project. Nulliparous dams (e.g. previous births increase likelihood of successful breeding for this study), dams of extreme ages, either younger or older, and those dams having fewer than 20 teeth were excluded.
The animals were sampled prospectively at three time-points during the study. The study design has been described previously [46]; briefly, however, the experimental animals were sampled at baseline (clinical examination, serum) and teeth in quadrants one and four were ligated. A second sampling took place at mid-gestation (∼3 months) into the pregnancy and ligatures were tied on the contralateral maxillary and mandibular quadrants (quadrants two and three). The third sample was obtained from 2 to 10 days after delivery and the ligatures were removed. The controls animals went through the same protocol and sampling processes, without the placement of ligatures.
A complete periodontal evaluation was performed at each of the three sampling intervals for supragingival plaque, pocket depth, recession and bleeding upon probing [47,48] at four sites on each tooth: distobuccal, buccal, mesiobuccal and lingual (premolar, first and second molar) in each quadrant. Attachment level values were calculated from the pocket depth and recession measures [47,49]. Missing teeth or teeth that could not be scored were noted. A gingival bleeding score, following determination of the pocket depth measure, was obtained. Ligatures were tied on the first and second molar and second premolar teeth (teeth five, six and seven) using 3–0 silk sutures. To promote inflammation, the animals in the experimental group were placed on a soft chow diet, consisting of commercial chow biscuits soaked in warm water for 10 min and drained [50].
The Composite Index of Periodontal Disease (CIPD) was developed to provide a single index value that would incorporate measures of both disease extent and severity and included weighted measures of gingival bleeding and attachment loss (unpublished data). For the CIPD we weighted the variables such that the measure of destructive disease (CAL) and the extent of destruction (% of sites with CAL >2 mm) were increased in contribution to the CIPD. The CIPD results demonstrated substantial heterogeneity of clinical presentation of the baboons, not dissimilar from that reported in human populations. A CIPD of <20 is consistent with relative gingival health in non-human primates; 20–<50 represents gingivitis; 50–<75 mild periodontitis; 75–<100 moderate periodontitis; and >100 severe periodontitis.
Inflammatory mediators
Blood (approximately 10 ml) was obtained by femoral venipuncture into red-topped vacutainer tubes. The blood was allowed to clot for 1 h, centrifuged for 15 min at 3000 g and the serum removed and the serum prepared and stored at −70°C after separation into 0·5–0·75-ml aliquots.
A panel of acute phase reactants, including C-reactive protein (CRP), bactericidal permeability inducing factor (BPI) and lipopolysaccharide binding protein (LBP) were quantified using an enzyme-linked immunosorbent assay (ELISA) developed in our laboratory (i.e. CRP) or obtained commercially (BPI, LBP; Hycult Biotechnology, Cell Sciences, Canton, MA, USA). Various serum cytokines/chemokines, including interleukin (IL)-1β, IL-6, IL-8, tumour necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α (CCL3), regulated upon activation, normal T cell expressed and secreted (RANTES) (CCL5), IL-12p40 and MCP-1 (CCL2) were measured using a multiplex beadlyte assay on a Luminex IS-100 (Millipore, Billerica, MA, USA). PGE2 levels were assessed using a commercial ELISA kit (Assay Design, Ann Arbor, MI, USA).
Serum antibody levels
Immunoglobulin (Ig)G antibody levels to 20 bacteria were determined by quantitative ELISA using formalin-killed whole bacterial antigens, as described previously [51,52]: Actinomyces naeslundii ATCC49340, Actinobacillus actinomycetemcomitans JP2, Campylobacter rectus ATCC33238, Capnocytophaga gingivalis ATCC33624, C. ochracea ATCC33596, C. sputigena ATCC33624, Eikenella corrodens ATCC23834, Eubacterium nodatum ATCC33099, Fusobacterium nucleatum ATCC49256, Micromonas micros ATCC33270, Porphyromonas gingivalis FDC381, Prevotella intermedia ATCC25611, P. loeschii ATCC15930, P. nigrescens ATCC33563, Streptococcus gordonii ATCC49818, S. mutans ATCC25175, S. sanguis ATCC10556, Treponema denticola ATCC35405, Tannerella forsythia ATCC49307 and Veillonella parvula ATCC10790.
Statistical analyses
Due to the extensive variability in mediator levels across the population, the data were all transformed using a log10 transformation and the antibody data were transformed using a log2 transformation. Antibody data were standardized using the antibody baseline mean and standard deviation to create a Z-statistic for each individual animal [46]. An analysis of variance (ANOVA) was used to determine differences among the baseline disease categories with a post-hoc Holm–Sidak assessment for individual group differences. Spearman's correlation on ranks was used to determine relationships between the various host response variables, as well as to the periodontal presentation of the animals.
Results
Serum inflammatory mediators in pregnant baboons
Figure 1 shows the levels of these mediators in the control and experimental population during pregnancy, at baseline and after ligation of teeth in two quadrants (MP) or four quadrants (D). The results in Fig. 1a show substantial elevations in IL-6 occurring in the experimental animals at the time of delivery, while PGE2 and BPI were both increased over baseline, particularly at MP. IL-8, MCP-1 and LBP all decreased from baseline through the ligation phase of the study. The only change noted in the control animals (Fig. 1b) was an increased level of PGE2 at MP. IL-1β, MIP-1α, TNF-α and IL-12p40 were detected in <5% of the serum samples tested and thus are not included in the data presentation. Comparisons of the various mediator levels between the experimental and control groups at each time-point also demonstrated that levels of IL-6, IL-8 and MCP-1 were significantly different at delivery, while only LBP was significantly different at baseline between these groups.
Fig. 1.
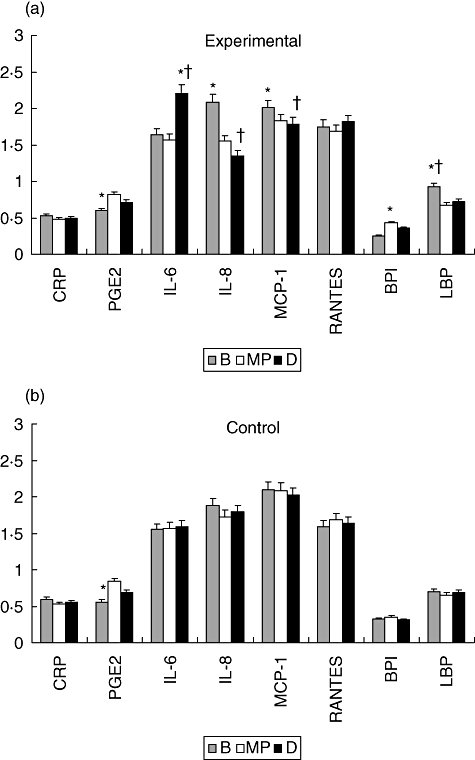
Levels of systemic inflammatory mediators in baboons at baseline (B), mid-pregnancy (MP) and delivery (D) in experimental (a) and control (b) animals. The bars denote the group means and the vertical brackets enclose 1 standard error of the mean. The asterisk (*) denotes significantly different from other time-points at least at P < 0·05. The dagger (†) denotes significantly different from the control animals at the same time-point at least at P < 0·05.
Serum inflammatory mediators and oral clinical presentation
Due to the inherent clinical variation in the animals as they entered the study, Fig. 2a,b stratifies the baboons based upon clinical presentation at baseline into healthy (H) (CIPD <20), gingivitis (G) (CIPD 20–<50) and periodontitis (P) (CIPD >50) subgroups and depicts the levels of the various mediators in serum from these subgroups of animals. The results compare changes in the levels of the various inflammatory mediators during the 6 months of ligature-induced disease. No differences were observed in the levels of any of the analytes in serum comparing these experimental subgroups to the control animals at baseline. Similarly, no differences were noted in CRP levels across the subgroups and sampling times. PGE2 levels were elevated throughout ligation in all the clinical subsets of animals. In contrast, BPI was increased significantly at mid-pregnancy in the animals that were healthy or had gingivitis at baseline, with significantly lower levels at delivery in the subset with periodontitis at baseline. A pattern of decreasing levels of LBP was noted in all groups during the ligation phase of the study. IL-8 and MCP-1 demonstrated patterns similar to the LBP, with decreasing levels of these inflammatory mediators in all subsets of animals throughout the entire 6 months of ligature-induced disease. The levels of IL-6 were increased significantly in all subsets at delivery, following 6 months of periodontal disease, while RANTES levels were generally similar across groups and times.
Fig. 2.
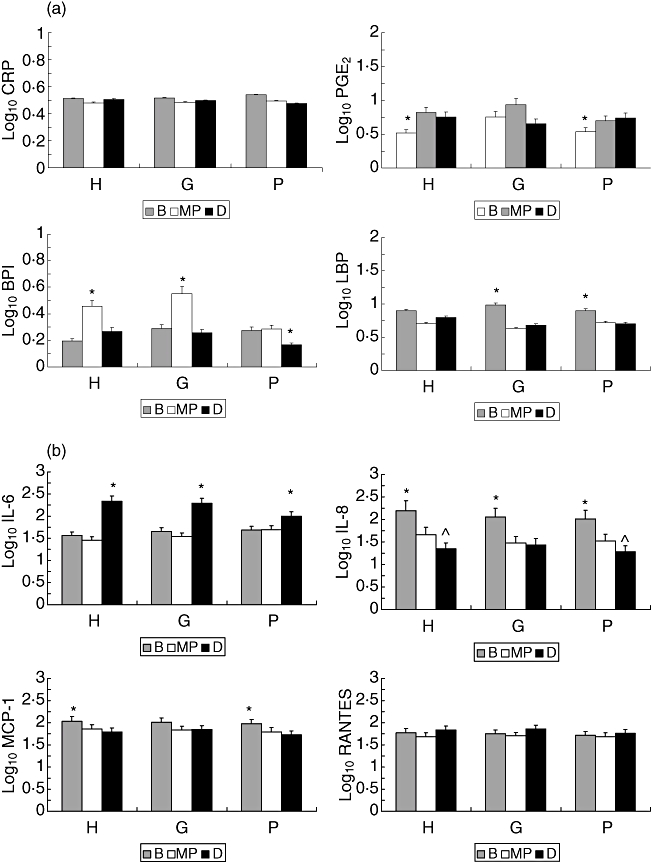
(a,b) Levels of systemic inflammatory mediators at baseline (B), mid-pregnancy (MP) and delivery (D) in experimental animals stratified into clinical presentation at baseline (H: health; G: gingivitis; P: periodontitis). The bars denote the group means and the vertical brackets enclose 1 standard error of the mean. The asterisk (*) denotes significantly different from other time-points at least at P < 0·05.
Serum inflammatory mediators and response to periodontitis
Figure 3a–c provides a comparison of the mediator levels at baseline, mid-pregnancy and delivery between clinical subsets of animals. In this figure, each animal is grouped into a subset based upon their particular disease presention (i.e. CIPD value) at the baseline, mid-pregnancy and delivery time-points. Thus, this approach focuses directly upon clinical presentation and systemic inflammatory response relationships at the time-points. The results demonstrated increased levels of IL-6 and BPI in the gingivitis and periodontitis groups at baseline. In contrast, IL-8, MCP-1 and RANTES showed decreasing levels comparing health to gingivitis to periodontitis in this population (Fig. 3a). PGE2 was elevated significantly in the gingivitis subset of animals at baseline. The data also indicate that IL-8 and LBP levels are elevated significantly in experimental animals presenting with health and/or gingivitis at baseline compared to the control group of animals.
Fig. 3.
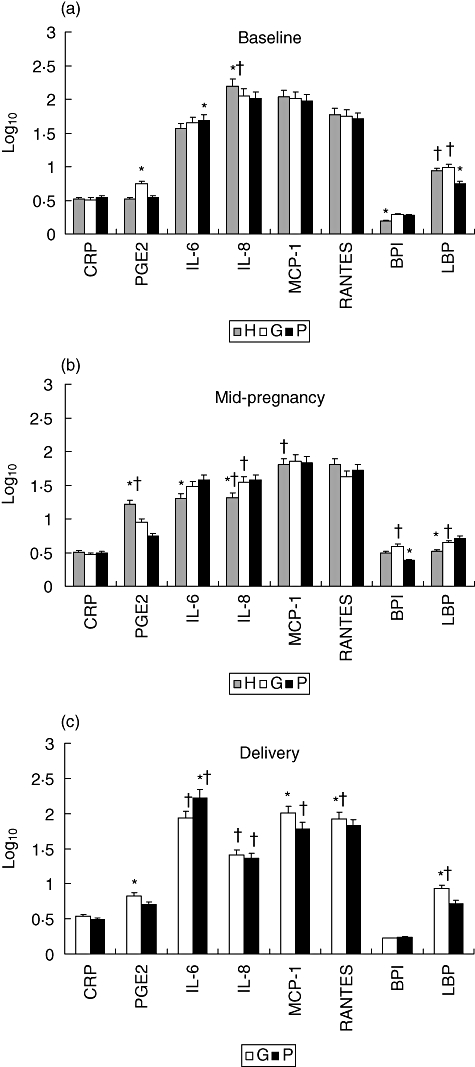
(a–c) Levels of systemic inflammatory mediators at baseline (a), mid-pregnancy (b) and delivery (c) in experimental animals stratified based upon clinical presentation (health, gingivitis, periodontitis) at each sampling interval. The bars denote the group means and the vertical brackets enclose 1 standard error of the mean. The asterisk (*) denotes significantly different from other subgroups at least at P < 0·05. The dagger (†) denotes significantly different from the control animals at the same time-point at least at P < 0·05.
Interestingly, at mid-pregnancy (Fig. 3b), IL-6, IL-8 and LBP were significantly lower, primarily in the subgroup that demonstrated the least clinical response to ligation (i.e. H), indicative of progressing periodontal disease. In contrast, PGE2 demonstrated a significant difference, with lowest levels in the periodontitis group. BPI levels were also significantly lower in the periodontitis group at mid-pregnancy. It can also be noted that the health and/or gingivitis animals exhibited levels of PGE2, IL-8, MCP-1, BPI and LBP that were significantly different from the control animal levels at mid-pregnancy.
By delivery (Fig. 3c), as expected, no animals in the experimental ligature group were determined to be periodontally healthy (i.e. CIPD <20). IL-6 was the only mediator that was increased in the periodontitis animals at this time-point. In addition, serum IL-6 levels were increased significantly and IL-8 levels were decreased significantly in both subsets of experimental animals compared to the control animals at delivery. PGE2, MCP-1, RANTES and LBP were all decreased in the most diseased subset of animals. Both RANTES and LBP levels were increased significantly in the gingivitis group compared to control animals, while MCP-1 was decreased in the periodontitis group compared to the control values.
Serum inflammatory mediators, antibody levels and periodontal disease
Table 1 provides a summary of the relationships among the levels of various inflammatory mediators throughout the protocol. The results demonstrated significant correlations among selected mediators, with CRP levels being independent of the other inflammatory biomarkers. However, the acute phase reactants, BPI and LBP, showed the most consistent relationship as markers of systemic inflammation throughout the pregnancy and ligature-induced disease.
Table 1.
Relationship of serum inflammatory mediators in the baboons at each time-point of the study
| Inflammatory analyte | Baseline | Mid-pregnancy | Delivery |
|---|---|---|---|
| CRP | LBP↓ | n.s. | MCP-1↑ |
| PGE2 | RANTES↑ | RANTES↑, BPI↑ | BPI↑, IL-6↓ |
| IL-6 | MCP-1↑ | n.s. | PGE2↓ |
| IL-8 | LBP↓ | MCP-1↑, BPI↓ | LBP↑, BPI↓ |
| MCP-1 | BPI↓, LBP↓ | n.s. | CRP↑ |
| RANTES | PGE2↑ | PGE2↑ | n.s. |
| BPI | MCP-1↓, LBP↑ | PGE2↑, LBP↑, IL-8↓ | PGE2↑, LBP↑, IL-8↓ |
| LBP | BPI↑, CRP↓, | BPI↑ | BPI↑, IL-8↑ |
| MCP-1↓, IL-8↓ |
The arrows (↓ negative; ↑ positive) denote the direction of relationship of the mediators. Only mediators with correlations with P < 0·001 are included. BPI, bactericidal permeability inducing factor; CRP, C-reactive protein; IL, interleukin; LBP, lipopolysaccharide binding protein; MCP, macrophage chemotactic protein; n.s., no significant relationship between any of the mediators; PGE2, prostaglandin E2; RANTES, regulated upon activation, normal T cell expressed and secreted.
Table 2 provides a similar assessment in relating the inflammatory mediators to serum antibody levels to oral bacteria at baseline, mid-pregnancy and delivery. Using a forward stepwise regression to assess the level of antibody specificity that best predicted the individual systemic inflammatory analyte levels, the results provided some interesting outcomes. Generally PGE2, RANTES and BPI levels were unrelated to the antibody responses. CRP, IL-8, MCP-1 and LBP showed significant relationships to antibody response profiles at baseline. IL-8 and MCP-1 levels maintained a relationship to specific antibody profiles through mid-pregnancy, including both antibody specificities and direction. IL-6 levels were related to specific antibody patterns at mid-pregnancy and delivery. Examination of the overall systemic antibody responses indicated that responses to F. nucleatum, P. gingivalis, A. actinomycetemcomitans and C. rectus were associated most frequently with variations in inflammatory mediator levels.
Table 2.
Relationship of serum inflammatory mediators to levels of serum antibody in the baboons at each time-point of the study
| Inflammatory analyte | Baseline | Mid-pregnancy | Delivery |
|---|---|---|---|
| CRP | 0·621 | n.s. | n.s. |
| Pg↑, En↑, Ec↓ | |||
| PGE2 | n.s. | n.s. | n.s. |
| IL-6 | n.s. | 0·452 | 0·573 |
| Fn↑, Pg↑, Mm↓ | Aa↑, Cr↓ | ||
| IL-8 | 0·607 | 0·562 | n.s. |
| Aa↑, Fn↑, Cr↓, An↓ | Pg↑, Fn↑, Tf↓ | ||
| MCP-1 | 0·545 | 0·453 | n.s. |
| Aa↑, Pn↑, Cr↓,Sg↓ | Aa↑, Fn↑, Ec↓, An↓ | ||
| RANTES | n.s. | n.s. | n.s. |
| BPI | n.s. | n.s. | n.s. |
| LBP | 0·457 | n.s. | n.s. |
| Cr↑, Pg↓, Fn↓, |
The numbers denote an adjusted R2 value. The arrows denote the direction of antibody relationship to the mediator. BPI, bactericidal permeability inducing factor; CRP, C-reactive protein; IL, interleukin; LBP, lipopolysaccharide binding protein; MCP, macrophage chemotactic protein; n.s., no significant relationship between antibody and any of the mediators; PGE2, prostaglandin E2; RANTES, regulated upon activation, normal T cell expressed and secreted.
Finally, we attempted to model the oral clinical disease expression via a specific profile of systemic inflammatory responses. The results in Table 3 provide a summary of these analyses. Levels of IL-6, IL-8 and LBP demonstrated a significant profile across the entire population irrespective of the sampling interval. Separating these comparisons into the individual sampling times demonstrated that none of the analytes profiled the oral clinical presentation at baseline. However, at mid-pregnancy, PGE2 and RANTES were related significantly to the oral disease, with only BPI levels as a significant correlation of oral disease at delivery. Interestingly, throughout the experimental protocol, the profiles of serum mediators were less dependent upon the sampling interval, and patterned more closely the actual clinical presentation of the animals throughout the course of the study (Table 3).
Table 3.
Multiple linear regression analysis of serum inflammatory mediators and CIPD values as dependent clinical measure
| Grouping | Model (P =) | Analytes |
|---|---|---|
| Total | <0·001 | IL-6; <0·001↑ |
| IL-8; 0·046↓ | ||
| LBP; 0·009↓ | ||
| Baseline | n.s. | n.s. |
| Mid-pregnancy | 0·013 | RANTES; 0·031↑ |
| Delivery | 0·043 | BPI; 0·022↓ |
| Healthy | <0·001 | IL-6; <0·001↑ |
| MCP-1; 0·047↓ | ||
| BPI; 0·046↓ | ||
| Gingivitis | n.s. | CRP; 0·018↓ |
| Periodontitis | 0·008 | PGE2; <0·001↑ |
| IL-8; 0·046↓ |
The numbers denote P-values for the analytes and model. The arrows denote the direction of mediator relationship to the Composite Index of Periodontal Disease (CIPD) values representing the oral clinical presentation. BPI, bactericidal permeability inducing factor; CRP, C-reactive protein; IL, interleukin; LBP, lipopolysaccharide binding protein; MCP, macrophage chemotactic protein; n.s., no significant relationship between CIPD and any of the mediators; PGE2, prostaglandin E2; RANTES, regulated upon activation, normal T cell expressed and secreted.
Discussion
As more attention is being directed towards chronic diseases in the human population, new concepts of aetiology and resulting loss of tissue/organ function continue to develop. Many of these chronic diseases have been identified to have components of chronic inflammation, both locally and systemically, that contribute to inducing collateral damage of the host resulting in the clinical symptoms. The systemic acute phase response with increases in various inflammatory mediators is an important component of innate immunity and is fundamentally designed to protect the non-immune host from noxious challenge and to re-establish homeostasis [2]. However, it is now recognized that the chronic stimulation of this systemic inflammatory response provides markers for risk of disease, as well as the probability that the biomolecules of this response can actually contribute to the disease processes.
Numerous studies have reported that chronic periodontal infections trigger chronic inflammation that is expressed locally as periodontitis [12,13], and systemically by elevations in various inflammatory mediators [2]. The levels of these mediators are associated generally with the severity/extent of periodontal disease, frequently decrease significantly with periodontal therapy and are decreased in patients who become edentate (Cunningham LL, Novak MJ, Stevens J, Abadi B and Ebersole JL. The oral-systemic link: a bidirectional relationship. submitted.). Thus, while the ‘cause and effect’ relationship between the systemic inflammatory mediators and periodontitis is difficult to document unequivocally, the breadth of evidence indicates that chronic periodontal infections may be a contributor to the burden of risk for initiating and/or sustaining symptoms associated with chronic inflammatory diseases.
We have described a non-human primate model of a chronic polymicrobial periodontal infection and have demonstrated a pattern of host responses similar to those which occur in human disease [53–55]. The baboon model of ligature-induced periodontitis and pregnancy can be used to assess the host response profiles during disease and to identify some biological links with adverse pregnancy outcomes [46]. Periodontitis in the non-human primates elicited by ligature placement is accompanied by changes in the subgingival microbial ecology with bacterial species similar to those in human disease [47,56,57]. This chronic oral infection elicits elevated levels of local inflammatory, innate and acquired immune mediators [12,13,58,59]. The results of this report focused upon the capacity of the oral infection and disease to trigger changes in the systemic host response apparatus, manifested by changes in various acute phase reactants, and inflammatory mediators and cytokines/chemokines.
Our previous results have demonstrated extensive variability in periodontal clinical presentation of the group of female baboons, not dissimilar from the heterogeneity reported in human populations, with some animals showing pre-existing naturally occurring mild to moderate periodontitis [46]. Additionally, while all the experimental animals subjected to tooth ligation developed significant increases in gingival inflammation and destructive disease following placement of ligatures during pregnancy, the changes in disease in response to ligation exhibited individual variation. Thus, it would be predicted that baseline analyte values, and changes in these biomolecules during disease and pregnancy, would be expected to reflect the clinical variability.
The results demonstrated significant differences in selected serum inflammatory mediators during the ligation phase of the study related to the time-point of the study and associated with ligation of teeth in two quadrants (MP) or four quadrants (D). Interestingly, the profile of inflammatory mediators at the various time-points of disease was not associated consistently with increasing disease, with only IL-6 levels demonstrating a significant increase after 6 months of periodontal disease. The results suggested that although there were variations in systemic analyte measures related to periodontitis, individual variation in the clinical responses of the animals may have a substantial impact upon interpreting the direct link between oral disease and systemic responses. Moreover, while previous studies in human periodontitis have suggested local involvement of a range of mediators, including IL-1β and TNF-α, expression of these proinflammatory response molecules were not observed in the systemic responses of the baboons to periodontal disease progression. This is consistent with differences in local versus systemic cytokine/chemokine response profiles observed with this disease in humans [13].
Therefore, we evaluated changes in the inflammatory mediators through the 6-month ligation in subsets of the animals based upon clinical presentation at baseline. These results demonstrated consistent patterns of systemic inflammation related to progressing periodontitis. PGE2 levels increased significantly by MP and remained elevated throughout the entire pregnancy. Similarly, BPI levels were also increased significantly by MP in most of the animals and generally decreased substantially by delivery. LBP levels were elevated generally at baseline and decreased significantly throughout the disease process. As was noted with the population as a whole, IL-6 levels were increased significantly by delivery, irrespective of the baseline clinical characteristics of the animals. Both IL-8 and MCP-1 decreased from baseline throughout the study, with the lowest levels of IL-8 in serum samples obtained at delivery, unrelated to the clinical presentation of the animals at baseline. A summary of these outcomes was that the clinical presentation at baseline had less impact on the systemic inflammatory mediator levels than the effect of the continued disease over 6 months induced by ligation and creation of chronic periodontitis in the animals.
Finally, based upon these findings, we evaluated response differences in subsets of animals as they progressed through the experimental challenge during pregnancy. Thus, at baseline, stratification of the animals related to naturally occurring oral health/disease showed some distinct differences in serum inflammatory mediators that differentiated the healthy from gingivitis from the periodontitis groups. At mid-pregnancy after 3 months of ligature-induced challenge, the animals were restratified based upon their clinical response to the ligatures. Substantial differences in the analyte profiles were notable, with the group demonstrating the highest level of periodontitis showing elevated levels of IL-6, IL-8 and LBP and significantly decreased levels of PGE2 and BPI. By the time of delivery, and following ligation of teeth in four quadrants, all animals had a CIPD >20 (not periodontally healthy). Again, the most diseased animals provided a profile of serum analytes that was distinctive from animals expressing primarily gingival inflammation, with a lower level of destructive disease. These data suggested that the variation in naturally occurring periodontal inflammation and disease in the female baboons was reflected by patterns of systemic inflammation. Moreover, those animals that responded more robustly to the infection burden accompanying ligation generally demonstrated a unique profile of mediator levels. As we have observed previously, these findings are consistent with a subset of these non-human primates that show an increased susceptibility to dysregulated local responses eliciting greater disease and allowing a more substantial challenge to the systemic inflammatory apparatus. These outcomes would also suggest that animals with a more effective adaptive immune response to the microbial challenge would demonstrate less disease, as we have reported previously [46,55], and less systemic challenge with lower serum inflammatory responses. Examination of the relationships between the inflammatory mediators and antibody in serum showed that elevated or decreased antibody specificities were coincident with levels of selected mediators. However, identification of a particular pattern of antibodies that best described the systemic inflammatory response profiles was somewhat complex. Generally, the acute phase reactants were delineated by unique patterns of antibody responses that were observed at specific time-points during the study. The chemokines IL-8 and MCP-1 demonstrated some similarity in the patterns of antibody correlations, particularly at baseline and mid-pregnancy. IL-6 levels were best described by distinctive antibody specificities during the protocol. However, of the 20 antibody specificities that were evaluated, levels of F. nucleatum, P. gingivalis, A. actinomycetemcomitans and C. rectus showed some consistency in contributing to relationships with the range of inflammatory mediators analysed.
However, within the model system, a pattern of the serum analytes provided some insight into describing the expression of disease. We observed a clear association of IL-6, IL-8 and LBP levels across disease expression and throughout pregnancy. When broken down further, we observed that these relationships were related primarily to the characteristics of the disease expression in the individual animal, and generally related less to the stage of pregnancy at which the sample was obtained. That is, the mediators appear to reflect most clearly the clinical parameters of the oral disease, with minimal impact of the time–course of pregnancy in this model.
This study provides an evaluation of the systemic response characteristics of female baboons to ligature-induced periodontitis during pregnancy. Our findings support that ligature-induced periodontitis in baboons elicits changes in systemic inflammatory mediators. Moreover, a subset of the population of baboons that demonstrated a greater clinical response to ligation during pregnancy exhibited a discrete systemic inflammatory response. This model of periodontitis and pregnancy resulted in alterations in the level of serum inflammatory mediators throughout the pregnancy and will provide an opportunity to delineate risk factors for oral–systemic disease linkages.
Acknowledgments
This work was supported by USPHS grant DE13958 from the National Institute of Dental and Craniofacial Research. We would like to thank Scott Eddy, Robert Ayala and Malini Bharadwaj for technical support in developing and managing these data. We acknowledge the crucial contribution of Drs Kathleen Brasky, Karen Rice and the scientific and technical staff at the Southwest Foundation for Biomedical Research and contribution from USPHS grant 13986 in support of the Southwest National Primate Research Center at the Foundation.
Disclosure
The authors claim no conflict or financial interests related to the research reported.
References
- 1.Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ, van Dijk E, Meloen RH. Monitoring health by values of acute phase proteins. Acta Histochem. 2006;108:229–32. doi: 10.1016/j.acthis.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 3.Nibali L, D'Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007;34:931–7. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 4.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21:256–60. doi: 10.1111/j.1399-302X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki K, Honda T, Oda T, et al. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res. 2005;40:53–8. doi: 10.1111/j.1600-0765.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 6.Nibali L, Tonetti MS, Ready D, et al. Interleukin-6 polymorphisms are associated with pathogenic bacteria in subjects with periodontitis. J Periodontol. 2008;79:677–83. doi: 10.1902/jop.2008.070453. [DOI] [PubMed] [Google Scholar]
- 7.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–7. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 8.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–43. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr. 2006;18:125–31. doi: 10.1097/01.mop.0000193293.87022.4c. [DOI] [PubMed] [Google Scholar]
- 11.Pussinen PJ, Paju S, Mantyla P, Sorsa T. Serum microbial- and host-derived markers of periodontal diseases: a review. Curr Med Chem. 2007;14:2402–12. doi: 10.2174/092986707781745604. [DOI] [PubMed] [Google Scholar]
- 12.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 2007;43:278–93. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 14.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4:242–50. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Farasat S, Aksentijevich I, Toro JR. Autoinflammatory diseases: clinical and genetic advances. Arch Dermatol. 2008;144:392–402. doi: 10.1001/archderm.144.3.392. [DOI] [PubMed] [Google Scholar]
- 17.Bistrian B. Systemic response to inflammation. Nutr Rev. 2007;65:S170–2. doi: 10.1301/nr.2007.dec.s170-s172. [DOI] [PubMed] [Google Scholar]
- 18.Craig RG. Interactions between chronic renal disease and periodontal disease. Oral Dis. 2008;14:1–7. doi: 10.1111/j.1601-0825.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 19.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 20.Wouters EF, Groenewegen KH, Dentener MA, Vernooy JH. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc. 2007;4:626–34. doi: 10.1513/pats.200706-071TH. [DOI] [PubMed] [Google Scholar]
- 21.Sacre SM, Drexler SK, Andreakos E, Feldmann M, Brennan FM, Foxwell BM. Could Toll-like receptors provide a missing link in chronic inflammation in rheumatoid arthritis? Lessons from a study on human rheumatoid tissue. Ann Rheum Dis. 2007;66(Suppl 3):iii81–6. doi: 10.1136/ard.2007.079012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 23.Hisamatsu T, Ogata H, Hibi T. Innate immunity in inflammatory bowel disease: state of the art. Curr Opin Gastroenterol. 2008;24:448–54. doi: 10.1097/MOG.0b013e3282ff8b0c. [DOI] [PubMed] [Google Scholar]
- 24.Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs. 2008;17:1051–65. doi: 10.1517/13543784.17.7.1051. [DOI] [PubMed] [Google Scholar]
- 25.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–63. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Boggess KA. Pathophysiology of preterm birth: emerging concepts of maternal infection. Clin Perinatol. 2005;32:561–9. doi: 10.1016/j.clp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Nygren P, Fu R, Freeman M, Bougatsos C, Klebanoff M, Guise JM. Evidence on the benefits and harms of screening and treating pregnant women who are asymptomatic for bacterial vaginosis: an update review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:220–33. doi: 10.7326/0003-4819-148-3-200802050-00008. [DOI] [PubMed] [Google Scholar]
- 28.Guaschino S, De Seta F, Piccoli M, Maso G, Alberico S. Aetiology of preterm labour: bacterial vaginosis. Br J Obstet Gynaecol. 2006;113(Suppl 3):46–51. doi: 10.1111/j.1471-0528.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- 29.Hansen-Pupp I, Hallin AL, Hellstrom-Westas L, et al. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr Res. 2008;64:183–8. doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- 30.Blank V, Hirsch E, Challis JR, Romero R, Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour – a workshop report. Placenta. 2008;29S:102–4. doi: 10.1016/j.placenta.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Shoji T, Yoshida S, Mitsunari M, et al. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro- and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol. 2007;75:82–90. doi: 10.1016/j.jri.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294 e1–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–78. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005;3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmerman MF, van der Weijden GA. Risk factors for periodontitis. Int J Dent Hyg. 2006;4:2–7. doi: 10.1111/j.1601-5037.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 37.Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–66. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 40.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–41. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 41.Ebersole JL, Cappelli D, Holt SC. Periodontal diseases: to protect or not to protect is the question? Acta Odontol Scand. 2001;59:161–6. doi: 10.1080/000163501750266756. [DOI] [PubMed] [Google Scholar]
- 42.Ebersole JL. Immune responses in periodontal diseases. In: Wilson TG Jr, Kornman KS, editors. Fundamentals of periodontics. Chicago: Quintessence Publishing Co., Inc.; 2003. pp. 111–43. [Google Scholar]
- 43.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 44.Boggess KA, Beck JD, Murtha AP, Moss K, Offenbacher S. Maternal periodontal disease in early pregnancy and risk for a small-for-gestational-age infant. Am J Obstet Gynecol. 2006;194:1316–22. doi: 10.1016/j.ajog.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 45.Boggess KA. Pathogenicity of periodontal pathogens during pregnancy. Am J Obstet Gynecol. 2005;193:311–12. doi: 10.1016/j.ajog.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 46.Cappelli D, Steffen MJ, Holt SC, Ebersole JL. Periodontitis in pregnancy: clinical and serum antibody observations from a baboon model of ligature-induced disease. J Periodontol. 2009;80:1154–65. doi: 10.1902/jop.2009.080199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappelli D, Holt SC, Singer RE, Pickrum HM, Ebersole JL. Effects of 0·12% chlorhexidine gluconate on experimental gingivitis in non-human primates: clinical and microbiological alterations. Oral Dis. 2000;6:124–31. doi: 10.1111/j.1601-0825.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds MA, Dawson DR, Novak KF, et al. Effects of caloric restriction on inflammatory periodontal disease. Nutrition. 2009;25:88–97. doi: 10.1016/j.nut.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebersole JL, Cappelli D, Holt SC, Singer RE, Filloon T. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol Immunol. 2000;15:19–26. doi: 10.1034/j.1399-302x.2000.150104.x. [DOI] [PubMed] [Google Scholar]
- 50.Kornman KS, Holt SC, Robertson PB. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodont Res. 1981;16:363–71. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 51.Ebersole JL, Cappelli D, Steffen MJ. Characteristics and utilization of antibody measurements in clinical studies of periodontal disease. J Periodontol. 1992;63:1110–16. doi: 10.1902/jop.1992.63.12s.1110. [DOI] [PubMed] [Google Scholar]
- 52.Ebersole JL, Kornman KS. Systemic antibody responses to oral microorganisms in the cynomolgus monkey: development of methodology and longitudinal responses during ligature-induced disease. Res Immunol. 1991;142:829–39. doi: 10.1016/0923-2494(91)90128-6. [DOI] [PubMed] [Google Scholar]
- 53.Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clin Vaccine Immunol. 2008;15:1067–75. doi: 10.1128/CVI.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebersole JL, Cappelli D, Mathys EC, et al. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann Periodontol. 2002;7:102–11. doi: 10.1902/annals.2002.7.1.102. [DOI] [PubMed] [Google Scholar]
- 55.Ebersole JL, Steffen MJ, Reynolds MA, et al. Differential gender effects of a reduced-calorie diet on systemic inflammatory and immune parameters in nonhuman primates. J Periodont Res. 2008;43:500–7. doi: 10.1111/j.1600-0765.2008.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–97. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- 57.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–7. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 58.Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:637–46. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 59.Kinane DF, Podmore M, Murray MC, Hodge PJ, Ebersole J. Etiopathogenesis of periodontitis in children and adolescents. Periodontol 2000. 2001;26:54–91. doi: 10.1034/j.1600-0757.2001.2260104.x. [DOI] [PubMed] [Google Scholar]


