Abstract
Pulmonary fibrosis is characterized by progressive worsening of pulmonary function leading to a high incidence of death. Currently, however, there has been little progress in therapeutic strategies for pulmonary fibrosis. There have been several reports on cytokines being associated with lung fibrosis, including interleukin (IL)-6 and transforming growth factor (TGF)-β1. We reported recently that two substances (ATRA and thalidomide) have preventive effects on pulmonary fibrosis by inhibiting IL-6-dependent proliferation and TGF-β1-dependent transdifferentiation of lung fibroblasts. Rheumatoid arthritis is a chronic autoimmune disorder, and its pathogenesis is also characterized by an association with several cytokines. It has been reported that calpain, a calcium-dependent intracellular cysteine protease, plays an important role in the progression of rheumatoid arthritis. In this study, we examined the preventive effect of Calpeptin, a calpain inhibitor, on bleomycin-induced pulmonary fibrosis. We performed histological examinations and quantitative measurements of IL-6, TGF-β1, collagen type Iα1 and angiopoietin-1 in bleomycin-treated mouse lung tissues with or without the administration of Calpeptin. Calpeptin histologically ameliorated bleomycin-induced pulmonary fibrosis in mice. Calpeptin decreased the expression of IL-6, TGF-β1, angiopoietin-1 and collagen type Iα1 mRNA in mouse lung tissues. In vitro studies disclosed that Calpeptin reduced (i) production of IL-6, TGF-β1, angiopoietin-1 and collagen synthesis from lung fibroblasts; and (ii) both IL-6-dependent proliferation and angiopoietin-1-dependent migration of the cells, which could be the mechanism underlying the preventive effect of Calpeptin on pulmonary fibrosis. These data suggest the clinical use of Calpeptin for the prevention of pulmonary fibrosis.
Keywords: calpain, cell migration, cell proliferation, cytokines, pulmonary fibrosis
Introduction
Pulmonary fibrosis is a progressive and lethal lung disease characterized by the proliferation of fibroblasts and the deposition of extracellular matrix (ECM) materials. It is frequently associated with collagen diseases, rheumatoid arthritis (RA), radiotherapy to the thoracic region, and drugs including anti-cancer agents. Idiopathic pulmonary fibrosis (IPF) is the most common type with a prevalence of 16–18 per 100 000 people, leading to a high incidence of death (>50% 5-year mortality rate) due to eventual respiratory failure [1]. There are currently few effective therapeutic and preventive strategies for pulmonary fibrosis [2–4].
Calpain, a calcium-dependent intracellular cysteine protease, plays an important role in various cellular processes including cell growth, differentiation and apoptosis [5]. In RA patients, over-expression of calpains are found in arthritic synovial fluid and are thought to play important roles in various cellular processes including cell growth, differentiation and apoptosis [5–7]. RA is a chronic autoimmune disorder characterized by an association with several cytokines, including interleukin (IL)-6, IL-1 and tumour necrosis factor (TNF)-α[8,9]. It has been reported that calpain-inhibitory compound ameliorates experimental arthritis via inhibitory effects on cytokine production such as IL-6 and IL-1β[10]. Such cytokines are also associated with pulmonary fibrosis, including transforming growth factor (TGF)-β1, IL-1, IL-6 and TNF-α[11–16]. We have also shown the important roles of IL-6 and TGF-β1 in our previous reports demonstrating the preventive effects of two substances (ATRA and thalidomide) on pulmonary fibrosis [17–19]. Moreover, inhibition of calpain has been demonstrated to prevent myocardial fibrosis [20]. Therefore, we focused upon the relationship between calpain and pulmonary fibrosis. In this study we investigated whether a calpain inhibitor, Calpeptin, had preventive or therapeutic effects on pulmonary fibrosis, and show that Calpeptin prevents bleomycin-induced pulmonary fibrosis in mice through the inhibition of the production of proinflammatory and profibrotic cytokines and the reduction of collagen synthesis.
Materials and methods
Cell culture
WI38VA-13, a human lung fibroblastic cell line transformed by SV40, IMR-90, a primary human fetal lung fibroblast line and A549, a human alveolar type II epithelial cell line, were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma Chemical Co., St Louis, MO, USA) supplemented with 10% heat-inactivated fetal calf serum. Cells were cultured with antibiotics in a humidified incubator with 5% CO2 at 37°C. In some experiments, the cells were stimulated with recombinant human IL-6 and recombinant human angiopoietin-1 (Ang-1) (R&D Systems, Oxford, UK). Calpeptin (Calbiochem, San Diego, CA, USA) was diluted in dimethylsulphoxide (DMSO) and added to the growth medium to yield the final DMSO solvent concentration <0·01% (v/v). As a control, cells were treated with the same concentration of DMSO and all cultures in this study contained the same final concentration of DMSO. In preliminary experiments, this final concentration of DMSO had no gross effect on WI38VA13, IMR90 and A549 cells.
Animal studies
C57BL/6 female mice were purchased from Clea Japan (Tokyo, Japan) and maintained in our specific pathogen-free animal facility. All animals were kept according to the Animal Protection Guidelines of Hyogo College of Medicine. All protocols for animal use and euthanasia were reviewed and approved by the Institute of Laboratory Animals, Hyogo College of Medicine, Japan. Eight-week-old mice were injected intraperitoneally with bleomycin sulphate (Bleo) [2 mg/mouse (0·1 mg/g body weight)/day; Nippon Kayaku, Tokyo, Japan] on days 1, 8 and 15. In some experiments, the mice were injected intraperitoneally with 0·04 mg of Calpeptin (diluted in DMSO) in 0·2 ml of distilled water or 0·2 ml of distilled water alone (controls). The injections were repeated three times weekly [1] throughout the course (days 1–28) [2] for the first 14 days (days 1–14) or [3] for the last 14 days (days 15–28). All mice with or without Bleo and/or Calpeptin were injected in the same final concentration of DMSO.
Histological studies
Histological examination was performed by staining with haematoxylin and eosin (H&E) or Azan using an aniline blue method, as described previously [17–19]. For quantitative analysis of the severity of fibrosis, the level of fibrosis was measured using the number of pixels stained blue by Azan staining using the Analytical Digital Photomicroscopy technique and Adobe® Photoshop® (Adobe Systems Inc., San Jose, CA, USA) (magnification ×200) [18,19]. Sections were immunostained using mouse anti-human calpain monoclonal antibody (1:100; Abnova, Taipei, Taiwan) followed by biotin-conjugated goat anti-mouse antibody (1:250; Dako, Glostrup, Denmark). The tyramide signal amplification (TSA) biotin system (Perkin-Elmer, Boston, MA, USA) was used to enhance staining, and peroxidase activity was envisioned with the diaminobenzidine kit (Dako).
Quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR)
Quantitative real-time RT–PCR was performed using TaqMan Gene expression products as described previously [17–19,21]. The levels of mRNA are represented as the ratio to 18SrRNA (Applied Biosystems), an endogenous control.
Measurement of IL-6 and TGF-β1
The concentrations of IL-6 and TGF-β1 in the culture supernatants with or without Calpeptin (100 nM) for 24 h were measured using an enzyme-linked immunosorbent assay (ELISA) kit (BioSource, Camarillo, CA, USA), as described previously [17,18].
Cell proliferation assay
A cell proliferation assay was performed as described previously [17]. Cell Counting Kit-8 (Dojindo, Tokyo, Japan) was used to characterize the growth of the cells.
Cell apoptosis assay
Cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) were determined by Cell Death Detection ELISAPLUS (Roche, Mannheim, Germany).
Cell migration assay
In vitro migration assays were performed as described previously [21]. Briefly, WI38 VA13 cells were precultured overnight with or without Calpeptin (100 nM), and then suspended at a density of 1 × 106 cells/ml in DMEM and placed in the upper half of a Boyden chamber. The lower half of the Boyden chamber was filled with DMEM containing 100 ng/ml recombinant human Ang-1 or DMEM alone.
Collagen protein measurement
Collagen protein was measured using the Sircol soluble collagen assay Kit (Biocolor, Belfast, UK), as described previously [18].
Statistical analysis
Results are given as the mean ± standard deviation (s.d.) of values. Statistical analysis was performed using the Bonferroni/Dunn multiple comparisons test.
Results
Prevention of bleomycin-induced pulmonary fibrosis by Calpeptin
In the lung tissues from the bleomycin-treated mice without Calpeptin (Bleo + DMSO), the pulmonary interalveolar septa thickened and had been infiltrated by inflammatory cells, with collagen depositions and increased calpain expression in the interstitium disclosed by Azan and calpain staining. Intraperitoneal administration of Calpeptin three times a week (Bleo + Calpeptin) inhibited the collagen deposition and increase of calpain activity in the bleomycin-treated mouse lung tissues (Fig. 1a). Mice treated with Calpeptin but without bleomycin showed no changes, including body weight and other organs (data not shown). Moreover, the mRNA levels of Calpain1 and Calpain2 from mouse lung tissues at 28 days after the first injection of bleomycin (Bleo + DMSO) were elevated approximately 1·7-fold and 1·5-fold compared to those of the control mice (DMSO), which were suppressed significantly by the administration of Calpeptin (Bleo + Calpeptin) (P = 0·0007 and P = 0·0002, respectively) (Fig. 1b and c).
Fig. 1.
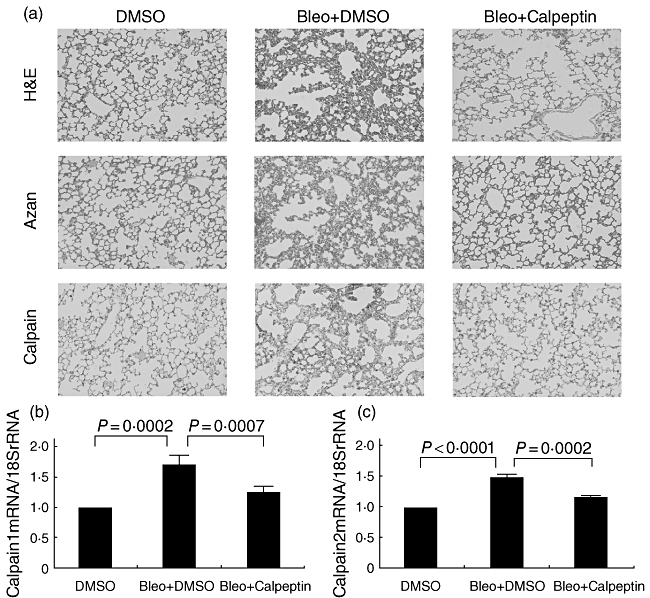
Effect of calpain inhibitor Calpeptin on bleomycin-induced pulmonary fibrosis. Eight-week-old mice were injected intraperitoneally with or without [dimethylsulphoxide (DMSO) only] Bleo on days 1, 8 and 15. Calpeptin (0·04 mg/mouse/day) (Bleo+ Calpeptin) or distilled water alone (Bleo + DMSO) was administered intraperitoneally three times a week during the time–course. On day 28, the mice (n = 5 in each experiments) were killed. Histological changes were demonstrated by haematoxylin and eosin (H&E), Azan and calpain staining (original magnification: ×200) (a). Real-time reverse transcription–polymerase chain reaction was performed to determine the changes in the lung tissues of mice in the mRNA levels of Calpain1 (b) and Calpain2 (c). All results are indicated as the mean ± standard deviation of three separate experiments.
Inhibitory effect of Calpeptin on the proliferation of lung fibroblasts
We first investigated the in vitro effect of Calpeptin on the growth of human lung fibroblasts. The addition of Calpeptin had a suppressive effect on the proliferation of both WI38 VA13 and IMR90 cells in a dose-dependent manner. The maximum inhibitory effect was observed at a concentration of 100 nM Calpeptin [8% decrease (P < 0·0001) and 10% decrease (P < 0·0001), respectively, Fig. 2a]; whereas lower concentrations (1 nM or 10 nM) of Calpeptin had a minor effect compared to 100 nM. The final concentration of DMSO [<0·01% (v/v)] had no gross effect on any cells (data not shown). The concentration of 100 nM Calpeptin had no effect on the cell viability of WI38 VA13 and IMR90 cells (with DMSO only: 94·7% and 93·7%, with 100 nM Calpeptin: 93·7% and 93·0%, respectively).
Fig. 2.
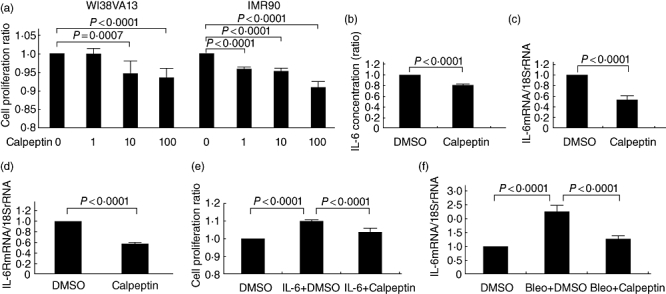
Effect of calpain inhibitor Calpeptin on the interleukin (IL)-6/IL-6R system. WI38VA-13 and IMR90 cells were cultured with or without Calpeptin (1–100 nM) for 48 h and cell proliferation was assayed (a). IL-6 concentration in the culture supernatants for 24 h (b), and the changes in mRNA levels for 7 h for IL-6 (c) and IL-6R (d) of WI38VA-13 cells in the presence or absence of 100 nM Calpeptin were measured by enzyme-linked immunosorbent assay and real-time reverse transcription–polymerase chain reaction (RT–PCR), respectively. WI38VA-13 cells were cultured in the presence or absence of IL-6 (1000 pg/ml) with or without Calpeptin (100 nM) for 96 h and cell proliferation was assayed (e). Real-time RT–PCR was performed to determine the changes in mRNA levels of IL-6 in the lung tissues of mice (f). All results are indicated as the mean ± standard deviation of three separate experiments.
Effect of Calpeptin on the IL-6/IL-6R-mediated proliferation of lung fibroblasts
As demonstrated previously [17,22], human lung fibroblasts secreted IL-6 and IL-6 stimulated the proliferation of the cells in an autocrine manner. Here, we examined the effect of Calpeptin on IL-6 production, IL-6/IL-6R expression in lung fibroblasts, and IL-6-mediated cell proliferation. The concentration of IL-6 in the culture supernatant was decreased by the addition of Calpeptin (P < 0·0001) (Fig. 2b). We next investigated the effect of Calpeptin on the expression of IL-6 and IL-6R mRNA by real-time RT–PCR analysis. Figure 2c and d shows that IL-6mRNA : 18SrRNA and IL-6RmRNA : 18SrRNA ratios were decreased in WI38VA-13 cells by Calpeptin at 7 h (P < 0·0001). According to our previous study, in which the addition of IL-6 stimulated fibroblast growth in a dose-dependent manner and reached a plateau at the concentration of 1000 pg/ml [17], we examined the effect of Calpeptin on 1000 pg/ml of IL-6-mediated cell proliferation and found that Calpeptin inhibited IL-6-induced cell proliferation of lung fibroblasts (P <0·0001) (Fig. 2e). Moreover, the mRNA levels of IL-6 from mouse lung tissues at 28 days after the first injection of bleomycin (Bleo + DMSO) were analysed by real-time RT–PCR and shown to be elevated approximately 2·3-fold compared to those of the control mice (DMSO), which were suppressed significantly by the administration of Calpeptin (Bleo+ Calpeptin) (P < 0·0001) (Fig. 2f).
Inhibitory effect of Calpeptin on the Ang-1-induced migration of lung fibroblasts
We next examined the impact of Calpeptin on Ang-1 and Tie-2 mRNA expression in lung fibroblasts. As shown in Fig. 3a and b, Calpeptin decreased the expression of both Ang-1 and Tie-2 mRNA by 45 and 52% in WI38 VA13 cells, respectively (P<0·0001). Fibroblast migration plays an important role in pulmonary fibrosis. We therefore examined whether Ang-1 plays a role in the migration of lung fibroblasts and whether Calpeptin has an effect on Ang-1 induced fibroblasts migration. We revealed that WI38 VA13 cell migration was induced (1·3-fold increase, P =0·0007) by Ang-1, which was inhibited in the presence of Calpeptin (13% decrease, P =0·0117, Fig. 3c). Moreover, the mRNA levels of Ang-1 and Tie-2 from mouse lung tissues at 28 days after the first injection of bleomycin (Bleo + DMSO) were both elevated approximately 1·7-fold compared to those of the control mice (DMSO), which were suppressed significantly by the administration of Calpeptin (Bleo + Calpeptin), respectively (P<0·0001, P =0·0008) (Fig. 3d and e).
Fig. 3.
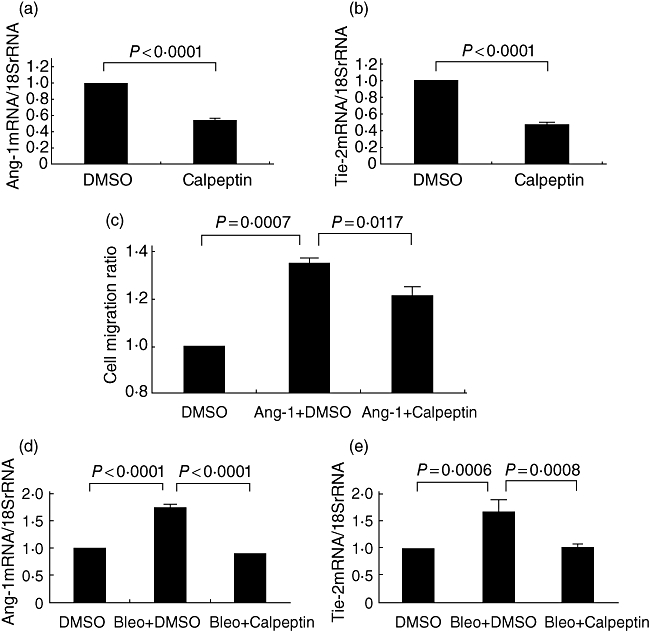
Inhibitory effect of calpain inhibitor Calpeptin on the Ang-1/Tie-2 system. WI38 VA13 cells were cultured in the presence or absence of 100 nM Calpeptin for 7 h and real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed to determine the changes in mRNA levels for Ang-1 (a) and Tie-2 (b). WI38 VA13 cells were precultured overnight with or without Calpeptin (100 nM) and cultured further in the presence or absence of Ang-1 (100 ng/ml) and a cell migration assay was performed (c). Real-time RT–PCR was performed to determine the changes in the lung tissues of mice in the mRNA levels of Ang-1 (d) and Tie-2 (e). All results are indicated as the mean ± standard deviation of three separate experiments.
Effect of Calpeptin on collagen synthesis and the production of TGF-β1 of lung fibroblasts
In a Sircol assay, as shown in Fig. 4a, collagen synthesis of WI38 VA13 cells was suppressed by Calpeptin (28% decrease, P<0·0001). TGF-β1 is known to promote collagen synthesis by fibroblasts [12,13]. We performed experiments to study the effect of Calpeptin on TGF-β1 production of lung fibroblasts. The concentration of TGF-β1 in the culture supernatant was decreased by 25% by the addition of Calpeptin (P< 0·0001) (Fig. 4b), and Calpeptin decreased the COL1A1 and TGF-β1 mRNA expression levels by 27 and 16% in WI38 VA13 cells, respectively (P<0·0001, P =0·0043) (Fig. 4c and d). Moreover, the mRNA levels of COL1A1 and TGF-β1 from mouse lung tissues at 28 days after the first injection of bleomycin (Bleo + DMSO) were elevated approximately 2·5-fold and 1·6-fold compared to those of the control mice (DMSO), which were suppressed significantly by the administration of Calpeptin (Bleo + Calpeptin) (P< 0·0001) (Fig. 4e and f).
Fig. 4.
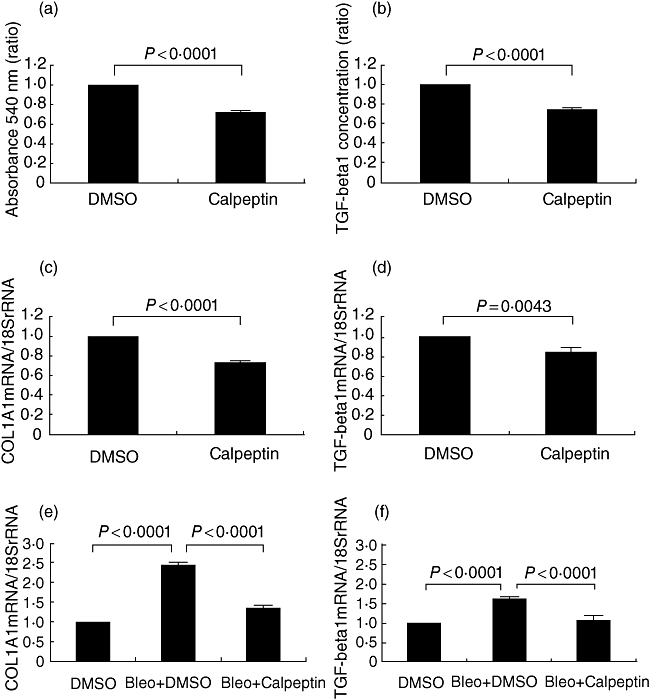
Effect of calpain inhibitor Calpeptin on collagen synthesis and production of transforming growth factor (TGF)-β1. WI38 VA13 cells were cultured in the presence or absence of 100 nM of Calpeptin for 24 h, and the supernatants of the cells were collected and examined for the amount of collagen (a) using a Sircol soluble collagen assay and TGF-β1 concentration (b) was measured by enzyme-linked immunosorbent assay. WI38 VA13 cells were cultured in the presence or absence of 100 nM Calpeptin for 7 h and real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed to determine the changes in mRNA levels for COL1A1 (c) and TGF-β1 (d). Real-time RT–PCR was performed to determine the changes in the lung tissues of mice in the mRNA levels of COL1A1 (e) and TGF-β1 (f). All results are indicated as the mean ± standard deviation of three separate experiments.
Both early and late preventive effects of Calpeptin on bleomycin-induced lung fibrosis
To study the ‘preventive’ and ‘therapeutic’ effects of Calpeptin on lung fibrosis, we examined the early (inflammatory responses were probably mainly demonstrated) and late (post-inflammatory fibrotic changes were probably mainly observed) effects of Calpeptin by transient administration to bleomycin-treated mice. The administration of Calpeptin for the first 14 days (days 0–14) or the last 14 days (days 14–28) prevented bleomycin-induced pulmonary fibrosis equally (35·4% decrease, 31·3% decrease) (P<0·0001). Although the administration of Calpeptin throughout the course (days 0–28) of the experiment demonstrated more effective preventive effect (38·6% decrease) (P<0·0001) than that for the first or last 14 days, there was no statistical significance.
Discussion
In this study, we demonstrated the relationship between calpain and pulmonary fibrosis. We treated mice with bleomycin by intraperitoneal administration instead of intratracheal administration, as usually carried out in many reports, because we wished to study the effect of the drug when added to the whole body as this administration resembled more clearly the use of the drug in human cancer therapy. Here, we showed that calpain expression was increased in the thickened pulmonary interalveolar septa in bleomycin-treated mouse lung tissues. We also showed that a calpain inhibitor, Calpeptin, prevented bleomycin-induced pulmonary fibrosis in mice and that the markedly increased mRNA levels of IL-6, Ang-1, TGF-β1 and COL1A1 (a gene for collagen synthesis) in the bleomycin-treated mouse lung tissues were decreased by the addition of Calpeptin.
In an in vitro study, we found that Calpeptin inhibited lung fibroblast proliferation in a dose-dependent manner. As reported previously [17,22], human lung fibroblasts were induced to proliferate by IL-6 in a dose-dependent manner. In this report, Calpeptin inhibited both IL-6/IL-6R expression and IL-6-induced cell proliferation of lung fibroblasts, indicating an autocrine/paracrine loop involving IL-6 and IL-6R. TNF-α and IL-1β, both of which are reported to stimulate IL-6 production [23,24], were not detected in the WI38 VA13 cell culture supernatants (data not shown). The IL-6/IL-6R system is a possible mechanism for the inhibitory effect of Calpeptin on pulmonary fibrosis, which involves decreased lung fibroblast proliferation.
CCAAT/enhancer-binding protein β (C/EBPβ), also known as nuclear factor-IL-6 (NF-IL-6), is a transcription factor that plays an important role in the regulation of cell growth and differentiation. C/EBPβ has been reported to play an essential role in bleomycin-induced pulmonary fibrosis [25]. We demonstrated that the C/EBPβ : 18SrRNA ratio was decreased in WI38VA-13 cells by Calpeptin at 7 h (23·7% decrease, P < 0·0001). Moreover, the mRNA levels of C/EBPβ from mouse lung tissues at 28 days after the first injection of bleomycin was shown to be elevated approximately 2·2-fold compared to those of the control mice, which were suppressed significantly by the administration of Calpeptin (52% decrease, P < 0·0001).
Although the effect of Calpeptin on expression of C/EBPβ is controversial [26,27], here we observed the inhibitory effect of Calpeptin on C/EBPβ mRNA expression, suggesting that inhibition of C/EBPβ is also one possible mechanism for the inhibitory effect of Calpeptin on pulmonary fibrosis, which is compatible with the previous report [25].
Ang-1 and Ang-2 are counteracting ligands for the endothelial specific receptor, tyrosine kinase Tie-2, and important regulators of blood vessel growth, maturation and function. Ang-1 promotes angiogenesis, induces vascular maturation and decreases vascular permeability. Conversely, Ang-2 has the ability to destabilize blood vessels, enhance vascular leaking and antagonize Ang-1 [28–30]. Fibroblast migration plays an important role in pulmonary fibrosis, and a recent report showed that Ang-1 induced endothelial cell migration [31]. On the other hand, we found that Ang-1 induced malignant pleural mesothelioma cell migration, which has a mesenchymal origin similar to lung fibroblasts [21]. Therefore, we examined whether Calpeptin had an effect on Ang-1/Tie2 expression and Ang-1-dependent migration of lung fibroblasts and showed that Calpeptin decreased both the Ang-1 and Tie-2 mRNA expression of cultured lung fibroblasts as well as the Ang-1-induced migration of these cells. Conversely, no Ang-2 production was detected in the supernatant of these cells (data not shown). Furthermore, the association between pulmonary fibrosis and neovascularization has been demonstrated recently in the lung tissues of patients with IPF [32] and in a rat bleomycin-treated model [33]. In addition to its inhibitory effect on cell migration, the suppression of angiogenesis might be another mechanism behind the preventive effect of Calpeptin on pulmonary fibrosis via the Ang-1/Tie-2 systems.
Pulmonary fibrosis is characterized by the deposition of ECM materials such as type I collagen. TGF-β1 is a key cytokine in human pulmonary fibrogenesis [12–14]. In this study, we demonstrated that Calpeptin decreased collagen synthesis from lung fibroblasts using a Sircol assay and by measuring the COL1A1 mRNA expression of these cells. We also found that the addition of Calpeptin decreased TGF-β1 production from cultured fibroblasts. In addition, in in vivo studies, bleomycin treatment increased the levels of COL1A1 and TGF-β1 mRNA in mouse lung tissues, which were decreased by the addition of Calpeptin.
Although some other cells may contribute to fibrogenesis in the lung, such as alveolar epithelial cells, the concentration of IL-6 or TGF-β1 in culture supernatants of A549 cells was not affected by addition of Calpeptin (data not shown), suggesting specific effects of Calpeptin to fibroblasts.
Hepatocyte growth factor (HGF) is a potent mitogenic factor for alveolar epithelial cells. It has been reported that HGF increases alveolar epithelial repair and improves pulmonary fibrosis, and TGF-β1 is involved in HGF-induced reduction of lung fibrosis [34]. However, Calpeptin had no effect on the expression of HGF or its receptor (data not shown).
It seems that the inhibitory effects of Calpeptin on many factors, including IL-6 and TGF-β1, are not derived from its toxic effect, because Calpeptin had no influence on the viability of both WI38 VA cells and IMR90 cells by trypan blue staining or their cell apoptotic rate (data not shown). Calpeptin also had no effect on the expression of HGF or its receptor, suggesting its specific effects on such factors.
It is worth noting that in this report we showed the ‘late’, i.e. ‘therapeutic’, effect of Calpeptin in bleomycin-induced lung fibrosis models in addition to the ‘early’, i.e. ‘preventive’, effect because in clinical use the ‘therapeutic’ effect is often more important when clinicians find that the fibrotic changes of various aetiologies are already apparent in their patients. The histopathological fibrosis in our animal studies was entirely interstitial and without the significant consolidation or architectural remodelling found in even the earliest examples of human IPF. Whether the changes we have documented for bleomycin-induced injury after 28 days translate to potential benefits in patients with IPF remains to be shown in clinical trials.
In summary, we report that Calpeptin histologically ameliorated bleomycin-induced pulmonary fibrosis in mice and that Calpeptin decreased the expression of IL-6, TGF-β1, Ang-1 and COL1A1 mRNA in mouse lung tissues. Using in vitro studies, we demonstrated the preventive effect of Calpeptin on bleomycin-induced pulmonary fibrosis in mice through the following possible mechanisms: [1] inhibition of lung fibroblast proliferation via the IL-6/IL-6R system [2], inhibition of lung fibroblast migration and neovascularization via the Ang-1/Tie-2 system and [3] inhibition of collagen synthesis and TGF-β1 production. Although the precise cellular suppressive mechanism of Calpeptin in pulmonary fibrosis has not been investigated fully and needs to be studied further to aid its clinical use, our data may lead to the development of novel strategies incorporating Calpeptin for the prevention and treatment of various types of lung fibrosis.
Acknowledgments
We thank Ms Hidemi Kitai (Division of Respiratory Medicine, Department of Internal Medicine, Hyogo College of Medicine) for technical assistance. This work was supported by KAKENHI, a Grant-in-Aid for Scientific Research (C) [20590936] (Tokyo), Funds for Cancer Research from the Hyogo Prefecture Health Promotion Association (Hyogo), and Special Coordination Funds for Promoting Science and Technology (H18-1-3-3-1) (Tokyo).
Disclosure
None.
References
- 1.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–72. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society. International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. This joint statement of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. [DOI] [PubMed] [Google Scholar]
- 3.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 4.Mason RJ, Schwarz MI, Hunninghake GW, Musson RA. NHLBI Workshop Summary. Pharmacological therapy for idiopathic pulmonary fibrosis. Past, present, and future. Am J Respir Crit Care Med. 1999;160:1771–7. doi: 10.1164/ajrccm.160.5.9903009. [DOI] [PubMed] [Google Scholar]
- 5.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Shimizu K, Shimizu K, Suzuki K, Nakagawa Y, Yamamuro T. Calcium-dependent cysteine proteinase (calpain) in human arthritic synovial joints. Arthritis Rheum. 1992;35:1309–17. doi: 10.1002/art.1780351111. [DOI] [PubMed] [Google Scholar]
- 7.Ménard HA, el-Amine M. The calpain–calpastatin system in rheumatoid arthritis. Immunol Today. 1996;17:545–7. doi: 10.1016/s0167-5699(96)30064-9. [DOI] [PubMed] [Google Scholar]
- 8.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989;83:1267–76. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letavernier E, Perez J, Bellocq A, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–8. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida M, Sakuma J, Hayashi S, et al. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor β1, or platelet-derived growth factor B gene. Proc Natl Acad Sci USA. 1995;92:9570–4. doi: 10.1073/pnas.92.21.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor-β is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–79. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 14.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–36. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki Y, Araki K, Vesin C, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–9. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabata C, Kubo H, Tabata R, et al. All-trans retinoic acid modulates radiation-induced proliferation of lung fibroblasts via IL-6/IL-6R system. Am J Physiol Lung Cell Mol Physiol. 2006;290:597–606. doi: 10.1152/ajplung.00282.2005. [DOI] [PubMed] [Google Scholar]
- 18.Tabata C, Kadokawa Y, Tabata R, et al. All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:1352–60. doi: 10.1164/rccm.200606-862OC. [DOI] [PubMed] [Google Scholar]
- 19.Tabata C, Tabata R, Kadokawa Y, et al. Thalidomide prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol. 2007;179:708–14. doi: 10.4049/jimmunol.179.1.708. [DOI] [PubMed] [Google Scholar]
- 20.Yoshifuji H, Umehara H, Maruyama H, et al. Amelioration of experimental arthritis by a calpain-inhibitory compound: regulation of cytokine production by E-64-d in vivo and in vitro. Int Immunol. 2005;17:1327–36. doi: 10.1093/intimm/dxh311. [DOI] [PubMed] [Google Scholar]
- 21.Tabata C, Tabata R, Hirayama N, et al. All-trans-retinoic acid inhibits tumor growth of malignant pleural mesothelioma in mice. Eur Respir J. 2009;34:1159–67. doi: 10.1183/09031936.00195708. [DOI] [PubMed] [Google Scholar]
- 22.Fries KM, Felch ME, Phipps RP. Interleukin-6 is an autocrine growth factor for murine lung fibroblast subsets. Am J Respir Cell Mol Biol. 1994;11:552–60. doi: 10.1165/ajrcmb.11.5.7946384. [DOI] [PubMed] [Google Scholar]
- 23.Brach MA, Gruss HJ, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. J Biol Chem. 1993;268:8466–72. [PubMed] [Google Scholar]
- 24.Shibanuma M, Kuroki T, Nose K. Inhibition by N-acetyl-L-cysteine of interleukin-6 mRNA induction and activation of NF kappa B by tumor necrosis factor alpha in a mouse fibroblastic cell line, Balb/3T3. FEBS Lett. 1994;353:62–6. doi: 10.1016/0014-5793(94)01014-5. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Ullenbruch MR, Jin H, Gharaee-Kermani M, Phan SH. An essential role for CCAAT/enhancer binding protein beta in bleomycin-induced pulmonary fibrosis. J Pathol. 2007;211:455–62. doi: 10.1002/path.2119. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Yang H, Cao P, et al. Degradation of C/EBPbeta in cultured myotubes is calpain-dependent. J Cell Physiol. 2006;208:386–98. doi: 10.1002/jcp.20684. [DOI] [PubMed] [Google Scholar]
- 27.Patel YM, Lane MD. Role of calpain in adipocyte differentiation. Proc Natl Acad Sci USA. 1999;96:1279–84. doi: 10.1073/pnas.96.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 30.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Malak NA, Mofarrahi M, Mayaki D, Khachigian LM, Hussain SN. Early growth response-1 regulates angiopoietin-1-induced endothelial cell proliferation, migration, and differentiation. Arterioscler Thromb Vasc Biol. 2009;29:209–16. doi: 10.1161/ATVBAHA.108.181073. [DOI] [PubMed] [Google Scholar]
- 32.Turner-Warwick M. Precapillary systemic–pulmonary anastomoses. Thorax. 1963;18:225–37. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peao MN, Aguas AP, de Sa CM, Grande NR. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec. 1994;238:57–67. doi: 10.1002/ar.1092380108. [DOI] [PubMed] [Google Scholar]
- 34.Gazdhar A, Fachinger P, van Leer C, et al. Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:529–36. doi: 10.1152/ajplung.00082.2006. [DOI] [PubMed] [Google Scholar]


