Abstract
Activated ERK signaling mediated plasticity-related gene transcription has been proposed for one possible mechanism by which 17β-estradiol (E2) enhances synaptic plasticity and memory. Because activated ERK also enhances plasticity-related mRNA translation in the dendrites of neurons, we sought to determine the effects of E2 on activation of ERK, phosphorylation of translation initiation factors, and dendritic mRNA translation in hippocampal neurons. Acute E2 application resulted in a rapid, transient increase in phosphorylation of translation initiation factors, ribosomal protein (S6) and eIF4E binding protein1 (4EBP1), in an activated ERK-dependent manner. Since phosphorylation of these translation factors enhance mRNA translation, we tested E2's effects on dendritic mRNA translation. Using a green fluorescent protein (GFP)-based dendritic mRNA translation reporter (reporter plasmid construct consisted of a GFP gene fused to the 3′UTR from CAMKIIα, which contains dendritic resident mRNA targeting and mRNA translational regulatory elements) we showed that E2 treatment resulted in increased somatic and dendritic GFP mRNA translation in GFP- reporter transfected hippocampal neurons. Translation inhibitor anisomycin and ERK inhibitor U0126 blocked E2 effects. Taken together, our results provide a novel mechanism by which E2 may trigger local protein synthesis of α-CaMKII in the dendrites, which is necessary for modulation of synaptic plasticity.
Introduction
The steroid hormone 17β-estradiol (E2) enhances the magnitude of hippocampal synaptic transmission and synaptic plasticity, which includes both long-term potentiation (LTP) and long-term depression (LTD). Further, E2-induced enhancement of synaptic plasticity correlates well with the improvement of cognitive tasks performed by rodents and primates. (Spencer,et al., 2008; Sinopoli et al., 2006; Day & Good, 2005). Various mechanisms have been identified through which E2 may modulate synaptic plasticity. Specifically, it has been reported that E2 increases the expression of the NMDA receptor (NMDAR) subunit NR2B (Adams et al., 2004) and enhances the magnitude of NMDAR-dependent LTP (Smith CC et al., 2006). Induction and persistence of LTP and LTD require new protein synthesis. Studies of the regulation of protein synthesis in the context of memory formation and LTP have focused mainly on transcription, especially the transcription factor CREB (Silva et al., 1998). Since it has been demonstrated that E2 increases activation of CREB in hippocampal neurons (Lee, SJ et al., 2004), it was proposed that persistence of plasticity originates through the E2-induced transcription of synaptic plasticity-related genes (Spencer et al., 2008).
Recent genetic, physiological, pharmacological, and biochemical studies provide strong evidence that translational control plays a crucial role in modulating synaptic plasticity and memory via the regulation of translation of dendritic resident mRNAs (Costa-Mattioli M et al., 2009). The evidence that rapid trafficking of mRNA to the dendrites and dendritic resident mRNA translation is necessary in synaptic plasticity and memory comes from the α-CaMKII gene. In particular, it has been reported that genetic disruption of the dendritic mRNA localization signals of α-CaMKII in the hippocampus of mice impairs stabilization of LTP and memory consolidation (Millers et. al., 2002). Also, it has been reported that induction of LTP and LTD trigger activation of mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoionositide3-kinase-mammalian target of rapamycin (PI3K-mTOR) pathways which enhance phosphorylation of translation initiation factors (Banko JL, et al., 2006; Kelleher RJ et al., 2004a). The same authors have also demonstrated that the increased phosphorylation of translation initiation factors is required for translation dependent LTP, LTD, and learning (Kelleher et al, 2004b; Banko et al., 2007). The possibility that E2-induced signaling regulates translation and may play a role in synaptic plasticity has yet to be explored.
It is now well established that E2, by interacting with surface membrane, rapidly modulates various signaling pathways including cAMP dependent protein kinase A (PKA), ERK, and protein kinase C (PKC) (Zhou et al., 1996; Singh et al., 1996; Alzamora et al., 2007). Synaptic activation of some of the same kinases are crucial for maintainence of LTP as well as learning and memory (Malenka and Bear, 2004). Recently, we have shown that E2 directly potentiates L-type voltage gated calcium channels (L-type VGCC) in hippocampal neurons (Sarkar, et al., 2008). However in neostriatum, which consists of caudate and putamen, E2 reduces L-type calcium currents (Mermelstein, et al., 1996). E2-induced calcium influx via L-type VGCC activates the ERK signaling cascade (Wu, et al., 2005). Since an E2 surge during the estrous cycle causes polyribosomes to accumulate in the dendrites (McCarthy et al., 2003) and translation factors and plasticity-related mRNAs are present in dendrites and dendritic spines (Steward and Schuman, 2001; Sutton and Schuman, 2006), we reasoned that by activating the ERK pathway, E2 may mechanistically link phosphorylation of translation factors to synaptic plasticity related dendritic resident mRNA translation.
Here we demonstrated that E2-induced signaling resulted in enhanced phosphorylation of ribosomal protein (S6) and eIF4E binding protein 1 (4EBP1), in an ERK-dependent manner. Using a green fluorescent protein (GFP) translational reporter, we demonstrated that E2 induces ERK-dependent somatic and dendritic synthesis of GFP. Taken together, our results suggest a mechanism by which E2-induced ERK signaling may trigger local protein synthesis of αCaMKII in the dendrites that are necessary for persistent forms of LTP.
Materials & Methods
Primary Neuronal Cultures
At embryonic day 17–18, rats (Sprague-Dawley Charles Rivers, USA) were anesthetized and cervically dislocated. The brains of pups were removed and placed into Mg2+ free HBSS. Hippocampi and cortical regions were removed under a dissecting microscope, washed, and placed into Neurobasal culture media (without phenol red) supplemented with B27 and Pen-Strep (Invitrogen Carlsbad, CA, USA). The hippocampi were triturated by using a graded series of fine polished Pasteur pipettes, and subsequently filtered through a 40μm nylon cell strainer (Becton Dickinson Labware). The neurons were plated on poly-L-lysine-coated glass cover slips and cultured in vitro in 95% humidity and 5% CO2 atmosphere. At day 2, cells were treated with 5μM arabinofuranosylcytosine (AraC) to inhibit glial cell growth. At 12–13 days in vitro, the age of cultures used in these studies, 99% of the cell population was neurons as determined by MAP2 and GFAP immunofluoresence staining and confocal microscopy.
Transfection
Neurons were plated at a density of 200–400 cells/mm2 on glass coverslips (coated with poly-L-lysine). Cells were cultured in 6-well plates with neurobasal medium supplemented with B-27 and 0.5 mM glutamax (Invitrogen Carlsbad, CA, USA) for 12 days before use. Neurons were transfected with pcDNA3.1-5′untranslated region (UTR) of CaMKII-α-dGFP-3′UTR of CaMKII plasmids (1 μg/well), using Lipofectamine 2000 (Invitrogen,Carlsbad, CA, USA) according to the manufacturer's instructions.
Confocal image acquisition and analysis
Forty eight hours after transfection, neurons on coverslips were placed inside the chamber of the confocal microscope and maintained at 36.5°C and 5% CO2 throughout the image acquisition period. Healthy pyramidal neurons that expressed GFP were chosen for the experiment. Confocal images were acquired in 0.5 μm sections; image analysis was conducted on z-compressed image stacks that contained the entire neuron of interest. GFP was excited at 488 nm and emitted light was collected between 510–550 nm. Images were acquired with parameters that maximized the dynamic range of pixel intensity for the dendritic signal. Using these parameters, the cell body fluorescence intensity was saturated. In all experiments, identical acquisition parameters and settings were used for both control and reagent treated dendrites on a given experimental day. For the photobleaching experiment, a segment of dendrites was photobleached by exciting GFP at 494 nm for 10–60 s until GFP signals were barely seen. Appropriate experimental reagents were added to the medium immediately after photobleaching. Time lapse images were acquired using Zeiss LSM 510 confocal microscope. The same acquisition parameters and settings were applied to controls and experimental groups. Fluorescence intensity in the photobleached distal dendrites was quantified using Image J (National Institutes of Health). Changes in fluorescence intensity in dendrites over time were determined by [DELTA] F= (Ft−F0)/F0 (Ft is the mean fluorescent intensity of photobleached dendrites at time t after treatment, and F0 is the mean fluorescence intensity of the dendritic segment immediately after photobleaching). Statistical significance of differences between groups was determined by analysis of variance (Graphpad Prism).
Immunoblotting
Cells were lysed using cold radioimmunoprecipitaion assay (RIPA) buffer containing protease and phosphatase inhibitors (1% NP40 containing 150 mM NaCl, 2 mM EDTA, 1% deoxycholate, 200 μg/mL aprotinin, 100 μg/mL pepstatin, 50 μg/mL leupeptin, 10 mM benzamidine, 20 mM NaF, and 10 mM Na-ortho vanadate). Before electrophoresis, protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples (30 μg total protein) were resolved using SDS-polyacrylamide gel electrophoresis, and transferred onto Immobilon-P membranes. Rabbit polyclonal antisera against phosphorylated ERK1/2 (Thr202/Tyr204), S6 (S235/S236), and 4EBP1 (S65) [Cell Signaling, USA], were used for detection. Blots were stripped and reprobed with antisera directed against total ERK1/2, S6, and 4EBP1 (Cell Signaling, USA). Results were quantified using Image J (National Institute of Health), calculated as the ratio of phosphorylated species to total, and then normalized to the untreated control condition.
Results
Estrogen Induces ERK-Dependent Phosphorylation of Translation Initiation Factors
Estrogens exert multiple effects on central nervous system neurons. E2 enhances synaptic transmission through both NMDA and AMPA receptors (Smith et al., 2006), and modulates LTP and LTD (Day et al., 2005). E2 activates kinases such as ERK, and protein kinase B (Akt), and also increases dendritic spine size and synapse density (Spencer, et al., 2008). These observations indicate that E2 potentially acts as a modulator of synaptic plasticity. Modulation of synaptic plasticity requires new protein synthesis.
Recent work has implicated local dendritic protein synthesis in LTP and LTD. LTP- and LTD-inducing stimuli enhance translation of a diverse set of dendritic resident mRNAs such as arc, α-CaMKII, AMPAR (GluR1 and GluR2 subunits), and eukaryotic translation initiation factor 1A (eIF1A) ((Tang, et al., 2002; Smith. et al., 2005; Kelleher, et al., 2004a; Sutton, et al., 2006). Mechanistically, the increase in mRNA translation is characterized by ERK- and/or mTOR-dependent increases in phosphorylation of eukaryotic translation initiation and elongation factors. We reasoned that E2-induced ERK signaling enhances phosphorylation of translation initiation factors via activation of ERK, and therefore E2-induced ERK-activation of translation initiation factors may be mechanistically linked to dendritic mRNA translation.
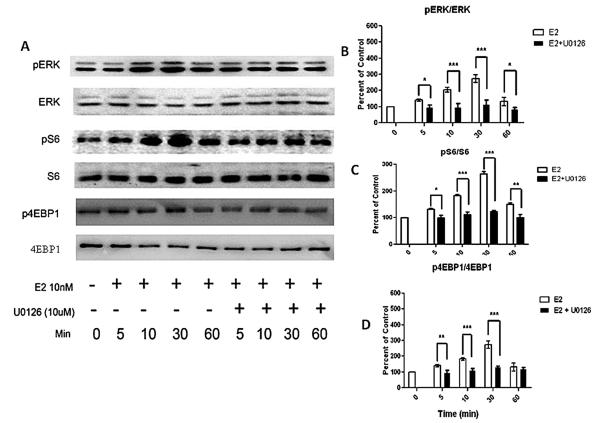
We tested this hypothesis by first determining the correlation between E2-induced phosphorylation of ERK with the phosphorylation states of translation initiation factors 4EBP1 and S6, in primary rat hippocampal neuronal cultures. As shown in Figure 1A, E2 (10 nM) induced phosphorylation of ERK within 5 min (140±7.9% compared to control 100%, n=3, p<0.05). Maximum phosphorylation of ERK was observed at 30 min (274±24% compared to control 100%, n=3, p<0.001) but at 60 min phosphorylation decreased to near basal level (131±25% compared to control 100%, n=3). E2-induced ERK phosphorylation was partially abolished by an inhibitor of ERK, U0126 (Fig.1B). Parallel with ERK phosphorylation, E2 treatment in hippocampal neurons temporally increased phosphorylation at specific sites of S6 and 4E-BP1 proteins. Like ERK phosphorylation, the increased phosphorylation of S6 and 4EBP1 was highest at 30 min (phospho-S6, 263±10% compared to control 100%, n=3, p<0.05), and phospho-4EBP1 (274±13% compared to control 100%, n=3, p<0.05). E2-induced phosphorylation of both S6 and 4EBP1 was inhibited by U0126. In our experiment we used 10μM of U0126. This concentration of U0126 is 100-fold greater than the IC50 for Mek inhibition. In our experiments we used U0126 in presence of E2. Therefore, activation of other kinase(s) by E2 could account for U0126's inability to reduce Erk phosphorylation below the level of control.
Fig 1. E2-induced ERK signaling temporally regulates the phosphorylation state of translation initiation factors.
(A) Treatment of hippocampal neurons with E2 enhances phosphorylation of ERK, S6, and 4EBP1 in an ERK-dependent manner. (B-D) Quantification of normalized levels of phosphorylated ERK, S6, and 4EBP1 in hippocampal neurons after E2 and the MEK inhibitor (U0126) treatment. Statistically significant differences were determined by 2 way ANOVA, followed by Bonferroni post-tests and are denoted as follows: * p<0.05, ** p<0.01, *** p>0.001 for n= 3 independent experiments.
E2 Induces Dendritic mRNA Translation
Our observation of E2-induced S6 phosphorylation, and the recent report that an E2 surge during the estrous cycle causes polyribosomes to accumulate in the dendrites of the hippocampus potentially indicate that E2 may induce dendritic mRNA translation (McCarthy and Miller, 2003). The mRNA for α-CaMKII is one of the most prominent dendritic mRNAs identified to date, and rapid synthesis of α-CaMKII has been observed during synaptic plasticity. We examined dendritic protein synthesis in living neurons by using a green fluorescent protein (GFP) reporter flanked by 5′ and 3′ untranslated regions (UTR) from α-CaMKII mRNA. The UTRs of α-CaMKII mRNA contain information sufficient for its dendritic localization (Mori, et al., 2000; Aakalu, et al., 2001). Also, it has been reported that E2, by activating PI3K-Akt-mTOR kinase, phosphorylates translation initiation repressor 4EBP1 and increases post synaptic density-95 (PSD-95) protein synthesis without increasing mRNA levels (Li, et al., 2004). These results raised the possibility that E2 may induce dendritic resident mRNA translation. We examined this possibility by using a translation reporter in which the coding sequence of GFP is fused with 5′ and 3′ untranslated regions from α-CaMKII, providing both dendritic mRNA localization and translational regulation (Mori, et al., 2000; Aakalu, et al., 2001).
In order to determine the effects of E2 on localization and levels of GFP-reporter expression hippocampal neurons were transfected with translation reporter plasmid DNA, and forty eight hrs after transfection, time lapse confocal images were acquired. As shown in Figure 2A, E2 induced spatial-temporal increments of GFP synthesis in the soma as well as in the dendrites. Figure 2B shows a time-lapse montage of a straightened dendrite taken from Figure 2A. It is evident that GFP fluorescence intensity increased in the distal area as time lapsed (also see supplemental video). In the 3-D plot of fluorescence from the montage, a spatial-temporal increase in dendritic reporter expression was evident as a result of E2 treatment (Figure 2C). We next used a myristoylated GFP translation reporter that limits the diffusion of somatically synthesized GFP to the dendrites. 48 hrs after transfection the myristoylated GFP construct (Aakalu, et al., 2001), healthy live neurons were subjected to time-lapse imaging. First the dendritic processes with GFP expression were photobleached, then recovery of GFP in the respective photobleached dendrites was recorded by time-lapse imaging. Compared to sham treatment (Figure 2D), E2-stimulated photobleached GFP recovery was significantly enhanced as time lapsed (Figure 2E). E2-enhanced GFP recovery in the dendrites was blocked by the application of a translation inhibitor, anisomycin (10μM), but not completely in the soma (Figure 2F), implying that new GFP synthesis had occurred in the distal part of the dendrites. Anisomycin inhibits protein synthesis by reversibly binding with peptidyl transferase or 80S ribosomal complex, and over time this reversibility becomes prominent especially at suboptimal doses (10μM) as used in our experiment and, this may result in incomplete inhibition of protein synthesis as evidenced in the presence of E2 with anisomycin (Figure 2F).
Fig 2. E2 temporally and spatially increases translation of a GFP reporter.
E2 treated neurons showed increased GFP fluorescence in the soma as well as in the dendrites (Fig. 2A, Supplemental VideoS1, and snap shots, Supplemental Fig. 1). A time lapse montage of the dendrite (marked B in the neuron, Fig. 2A) shows increased GFP translation as time lapsed from 0-75 min after E2 treatment. (2B) An XYZ plot showing fluorescence intensity profile of the time lapse montage of the dendrite. Left, fluorescence intensity peak represents the somatic GFP, and left to right represents GFP fluorescence along the length of the dendrite. (2C) A time lapse montage of GFP signals in photobleached dendrites after treatment with sham (2D), E2 (2E), E2 in presence of anisomycin (2F), and E2 in presence of U0126 (2G). Drugs were applied immediately after photobleaching. It is evident that E2 treatment triggers GFP recovery but not in U0126 or anisomycin treated dendrites, although inhibition by anisomycin was not complete in the soma. Quantification of GFP reporter signals in photobleached dendrites (2H). Depicted are mean ± SEM. 2 Way ANOVA revealed significant differences among the groups.
The fluorescence intensity of dendrites shown in Figure 2 D, E, F and G were quantified at 60 minutes as distance from the soma for the single dendrites as shown (2I)
To determine the role of E2-induced ERK signaling in dendritic protein translation, we used U0126. Figure 2G shows that U0126 (10μM) treatment abolished E2-induced GFP synthesis in the dendrites of hippocampal neurons. Quantification of GFP signals in photobleached dendrites revealed statistical significances among the groups shown in Figure 2H. Further, the analysis of reporter GFP protein synthesis in the dendrites (those > 50μm from the soma) demonstrated that the fluorescent signal is mainly derived from local translation (Figure 2I). Taken together, these results demonstrate that E2 activation of the ERK signaling pathway induces phosphorylation of translation initiation factors which may mechanistically link estrogen-induced increased mRNA translation and LTP in hippocampal neurons.
Discussion
Protein synthesis is required for persistent forms of synaptic plasticity and memory formation. Key regulators of LTP-related protein synthesis are ERK and mTOR, which are believed to modulate the translational capacity by facilitating the synthesis of particular components of the protein synthesis machinery (Meyuhas et al., 2000).
It has been shown that E2 exerts multiple effects on central nervous system neurons, including potentiation of LTP and LTD. In both animals and humans, experimental results have shown that estrogens improve cognitive function. E2-induced modulation of synaptic plasticity and memory formation require new protein synthesis, but little is known about the underlying regulatory mechanisms. We investigated the role of E2-induced ERK signaling in these processes.
In our current study, we demonstrated that E2-induced ERK activation was required for inducible phosphorylation of multiple factors that play a critical role in the process of translation initiation. In neurons, the ERK signaling pathway is activated by stimuli associated with synaptic activity and plasticity. ERK-dependent specific phosphorylation of ribosomal protein S6 and 4EBP1 has been mechanistically linked to increases in translational efficiency in response to neuronal activity (Kelleher, et al., 2004b). Our observation is that these phosphorylation events are coordinately regulated with the E2-induced signaling mechanism. Our results indicate that E2-induced ERK activation induces phosphorylation of specific residues in the ribosomal protein S6 (serine 235/236), and the inhibitor of cap binding factors eIF4E and 4EBP1 (serine 65). This E2-induced phosphorylation was significantly inhibited by U0126 (MEK inhibitor). Thus, E2-induced phosphorylation of translation initiation factors S6 and 4EBP1 maybe mechanistically linked to increased translation efficiency in E2-treated neurons.
ERK and mTOR-dependent synthesis of translational machinery is observed in many cell types as an early response to stimuli that induce cell growth, and this process depends on transcriptional and translational control over the expression of very specific classes of mRNAs (Meyuhas, 2000). Because synaptic plasticity and memory require new protein synthesis, the inducible translation of mRNAs in dendrites has been proposed as a control point in neuronal plasticity. It has been demonstrated that various mRNAs that encode proteins involved in synaptic plasticity (including: α-CAMKII, MAP2, arc/ARG3.1, and the GluR1 subunit of AMPA receptors), are locally translated after neuronal activation (Huber et al., 2000). Importantly, dendritic synthesis of α-CaMKII protein is essential for LTP and various forms of memory (Miller, et al., 2002). We used the α-CAMKII GFP reporter (Aakalu, et al., 2000) to investigate the role of E2 in induction of protein synthesis in dendrites of cultured neurons. The E2-induced ERK signaling pathway is restricted to α-CaMKII specific, cis-acting regulatory elements present in the translation reporter construct used in this study. Others have shown that the translation enhancing activity of the ERK pathway applies to almost all neuronal mRNA involved in long-term synaptic plasticity (Kelleher, et al., 2004b). Thus, our results provide a possible mechanism by which E2-induced ERK signaling triggers translation of a broad range of neuronal mRNAs, which are required for long-term synaptic plasticity and memory formation.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Jansen W, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Alzamora R, Brown LR, Harvey BJ. Direct binding and activation of protein kinase C isoforms by aldosterone and 17â-estradiol. Mol Endocrinol. 2007;21:2637–2650. doi: 10.1210/me.2006-0559. [DOI] [PubMed] [Google Scholar]
- Banko JL, Marhav M, Stern E, Sonenberg N, Rosenblum K, Klann E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. J Neurosci. 2006;26:2167–2173. doi: 10.1016/j.nlm.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hon L, Paulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. Neurobiol Learn Mem. 2007;87:248–256. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klan E, Sonenberg S. Translational control of long-lasting Synaptic Plasticity and Memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Good M. Ovariectomy-induced disruption of long-term synaptic depression in the hippocampal CA1 region in vivo is attenuated with chronic estrogen replacement. Neurobiol Learn and Memory. 2005;83:13–21. doi: 10.1016/j.nlm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Gong R, Tang SJ. Mitogen-activated protein kinase signaling is essential for activity-dependent dendritic protein synthesis. Neuroreport. 2006;17:1575–1578. doi: 10.1097/01.wnr.0000234742.42818.ff. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004a;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004b;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neurosci. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Milner TA. Dendritic ribosomes suggest local protein synthesis during estrous synaptogenesis. Neuroreport. 2003;14:1357–1360. doi: 10.1097/01.wnr.0000078380.40088.99. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Sumeier J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3′ untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea LA. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. Creb and Memory Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1178–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic Plasticity, and memory. Neuron. 2002;36:507–519. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Waters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.