To the Editor
Several epidemiologic studies have found an association between the presence of an apolipoprotein E (APOE) ε4 allele and increased risk for Alzheimer disease (AD). Although 2 brain imaging studies of middle-aged and elderly individuals have found that the ε4 allele is associated with an AD-like pattern on positron emission tomogrophy (PET) brain scans,1,2 we are not aware of any study has that has examined this association in young individuals.
Methods
We recruited 18 college-age individuals who were without significant medical problems, as determined by medical, neurologic, psychiatric, and neuropsychological evaluations, as well as by magnetic resonance imaging of the brain. After obtaining informed consent, we determined the ε4 allele status of each individual. After providing informed consent, all participants waived their right to be informed of these results. Participants then underwent resting H215O PET scans of the brain to determine resting relative cerebral blood flow (rCBF). The Statistical Parametric Mapping 1999 program was used to implement standard steps for the PET images of each participant. PET counts, proportionally scaled by the global mean, were used as a surrogate of resting rCBF. Voxel-wise t statistics for between-group comparisons were computed, with P values for multiple comparisons adjusted by the Bonferroni correction.
Results
Three of the 18 participants were found to be carriers of the ε4 allele, while the other 15 did not have the allele. Carriers were of similar age than noncarriers (26 vs 23 years, P=.07), education (17.3 vs 16.5, P=.56) and sex (P=.13). Neuropsychological performance did not differ between the groups for the Mini-Mental State Examination (29.2 vs 28.9, P=.46), Nelson Adult Reading Test intelligence quotient (121.3 vs 119.9, P=.60), Wechsler Adult Intelligence Scale-Revised vocabulary subtest (12.0 vs 12.8, P=.51), digit symbol subtest (11.7 vs 12.7, P=.62), and Selective Reminding Test delayed recall score (9.7 vs 10.5, P=.35).
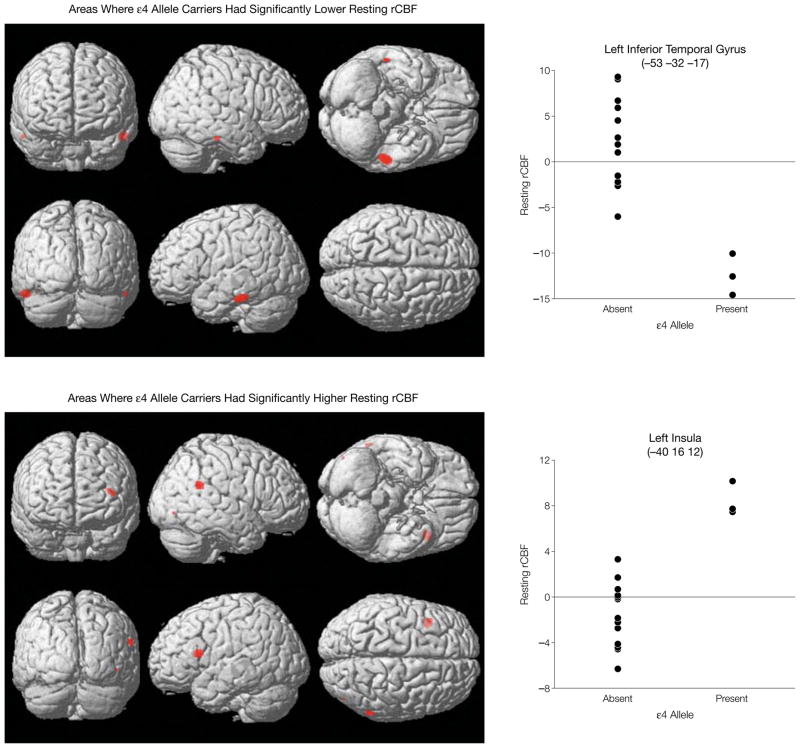
Compared with individuals without the ε4 allele, ε4 carriers exhibited significantly lower resting rCBF in the left and right inferior temporal gyri, while resting rCBF was higher in the left insula, right supramarginal gyrus, and the inferior occipital gyrus (Figure). The results were unchanged after controlling for age.
Figure.
Resting Relative Cerebral Blood Flow (rCBF) in Individuals With and Without an Apolipoprotein ε4 Allele
Three-dimensional brain-rendering representation of statistical parametric mapping. Areas of red depict areas in which mean resting rCBF is significantly (P<.05, Bonferroni corrected) different between individuals with and without an ε4 allele. The graphs plot the rCBF values for the 2 groups in regions in which differences were most significant—the left inferior temporal gyrus and the left insula. The values in parentheses in the graph panel labels are Talairach coordinates denoting the exact brain locations of the statistically significant areas.
Comment
Reiman et al2 found that the left and right inferior temporal regions were among the 7 regions with the greatest reductions in cerebral glucose metabolism among adults with the ε4 allele. These bilateral inferior temporal regions that they noted in the older individuals overlap with the areas of reduced rCBF in our younger individuals. The significant associations in our data indicate that despite the small number of individuals in the ε4 group (which is consistent with the proportions of the APOE polymorphisms in the population) the effects were strong enough to be demonstrable.
The ε4 allele has been implicated in impaired brain repair mechanisms3 that place individuals at risk for either AD or other brain diseases (eg, worse cognitive sequelae after traumatic brain injury). The observed rCBF pattern of ε4 carriers (which were cognitively indistinguishable from the non–ε4 carriers) may be the early signature of an APOE-dependent alteration in brain function. This might result in greater vulnerability to genetic or environmental effects later in life. Therefore it is conceivable that some of these young participants carrying the ε4 allele may be more susceptible to AD, traumatic brain injury, or other brain insult.
Alternatively, it is conceivable that brain regions where significant hypoperfusion was detected already may have been affected by AD pathology. Abnormally high levels of Aβ in the brain have been reported among carriers of the ε4 allele as young as 40 years,4 and neurofibrillary tangles have been found in carriers as young as 22 years.5 The more spatially extended PET deficits that have been noted in elderly carriers of the ε4 allele1,2 may suggest a progressive pattern.
It also is possible that methodological differences between the studies may account for the somewhat inconsistent pattern of brain findings between our study and previous imaging studies of older ε4 carriers. These might include different imaging modalities, different methods of analysis (region of interest vs voxel-wise), differences in sample size, and differences other than age among the studied populations (eg, demographic factors, neuropsychological performance, recruitment methods). In addition to hypoperfusion in some areas, we found that the presence of an ε4 allele was associated with increased rCBF in other areas. Although we studied rCBF at rest, this hyperperfusion may be compensatory, similar to the hyperperfusion noted in a functional study of healthy middle-aged and elderly carriers of the ε4 allele during performance of a memory task.6
Contributor Information
Nikolaos Scarmeas, Department of Neurology.
Christian G. Habeck, Cognitive Neuroscience Division, Taub Institute for Research in Alzheimer’s Disease and the Aging Brain, College of Physicians and Surgeons of Columbia University New York, NY.
Yaakov Stern, Cognitive Neuroscience Division, Taub Institute for Research in Alzheimer’s Disease and the Aging Brain, College of Physicians and Surgeons of Columbia University New York, NY.
Karen E. Anderson, Department of Psychiatry, University of Maryland, Baltimore.
References
- 1.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 2.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 3.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morishima-Kawashima M, Oshima N, Ogata H, et al. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp Neurol. 1998;153:152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- 6.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]