Abstract
Background
tDCS appears to have modulatory effects on the excitability of cortical brain tissue. Though tDCS as presently applied causes no apparent harm to brain structure or function, a number of uncomfortable sensations can occur beneath the electrodes during stimulation, including tingling, pain, itching, and burning sensations. Therefore, we investigated the effect of topically applied Eutectic Mixture of Local Anesthetics (EMLA) on tDCS-related discomfort.
Methods
Nine healthy adults received both anodal and cathodal 2.0 mA tDCS for 5 minutes over the prefrontal cortex with the skin pretreated for 20 minutes with either EMLA or placebo cream. Participants rated procedural discomfort 6 times across 8 dimensions of sensation.
Results
On average, the mean sensation ratings for EMLA-associated tDCS stimulation were significantly lower than placebo-associated stimulation for every cutaneous sensation evaluated. Cathodal stimulation was associated with higher ratings of “sharpness” and intolerability than anodal stimulation.
Conclusions
Topical EMLA may reduce tDCS-related discomfort.
Keywords: transcranial direct current stimulation, tDCS, EMLA, pain reduction, anode, cathode
Introduction
During the past decade, multiple studies have demonstrated that transcranial application of weak direct current (tDCS) has modulatory effects on the excitability of cortical brain tissue [1–2]. The ease of producing this current—via the simple placement of two electrodes (anode and cathode) on a subject’s scalp—has prompted investigations into the potential clinical uses of transcranial direct current stimulation (tDCS). As these studies progress, the exact methods and safety parameters for applying tDCS are also adapting. Though tDCS as presently applied causes no apparent harm to brain structure or function [3], a number of uncomfortable sensations often occur beneath both electrodes at the onset and sometimes throughout the duration of stimulation [4–5]. While some subjects have complained of tDCS-induced headaches, nausea, and other discomfort [4], the large majority of reported irritations are skin-related, including sub-electrode tingling, pain, itching, and burning sensations [4–5].
Therefore, it is important to determine if a patient’s discomfort can be reduced. Recent research indicates that increasing ionic concentration within the NaCl solution used to soak the sponge-electrodes correlates with increasing discomfort for tDCS recipients [6]. However, another study suggests that while de-ionized solutions may reduce discomfort, they also increase impedance at the scalp and may account for the appearance of skin lesions [7]. Trading tissue damage for lessened procedural discomfort is not a safe or clinically viable option. Therefore, we investigated the effect of topically applied Eutectic Mixture of Local Anesthetics (EMLA) cream at specific locations of electrode placement without reducing NaCl solution concentration.
Materials and Methods
1. Participants
Nine healthy adults (7 men; Age (years): average = 28.56, range = 20–51) volunteered to participate in this study, which was approved by the MUSC institutional review board.
2. Direct Current Stimulation
A battery-powered constant current device (Phoresor II Auto, Model PM850) was used to deliver transcranial direct current stimulation to subjects. The device was capable of delivering a constant current at any level ranging from 0–4.0 mA and took roughly 30 seconds to “ramp up” to the experimental level of 2.0 mA. Current was directed from the machine into the anode (positive electrode), through the subject’s head, into the cathode (negative electrode), and back to the machine. Both 4×4 cm sponge-electrodes were soaked in 5 mL of standard isotonic 0.9% NaCl saline solution and were held in place on the subject’s scalp by a Velcro head band. The current density was estimated to be 0.125 mA/cm2, assuming that residual from either cream did not interfere with the electrical properties of the circuit.
3. EMLA and Placebo Cream
Two different creams were applied to specific locations on a subject’s forehead over locations corresponding with F3 and F4 from the EEG 10–20 system. EMLA (Eutectic Mixture of Local Anesthetics) was used as the topical anesthetic. EMLA is an emulsion preparation containing 2.5% lidocaine and 2.5% prilocaine by weight. Signa Gel, a standard EEG conductive gel, was used as a placebo cream. Both creams were applied and covered with an occlusive dressing for 20 minutes. The creams were then removed.
4. Face Locator Program
Participants rated eight specific sensations related to procedural discomfort (painfulness, tingling quality, sharpness, piercing quality, electrical quality, sense of tugging, pinching quality, and intolerability) via a custom-designed software program named “Face Locator” [8–9]. The program allows subjects to use a mouse to rate their perception of each sensation on separate visual analogue scales (VAS). These ratings are stored and translated into numerical values ranging from 0 to 100 by the computer.
5. Experimental Procedure
2.5 g EMLA and 2.5 g placebo gel were placed on separate, randomly selected sides of the subject’s forehead (F3 and F4), each in an area of 4×4 cm2. Occlusive dressings were placed over these sites, and subjects were kept blind to the specific location of each cream. After 20 minutes, the occlusive dressings were removed, and the creams were wiped off. Then the electrode to be tested (either anode or cathode) was randomly placed over one of the two former cream sites. The untested electrode was placed over the ipsilateral motor cortex area. Once the subject was placed in a comfortable chair with easy access to the Face Locator program, we turned on the stimulation device and “ramped up” the current to a level of 2.0 mA. The experiment’s 5-minute duration officially began at the conclusion of the 30-second “ramping period.” At this zero time point and at the end of each subsequent minute, participants used Face Locator to rate their perception of the eight sensations specifically under the forehead electrode (6 total ratings). Subjects were asked to ignore and not rate any feelings underneath the posterior electrode. At the end of the 5 minute period, the direct current was manually ramped down, and the electrodes were removed. The electrodes were then moved to their respective locations on the opposite side of the head, with the tested electrode over the opposite cream site and the untested electrode over the opposite motor cortex. The stimulation and sensation-recording process was then repeated. Two days to two weeks later, the subjects returned to be tested again with the roles of the previously tested and untested electrodes reversed (i.e. the anode and cathode were switched).
Statistical Analysis
We examined the effects of EMLA and placebo cream on cutaneous sensations evoked by anodal and cathodal tDCS using hierarchical linear modeling (HLM). HLM has been shown to handle nested models with serially dependent data points and randomly distributed missing values appropriately [10–13]. Additionally, this approach is growing in popularity among clinical and educational researchers due to its flexibility and appropriateness for data typically encountered in clinical and social science research [14–16]. For this study, 6 rating periods (one per minute) were conducted for each of the 8 sensory dimensions in question. Further, this study employed a 2 (EMLA versus Placebo) X 2 (anode frontal versus cathode frontal) design. Each participant’s time-series (VAS ratings over time during each of the 4 conditions) intercepts and slopes were entered into the model as subject-level random effects in the model. The covariance structure was set to “unstructured.” Critical alpha was Bonferroni adjusted to .00625 to correct for multiple comparisons.
III. Results
EMLA v. Placebo
EMLA-associated stimulation produced a statistically significant reduction in each of the self-rated sensations when compared to placebo-associated stimulation. See table 1 for mean ratings differences and statistical results.
Table 1.
Mean (standard error) Visual Analogue Scale (VAS) ratings across 8 sensory dimensions during tDCS with and without pretreatment with EMLA cream (Bonferroni-corrected critical alpha = .006 to correct for multiple comparisons).
| Sensation | EMLA | Placebo | Difference | Statistical Significance |
|---|---|---|---|---|
| Pain | 7.32(1.34) | 14.84(2.88) | −7.52 | (F(1,195)=31.64, p<.0001) |
| Tingling | 7.76(1.70) | 17.98(3.61) | −10.22 | (F(1,195)=36.07, p<.0001) |
| Sharp | 4.39(1.41) | 11.64(2.67) | −7.25 | (F(1,195)=26.34, p<.0001) |
| Piercing | 3.65(1.36) | 10.59(2.62) | −6.95 | (F(1,195)=26.01, p<.0001) |
| Electric | 7.09(1.44) | 16.66(3.36) | −9.58 | (F(1,195)=43.94, p<.0001) |
| Tugging | 2.33(0.99) | 6.08(2.20) | −3.75 | (F(1,195)=14.41, p=.0002) |
| Pinching | 4.78(0.96) | 8.44(3.68) | −3.67 | (F(1,195)=14.50, p=.0002) |
| Intolerability | 3.52(1.30) | 8.99(2.89) | −5.47 | (F(1,195)=17.68, p<.0001) |
Anode v. Cathode
There was a significant difference between anode and cathode on “intolerability” ratings F(1,197)=5.61, p=0.0188. Cathode was associated with an average intolerability rating that was 3.27 points higher than anode. There was also a significant difference between anode and cathode on “sharp” ratings F(1,198)=5.46, p=0.0205. Cathode was associated with an average sharp rating that was 3.57 points higher than anode. There was no significant difference between anode and cathode on pain, tingling, piercing, electric, tugging and pinching ratings, although cathode ratings were consistently higher mathematically than the anode ratings for each of these sensations.
IV. Discussion
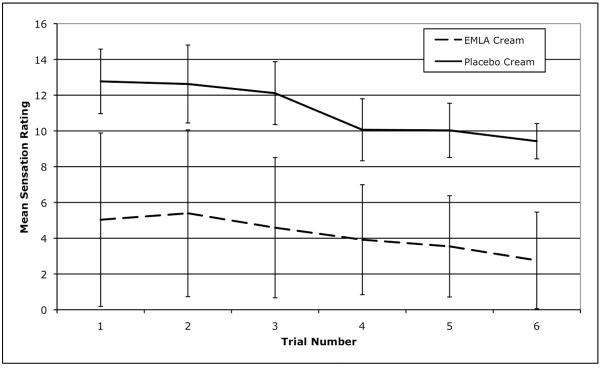
These results suggest that using topical EMLA prior to tDCS may reduce pain and unpleasantness during prefrontal stimulation. On average, the mean VAS ratings for EMLA-associated stimulation were significantly lower than placebo-associated stimulation for every category, ranging from 3.67 points lower in “pinching” to 10.22 points lower in “tingling.” However, it is important to consider the clinical practicality and experimental relevancy of EMLA-associated tDCS. Clinicians might suggest that the anesthetic benefits of EMLA may not outweigh its cost in increased procedural time as these unpleasant sensations are often minimal and quickly diminish in the majority of tDCS participants (our data confirms the mild levels of sensation, as well as a statistically insignificant decrease in the sensations over time; see Figure 1). However, if the clinically acceptable and recommended current density levels of tDCS are increased in the future, it is plausible that the associated cutaneous sensations might also increase accordingly. Should this situation occur, the anesthetic properties of EMLA cream would likely become more important in the clinical setting. EMLA may also currently have relevance for patients whose pain tolerance is lower than that of the general population.
Figure 1.
In regards to the experimental setting, EMLA’s effect on tDCS sensations may prove to have even more immediate importance. In our experience, during sham stimulation, many patients feel an initial sensation of electrical activity followed by an absence of this sensation. Some have then asked if the machine has been turned off, regardless of its actual “on” or “off” setting. Decreasing these sensations considerably via the use of EMLA cream may actually make true tDCS more indistinguishable from placebo, as the patient will sense less electrical activity in either case.
There are also important procedural considerations for the topical use of EMLA with tDCS. It is important to thoroughly wipe the cream from the skin prior to stimulation to avoid any negative effects from electrophoresis. We observed no negative side effects along these lines. One subject did experience a visual sensation of light flashes during the “ramp-in” phase of anodal stimulation of the right prefrontal cortex, and the stimulation was promptly stopped. The subject experienced no further side effects post-stimulation, and the sensation was likely related to electrode position rather than the EMLA or placebo creams. We believe that the angle of the direct current may have been such that it intersected and stimulated the optic nerve, and in later experiments we have found that by slightly adjusting the position of the posterior electrode, these flash sensations can be eliminated if they are initially present.
The differences between cathodal and anodal discomfort appeared rather minimal when applied over the forehead of healthy adult volunteers at this low intensity and duration. The significant differences between the electrodes that were observed in the “intolerability” and “sharp” categories are consistent with previous findings in which unidirectional electrical stimulation of the scalp yielded a significantly higher sensation of pain near the cathode than the anode [9]. We hypothesize that the mechanism behind this may be associated with a concentrated exiting of current from the scalp at the cathode. Nonetheless, it seems that any means of reducing anodal and cathodal discomfort can likely be the same. At most, cathodal stimulation may require slightly greater attention.
It is also unclear if these results obtained over the prefrontal cortex in healthy adults would generalize to tDCS applied over other scalp locations or in different patient populations. Finally, we do not know how long the anti-nociceptive effects of the EMLA cream might last, and whether additional dosing would be needed for longer tDCS sessions. Further studies are needed to address these questions.
In conclusion, our findings suggest that topical EMLA may reduce procedural pain and discomfort associated with tDCS without modifying NaCl solution concentration.
Acknowledgments
The authors would like to thank the following programs and organizations: Mr. McFadden was funded through a grant from the MUSC College of Graduate Studies Summer Health Professionals Research Program. Dr. George was funded through grants from the NIH (NIMH, NIDA, NI), the DOD and VA. Dr. Borckardt was funded through grants from the NIH (NINDS, NINR) and the Robert Wood Johnson Foundation.
Footnotes
Conflict of interest: The authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Priori A, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 2.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulation. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Poreisz C, et al. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72(4–6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118(5):1166–70. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Palm U, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain Stimulation. 2008;1(4):386–387. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Borckardt JJ, et al. Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. J ECT. 2006;22(4):259–64. doi: 10.1097/01.yct.0000244248.40662.9a. [DOI] [PubMed] [Google Scholar]
- 9.Borckardt JJ, et al. Focal electrically administered therapy: device parameter effects on stimulus perception in humans. J ECT. 2009;25(2):91–8. doi: 10.1097/YCT.0b013e318183c6a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101(1):147–158. [Google Scholar]
- 11.Bryk AS, Raudenbush SW. Advanced qualitative techniques in the social sciences. Vol. 1. Thousand Oaks, CA, US: Sage Publications, Inc; 1992. Hierarchical linear models: Applications and data analysis methods; p. xvi.p. 265. [Google Scholar]
- 12.Draper D. Inference and Hierarchical Modeling in the Social Sciences. Journal of Educational and Behavioral Statistics. 1995;20(2):115–147. [Google Scholar]
- 13.Hofmann DA, Gavin MB. Centering Decisions in Hierarchical Linear Models: Implications for Research in Organizations. Journal of Management. 1998;24(5):623–641. [Google Scholar]
- 14.de Ruijter JMP, Huffman ML. Gender composition effects in the Netherlands: a multilevel analysis of occupational wage inequality. Social Science Research. 2003;32(2):312–334. [Google Scholar]
- 15.Griffin MA. Interaction Between Individuals and Situations: Using HLM Procedures to Estimate Reciprocal Relationships. Journal of Management. 1997;23(6):759–773. [Google Scholar]
- 16.Guo S, Hussey D. Note on research methodology. Analyzing longitudinal rating data: a three-level hierarchical linear model. Social Work Research. 1999;23(4):258–268. [Google Scholar]