Abstract
The goal of this research is to utilize a transdisciplinary framework to guide the selection of putative moderators of the effectiveness of an intervention to promote physical activity behavior adoption and maintenance in the context of a randomized controlled intervention trial. Effective interventions to increase physical activity are sorely needed, and one barrier to the identification and development of such interventions is the lack of research targeted at understanding both the mechanisms of intervention efficacy and for whom particular interventions are effective. The purpose of this paper is to outline our transdisciplinary approach to understanding individual differences in the effectiveness of a previously successful exercise promotion intervention. We explain the rationale for and operationalization of our framework, characteristics of the study to which we apply the framework, and planned analyses. By embracing a transdisciplinary orientation for individual differences important in the prediction of physical activity (spanning molecular approaches, animal models, human laboratory models, and social psychological models), we hope to have a better understanding of characteristics of individuals that are important in the adoption and maintenance of physical activity.
Keywords: Physical Activity, Exercise, Transdisciplinary, Genetic, COSTRIDE, Affect, Motivation
It is well known that regular physical activity has enormous health benefits and that sedentary lifestyle leads to increased morbidity and mortality (USDHHS, 2008). Yet an astounding number of individuals remain sedentary (e.g., Pate et al., 1995; Penedo & Dahn, 2005). Recent reports show that less than half of U.S. adults self-report engaging in regular leisure-time physical activity (Macera et al., 2005) while a study using accelerometer-acquired data showed that less than 5% of adults met physical activity recommendation guidelines (Troiano et al., 2008). The development of successful physical activity promotion interventions is thus crucially important.
At the outset, we acknowledge that there are multiple levels that impinge on energy balance, broadly defined, from individual difference factors to factors existing outside of the person including real and perceived social support (Courneya, Plotnikoff, Hotz, & Birkett, 2000), cultural norms (King, Toobert et al., 2006; King, Marcus et al., 2006), the built environment (King, Marcus et al., 2006), the availability of and information about healthy food (Matson-Koffman, Brownstein, Neiner, & Greaney, 2005), and the safety of neighborhoods (King, Marcus et al., 2006; Foster & Giles-Corti, 2008) among many others. The examination of each of these levels of influence will be crucial in the ultimate prediction of physical activity as well as the development of interventions to increase physical activity, and a multidisciplinary approach will be key to examining each of these levels in concert. While transdisciplinary approaches to date often combine multiple levels of influence (e.g., Lytle, 2009; Satariano & McAuley, 2003), little research has focused on the interplay of disciplines within a level, and this is a unique contribution of the transdisciplinary framework we have proposed. To wit, we view our framework as a broad conceptualization of an approach to individual difference factors important for the adoption and maintenance of physical activity that crosses disciplines within the same level of influence.
There are exercise promotion interventions that successfully encourage initial adoption of physical activity, but these effects are not universal, and the maintenance of regular physical activity has proven difficult (Bock, Marcus, Pinto, & Forsyth, 2001; Marcus, Nigg, Riebe, & Forsyth, 2000; Rothman, Baldwin, & Hertel, 2004). Thus, a better understanding of individual characteristics that account for variability in both successful initial change from sedentary to active lifestyle and the maintenance of an active lifestyle are important areas for research. Such knowledge will facilitate the development of a variety of intervention modalities that are targeted for optimal success. Our perspective, consistent with many in the exercise promotion field (e.g., Marcus et al., 2006; Rosenfield, 1992) is that a full understanding of the within-person determinants of physical activity behavior change and maintenance requires a transdisciplinary approach. Put simply, changing and maintaining physical activity requires consideration of the complex interplay among psychological, behavioral, genetic, and physiological determinants and consequences of the behavior (Dishman et al., 2006; Marcus et al., 2006).
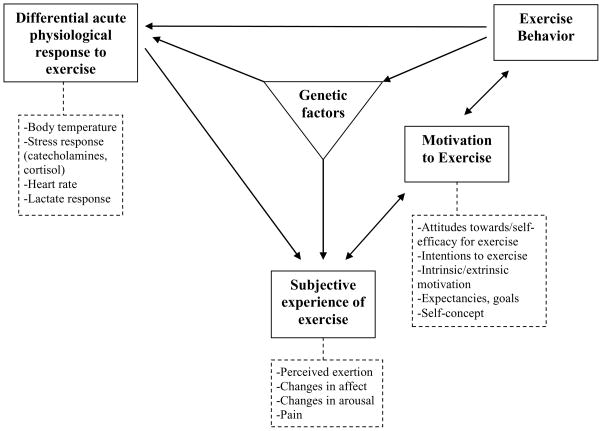
The framework that guides our research is a transdisciplinary conceptualization of potential relationships among genetic, physiological, and psychological factors and exercise behavior (Bryan, Hutchison, Seals, & Allen, 2007), an approach suggested in recent editorials regarding health psychology in general (e.g., Taylor, 2008) and reviews of the exercise literature in particular (e.g., King, Bauman, & Abrams, 2002; Marcus et al., 2006). We believe this framework is potentially applicable to both voluntary physical activity (e.g., purposeful activity meant to increase heart rate) as well as lifestyle activity (e.g., taking the stairs, parking farther from the door). We view our framework (see Figure 1) not as a hard and fast causal model, but as a comprehensive ordered clustering of domains that should be implicated in tests of individual difference variables that may explain for whom a particular intervention to increase physical activity is successful and who is able to maintain those successes. The purpose of this paper is to illustrate one potential utility of the framework by explaining how it was used to guide the selection of putative moderators of the efficacy of an intervention to increase physical activity.
Figure 1.
An expanded and revised transdisciplinary model of exercise behavior.
A Transdisciplinary Framework
According to the transdisciplinary framework of Bryan et al. (2007), genetic factors (e.g., genes that influence pain tolerance or cardiopulmonary systems) influence both differential physiological response to exercise (e.g., lactate concentration, temperature regulation) and the subjective experience of exercise (e.g., affective response to exercise, perceived exertion). The subjective experience of exercise is, in part, the psychological interpretation of physiological changes to the body that occur during exercise (e.g., perceiving pain due to lactate increase). In turn, the subjective experience of exercise influences motivation to exercise (e.g., those who experience exercise as pleasurable will be more highly motivated to do it again). This motivation is translated into future exercise behavior, and future exercise behavior recapitulates the model, leading to changes in and improvements in physiological responding.
This framework is meant to be flexible and dynamic with respect not only to the factors included in the model, but in the direction of association between those factors. In fact, as a result of recent findings in the literature and our own work, we have expanded and reconceptualized our framework in order to more accurately capture the intended dynamic structure. Our initial version focused on unidirectional influences; we have since added bidirectional arrows to indicate the often complex interactions among physiological, psychological, and genetic variables. For example, while many studies indicate that positive affective responses to exercise lead to greater motivation (e.g., Bryan et al., 2007; Kwan & Bryan, 2010a; Kwan & Bryan, 2010b) other studies show that this relationship can also accrue in the opposite direction, i.e., that one’s motivations regarding exercise can influence affective responses (e.g., Annesi, 2005). Our original version also conceptualized genes as the sole exogenous variable. Given recent developments in the field of epigenetics, we now allow for the possibility of effects of exercise behavior not only on physiological response, but on gene expression (e.g., epigenetic effects via DNA methylation; Booth & Neufer, 2005).
The advantage of this type of multifaceted approach is its novelty, the incorporation of multiple disciplines within the same level of influence, and the extent to which the focus is on the overarching “big picture” of individual differences impinging on a complex behavior (physical activity). Another strength of this approach is that there is no restriction on the particular operationalization of the constructs in this framework. For example, we have generally focused our own work on the motivation construct around the Theory of Planned Behavior from the social psychological literature (Ajzen, 1991). But there are any number of other conceptualizations that would also be subsumed under motivation, including quality of motivation (e.g., self-regulation ability, intrinsic versus extrinsic motivation), motivations specific to a behavioral outcome (e.g., the motivation to lose weight) or to achieve a particular goal (e.g., the motivation to finish a 10K). As an example, the Health Action Process Approach (HAPA; Schwarzer, 1999) emphasizes the role self-efficacy plays in regulatory processes involved in exercise behavior. According to this approach, the amount of effort invested in maintaining exercise is dependent upon self-efficacy: Individuals who believe they will be successful will be more likely to anticipate possible obstacles and plan how to overcome them whereas individuals who believe they will fail will give up in the face of hardship.
This definitional generality applies to each of the constructs in our framework, and as such it is useful as a foundation for stimulating and encouraging innovative research. The drawback, of course, is that such an approach cannot have the specificity or detail in any one domain (i.e., there are entire review articles on the role of lactate, or on one particular facet of affective response) of more narrow perspectives. Thus, we give a broad overview and justification for the domains we have focused on as potentially important in understanding the intra-individual determinants of response to an intervention to increase physical activity, with the caveat that the individual moderators listed under each domain are not meant to be exhaustive or inclusive. Finally, while this framework represents an hypothesized meditational chain and we have tested these relationships in other work (e.g., Bryan et al., 2007; Magnan et al., 2010), it is not this aspect we explore in the study presented here. The goal of this study is to use our framework as a basis for the selection of potential moderators of the effects of an intervention to increase physical activity. These are clearly two separate issues, for while each of the variables exist in our transdisciplinary (meditational) framework, it is possible that only a subset might exert a strong enough direct influence to serve as moderators of intervention efficacy.
Genetic Factors
Research on the human genome is expanding exponentially, and there have been calls for the addition of genetic data in studies of health behavior generally (e.g., Plomin, 1998; Taylor, 2008) and physical activity behavior in particular (Dubbert, 2002; Dishman et al., 2006; Marcus et al., 2006). We thus view genetic factors as the “core” of the transdisciplinary framework from which, to some degree, all other constructs emanate. Genetic factors appear to account for a considerable amount of the variance in daily exercise behavior (e.g., Bouchard & Perusse, 1994; Eriksson, Rasmussen, & Tynelius, 2006), and our position is that these genetic effects on behavior accrue not directly, but rather through their influence on physiological and subjective responses to exercise. As mentioned, there is emerging evidence that environmental and behavioral variables (e.g., exercise) can have a profound regulatory influence on the expression of the genes via DNA methylation and other epigenetic mechanisms (Booth & Neufer, 2005). Consistent with this work, the updated framework allows for the possibility that exercise behavior influences gene expression, as indicated by the path from exercise behavior to genetic factors.
Our pilot study (Bryan et al., 2007) focused on a single nucleotide polymorphism in the Brain Derived Neurotrophic Factor (BDNF) gene as a putative genetic factor involved in voluntary exercise. The BDNF gene was chosen due to evidence linking the neurotrophic factor it codes for to exercise in animal models (e.g., Donovan et al., 2000; Adlard, Perreau, & Cotman, 2005; Johnson & Mitchell, 2003; Olson, Eadie, Ernst, & Christie, 2006) and we showed some support for the importance of this genetic factor and its association with physiological and affective responses to exercise. As the BDNF gene is just one of many genetic factors that influences exercise behavior, additional exploration is needed to expand our knowledge of genetic impacts on physical activity. In addition, the field of genomics is quickly moving beyond testing one or even a few loci at a time, and we are now conducting genome wide analyses with up to 1 million genotypes per subject and examining the data through multivariate approaches designed to capture groups of genetic markers that may be important at various levels of this transdisciplinary framework (e.g., groups of single nucleotide polymorphisms associated with temperature regulation or affective change in response to exercise). The goal for the future is thus to find panels of markers that are associated with a narrow phenotype of interest (e.g., affective response to exercise) as any one particular gene is unlikely to account for substantial variance on its own (c.f., Hutchison, in press).
Physiological Factors
Our framework suggests that variations in genetic composition and in prior exercise history will influence the acute physiological effects of exercise on the body. Body temperature, stress regulation (e.g., cortisol release), heart rate, and lactate production are examples of mechanisms that might in turn influence acute subjective responses to exercise (e.g., Ekkekakis & Acevedo, 2006; Kiviniemi, Voss-Humke, & Seifert, 2007; Parfitt, Rose, & Burgess, 2006). Consistent with our framework, our initial work showed that the BDNF gene moderated the effect of exercise on heart rate and perceived exertion, and that perceived exertion was then a significant predictor of acute affective response to exercise (Bryan et al., 2007). In a more recent study we demonstrated that increases in blood lactate levels during submaximal exercise resulted in less improvement in positive affect and that larger increases in salivary cortisol during submaximal exercise were associated with less reduction in negative affect (Kwan et al., unpublished data).
Subjective Experience of Exercise
While there are a multitude of variables that contribute to sustaining one’s motivation over time, the positive or negative subjective experience of exercise—and in particular the acute affective response—may be particularly important (Carels, Berger, & Darby, 2006; Dishman, 1990; Kiviniemi, Voss-Humke, & Seifert, 2007; Lox, Martin, & Petruzzello, 2003). Affective responses to an acute bout of moderate intensity physical activity are a common target for investigation in exercise psychology (Ekkekakis & Petruzzello, 1999; Reed, 2005; Yeung, 1996), and have been explored as predictors of future exercise behavior (e.g., Ekkekakis, Hall & Petruzzello, 2005; Wankel, 1993; Kwan & Bryan, 2010a; 2010b). Because exercise, on average, leads to improvements in affect, it has been suggested that participation in exercise could be self-reinforcing (e.g., Annesi, 2005). Consistent with this logic, evidence suggests that more positive affective responses to exercise are related to better adherence to exercise programs (Annesi, 2002, 2005; Hardy & Rejeski, 1989). However, these effects are not universal; studies have shown that some people experience no change or, worse, deterioration of affect during exercise, even at moderate intensity (Ekkekakis et al., 2005; Parfitt et al., 2006; Van Landuyt, Ekkekakis, Hall & Petruzzello, 2000). These differences in immediate response are hypothesized to underlie differences in motivation to exercise in the future.
Motivation
There are a multitude of potential operationalizations of motivation that would be consistent with our framework, ranging from fundamental drives (e.g., to increase one’s mate value; Caldwell 2009), to desire for intrinsic reward (e.g., to enjoy the feeling of exerting oneself; Kilpatrick et al., 2005), to broad behavioral outcomes (e.g., exercising to lower one’s cholesterol), to concrete goals (e.g., to run the Turkey Trot 5K on Thanksgiving). The basic theoretical conceptualization we have appealed to in our initial work is based on the Theory of Planned Behavior (TPB; Ajzen, 1991), which, while certainly not exhaustive or ideal, has been extensively and successfully applied in the exercise domain (e.g., Hagger, Chatzisarantis, & Biddle, 2002; Symons Downs & Hausenblas, 2005). The TPB asserts that ones motivation to engage in a behavior is the result of intentions to engage in the behavior, which is itself the result of positive attitudes towards, normative support for, and perceived self-efficacy for the behavior. And increased motivation is, according to the TPB, the most proximal determinant of behavior.
Use of the Framework in a Study of Physical Activity Behavior Change
As an example of one potential use for the transdisciplinary framework outlined by Bryan et al. (2007), we recently completed an NCI-funded randomized controlled intervention trial in the Denver Metropolitan area comparing an exercise promotion intervention to a health and wellness contact control. We refer to the study as Colorado STRIDE (COSTRIDE). Both the exercise and health and wellness control interventions were developed by our collaborator Bess Marcus and her colleagues (Bock et al., 2001; Marcus, Emmons et al., 1998; Marcus, Bock et al., 1998; Marcus, Napolitano King et al., 2007; Marcus, Napolitano, Lewis et al., 2007). Marcus and colleagues have developed an individually-tailored, print-based intervention to increase physical activity behavior that has demonstrated effectiveness in helping sedentary individuals to increase their levels of physical activity. The details of our study and the characteristics of the baseline sample of participants are described elsewhere (Magnan et al., 2010). In addition to replicating Marcus and colleagues’ study in a demographically and geographically different area, our goal in this work was to understand for whom the exercise promotion intervention is successful. We utilized this transdisciplinary framework to guide our selection of genetic, physiological, and psychological individual difference variables that might moderate the success of the intervention for the initiation and maintenance of physical activity.
Study Design
The study sample consists of 238 participants who were recruited over the course of four years from the Denver Metro area. Participants are relatively inactive (e.g., <90 minutes of voluntary moderate intensity physical activity per week in the past three months), but otherwise healthy men and women between the ages of 18 and 45. Final attrition was 23% (181 of the original 238 successfully completed the 12-month follow-up) consistent with similar exercise intervention studies (Yancey et al., 2006; Foreyt et al., 1993; Marcus, Bock et al., 1998). Each participant completed the informed consent process, a VO2 max test (Christou, Gentile, DeSouza, Seals, & Gates, 2005; Bryan et al., 2007), and submaximal treadmill test (30 minutes at 65% of VO2 max) during three separate laboratory visits within the first month of participating. Following the submaximal test, participants were randomized into either the COSTRIDE exercise intervention or the health and wellness (HW) contact control. Participants then came back to the clinic for two separate follow-up visits at 6 and 12 months post-randomization. The intervention was delivered in the form of direct mailings to participants’ homes, and the informational mailings for the two conditions differ in content but are matched for frequency and quantity. Participants who were randomized into the COSTRIDE exercise intervention received mailings with personally tailored feedback based on responses to recent assessments. These mailings also contained information on different topics related to exercise such as safety tips for exercising outdoors, purchasing appropriate athletic shoes, and overcoming obstacles and barriers that hinder physical activity behavior. Participants who were randomized into the HW condition did not receive any personalized feedback but they did receive information related to general health and wellness topics such as stress management, healthy diet, and improved sleep.
Selection and Operationalizations of Moderators
To assess genetic factors that might moderate intervention efficacy, saliva was collected for DNA analyses at baseline and 12 months. The second sample was collected in order to examine epigenetic changes (e.g., DNA methylation) that may covary with exercise behavior. It is important to note, however, that whether or not methylation of DNA assessed via saliva is indicative of or even associated with methylation in other structures in the body (e.g., brain, muscle, etc.) is an empirical question. So this particular hypothesis represents an innovative empirical question being asked in this research. A primary genetic factor of interest is the BDNF single nucleotide polymorphism identified in our prior work, and we have already shown that this genetic factor moderates the effectiveness of the intervention on some psychological outcomes (e.g., depressive symptoms; Caldwell Hooper, Feldstein Ewing, & Bryan, 2010).
A number of physiological response to exercise moderators were assessed during the submaximal exercise session, and full details of the procedure for acquiring each of these measures can be found in Magnan et al. (2010). To summarize briefly, we assessed blood lactate response, heart rate response, temperature regulation response, and catecholamine (epinephrine and norepinephrine) response. The blood borne assessments were collected via an intravenous catheter inserted for the duration of the submaximal exercise session, and samples were collected by a registered nurse before (11.5ml), at 10 minutes into the activity (5.5ml) and at the conclusion of the activity (30 minutes; 11.5ml). Heart rate was acquired via a commercially available heart rate monitor worn continuously throughout the bout, and core temperature was acquired by taking the average of 2–3 tympanic temperature readings before activity, three times during activity, and twice post-activity. We acknowledge that tympanic thermometry is an imperfect assessment of core temperature (see Sato, Kane, Gisolfi, Kondo, & Sato, 1996) but it was the most pragmatic for our context.
Physiological responses related to body temperature, catecholamine response, and lactate production may be influential in acute affect changes after exercise and exercise behavior itself. For example, increased body temperature is associated with increases in negative affect (e.g., Petruzzello, Landers, & Salazar, 1993) and higher lactate concentration is associated with greater perceived exertion and fatigue during exercise (Lagally et al., 2002). Thus, we hypothesize that individuals who experience better temperature regulation, lower levels of lactate production, and have better catecholamine regulation will be more likely to maintain a moderate intensity physical activity regimen.
Subjective experience of exercise was assessed in a number of ways with assessments at six points during the submaximal exercise session: five minutes prior to activity, immediately before activity begins, 10, 20, and 30 minutes into activity, and five minutes post activity. Affective response to exercise was measured with the Physical Activity Affect Scale (PAAS; Lox, Jackson, Tuholski, Wasley, & Treasure, 2000), which includes 12 items representing four subscales (positive affect, negative affect, exhaustion, and tranquility), the Feelings Scale (Hardy & Rejeski, 1989) and the Felt Arousal Scale (Svebak & Murgatroyd, 1985), single-item measures of affective valence and arousal, respectively. Ratings of perceived exertion (RPE) were measured with the commonly used Borg RPE scale (Borg, 1985). Pain sensitivity was assessed with an 11-point (0 = no pain at all to 10 = extremely intense pain) single-item subjective measure reflecting an individual’s level of current pain during exercise.
Increasing positive affect and decreasing negative affect is an often reported effect of acute exercise (e.g., see reviews by Peluso & Guerra de Andrade, 2005 and Reed, 2005). However, a positive affect increase is not universal – some individuals will experience no change or even deterioration in affect (Ekkekakis et al., 2005; Parfitt et al., 2006; Van Landuyt et al., 2000). There is some evidence to suggest that positive affect responses during exercise result in regular exercise activity (Bryan, et al., 2007; Petruzzello, Hall, & Ekkekakis, 2001). Thus, we hypothesize that individuals who experience more positive affect during exercise and less perceived exertion will be more likely to maintain a moderate intensity physical activity regimen.
Measures of motivation to exercise were assessed in questionnaire format administered at baseline, 3, 6, 9, and 12 months. Behavioral beliefs representing attitudes towards exercise were measured using the 18-item physical activity enjoyment scale (PACES; Kendzierski & DeCarlo, 1991; sample items “I enjoy it” and “I hate it”, α=.91). Remaining motivational factors were drawn from our previous work (Bryan & Rocheleau, 2002; Bryan et al., 2007). Social norms regarding exercise were measured with nine items (sample item “Most people who are important to me think I should do aerobic exercise.” α=.86). Self-efficacy was measured with nine items assessing confidence in one’s abilities to engage in aerobic exercise (sample item “I feel confident that I could do many different kinds of aerobic exercise.” α=.86). Intention to engage in aerobic exercise was measured with four items assessing the perceived likelihood of exercise behavior in the next three months (e.g., “How likely is it that you will go to a recreation center or a health club to do aerobic exercise in the next three months?” α=.68).
Support for TPB in predicting exercise behavior has been established across populations including older adults (e.g., Brenes, Strube, & Strandt, 1998), college students (e.g., Bryan & Rocheleau, 2002) and pregnant women (Godin, 1993), to name a few. Additionally, Bryan and Rocheleau (2002) found prospective relationships between TPB variables and exercise behavior measured at a three-month assessment and in a multivariate prediction model, and TPB variables accounted for 47% of variance in exercise intentions. Thus, we hypothesize that more positive attitudes, higher self-efficacy, stronger normative support, and stronger intentions will be associated with better maintenance of exercise behavior.
Exercise behavior was assessed via an interviewer administered measure (7-Day physical activity recall (PAR); Blair et al., 1985) completed at baseline, 6 and 12 months. At baseline, 3, 6, 9, and 12 months, we also assessed behavior with a set of three self-report questions which have shown adequate reliability and validity in our prior work (e.g., Bryan et al., 2007; Bryan & Rocheleau, 2002). Participants indicated how often they had engaged in aerobic activity in the past three months (1 = never to 7 = often), the average number of days per week they engaged in aerobic exercise over the past three months, and the number of days in the past week they had engaged in aerobic exercise for at least 30 minutes.
Planned Analyses
This study essentially involves a one-factor between-subjects design, where assignment to intervention condition is the sole manipulated between-subjects factor. Our plan is to systematically explore the influence of genetic, physiological, subjective response, and psychological variables consistent with our transdisciplinary framework as potential moderators of the effectiveness of the intervention. These analyses will allow us to begin to characterize the types of individuals who respond well to the individually-tailored print-based intervention versus those for whom a different intervention modality may be warranted. The primary analyses will involve a random coefficient regression (RCR) approach (c.f., Cohen, Cohen, West, & Aiken, 2003) where intervention condition, time (ranging from baseline to 12 months), and the moderator of interest serve as main effects, and all possible interaction terms are explored. Of key interest will be the condition×time×moderator effect where we will look for differential responsiveness over time to the exercise versus health and wellness conditions, as a funtion of level of the moderator. RCR is appropriate as it allows us to model the intercept and time factors as both fixed and random effects, to account for individual variability in both exercise behavior at baseline as well as trajectory of change in response to the intervention (Cohen et al., 2003). Further, in contrast to more traditional repeated measures ANOVA approaches, RCR allows us to retain the continuous nature of our moderators for optimal power (MacCallum, Zhang, Preacher, & Rucker, 2002).
Summary
We hope that we have demonstrated the potential utility of our transdisciplinary framework for the assessment of moderators of physical activity behavior adoption and maintenance. We view this as only one of many potential uses of the framework, and encourage broad exploration of the relationships proposed in different populations (e.g., sedentary adults, children, obese individuals, highly trained athletes) and under different circumstances (e.g., longitudinal studies of physical activity, physical activity post-partum, physical activity after cancer treatment).
A particular strength of the research study we described here is its focus on relatively inactive individuals, a group for whom tests of the effects of genetic factors, physiological indicators, and most importantly subjective responses to exercise are rare, but for whom the data are crucially important. While prior research has shown support for specific relationships in our framework (e.g., affective responses to physical activity being predictive of future behavior (Bryan et al., 2007; Kiviniemi et al., 2007; Kwan & Bryan, 2010a; 2010b), it has focused primarily on active participants (e.g., highly fit individuals and trained athletes; Bulbulian & Darabos, 1986; Farrell, Gustafson, Morgan, & Pert, 1987) in studies of small sample size [e.g., Backhouse, Ekkekakis, Biddle, Foskett, & Williams, 2007 (N = 12); Parfitt et al., 2006 (N = 12)]. Given the health risks associated with a sedentary lifestyle, and the potential benefits of engagement in some level of activity, it seems crucial to understand these factors in this understudied group and with adequate sample size to detect moderated effects of behavior change intervention success.
An advantage of our transdisciplinary framework, more generally, is that it is in no way “set in stone” and we encourage others to test other potential moderators and relationships not specifically detailed in the framework. We view the strengths of our framework as: 1) the categories of variables (genetic, psychological, physiological) within the same level of influence that it brings together and 2) the notion that we ought to be looking at how these variables are related to one another so that we know better where, how, and with whom to intervene. For example, with different populations the processes involved might work differently. So, for sedentary individuals there may be a larger genetic component associated with the subjective experience of exercise, so in sedentary individuals genetic factors might be a larger moderator of exercise uptake. But once one is fit and experiences consistent rewards, then the motivational variables may take precedence as moderators of maintenance in response to an intervention. While we cannot address these questions in this particular study, such explorations in future research would give us important data for the targeting of different intervention approaches to different types of individuals.
There are certainly limitations to our approach. A major limitation of our transdisciplinary framework is that we do not address the important social, cultural, and environmental variables that are crucial to understanding the range of impacts on sedentary lifestyle. Further, our broad approach that necessitates extensive measurement across a number of domains from each participant precludes detailed and controlled examinations of any one section of the model. For example, recent research on core temperature measurement recommends the use of ingested temperature sensors to record core body temperature (e.g., Gant, Atkinson, & Williams, 2006; Goodman, Kenefick, Cadarette, & Cheuvront, 2009). But the logistical issues and expense of such an approach mean that sample sizes for such studies are as small as 7 to 10 participants. Since temperature is only one of a large battery of assessments in transdisciplinary studies, and much larger samples are necessary to have the power to detect effects in intervention research, sometimes the objectively “best” measurement strategy is not feasible in transdiscplinary approaches.
In sum, it is our hope that the “big picture” approach that we emphasize will elucidate relationships among genetic, physiological, and psychological variables that will help us to better understand individual differences in the initiation and maintenance of physical activity behavior and develop effective interventions to increase physical activity, and decrease morbidity and mortality due to sedentary lifestyle.
Acknowledgments
The research was supported by grants from the National Cancer Institute (RO1 CA109858), and the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health (M01-RR00051).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela D. Bryan, Department of Psychology, MSC03 2220, University of New Mexico, Albuquerque, NM 87131, Phone: 505-277-5676, Fax: 505-277-1394
Renea Nilsson, Department of Psychology, 345 UCB, University of Colorado, Boulder, CO 80305.
Sara Anne Tompkins, Evaluation Coordinator, Colorado State University, Fort Collins, CO 80523-4040.
Renee E. Magnan, Department of Psychology, MSC03 2220, University of New Mexico, Albuquerque, NM 87131
Bess H. Marcus, Brown University Program in Public Health, 121 South Main Street, 8th Floor, Providence, RI 02903
Kent E. Hutchison, Mind Research Network and Departments of Psychology and Neurosciences, University of New Mexico, 1101 Yale Blvd NE, Albuquerque, NM 87106
References
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Annesi JJ. Relationship between changes in acute, exercise-induced feeling states, self-motivation, and adults’ adherence to moderate aerobic exercise. Perceptual and Motor Skills. 2002;94:425–439. doi: 10.2466/pms.2002.94.2.425. [DOI] [PubMed] [Google Scholar]
- Annesi JJ. Relations of self-motivation, perceived physical condition, and exercise-induced changes in revitalization and exhaustion with attendance in women initiating a moderate cardiovascular exercise regimen. Women & Health. 2005;42:77–93. doi: 10.1300/j013v42n03_05. [DOI] [PubMed] [Google Scholar]
- Backhouse SH, Ekkekakis P, Biddle SJH, Foskett A, Williams C. Exercise makes people feel better but people are inactive: Paradox or artifact? Journal of Sport and Exercise Psychology. 2007;29:498–517. doi: 10.1123/jsep.29.4.498. [DOI] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Annals of Behavioral Medicine. 2001;23:79–87. doi: 10.1207/S15324796ABM2302_2. [DOI] [PubMed] [Google Scholar]
- Booth FW, Neufer PD. Exercise Genomics and Proteomics. In: Tipton CM, Tate C, editors. ACSM Advanced Exercise Physiology. Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- Borg G. An Introduction to Borg’s RPE-Scale. Ithaca, NY: Mouvement; 1985. [Google Scholar]
- Bouchard C, Perusse L. Heredity, activity level, fitness, and health. In: Bouchard C, Shepard RJ, editors. Physical activity, fitness, and health: International proceedings and consensus statement. Champaign, IL: Human Kinetics Publishers; 1994. pp. 106–118. [Google Scholar]
- Brenes GA, Strube MJ, Storandt M. An application of the theory of planned behavior exercise among older adults. Journal of Applied Social Psychology. 1998;28:2274–2290. [Google Scholar]
- Bryan A, Hutchison KE, Seals DR, Allen DE. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychology. 2007;26:30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AD, Rocheleau CA. Predicting aerobic versus resistance exercise using the Theory of Planned Behavior. American Journal of Health Behavior. 2002;26:83–94. doi: 10.5993/ajhb.26.2.1. [DOI] [PubMed] [Google Scholar]
- Bulbulian R, Darabos BL. Motor neuron excitability: The Hoffmann reflex following exercise of low and high intensity. Medicine & Science in Sports & Exercise. 1986;18:697–702. [PubMed] [Google Scholar]
- Caldwell AE. Unpublished Masters Thesis. University of New Mexico; 2008. What Gets a Body Moving? Examining Individual Differences in Exercise Motivation. [Google Scholar]
- Caldwell Hooper AE, Feldstein Ewing SW, Bryan AD. BDNF SNP moderates the effect of an exercise intervention on depression outcomes. 2010 Manuscript under review. [Google Scholar]
- Carels RA, Berger B, Darby L. The association between mood states and physical activity in postmenopausal, obese, sedentary women. Journal of Aging and Physical Activity. 2006;14:12–28. doi: 10.1123/japa.14.1.12. [DOI] [PubMed] [Google Scholar]
- Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111:1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Courneya KS, Plotnikoff RC, Hotz SB, Birkett NJ. Social support and the theory of planned behavior in the exercise domain. American Journal of Health Behavior. 2000;24:300–308. [Google Scholar]
- Dishman RK. Determinants of participation in physical activity. In: Bouchard C, Shephard RJ, Stephens T, Sutton JR, McPherson BD, editors. Exercise, Fitness, and Health. Champaign, IL: Human Kinetics; 1990. pp. 75–102. [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR. Neurobiology of exercise. Obesity. 2006;14:345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramycardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- Dubbert PM. Physical activity and exercise: Recent advances and current challenges. Journal of Consulting and Clinical Psychology. 2002;70:526–536. [PubMed] [Google Scholar]
- Ekkekakis P, Acevedo E. Affective responses to acute exercise. In: Acevedo E, Ekkekakis P, editors. Psychobiology of Physical Activity. Champaign, IL: Human Kinetics; 2006. pp. 91–109. [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. Some like it vigorous: Measuring individual differences in the preference for and tolerance of exercise intensity. Journal of Sport & Exercise Psychology. 2005;27:350–374. [Google Scholar]
- Ekkekakis P, Petruzzello SJ. Acute aerobic exercise and affect: Current status, problems and prospects regarding dose-response. Sports Medicine. 1999;28:337–374. doi: 10.2165/00007256-199928050-00005. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Rasmussen F, Tynelius P. Genetic factors in physical activity and the equal environment assumption—The Swedish Young Male Twins Study. Behavioral Genetics. 2006;36:238–247. doi: 10.1007/s10519-005-9018-7. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Gustafson AB, Morgan WP, Pert CB. Enkephalins, catecholamines, and psychological mood alterations: Effects of prolonged exercise. Medicine & Science in Sports & Exercise. 1987;19:347–353. [PubMed] [Google Scholar]
- Foreyt JP, Goodrick GK, Reeves RS, Raynaud AS, Darnell L, Brown AH, et al. Response of free-living adults to behavioral treatment of obesity: Attrition and compliance to exercise. Behavior Therapy. 1993;24:659–669. [Google Scholar]
- Foster S, Giles-Corti B. The built environment, neighborhood crime and constrained physical activity: An exploration of inconsistent findings. Preventive Medicine. 2008;47:241–251. doi: 10.1016/j.ypmed.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Gant N, Atkinson G, Williams C. The validity and reliability of intestinal temperature during intermittent running. Medicine & Science in Sports & Exercise. 2006;38:1926–1931. doi: 10.1249/01.mss.0000233800.69776.ef. [DOI] [PubMed] [Google Scholar]
- Godin G. The theories of reasoned action and planned behavior: Overview of findings, emerging research problems and usefulness for exercise promotion. Journal of Applied Sport Psychology. 1993;5:141–157. [Google Scholar]
- Goodman DA, Kenefick RW, Cadarette BS, Cheuvront SN. Influence of sensor ingestion timing on consistency of temperature measures. Medicine & Science in Sports & Exercise. 2009;41:597–602. doi: 10.1249/MSS.0b013e31818a0eef. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis N, Biddle SJH. The influence of autonomous and controlling motives on physical activity intentions within the theory of planned behaviour. British Journal of Health Psychology. 2002;7:283–297. doi: 10.1348/135910702760213689. [DOI] [PubMed] [Google Scholar]
- Hardy CJ, Rejeski WJ. Not what, but how one feels: The measurement of affect during exercise. Journal of Sport & Exercise Psychology. 1989;11:304–317. [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. doi: 10.1146/annurev.clinpsy.121208.131441. (in press) [DOI] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Research. 2003;983:108–114. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Kendzierski D, DeCarlo KJ. Physical Activity Enjoyment Scale: Two validation studies. Journal of Sport & Exercise Psychology. 1991;13:50–64. [Google Scholar]
- Kilpatrick M, Hebert E, Bartholomew J. College students’ motivation for physical activity: Differentiating men’s and women’s motives for sport participation and exercise. Journal of American College Health. 2005;54:87–94. doi: 10.3200/JACH.54.2.87-94. [DOI] [PubMed] [Google Scholar]
- King AC, Bauman A, Abrams DB. Forging transdisciplinary bridges to meet the physical inactivity challenge in the 21st century. American Journal of Preventive Medicine. 2002;23(2S):104–106. [Google Scholar]
- King AC, Marcus B, Ahn D, Dunn AL, Rejeski WJ, Sallis JF, et al. Identifying subgroups that succeed or fail with three levels of physical activity intervention: The activity counseling trial. Health Psychology. 2006;25:336–347. doi: 10.1037/0278-6133.25.3.336. [DOI] [PubMed] [Google Scholar]
- King AC, Toobert D, Ahn D, Resnicow K, Coday M, Riebe D, et al. Perceived environments as physical activity correlates and moderators of intervention in five studies. American Journal of Health Promotion. 2006;21:24–35. doi: 10.1177/089011710602100106. [DOI] [PubMed] [Google Scholar]
- Kiviniemi MT, Voss–Humke AM, Seifert AL. How do I feel about the behavior? The interplay of affective associations with behaviors and cognitive beliefs as influences on physical activity behavior. Health Psychology. 2007;26:152–158. doi: 10.1037/0278-6133.26.2.152. [DOI] [PubMed] [Google Scholar]
- Kwan BM, Bryan A. In-task and post-task affective response to exercise: Translating exercise intentions into behavior. British Journal of Health Psychology. 2010a;15:115–131. doi: 10.1348/135910709X433267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: Self-efficacy, outcome expectations and temporal stability of intentions. Psychology of Sport and Exercise. 2010b;11:71–79. doi: 10.1016/j.psychsport.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagally KM, Roberts RJ, Gallagher KI, Goss FL, Jakicic JM, Lephart SM, et al. Perceived exertion, electromyography, and blood lactate during acute bouts of resistance exercise. Medicine & Science in Sports & Exercise. 2002;34:552–559. doi: 10.1097/00005768-200203000-00025. [DOI] [PubMed] [Google Scholar]
- Lox CL, Jackson S, Tuholski SW, Wasley D, Treasure DC. Revisiting the measurement of exercise-induced feeling states: The Physical Activity Affect Scale (PAAS) Measurement in Physical Education and Exercise Science. 2000;4:79–95. [Google Scholar]
- Lox CL, Martin KA, Petruzzello SJ. The psychology of exercise: Integrating theory and practice. Scottsdale, AZ: Holcomb Hathaway Publishers; 2003. [Google Scholar]
- Lytle LA. Examining the etiology of childhood obesity: The IDEA study. American Journal of Community Psychology. 2009 doi: 10.1007/s10464-009-9269-1. in press. Epub ahead of print retrieved January 10, 2010, from http://www.springerlink.com/content/h274p1121271l8v2/fulltext.pdf. [DOI] [PMC free article] [PubMed]
- Macera CA, Ham SA, Yore MM, Jones DA, Ainsworth BE, Kimsey CD, et al. Prevalence of physical activity in the United States: Behavioral Risk Factor Surveillance System, 2001. Preventing Chronic Disease. 2005;2:A17. [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher K, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Magnan RE, Nilsson RM, Marcus BH, Ciccolo J, Bryan AD. A transdisciplinary approach to physical activity behavior change. Manuscript under review 2010 [Google Scholar]
- Marcus BH, Bock BC, Pinto BM, Forsyth LH, Roberts MB, Traficante RM. Efficacy of an individualized, motivationally-tailored physical activity intervention. Annals of Behavioral Medicine. 1998;20:174–180. doi: 10.1007/BF02884958. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Emmons KM, Simkin-Silverman LR, Linnan LA, Taylor ER, Bock BC, et al. Evaluation of motivationally tailored vs. standard self-help physical activity interventions at the workplace. American Journal of Health Promotion. 1998;12:256–253. doi: 10.4278/0890-1171-12.4.246. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Napolitano MA, King AC, Lewis BA, Whiteley JA, Albrecht AE, et al. Telephone versus print delivery of an individualized motivationally-tailored physical activity intervention: Project STRIDE. Health Psychology. 2007;26:401–409. doi: 10.1037/0278-6133.26.4.401. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Napolitano MA, Lewis BA, King AC, Whiteley JA, Albrecht AE, et al. Examination of print and telephone channels for physical activity promotion: Rationale, design, and baseline data from project STRIDE. Contemporary Clinical Trials. 2007;28:90–104. doi: 10.1016/j.cct.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Nigg CR, Riebe D, Forsyth LH. Interactive communication strategies: Implications for population-based physical activity promotions. American Journal of Preventative Medicine. 2000;19:121–126. doi: 10.1016/s0749-3797(00)00186-0. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, et al. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114:2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- Matson-Koffman DM, Brownstein JN, Neiner JA, Greaney ML. A site-specific literature review of policy and environmental interventions that promote physical activity and nutrition for cardiovascular health: What works? American Journal of Health Promotion. 2005;19:167–193. doi: 10.4278/0890-1171-19.3.167. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Parfitt G, Rose EA, Burgess WM. The psychological and physiological responses of sedentary individuals to prescribed and preferred intensity exercise. British Journal of Health Psychology. 2006;11:39–53. doi: 10.1348/135910705X43606. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Journal of American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Peluso MAM, Guerra de Andrade LHS. Physical activity and mental health: The association between exercise and mood. Clinics. 2005;60:61–70. doi: 10.1590/s1807-59322005000100012. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, Hall EE, Ekkekakis P. Regional brain activation as a biological marker of affective responsivity to acute exercise: Influences of fitness. Psychophysiology. 2001;38:99–106. [PubMed] [Google Scholar]
- Petruzzello SJ, Landers DM, Salazar W. Exercise and anxiety reduction: Examination of temperature as an explanation for affective change. Journal of Sport & Exercise Psychology. 1993;15:63–76. [Google Scholar]
- Plomin R. Using DNA in health psychology. Health Psychology. 1998;17:53–55. doi: 10.1037//0278-6133.17.1.53. [DOI] [PubMed] [Google Scholar]
- Plomin R. Using DNA in health psychology. Health Psychology. 1998;17:53–55. doi: 10.1037//0278-6133.17.1.53. [DOI] [PubMed] [Google Scholar]
- Reed J. Acute physical activity and self-reported affect: A review. In: Clark AV, editor. Causes, Role and Influence of Mood States. Chicago, IL: Nova Science Publishers, Inc; 2005. pp. 91–113. [Google Scholar]
- Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between health and social sciences. Social Science Medicine. 1992;35:1343–1357. doi: 10.1016/0277-9536(92)90038-r. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Bladwin AS, Hertel AW. Self-regulation and behavior change. In: Baumeister RR, Vohs KD, editors. Handbook of self regulation: Research, theory and applications. New York: Guilford; 2004. pp. 130–148. [Google Scholar]
- Satariano WA, McAuley E. Promoting physical activity among older adults: From ecology to the individual. American Journal of Preventive Medicine. 2003;25(Suppl 2):184–192. doi: 10.1016/s0749-3797(03)00183-1. [DOI] [PubMed] [Google Scholar]
- Sato KT, Kane NL, Soos G, Gisolfi CV, Kondo N, Sato K. Reexamination of tympanic membrane temperature as a core temperature. Journal of Applied Physiology. 1996;80:1233–1239. doi: 10.1152/jappl.1996.80.4.1233. [DOI] [PubMed] [Google Scholar]
- Schwarzer R. Self-regulatory processes in the adoption and maintenance of health behaviors: The role of optimism, goals, and threats. Journal of Health Psychology. 1999;4:115–127. doi: 10.1177/135910539900400208. [DOI] [PubMed] [Google Scholar]
- Svebak E, Murgatroyd S. Metamotivational dominance A multimethod validation of reversal theory constructs. Journal of Personality and Social Psychology. 1985;48:107–116. [Google Scholar]
- Symons Downs D, Hausenblas HA. Applying the theories of reasoned action and planned behavior to exercise: A meta-analytic update. Journal of Physical Activity and Health. 2005;2:76–97. [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine & Science in Sports & Exercise. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Editorial: Bringing basic and applied research together to address underlying mechanisms. Psychology & Health. 2008;23:131–134. doi: 10.1080/08870440701747477. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. [Accessed November 4, 2008];Physical activity guidelines for Americans. 2008 Available at: http://www.health.gov/PAGuidelines/pdf/paguide.pdf.
- Van Landuyt LM, Ekkekakis P, Hall EE, Petruzzello SJ. Throwing the mountains into the lakes: On the perils of nomothetic conceptions of the exercise-affect relationship. Journal of Sport and Exercise Psychology. 2000;22:208–234. [Google Scholar]
- Wankel LM. The importance of enjoyment to adherence and psychological benefits from physical activity. International Journal of Sport Psychology. 1993;24:151–169. [Google Scholar]
- Yancey AK, McCarthy WJ, Harrison GG, Wong WK, Siegel JM, Leslie J. Challenges in improving fitness: Results of a community-based, randomized, controlled lifestyle change intervention. Journal of Women’s Health. 2006;15:412–429. doi: 10.1089/jwh.2006.15.412. [DOI] [PubMed] [Google Scholar]
- Yeung RR. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 1996;40:123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]