Abstract
Background
Food allergy is a common and serious health problem. A new herbal product, called food allergy herbal formula 2 (FAHF-2), has been demonstrated to have a high safety profile and potent long-term efficacy in a murine model of peanut-induced anaphylaxis.
Objective
To evaluate the safety and tolerability of FAHF-2 in patients with food allergy.
Methods
In this randomized, double-blinded, placebo-controlled, dose escalation, phase 1 trial, patients received 1 of 3 doses of FAHF-2 or placebo: 2.2 g (4 tablets), 3.3 g (6 tablets), or 6.6 g (12 tablets) 3 times a day for 7 days. Four active and 2 placebo patients were treated at each dose level. Vital signs, physical examination results, laboratory data, pulmonary function test results, and electrocardiogram data were monitored. Immunomodulatory studies were also performed.
Results
Nineteen food allergic participants were included in the study. Two patients (1 in the FAHF-2 group and 1 in the placebo group) reported mild gastrointestinal symptoms. One patient withdrew from the study because of an allergic reaction that was unlikely related to the study medication. No significant differences were found in vital signs, physical examination results, laboratory data, pulmonary function test results, and electrocardiogram data obtained before and after treatment visits. Significantly decreased interleukin (IL) 5 levels were found in the active treatment group after 7 days. In vitro studies of peripheral blood mononuclear cells cultured with FAHF-2 also demonstrated a significant decrease in IL-5 and an increase in culture supernatant interferon γ and IL-10 levels.
Conclusions
FAHF-2 appeared to be safe and well tolerated in patients with food allergy.
INTRODUCTION
Approximately 6% of children and 4% of adults have food allergies,1 which is now the leading cause of anaphylactic reactions treated in hospital emergency departments in the United States.2 Peanut and tree nut allergies account for approximately 80% of fatal and near-fatal anaphylactic reactions.3,4 Peanut, tree nut, fish, and shellfish allergies are lifelong disorders for most patients,1 and there is no definitive treatment for peanut or other food allergy. Aside from immediate access to postanaphylactic rescue medications, strict avoidance is the only strategy to manage this condition. Despite these measures, unintentional ingestions are common.1 Therefore, there is an urgent need to develop effective therapies.
There has been increasing interest in the role of herbal medications as a complementary treatment for various allergic disorders, including allergic rhinitis and asthma.5,6 Given the commonality between gastrointestinal symptoms experienced during food allergic reactions and those described as indications for the use of Wu Mei Wan, a classical 10-herb formula,7 our group developed the food allergy herbal formula 1 (FAHF-1).8 Ling-Zhi, one of the most commonly used dietary supplements in China and the United States, was added to enhance the antiallergic and anti-inflammatory properties of the formula.9–12 The herbal product FAHF-2 used in the present study eliminates Xi-Xin (Herba Asari or Asarum) and Zhi-Fu-Zi (Radix Lateralis Aconiti Carmichaeli Praeparata or Aconite), which were not found to contribute to the protective effect of the formulation. In addition, these 2 herbs are potentially toxic if processed improperly,13–15 and thereby their elimination increased the safety profile of FAHF-2.
Although FAHF-2 is a new herbal product, Wu Mei Wan and Ling-Zhi, components of FAHF-2, have a long history of human use and are frequently used today in China and Japan. Both are marketed in the United States as dietary supplements. Studies demonstrate that Wu Mei Wan, with or without modification, is effective for gastroenteritis and asthma.7,16 No adverse effects were reported in these clinical studies.17 Furthermore, Ling-Zhi has been shown to be beneficial for several chronic conditions, including chronic bronchitis, bronchial asthma, and allergic rhinitis.10
FAHF-2 completely blocks peanut-induced anaphylaxis in a murine model of peanut allergy,18 and subsequent studies found that FAHF-2–treated mice were protected against anaphylaxis for at least 6 months after discontinuing treatment.19 This effect is associated with sustained suppression of IgE and TH2 responses and increased IgG2a levels. Furthermore, there was a large margin of safety; it was shown that mice fed 24 times the effective daily dose showed no signs of acute toxic effects and had no evidence of abnormal liver and kidney functions, abnormal complete blood cell counts, or major organ disease. Human cell in vitro studies demonstrate a beneficial immunomodulatory effect of FAHF-2 on peripheral blood mononuclear cells (PBMCs) from children with peanut and multiple other food allergies.20 These results indicate that FAHF-2 is a potential candidate for developing a botanical drug for the treatment of food allergy.
On the basis of these observations, we initiated a phase 1 study to evaluate the tolerability and safety of FAHF-2 in patients with food allergy as a Food and Drug Administration (FDA) investigational new drug (IND) botanical drug product (IND 77,468).
METHODS
Study Participants
Food allergic individuals 12 through 45 years of age with a history of allergy to peanut, tree nut, fish, or shellfish as documented by a positive skin test result (mean wheal diameter ≥3 mm greater than the mean of the saline control) and/or food allergen specific IgE level (peanut, tree nut, fish, or shellfish specific IgE ≥0.7 kU/L) were eligible for the study. Females of childbearing potential were sexually inactive or using effective birth control measures, as deemed appropriate by the investigator, for the duration of the study.
Exclusion criteria included acute infection; history of systemic diseases; abnormal hepatic, bone marrow, or renal function; clinically significant abnormal electrocardiogram result; current uncontrolled moderate to severe asthma with a forced expiratory volume in 1 second (FEV1) of less than 80% predicted; drug or alcohol abuse; pregnancy or lactation; and participation in another research protocol within the previous 30 days.
This study was approved by the Mount Sinai Medical Center institutional review board. Written informed consent was obtained before enrollment.
Study Design
This study was a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 trial. Three doses of FAHF-2 were studied: 2.2 g (4 tablets), 3.3 g (6 tablets), and 6.6 g (12 tablets) 3 times a day for 7 days. Selection of the dose range was based on previous experience with FAHF-2 in animal models. Four active and 2 placebo patients were treated at each dose level. The FAHF-2 doses were increased after a review of the data from the 6 patients receiving the lower dose of medication by independent safety reviewers. Dose escalation was allowed if none of the 6 patients in a dosing group experienced a dose-limiting toxic effect as defined by a grade 3 adverse event (AE) attributable to the study medication. If 1 of 6 patients in the FAHF-2 group had experienced a dose-limiting toxic effect, then 6 additional patients (again 4 in the FAHF-2 group and 2 in the placebo group) were added to that group and the dose escalation delayed until the additional 6 patients completed the safety evaluation. If fewer than 2 of the 12 patients in the group experienced a dose-limiting toxic effect, the next 6 patients were enrolled at the next dose of FAHF-2. If 2 of the 12 patients experienced a dose-limiting toxic effect on any specified dose, no additional patients were to be enrolled at that dose or at a higher dose level until further discussions with the independent safety reviewers.
Initial evaluation consisted of a thorough medical history and physical examination, vital signs, skin prick testing and food specific IgE testing, baseline pulmonary function, electrocardiography, urinalysis, and routine laboratory blood tests (complete blood cell count, serum chemical analyses, renal function, liver function tests, and pregnancy test for female participants).
After initial screening, patients were prescribed either FAHF-2 or placebo for 7 days. Patients continued food allergen avoidance for the duration of the study and were asked to refrain from other herbal medication use. Investigators were in direct telephone contact with each patient on 2 occasions during the 7-day period to reinforce medication compliance and assess potential AEs. Patients were instructed to complete a symptom diary while they were participating in the trial. During the final visit, the intercurrent medical history was reviewed and physical examination, spirometry, electrocardiography, and laboratory testing were performed again.
Study Medication
The FAHF-2 product tested was in the form of tablets produced by Xiyuan Chinese Medicine Research and Pharmaceutical Manufacturer, Chinese Academy of Traditional Chinese Medicine Sciences (Beijing, China). The quality, safety, and consistency of FAHF-2 were established according to FDA guidance under a botanical drug title (Chemical, Manufacturing, and Control Data [21 CFR §312.23(a) (7)])21 and are summarized herein.
Raw medicinal herbs
Herbs used in this formula were of Chinese origin.17 All were inspected for identity and quality by pharmacists licensed to identify and process herbal medicines,13,22 and only herbs that met quality and safety standards were used.21 Voucher specimens of the raw herbs are archived in our laboratory.
Manufacturing process and final product quality control
FAHF-2 is composed of aqueous extracts of 9 herbs (Wu-Mei [Fructus Pruni Mume or Japanese apricot, 20.0%], Chuan-Jiao [Percarpium Zanthoxyli Bungeani or Pricklyash peel, 2.0%], Dang-Gui [Radix Angelicae Sinensis or Angelica root, 6.0%], Gan-Jiang [Rhizoma Zingiberis or ginger, 6.0%], Gui-Zhi [Ramulus Cinnamomi Cassiae or cassia cinnamon, 4.0%], Hong-Shen [Radix Ginseng or ginseng, 6.0%], Huang-Bai [Cortex Phellodendri or Phellodendron, 4.0%], Huang-Lian [Rhizoma Coptidis or Goldthread, 6.0%], and Ling-Zhi [Ganoderma or Reishi mushroom, 46.0%]), followed by ethanol purification (Figure 1). The extract powder was made into granules and then compressed to tablets following standard procedures (0.55 g per tablet). The final product, FAHF-2 tablets, was analyzed for contaminants. Levels of heavy metals, microbial pesticide residues, and ethanol residues were well below the recommended limits23–25
Figure 1.
Manufacturing process. Ling-Zhi were cut into small pieces, soaked in water overnight, and then boiled twice for 2 hours each time. The decoctions were combined, dried, and ground into fine powder. Hong-Shen was extracted twice with 80% aqueous ethanol. The ethanol extract was filtered, combined, and evaporated until no residual ethanol was present. The residue was combined with the rest of the 7 herbs and boiled twice in water for 1.5 hours each time. The decoction was collected, combined, and concentrated to 1.05 to 1.10 g/mL and purified by ethanol precipitation and then combined with the Hong-Shen ethanol extract. The extract was dried under reduced pressure (60°C). Both the 8-herb substance and Ling-Zhi powder were granulated and mixed together to form the food allergy herbal formula 2 (FAHF-2) extract.
High-performance liquid chromatographic fingerprint of FAHF-2
We generated a high-performance liquid chromatographic (HPLC) fingerprint of FAHF-2 (Figure 2) as a means of standardization of the FAHF-2 product to ensure batch to batch consistency. Two-dimensional HPLC profiles (at 254 nm) of FAHF-2 were obtained using the Waters Alliance 2695 HPLC system with photodiode array detector (Waters Corporation, Milford, Massachusetts). The separation was performed on a z ZORBAX SB-C18 (4.6 × 150 mm, 5 μm) column (Agilent, Santa Clara, California). The aqueous mobile phase A was 0.1% phosphoric acid, and the mobile phase B was acetonitrile. The separation gradient started at 98% of mobile phase A, linearly decreasing to 75% in 45 minutes, decreasing to 65% in the following 25 minutes, decreasing to 45% in the next 15 minutes, and further decreasing to 25% in another 10 minutes. We also generated HPLC fingerprints of the 9 individual herbs in FAHF-2 using the same method. The HPLC fingerprints of 3 key herbs, Wu-Mei, Huang-Bai, and Ling-Zhi, are shown in Figure 2. Peaks present in Wu-Mei (peaks 1–6), Huang-Bai (peaks 5–8 and 12–14), and Ling-Zhi (peaks 16–29, 31, 32, 35, and 36) were also present in FAHF-2. One peak corresponding to berberine was used as a marker to quantitatively compare batches to ensure consistency.
Figure 2.
High-performance liquid chromatography (HPLC) chromatogram. HPLC fingerprints of the food allergy herbal formula 2 (FAHF-2) (A) and 3 key individual herbs, Wu-Mei, Huang-Bai, and Ling-Zhi (B–D, respectively). HPLC conditions: column, Agilent Zorbax SB-C18 column (150 × 4.6-mm internal diameter; 5-mm particle size); flow rate, 1 mL/min; wavelength, 254 nm; column temperature, 27°C; mobile phase A, 0.10% H3PO4; and mobile phase B, acetonitrile. Data were processed using Waters Empower software.
Placebo capsules
Placebo tablets were identical in appearance but contained corn starch. The same excipients were used, and these tablets were manufactured by the same company as FAHF-2.
Study Procedures
Skin prick testing
Before enrollment and at the end of the week-long treatment, titrated skin prick tests were performed in duplicate with serial 10-fold dilutions (1:10 to 1:200,000) of stock peanut or individual tree nut, fish, and/or shellfish extracts (Greer Laboratories, Lenoir, North Carolina). Negative controls (phenol-saline solution) and positive controls (1-mg/mL histamine base) were also included. Skin prick tests were performed by pricking through a drop of extract with a bifurcated needle. A wheal of 3 mm or greater in diameter was considered a positive response.
Allergen specific IgE measurements
Specific IgE to peanut, tree nut, fish, and/or shellfish was detected using the ImmunoCAP (Phadia, Uppsala, Sweden).
Immunological Studies
Determination of cytokine profiles in patients before and after treatment (ex vivo study)
PBMCs from the patients were separated on a Ficoll gradient (GE Healthcare, Piscataway, New Jersey). For the ex vivo study, 4 × 105 cells per well were cultured in AIM-V (Invitrogen Corporation, Carlsbad, California) media in media alone, allergen (200 μg/mL), or phytohemagglutinin A (PHA) (Invitrogen Corporation; 2 μg/mL) in 96-well round bottom plates. The cultures were incubated in tissue culture conditions for 72 hours at baseline and after a week of treatment with FAHF-2 or placebo. The allergens used included peanut or pecan extracts generated in our laboratory.26 The supernatants were analyzed for the presence of interleukin (IL) 5, interferon γ (IFN-γ), and IL-10 levels by enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Diego, California). For IL-5, IL-10, and IFN-γ analyses, 9 patients from the treated group and 5 patients from the placebo group were included. Two patients from the treated group were not included because of lack of allergen for culturing (cashew and hazelnut). In addition, 1 patient from the treated group and 1 from the placebo group were not included in the analysis because of different culture conditions before and after treatment. Transforming growth factor β (TGF-β) analysis only included 7 treated and 4 placebo patients’ samples because of the previously mentioned reasons and lack of supernatants for analysis from 2 patients in the treated group and 1 from the placebo group.
Measurement of cytokine profiles in PBMCs in response to in vitro FAHF-2 treatment
For the in vitro study of the direct effect of FAHF-2 on cytokine profiles, PBMC samples were obtained from 6 patients enrolled in the phase 1 study at the baseline visit (because of availability of PBMCs after the cultures for the ex vivo experiments). An additional 5 samples were obtained from peanut allergic patients not enrolled in the phase 1 study, who (or whose parents) consented to participate in the in vitro study. The median age of these 11 patients was 12 years (range, 4–23 years), 8 were male, and the median peanut specific IgE level was 22.5 kIU/L (range, 2.33–100 kIU/L). PBMCs prepared as described herein were cultured with AIM-V media alone (Invitrogen Corporation), allergen (200 μg/mL), or allergen plus FAHF-2 (100 or 250 μg/mL) or PHA (2 μg/mL) for 72 hours. Supernatants were harvested and cytokine levels were measured by ELISA.
Statistical Analyses
Formal statistical methods were not used to calculate the sample size for the phase 1 study. The target number of patients was expected to minimize the risk by not exposing a large number of patients to treatment, while providing an ample size to gain impressions of safety and tolerability. Descriptive statistics are presented for demographic and routine laboratory data. Baseline laboratory values were defined as the pretreatment values obtained during the baseline screening visit. Statistical analyses for the immunologic studies were performed using the Wilcoxon signed rank test to assess the difference before and after treatment for the ex vivo studies and allergen- vs allergen plus FAHF-2–treated cultures in vitro.
Safety Monitoring
Safety monitoring for AEs was ongoing throughout the study. Our criteria were adapted from the World Health Organization (WHO) Recommendations for Grading of Acute and Subacute Toxicity27 with modifications; grade 1 AEs under the WHO grading were considered grade 3 AEs in our study, making our criteria for AEs much more stringent (Table 1). This grading system was approved by the FDA for our phase 1 clinical trial.
Table 1.
Grading of Adverse Events
| Adverse events | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Hematologic events | ||||
| Hemoglobin, g/dL | ≥11.0 | ≥11.0 | ≥11.0 | <11.0 |
| Leukocytes, ×103/μL | ≥4.0 | ≥4.0 | ≥4.0 | <4.0 |
| Granulocytes, ×102/μL | ≥2.0 | ≥2.0 | ≥2.0 | <2.0 |
| Platelets, ×103/μL | ≥100 | ≥100 | ≥100 | <100 |
| Metabolic and gastrointestinal | ||||
| Hyperglycemia, mg/dL | <116 | <116 | <116 | ≥116 |
| Hypoglycemia, mg/dL | ≥64 | ≥64 | ≥64 | <64 |
| Hypercalcemia, mg/dL | <10.6 | <10.6 | <10.6 | ≥10.6 |
| Hypocalcemia, mg/dL | ≥8.4 | ≥8.4 | ≥8.4 | <8.4 |
| Bilirubin | BUN <31 mg/dL | BUN <31 mg/dL | BUN <31 mg/dL | BUN >31 mg/dL |
| AST/ALT | ≤1.25 × N | ≤1.25 × N | ≤1.25 × N | >1.26 × N |
| Alkaline phosphatase | ≤1.25 × N | ≤1.25 × N | ≤1.25 × N | >1.26 × N |
| Abdominal distention | None | Mild | Mild to moderate, not requiring treatment | Moderate to severe, requiring treatment |
| Nausea/vomiting | None | None | Loss of appetite, nausea | Vomiting |
| Diarrhea | None | None | Transient, <3 days, <3 times per day | >3 days, >3 times per day |
| Renal | ||||
| Blood urea nitrogen | <1.25 × N | <1.25 × N | <1.25 × N | >1.26 × N |
| Creatinine | <1.25 × N | <1.25 × N | <1.25 × N | >1.26 × N |
| Proteinuria | None | None | None | >1+ (<3 g/L) |
| Hematuria | None | None | None | Microscopic uropathy |
| Fever with drug | None | None | None | Temperature ≥38.5 °C (>3 days) |
| Allergic | None | None | None | Drug rash or fever |
| Neurologic | None | None | None | Any perceived change in sensation, motor skills, mood |
| Dermatologic | None | None | None | Any dermatologic changes or alopecia |
| Cardiovascular | ||||
| Hypotension | None | None | None | Mild hypotension |
| Ischemia/arrhythmia | None | None | None | Any detectable electrocardiographic or symptomatic changes |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; N, upper limit of normal.
According to the study protocol, any AE of grade 3 in an FAHF-2–treated patient would result in immediate discontinuation of use of the study medication in that patient. If a grade 3 AE was observed in 2 active patients in any dose level, the maximum tolerated dose was defined to be 1 dose level lower.
RESULTS
Patients Characteristics
A total of 23 patients with food allergy underwent initial evaluation for the study. Of these, 4 patients met exclusion criteria; 2 patients had no evidence of food allergy on skin prick testing and serum specific IgE testing, and 2 had uncontrolled asthma with an FEV1 less than 80% predicted. Nineteen patients were enrolled in the study and randomized to FAHF-2 or placebo treatments; 1 patient withdrew from the study after the second day (sixth dose) because of an allergic reaction. Eighteen patients (12 patients in the FAHF-2 group and 6 in the placebo group) successfully completed 7 days of treatment with FAHF-2 in this acute phase 1 study and were included in the analyses evaluating the tolerability and safety of FAHF-2. Baseline characteristics of study patients are given in Table 2. No clinically significant differences were found between the FAHF-2 and placebo groups at baseline.
Table 2.
Baseline Allergic History of the Study Participantsa
| Patient No. | Age, y | Sex | Food allergies | Other allergies | Allergen specific IgE (all peanut, except where indicated), kIU/L | Total IgE, kIU/L |
|---|---|---|---|---|---|---|
| Active Treatment | ||||||
| Active treatment | ||||||
| 1 | 22 | M | PN, TN, sesame | Asthma | >100 | 2015 |
| 2 | 15 | M | PN, TN, sesame, SF, milk | Asthma, AD | 10.1 | 2127 |
| 3 | 18 | M | PN, TN, milk, egg, legumes | Asthma, AR | 2.96 | 191 |
| 4 | 14 | M | PN, TN, seeds, legumes | Asthma, AR | >100 | 899 |
| 5 | 16 | F | PN, TN, sesame | Asthma, AD | 80.1 | 348 |
| 6 | 18 | M | PN, TN, SF | Asthma | 3.53 | 131 |
| 7 | 22 | M | TN | None | 5.88 (hazelnut) | 108 |
| 8 | 12 | M | PN, TN | AR, AD | 23.0 | 133 |
| 9 | 23 | F | PN, TN | Asthma, AR, AD | 58 | 161 |
| 10 | 33 | M | TN | AR, AD | 2.45 (hazelnut) | 143 |
| 11 | 16 | M | PN, TN, seeds, egg | Asthma, AR, AD | 22.5 | 259 |
| 12 | 12 | M | TN | Asthma, AR, AD | 50.3 (cashew) | 959 |
| 13 | 12 | M | TN | Asthma AR | 2.33 (pecan) | 74 |
| Placebo | ||||||
| 1 | 17 | F | PN, TN, fish | Asthma, AR | >100 | 712 |
| 2 | 18 | F | PN, TN, sesame | Asthma, AR | 11.3 | 461 |
| 3 | 16 | M | PN, TN, soy, legumes | Asthma, AR, AD | 47.5 | 162 |
| 4 | 15 | M | PN, TN, fish, SF | Asthma, AR, AD | 4.61 | 1646 |
| 5 | 16 | M | PN, TN, seeds, egg | Asthma, AD | 4.1 | 816 |
| 6 | 17 | F | TN, seeds, fish, SF, egg | Asthma, AR, AD | 2.87 (pecan) | 773 |
Abbreviations: AD, atopic dermatitis; AR, allergic rhinitis; PN, peanut; SF, shellfish; TN, tree nut.
The median patient age was 16 years (range, 12–33 years) in the treatment group and 16.5 years (range, 15–18 years) in the placebo group. In the food allergy herbal formula 2 group, 54% had AD, 62% had asthma, 62% had AR, and 100% had multiple food allergies. In the placebo group, 67% had AD, 100% had asthma, 83% had AR, and 100% had multiple food allergies.
Adverse Events
No grade 3 AEs occurred in patients treated with FAHF-2. One patient receiving FAHF-2 reported diffuse urticaria 3 hours after the sixth dose of treatment (12 tablets). He reported no other associated symptoms. He was instructed to discontinue use of the study medication. The rash progressively worsened, and he was seen in a local emergency department 24 hours later. He returned for follow-up on day 7, and on physical examination, there was no urticaria or angioedema, but he had a maculopapular rash on his antecubital fossae and hands. He was referred to a dermatologist that same day and was diagnosed as having a flare of his underlying atopic dermatitis. He was prescribed oral and topical steroids, which led to resolution of his symptoms without sequelae. He had a history of tree pollen allergy; however, he had tolerated multiple Chinese herbal medicines in the past without reaction. He returned for skin prick testing with FAHF-2 one month later and showed no reaction to the herbal product, but he had a reaction to the positive control (histamine) and no reaction to the negative control (saline). Therefore, this patient’s reaction was deemed to be unlikely related to the study medication.
Of the 18 study participants who completed 7 days of treatment, 1 FAHF-2–treated patient (8%) reported loose bowel movements once a day on days 1 through 4 of treatment, which normalized thereafter, and 1 placebo-treated patient (17%) reported 1 episode of vomiting on day 4 of treatment. Neither patient required treatment for symptoms.
Results of laboratory testing for those who completed the study are given in Table 3. Overall, no significant differences were found in laboratory values obtained at baseline or after completing FAHF-2 treatment. Furthermore, no significant differences were found in pulmonary function tests and electrocardiogram findings before and after treatment.
Table 3.
Summary of Laboratory Results for Patients Completing 1 Week of Treatment With FAHF-2 or Placeboa
| Component | FAHF-2 (n = 12) |
Placebo (n = 6) |
Reference range | ||
|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | ||
| Glucose, mg/dL | 78 ± 12 | 80 ± 21 | 80 ± 17 | 69 ± 6 | 60–120 |
| Sodium, mEq/L | 139 ± 2.0 | 139 ± 1.5 | 140 ± 2.4 | 140 ± 1.8 | 135–145 |
| Potassium, mEq/L | 4.1 ± 0.3 | 4.0 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.5–5.0 |
| Chloride, mEq/L | 101 ± 2.5 | 101 ± 2.0 | 103 ± 1.8 | 103 ± 2.2 | 96–108 |
| Carbon dioxide, mEq/L | 26 ± 2 | 22 ± 7 | 25 ± 1.6 | 24 ± 1.5 | 22.0–32.0 |
| Urea, mg/dL | 13 ± 3.5 | 13 ± 3.4 | 14 ± 3.2 | 13 ± 3.0 | 11–25 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.4–1.2 |
| ALT, U/L | 16 ± 4.0 | 18 ± 7 | 15 ± 3.3 | 15 ± 4.8 | 1–53 |
| AST, U/L | 23 ± 4 | 23 ± 8 | 23 ± 4 | 21 ± 5 | 1–50 |
| WBC, × 103/μL | 6.4 ± 1.4 | 6 ± 1.1 | 7.7 ± 1.0 | 7 ± 1.4 | 4.5–11.0 |
| Hemoglobin, g/dL | 14.5 ± 1.6 | 14 ± 1.4 | 14 ± 0.7 | 14 ± 0.6 | 13.9–16.3 |
| Platelet, × 103/μL | 263 ± 44 | 248 ± 48 | 299 ± 71 | 253 ± 40 | 150–450 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FAHF-2, food allergy herbal formula 2; WBC, white blood cell count.
Data are presented as mean ± SD.
Immunologic Outcomes
No significant changes were found in allergen specific IgE or skin prick test results before or after 7 days of FAHF-2 treatment. No significant changes were found in serum cytokine levels before and after treatment with FAHF-2 in the active group (eFigure 1).
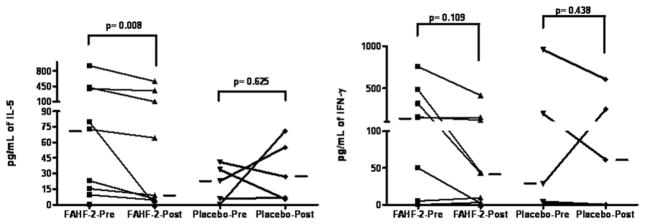
The ex vivo data demonstrated a statistically significant reduction in IL-5 levels after treatment with FAHF-2 and no change in the placebo group (Figure 3). However, IFN-γ, IL-10, and TGF-β did not show any difference in either group before or after treatment.
Figure 3.
Cytokine levels of patient peripheral blood mononuclear cells (PBMCs) before and after food allergy herbal formula 2 (FAHF-2) treatment in ex vivo. Patient PBMCs from the phase 1 study separated on a Ficoll gradient were cultured in AIM-V media with media alone, allergen, or phytohemagglutinin A for 72 hours at baseline and after a week of treatment with FAHF-2 or placebo. The supernatants were analyzed by enzyme-linked immunosorbent assay. Statistical analysis was performed with the Wilcoxon signed rank test. Bars indicate medians of each group (FAHF-2, n = 7–9; placebo, n = 4–5).
PBMCs from food allergic patients were cultured with 100 or 250 μg/mL of FAHF-2. At this optimally effective dose range, statistically significant decreases of IL-5 and increases of IFN-γ and IL-10 levels were observed (Figure 4).
Figure 4.

The in vitro effect of food allergy herbal formula 2 (FAHF-2) treatment on cytokine levels of patient peripheral blood mononuclear cells (PBMCs). Patient PBMCs separated on a Ficoll gradient were cultured in AIM-V media with media alone, allergen, allergen plus FAHF-2, or phytohemagglutinin A for 72 hours. The supernatants were analyzed by enzyme-linked immunosorbent assay. Closed circles indicate an FAHF-2 concentration of 250 μg/mL; open circles, FAHF-2 concentration of 100 μg/mL. Statistical analysis was performed with the Wilcoxon signed rank test. Bars indicate medians of each group (n = 11).
DISCUSSION
The use of complementary and alternative medicines (CAMs) is gaining interest in Western countries because of their reputed effectiveness, low cost, and favorable safety profiles. Patients are often interested in alternative therapy for chronic conditions either because conventional therapies are unsatisfactory or because of concerns about the adverse effects of synthetic drugs. The increasing prevalence and chronic nature of allergic diseases and the lack of preventive and curative therapy influence allergy and asthma patients in Western societies to seek CAM remedies.28,29 Studies have demonstrated the utility of Chinese herbal medicine for the treatment of a variety of diseases, including allergies, allergic rhinitis, and asthma.5,6 We have developed a 9-herb formula that has been shown to be highly effective in protecting peanut allergic mice from peanut-induced anaphylaxis. Furthermore, FAHF-2 has persistent effects and a high safety profile in this murine model.
Although there is strong public interest and increased use of CAMs, including traditional Chinese medicine, there are few phase 1 studies of CAM in the medical literature. A previous phase 1 study of ASHMI (a Chinese herbal treatment for asthma) demonstrated that CAM can be a safe treatment for asthma.30 There is currently no published data on phase 1 studies of CAM for the treatment of food allergy. A phase 1 study is the first step required by the FDA for developing new botanical drugs. Our group is the first to obtain an FDA IND approval to investigate a botanical product to treat food allergy. This phase 1 study was conducted to determine the safety and clinical tolerability of FAHF-2 in patients with food allergy. To our knowledge, this is the first clinical investigation of a botanical drug for multiple persistent food allergy, including peanut allergy, and the first botanical drug trial that includes children. Our study used a double-blind, placebo-controlled, dose escalation design that ensures safety and minimizes potential risks. A double-blind, placebo-controlled approach allowed us to exclude non–drug-related AEs. Our results support the usefulness of this design for testing new CAMs.
Nineteen patients were enrolled in this study. The only AE was minor gastrointestinal discomfort. However, these symptoms were reported equally by patients receiving placebo and active drug, and the symptoms were self-limited. One patient in the active treatment group experienced an allergic reaction; however, given his highly atopic status and negative skin test results to the herbal product, this reaction is unlikely due to the study medication. In addition, no significant differences were found in laboratory parameters, pulmonary functions, and electrocardiogram findings before and after treatment. Therefore, our results demonstrate that FAHF-2 is safe and well tolerated in food allergic patients.
Food allergy is associated with skewing of the immunologic response toward a TH2 profile. Earlier reports of favorable effects of immunotherapy in grass pollen, birch pollen, bee venom, and dust mite allergy have shown decreases in IL-4 or IL-5 levels and increases in IFN-γ and IL-10 after therapy.31–35 Results from this study indicate that 1 week of FAHF-2 treatment did not significantly alter cytokine levels in sera as determined by the Bio-Plex multiplex immunoassay system; however, ex vivo PBMC assays for IL-5 levels demonstrated significantly decreased levels after 7 days of treatment with FAHF-2. Furthermore, PBMCs treated with FAHF-2 in vitro demonstrated a significant reduction in IL-5 and increases in IFN-γ and IL-10 production, suggesting a beneficial immunomodulatory effect of FAHF-2. Of note, the pronounced results in in vitro experiments might be due to the direct effect of active compounds on the effector cells.
In conclusion, the results of this study demonstrate the safety and tolerability of FAHF-2 for food allergic individuals and preliminary beneficial immunologic results. Further research involving more patients studied for a longer duration is necessary to investigate the long-term safety and potential therapeutic effects of this new oral botanical drug for food allergy. A randomized, double-blind, placebo-controlled, phase 2 study to assess efficacy is planned.
Supplementary Material
Acknowledgments
Funding Sources: This publication was supported by the National Institutes of Health/National Center for Complementary and Alternative Medicine grant #1R01AT001495-01A1 and 2R01AT001495-05A1 and the Food Allergy Initiative to Dr. Xiu-Min Li, and in part by a grant from the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant # AI083883 to Dr. Julie Wang. We would also like to acknowledge the support from the CTSA: Clinical Translational Science Award UL1 RR 029887 (Mount Sinai School of Medicine).
Footnotes
Disclosures: Drs. Wang, Patil, Yang, Ko, Lee, and Ms. Noone, have no competing financial interests to disclose. Drs. Sampson and Li share the US Patent PCT/US05/08600 on FAHF-2 and are partners in Herbal Springs, LLC.
Additional figure accompany the on-line version of the article (available at http://www.annallergy.org).
Trial Registration: clinicaltrials.gov Identifier: NCT00602160.
References
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2006;117(2 suppl):S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37:651–660. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 5.Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483–495. doi: 10.1016/S1081-1206(10)60375-4. [DOI] [PubMed] [Google Scholar]
- 6.Li XM, Zhang TF, Sampson H, et al. The potential use of Chinese herbal medicines in treating allergic asthma. Ann Allergy Asthma Immunol. 2004;93:S35–S44. doi: 10.1016/s1081-1206(10)61485-8. [DOI] [PubMed] [Google Scholar]
- 7.Bensky D, Barolet R. Chinese Herbal Medicine: Formulas & Strategies. Seattle, WA: Eastland Press; 1990. [Google Scholar]
- 8.Li XM, Zhang TF, Huang CK, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108:639–646. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 9.Huang KC. The Pharmacology of Chinese Herbs. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 10.Hu KM, He YM. Correlation between complex prescription with lucidum ganoderma in treating rhinoallergitus and constitution. Shanghai J Traditional Chin Med. 2000;8:39–41. [Google Scholar]
- 11.Pierce A. The American Pharmaceutical Association Practical Guide to Natural Medicines. New York, NY: William Morrow & Co Inc; 1999. [Google Scholar]
- 12.Wen MC, Teper A, Srivistava KD, et al. Immunology of T cells by the Chinese herbal medicine Ling Zhi (Ganoderma lucidum) J Allergy Clin Immunol. 2003;111:S320. [Google Scholar]
- 13.Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. Seattle, WA: Eastland Press; 1993. [Google Scholar]
- 14.Towers GH. FAHF-1 purporting to block peanut-induced anaphylaxis. J Allergy Clin Immunol. 2003;111:1140. doi: 10.1067/mai.2003.1493. [DOI] [PubMed] [Google Scholar]
- 15.Li XM, Sampson HA. Reply. J Allergy Clin Immunol. 2003;111:1140–1141. [Google Scholar]
- 16.Wang YM, Huan GX. Utilization of Classical Formulas. Beijing: Chinese Medicine and Pharmacology Publishing Co; 1998. [Google Scholar]
- 17.Eight Famous Classical Traditional Chinese Medicine Formulas. Beijing: China Press of Traditional Chinese Medicine; 1997. [Google Scholar]
- 18.Srivastava KD, Kattan JD, Zou ZM, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava KD, Zhang T, Qu C, et al. Silencing peanut allergy: a Chinese herbal formula, fahf-2, completely blocks peanut-induced anaphylaxis for up to 6 months following therapy in a murine model of peanut allergy. J Allergy Clin Immunol. 2006;117:S328. [Google Scholar]
- 20.Ko J, Busse PJ, Shek L, et al. Effect of Chinese herbal formulas on T-cell responses in patients with peanut allergy or asthma. J Allergy Clin Immunol. 2005;115:S34. [Google Scholar]
- 21.US Food and Drug Administration (FDA) and Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. Washington, DC: FDA; 2004. [Google Scholar]
- 22.The State Pharmacopoeia Commission of The People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Beijing, China: People’s Medical Publishing House; 2005. [Google Scholar]
- 23.Raman P, Patino LC, Nair MG. Evaluation of metal and microbial contamination in botanical supplements. J Agric Food Chem. 2004;52:7822–7827. doi: 10.1021/jf049150+. [DOI] [PubMed] [Google Scholar]
- 24.Dolan SP, Nortrup DA, Bolger PM, et al. Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. J Agric Food Chem. 2003;51:1307–1312. doi: 10.1021/jf026055x. [DOI] [PubMed] [Google Scholar]
- 25.Caldas ED, Machado LL. Cadmium, mercury and lead in medicinal herbs in Brazil. Food Chem Toxicol. 2004;42:599–603. doi: 10.1016/j.fct.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Beyer K, Morrow E, Li XM, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107:1077–1081. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 27.Franklin HR, Simonetti GP, Dubbelman AC, et al. Toxicity grading systems: a comparison between the WHO scoring system and the common toxicity criteria when used for nausea and vomiting. Ann Oncol. 1994;5:113–117. doi: 10.1093/oxfordjournals.annonc.a058760. [DOI] [PubMed] [Google Scholar]
- 28.Ko J, Lee JI, Muñoz-Furlong A, et al. Use of complementary and alternative medicine by food-allergic patients. Ann Allergy Asthma Immunol. 2006;97:365–369. doi: 10.1016/S1081-1206(10)60802-2. [DOI] [PubMed] [Google Scholar]
- 29.Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009;123:297–306. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly-Pieper K, Patil SP, Busse P, et al. Safety and tolerability of an antiasthma herbal formula (ASHMI™) in adult asthmatics: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. J Altern Complement Med. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Secrist H, Chelen CJ, Wen Y, et al. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J Exp Med. 1993;178:2123–2130. doi: 10.1084/jem.178.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jutel M, Pichler WJ, Skrbic D, et al. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995;154:4187–4194. [PubMed] [Google Scholar]
- 33.Bellinghausen I, Metz G, Enk AH, et al. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27:1131–1139. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 34.Cosmi L, Santarlasci V, Angeli R, et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clin Exp Allergy. 2006;36:261–272. doi: 10.1111/j.1365-2222.2006.02429.x. [DOI] [PubMed] [Google Scholar]
- 35.Bohle B, Kinaciyan T, Gerstmayr M, et al. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.