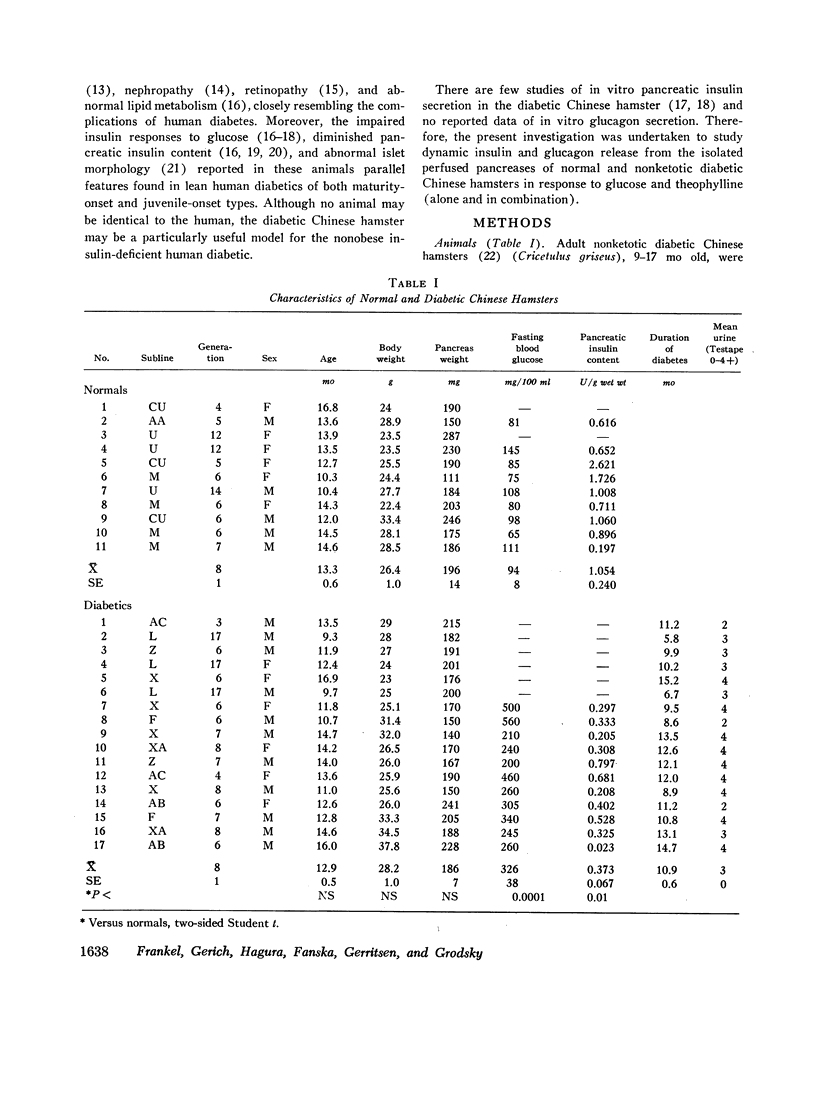
Abstract
Hereditary insulin-deficient diabetes mellitus occurs in certain sublines of nonobese Chinese hamsters. Several characteristics of this syndrome are similar to those seen in insulin-deficient human diabetics. Therefore, to characterize pancreatic islet function, dynamic insulin and glucagon release from normal and nonketotic diabetic hamster pancreases in response to glucose (300 mg/100 ml) and theophylline (10 mM), infused singly and together, was studied in vitro.
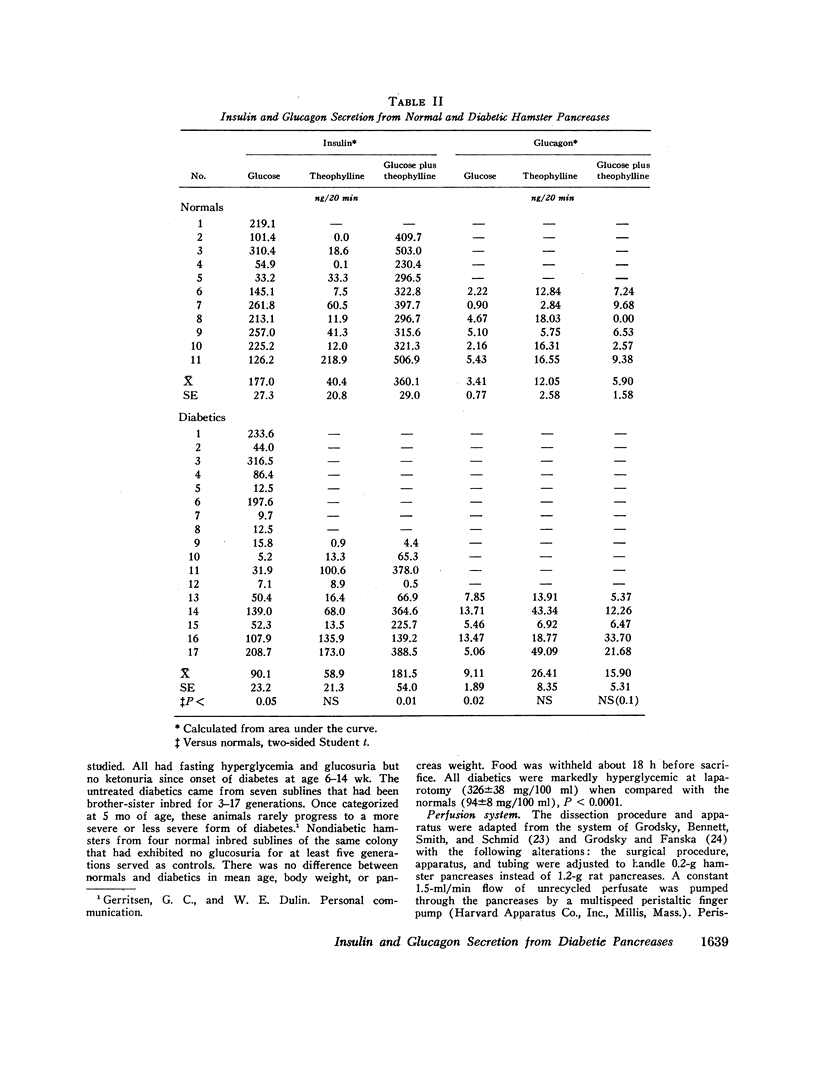
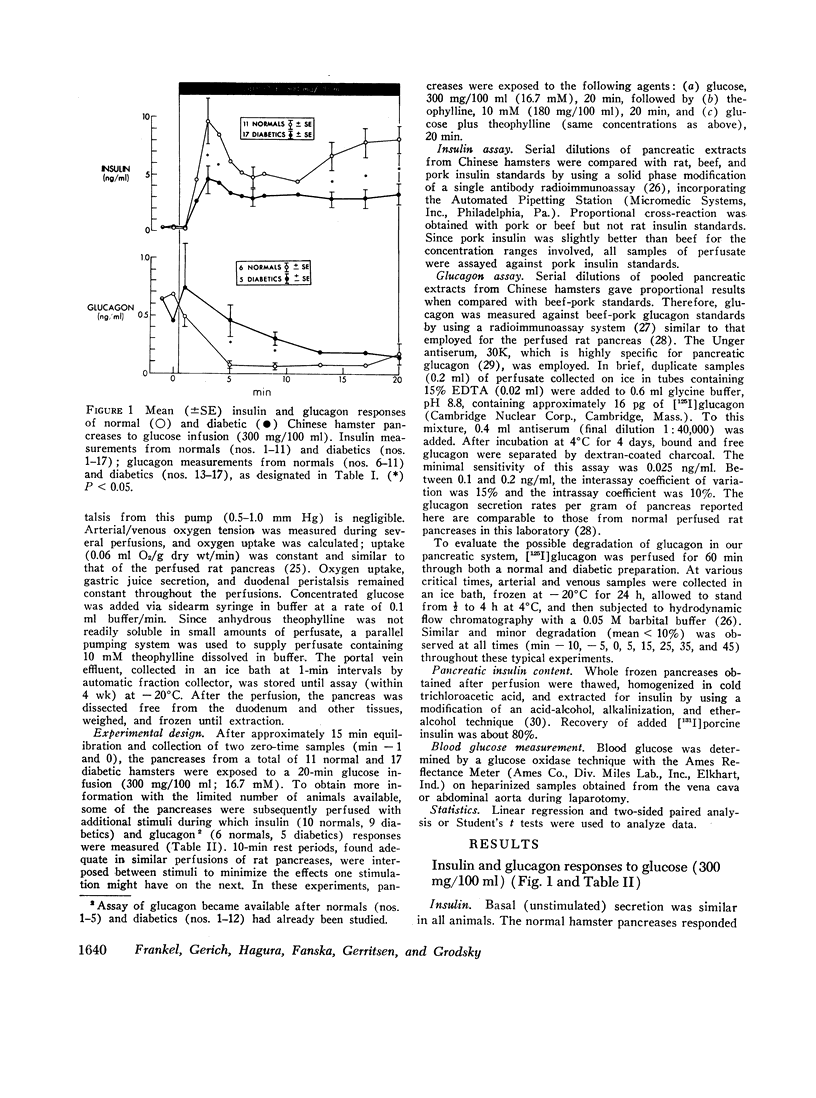
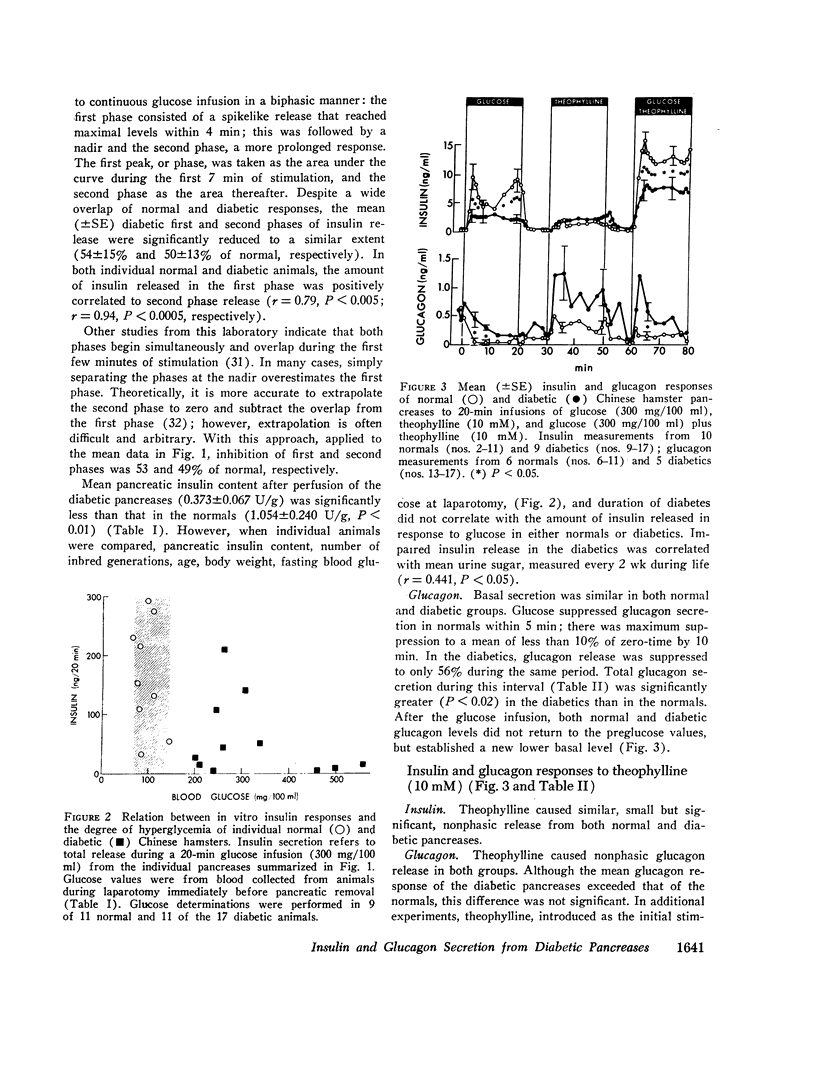
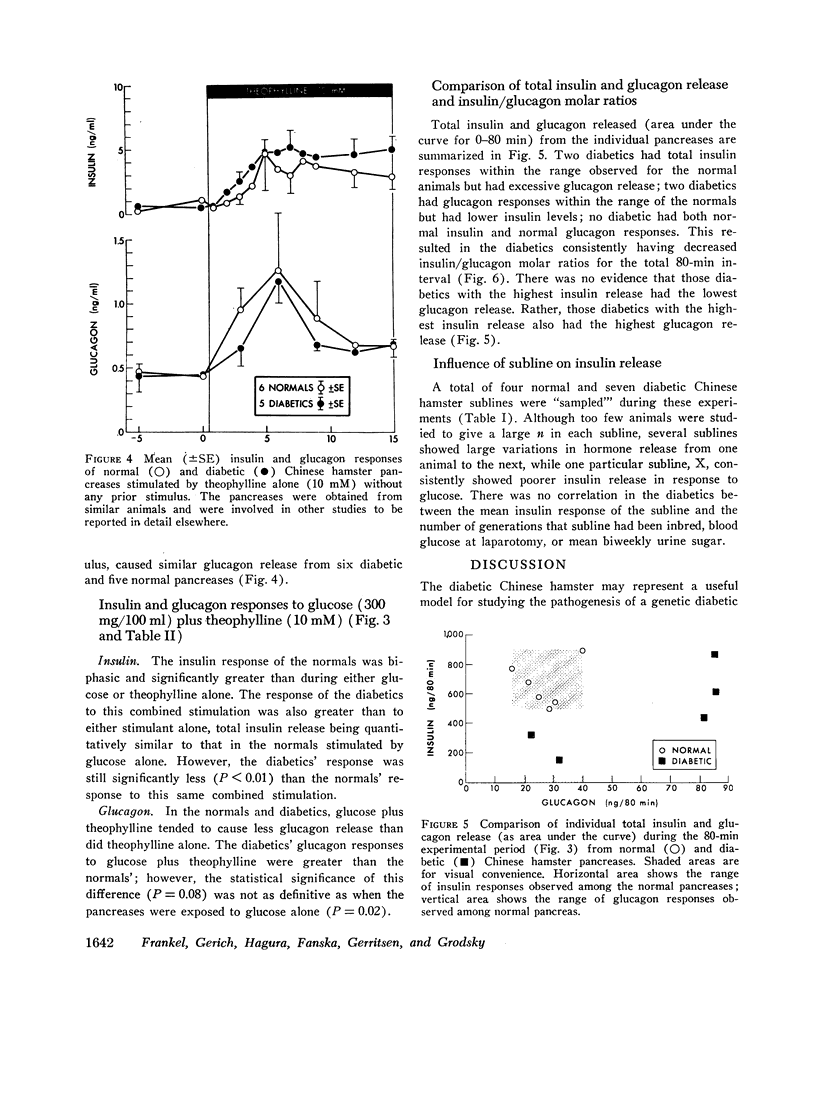
20-min glucose infusions of normal hamster pancreases caused biphasic insulin release, consisting of a rapid first peak and a gradually rising second phase, similar to that reported for man in vivo. Both phases were significantly reduced in the diabetic pancreases. Theophylline alone stimulated similar nonphasic insulin release in both the normal and the diabetic pancreases. Glucose and theophylline together caused greater insulin release than either stimulant alone in both normals and diabetics; however, the diabetic response was still subnormal.
Glucose suppressed glucagon release from normal pancreases; suppression was significantly impaired in diabetics. Theophylline stimulated nonphasic glucagon release in both the normals and diabetics. Glucose partially suppressed the theophylline-stimulated release in both groups.
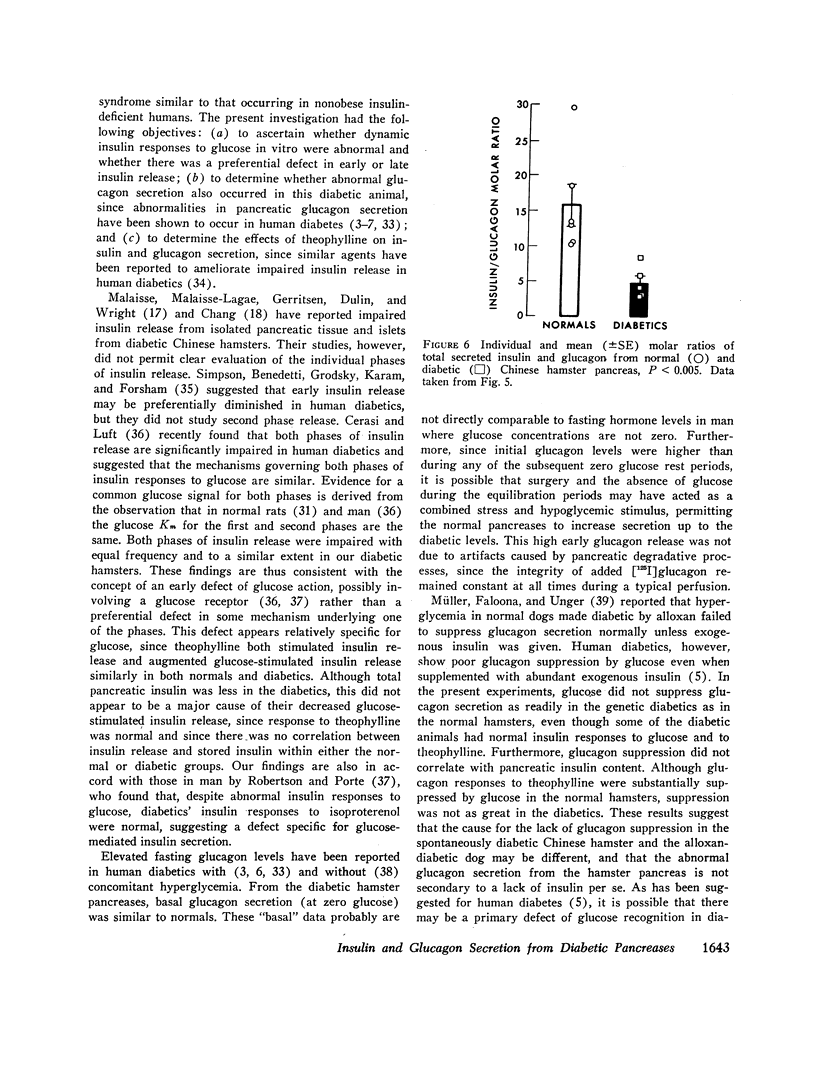
Insulin/glucagon molar ratios of the diabetics were consistently subnormal, although individual hormone levels often overlapped into the normal range.
In summary, the pancreases of genetically diabetic Chinese hamsters perfused in vitro showed: (a) decreased first and second phase insulin release in response to glucose-containing stimuli—only partially ameliorated by theophylline—, and (b) impaired suppression of glucagon in response to glucose, resulting in (c) a decreased insulin/glucagon molar ratio. These data support the suggestion that both alpha and beta cells of diabetic pancreases may be insensitive to glucose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerasi E., Luft R., Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972 Apr;21(4):224–234. doi: 10.2337/diab.21.4.224. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. The effect of an adenosine--3'5'--monophosphate diesterase inhibitor (aminophylline) on the insulin response to glucose infusion in prediabetic and diabetic subjects. Horm Metab Res. 1969 Jul;1(4):162–168. doi: 10.1055/s-0028-1095148. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Federman J. L., Gerritsen G. C. The retinal vasculature of the Chinese hamster: a preliminary study. Diabetologia. 1970 Jun;6(3):186–191. doi: 10.1007/BF01212228. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. An immunochemical assay of total extractable insulin in man. J Clin Invest. 1960 Jul;39:1070–1079. doi: 10.1172/JCI104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G., TARVER H., LIGHT A., SIMPSON M. V. Paper chromatography of insulin. Nature. 1956 Feb 4;177(4501):223–225. doi: 10.1038/177223a0. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Levin S. R., Forsham P. H., Grodsky G. M. In vitro inhibition of pancreatic glucagon secretion by diphenylhydantoin. J Clin Endocrinol Metab. 1972 Dec;35(6):823–824. doi: 10.1210/jcem-35-6-823. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerritsen G. C., Blanks M. C. Preliminary studies on food and water consumption of prediabetic Chinese hamsters. Diabetologia. 1970 Jun;6(3):177–179. doi: 10.1007/BF01212226. [DOI] [PubMed] [Google Scholar]
- Gerritsen G. C., Dulin W. E. Characterization of diabetes in the Chinese hamster. Diabetologia. 1967 Apr;3(2):74–84. doi: 10.1007/BF01222182. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin. II. Effect of calcium. Diabetes. 1972;21(2 Suppl):584–593. doi: 10.2337/diab.21.2.s584. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L., Smith D. F., Schmid F. G. Effect of pulse administration of glucose or glucagon on insulin secretion in vitro. Metabolism. 1967 Mar;16(3):222–233. doi: 10.1016/0026-0495(67)90171-0. [DOI] [PubMed] [Google Scholar]
- Heding L. G., Rasmussen S. M. Determination of pancreatic and gut glucagon-like immunoreactivity (GLI) in normal and diabetic subjects. Diabetologia. 1972 Dec;8(6):408–411. doi: 10.1007/BF01212168. [DOI] [PubMed] [Google Scholar]
- Jarrousse C., Rançon F., Rosselin G., Freychet P. Sécrétion de l'insuline et du glucagon par le pancréas du rat nouveau-né: effet du glucose et de l'adénosine 3'-5' cyclique monophosphate. C R Acad Sci Hebd Seances Acad Sci D. 1973 Jan 29;676(5):797–800. [PubMed] [Google Scholar]
- Kipnis D. M. Insulin secretion in diabetes mellitus. Ann Intern Med. 1968 Nov;69(5):891–901. doi: 10.7326/0003-4819-69-5-891. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Gerritsen G. C., Dulin W. E., Wright P. H. Insulin secretion in vitro by the pancreas of the Chinese hamster. Diabetologia. 1967 Apr;3(2):109–114. doi: 10.1007/BF01222186. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renold A. E., Burr I. The pathogenesis of diabetes mellitus. Possible usefulness of spontaneous hyperglycemic syndromes in animals. Calif Med. 1970 Apr;112(4):23–34. [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Welsh G. W., 3rd, Sims E. A. Diabetes mellitus in the Chinese hamster. II. The evolution of renal glomerulopathy. Diabetologia. 1967 Apr;3(2):266–286. doi: 10.1007/BF01222203. [DOI] [PubMed] [Google Scholar]
- Simpson R. G., Benedetti A., Grodsky G. M., Karam J. H., Forsham P. H. Stimulation of insulin release by glucagon in noninsulin-dependent diabetics. Metabolism. 1966 Nov;15(11):1046–1049. doi: 10.1016/0026-0495(66)90055-2. [DOI] [PubMed] [Google Scholar]
- Sims E. A., Landau B. R. Diabetes mellitus in the Chinese hamster. I. Metabolic and morphologic studies. Diabetologia. 1967 Apr;3(2):115–123. doi: 10.1007/BF01222187. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology. N Engl J Med. 1971 Aug 19;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Evaluation of alpha-cell function by infusion of alanine in normal, diabetic and obese subjects. N Engl J Med. 1973 Mar 8;288(10):487–490. doi: 10.1056/NEJM197303082881003. [DOI] [PubMed] [Google Scholar]




