Abstract
The key concepts and technologies developed in our laboratories in Purdue University for the miniaturization of mass spectrometry analysis systems are introduced. Mass analyzers of simple geometries with a novel atmospheric pressure interface were employed allowed reduction in the size of the ion trap mass spectrometer. Ambient ionization methods were developed and coupled to miniature mass spectrometers to allow direct MS analysis of complex samples without sample preparation and chemical separation. The performance of desorption electrospray ionization, low temperature plasma probe, paper spray as well as two handheld MS systems, Mini 10 and Mini 11, are described with demonstrations of capabilities for chemical analysis.
Keywords: mass spectrometry, miniaturization, ambient ionization
Mass spectrometry (MS) is a highly sensitive and selective analytical technology for both qualitative and quantitative chemical analysis. It is used widely in analytical laboratories for academic research, industrial product development and regulatory compliance with particular importance in proteomics, drug discovery, environmental monitoring, food regulation, forensics and homeland security. For analysis of complex chemical mixtures, mass spectrometers typically are used in conjunction with chromatographic chemical separation methods, such as gas chromatography (GC) and high performance liquid chromatography (HPLC). Conventionally, mass spectrometer analysis systems are lab scale instruments, for example a commercial HPLCMS system weights hundreds of pounds and consumes thousands of watts (Figure 1a), which limits the usage of MS systems for in-situ and/or in-field applications. The current procedures for analysis using MS systems are complex and time consuming. Although the final step in which the mass spectrum is recorded is highly automated and rapid (seconds), sample preparation, including the sample extraction, multi-step cleanup1, 2, and chromatographic separation typically requires much longer times and extensive human intervention. After the mass spectra are obtained, data processing and analysis also needs to be performed for analyte identification and concentration determination before the final analytical result can be reported.
Figure 1.
(a) Chemical analysis using lab scale mass spectrometry systems. (b) Conceptual design of a miniature mass spectrometry device for automated chemical analysis.
At Purdue University, we have been putting major effort into the miniaturization of the MS analysis systems with goals of not only developing MS systems of small size, lower power and lower cost, but also enabling rapid analysis using these systems with minimum requirements for special skills or knowledge from the operators3. As an idealized example shown in Figure 1b, a user could complete an analysis done simply by dropping the sample onto a cartridge and inserting it into a miniature MS analysis device. This type of device could be placed in a physician's office, at airport checking points or police stations, or even in a family home, wherever fast chemical analysis is needed. The function and capability of these MS devices would necessarily be highly specialized, as compared to the current laboratory MS systems that were developed to be versatile. Our strategy for the development for the miniature mass spectrometry analysis systems is to build simple small mass spectrometers with adequate MS analysis performance and with ambient ionization capability for direct analysis of raw samples. This strategy eliminates the requirement for complicated preparation, pretreatment or chromatographic separation.
Ambient ionization methods
The concept of ambient ionization has been developed and pursued to eliminate any extra steps before the MS analysis can be performed. When using a mass spectrometer for chemical analysis, the analyte molecules are first ionized and the mass-to-charge ratios of these ions are then measured using a mass analyzer which is operated in vacuum4. Many ionization methods have been developed over the years, including electron impact ionization (EI)5, chemical ionization (CI)6, matrix assisted laser desorption ionization (MALDI)7, electrospray ionization (ESI)8, atmospheric pressure chemical ionization (APCI), etc. The EI, CI and MALDI are implemented in vacuum while ESI and APCI are at atmospheric pressure, which is why they are also called “atmospheric pressure ionization” methods. To minimize matrix effects, especially for the analysis of complex mixtures, the analytes are extracted and samples with well controlled matrices are prepared before they are analyzed using these ionization methods for MS analysis. Ambient ionization methods differ from these methods in that analytes in raw samples are directly sampled and ionized for MS analysis, which enables high throughput analysis since they require no sample preparation. Desorption electrospray ionization (DESI)9 and direct analysis in real time (DART)10 , developed in 2004 and 2005, respectively, have been followed by more than 30 ambient ionization methods resulting in hundreds of publications in this area11. At Purdue we have developed several ambient ionization methods and applied them for a wide variety of applications. Here, we introduce three of them, desorption electrospray ionization (DESI)9, low temperature plasma (LTP) probe12, and paper spray (PS) ionization13, 14.
Desorption electrospray ionization
Desorption electrospray ionization uses charge droplets for ionizing the analyte molecules in a sample (Figure 2a). Electrospray with a high DC voltage (3-5 KV) and sheath gas (nitrogen normally) is used to generate the high velocity charged droplets that impinge on the sample, forming a thin solvent layer on the sample surface and generating secondary microdroplets which leave the surface15. The analytes are extracted into the liquid, ionized through process such as proton transfer, and are carried away from the surface in the secondary droplets. In the course of desolvation, dry ions of the analytes are formed in air and transferred into the mass spectrometer for MS analysis. As an example, 10 pg of TNT on paper can be directly analyzed using DESI (Figure 2b)16. The applicability of DESI to direct analysis of non-volatile compounds in condensed-phase samples has been demonstrated, including explosives on unclean surfaces16, ingredients in pharmaceuticals tablets17, 18, illicit drug in body fluids19, 20, drugs and lipids on biological tissues21, agrochemicals on fruits22, 23, among other examples.
Figure 2.
Desorption electrospray ionization (DESI) for direct sampling ionization for MS analysis. (a) Schematic setup of a DESI for MS analysis. (b) Mass spectrum recorded of desorption ionization of 10pg TNT on paper, negative MS. The inset shows the corresponding tandem mass spectrum. Reprinted with permission from ref. 16. (c) Automated 2D DESI source for high throughput analysis and imaging. (d) Imaging of rat brain tissue using DESI-MS, m/z = 834, cc: corpus callosum, LV: lateral ventricle. Reprinted with permission from ref. 27.
Chemical imaging capabilities have been developed using DESI24, 25 with a lateral resolution of about 200 um26. A 2D DESI imaging device is shown in Figure 2c, where the position of the DESI source is fixed relative to the inlet of the mass spectrometer, and the sample plate is precisely moved in the x and y directions using step motors. By programming the control system of the moving stage, a batch of samples can be analyzed automatically, which greatly increases the throughput of the analysis. A home-written program was developed to store the spectra recoded for each pixel and to retrieve them later for data analysis and image generation. The most common form of image is a 2D representation of the distribution of a particular compound (ions of a particular m/z value). DESI MS imaging has been applied for tissue analysis to acquire information on the distribution of lipids, including phospholipids (Figure 2d)27, 28 and cholesterol29, drugs30, etc, on raw tissue sections. The studies using DESI imaging of tumor tissues have shown that the boundaries between the tumor and non-tumor sections potentially can be identified using the profiles of the phospholipids21, 31.
Low temperature plasma ionization
Low temperature plasma (LTP) probe is another type of ambient ionization source that utilizes active species generated in a low power plasma to desorb and ionize the analytes in untreated samples12, 32. A low temperature plasma can be generated by dielectric barrier discharge33. The discharge gas, such as helium, argon, nitrogen or air, is passed through an alternating electric field, which is created by applying a high voltage AC (~3kV, 30 kHz) between two electrodes with dielectric barrier material layers in between to restrict the discharge current. A device shown in Figure 3a allows the plasma species to be extracted out of the discharge region, by a combination of the force from the gas flow and electric field, for sampling the chemicals on a surface. The temperature at the sampling spot on surface is around 30 °C12 and no macroscopic damage occurs to the materials to be analyzed. As shown in Figure 3b, the chemicals on a human finger can be directly analyzed using the LTP probe12.
Figure 3.
Low temperature plasma ionization. (a) Schematic setup of the LTP probe. Adapted with permission from ref. 12. (b) Detection of 1 ug cocaine on a human finger. Adapted with permission from ref. 12.
The LTP has been applied for analysis of explosives on surfaces12, ingredient in olive oil32, agrochemicals on fruits34, 35, illicit drugs in raw urine12, etc. Direct analysis of chemicals in bulk aqueous solutions has been achieved using LTP12. Interesting capabilities for fragmenting36 and modifying peptides 36 using LTP have also been developed. Some advantages of LTP, including low gas flow rate (<400 mL/min), the capability of using air as discharge gas, no stringent requirement of sampling angle, and capabilities for large area sampling, make it a good ionization candidate for portable MS instruments.
Paper spray ionization
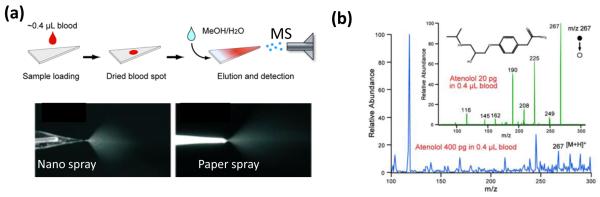
Paper spray ionization is a newly developed method of generating ions directly from samples on a paper substrate13, 14. As shown in Figure 4a, a simple two-step is performed for analysis of samples in extremely complex matrices, such as whole blood samples. First, the sample is loaded onto a chromatography paper of triangle shape, 5 mm in the base and 10 mm in height; then a high voltage DC signal (5kV for example) is applied to the paper while a small amount of solvent (10 ul of methonal/water, 1:1 in volume) is added onto the paper to generate ions. A spray plume similar to that for a nano-ESI is observed (Figure 4a)13 and the softness (amount of internal energy deposited controlling the degree of fragmentation in the mass spectrum) of paper spray is also found to be identical to that of nano-ESI.
Figure 4.
Paper spray ionization. (a) A typical operation process of applying paper spray for analysis of a dried blood spot and the comparison of sprays between paper spray and nano-ESI. Adapted with permission from ref. 13. (b) Analysis of therapeutic drug in dried blood spot, MS and MS/MS analysis of 400 pg atenolol and 20 pg atenolol, respectively, in 0.4 uL blood. Adapted with permission from ref. 14.
Paper is a good material for sample storage and has also been widely used in chromatographic separation. The capability of generating ions directly from the paper allows the development of a wide variety of applications for rapid analysis. During the characterization of paper spray, methanol/water solutions containing organic compounds, amino acids, peptides and proteins were sprayed and ESI-alike spectra were obtained14. Chemicals present on environmental surfaces can be collected onto the paper by wiping and subsequently analyzed with paper spray. Dried spots of biofluid samples including urine, serum and whole blood can be directly analyzed. The therapeutic drugs in dried blood spots (Figure 4b) can be quantitatively analyzed over their therapeutic ranges13. On-line reactions can also be implemented to improve the sensitivity for target analytes, which has been demonstrated in the analysis of cholesterol in human serum using a paper substrate pre-loaded with betaine aldehyde, which reacts with the hydroxyl groups on the cholestrerol14 to improve the ionization efficiency of cholesterol significantly.
As a summary, the DESI, LTP and paper spray represent a set of techniques with complementary characteristics for direct sampling and ionization for MS analysis. Their key features for implementation are listed in Table 1. While DESI serves as a versatile method for analysis of a wide range of compounds and has shown its capability of tissue imaging, the LTP is a top candidate for in-field applications due to the minimum requirement for consumables. Though analysis using paper spray might not be as instantaneous as DESI or LTP, in terms of sampling, its compatibility for sample cartridge design and potential for quantitative analysis make it very attractive for medical and other regulatory applications.
Table 1.
Summary of the characteristics of the ambient ionization methods.
| Ambient Ionization | Voltage | Consumable | Suitable Applications |
|---|---|---|---|
| DESI | DC, 5 kV | N2, 10 L/min; Solvent, 1 μL/min |
Small organic to large biomolecules |
| LTP | AC, 3 kV, 3kHz | Air or He2, <400mL/min |
Gas, organic compounds |
| Paper spray | DC, 3-4.5 kV | Paper substrate, Solvent, 10 μL |
Small organic to large biomolecules |
Miniature mass spectrometers
At Purdue, the miniaturization of mass spectrometers has gone through three stages, the miniaturization of the ion trap mass analyzer, the development of the integrated, miniature mass spectrometers and the development of self-sustained miniature MS analysis systems. The ion trap was selected as the mass analyzer for miniature systems due to its special capabilities of analyzing ions at relatively high pressure and performing tandem mass spectrometry analysis in a single, device of reduced size. The former results in a less stringent requirement on the pumping system, while the latter allows for confirmation of the chemical identity and improvement of signal-to-noise ratios in the analysis of target analytes. Mass analyzers of simplified geometry and small scale, cylindrical ion trap (CIT)37 and rectilinear ion trap (RIT)38, were developed and their performance were studied and optimized using theoretical modeling, numerical simulations and experimental characterization38, 39.
After several iterations in the design of the integrated instruments, two handheld mass spectrometers, the Mini 1040 and Mini 1141 , weighing 22lb and 8lb, respectively, have been developed with mass ranges up to m/z 800 at unit resolution. They have internal electron impact ionization capabilities, using a filament or a glow discharge electron source42, and can be coupled with GC or membrane sample introduction40 for analysis of volatile or semi-volatile organic compounds in gas or liquid samples. Their capability of coupling with atmospheric pressure ion sources was developed using the discontinuous atmospheric pressure interface (DAPI)43, which is critical for taking advantage of ambient ionization methods and thereby developing self-sustained chemical analysis systems. The DAPI allows the miniature mass spectrometers to provide sensitivity comparable to that for lab-scale mass spectrometers but requiring a much lower pumping capacity.
The performance of the Mini 10 and Mini 11 has been demonstrated with the traditional atmospheric pressure ionization methods (APCI and nano-ESI) as well as the ambient ionization methods, such as DESI, LTP and paper spray, etc. The direct analysis of benzene in air can be performed with a LOD of 0.6 ppb (Figure 5b); peptides and proteins (at extended mass ranges) can be analyzed using nano-ESI and MS/MS can be implemented to acquire peptide sequences (Figure 5c and 5d)44; direct sampling ionization using DESI and LTP allowed analysis of raw samples without pre-treatment, for example in the analysis melamine in whole milk with a LOD of 250 ppb 34.
Figure 5.
Handheld miniature mass spectrometers and their analytical performance. (a) Mini 11mass spectrometer, 4 kg and <35W. (b) Monitoring of benzene in air at different concentrations using Mini 10 (10kg, 70W) with APCI. Reprinted with permission from ref. 44. (c) MS/MS analysis of peptide, Mini 11 with nano-ESI, MRFA 1 ug/ml. Reprinted with permission from ref. 41. (d) Analysis of proteins using Mini 11 with nano-ESI and extended mass range, Cytochrome C, 200 ug/ml. Reprinted with permission from ref. 41.
Summary
As capable as mass spectrometers are for chemical analysis, their use for a much wider range of applications is limited not only by their size but also the sample workup required. The development of the miniature mass spectrometers reveals the possibility of the ultimate development of MS analysis systems at the consumer product level while the emergence of ambient ionization shifts the paradigm from simply shrinking traditional GC- or LC-MS systems to developing specialized MS units with direct sampling capabilities. The criteria for evaluating these systems will also not be the general set of rules used for the current laboratory MS systems, but by the adequacy of their performance for the target application for which they are designed.
Acknowledgement
Research supported by the National Science Foundation (CHE 0847205, CHE 0848650 and DBI 0852740), the National Institute of Health (1R21EB009459-01), and W. H. Coulter Foundation (Early Career Award for Translational Research in Biomedical Engineering).
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Hunt DF, Yates JR, Shabanowitz J, Winston S, Hauer CR. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shevchenko A, Wilm M, Vorm O, Mann M. Analytical Chemistry. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang Z, Cooks RG. Annual Review of Analytical Chemistry. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann E. d., Stroobant V. Mass Spectrometry: Principles and Applications. Second Edition John Wiley &Sons, LTD; 2002. [Google Scholar]
- 5.Lotz W. Journal Name: Z. Phys., 216: 241-7(1968).; Other Information: Orig. Receipt Date: 31-DEC-69. 1968 Medium: X. [Google Scholar]
- 6.Harrison AG. Chemical ionization mass spectrometry. 2nd ed. CRC Press; Boca Raton, FL: 1992. [Google Scholar]
- 7.Karas M, Bachmann D, Hillenkamp F. Analytical Chemistry. 1985;57:2935–2939. [Google Scholar]
- 8.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 9.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 10.Cody RB, Laramee JA, Durst HD. Analytical Chemistry. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang Z, Zhang X. The Analyst. 2010;135:659–660. doi: 10.1039/c003812c. [DOI] [PubMed] [Google Scholar]
- 12.Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, Ouyang Z. Analytical Chemistry. 2008;80:9097–9104. doi: 10.1021/ac801641a. [DOI] [PubMed] [Google Scholar]
- 13.He W, Jiangjiang L, Cooks RG, Zheng O. Angewandte Chemie International Edition. 2010;49:877–880. [Google Scholar]
- 14.Liu J, Wang H, Manicke NE, Lin J-M, Cooks RG, Ouyang Z. Analytical Chemistry. 82:2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 15.Costa AB, Cooks RG. Chemical Communications. 2007:3915–3917. doi: 10.1039/b710511h. [DOI] [PubMed] [Google Scholar]
- 16.Takats Z, Cotte-Rodriguez I, Talaty N, Chen H, Cooks RG. Chemical Communications. 2005:1950–1952. doi: 10.1039/b418697d. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Talaty NN, Takats Z, Cooks RG. Analytical Chemistry. 2005;77:6915–6927. doi: 10.1021/ac050989d. [DOI] [PubMed] [Google Scholar]
- 18.Tiina JK, Justin MW, Raimo AK, Tapio K, Cooks RG, Risto K. Rapid Communications in Mass Spectrometry. 2006;20:387–392. doi: 10.1002/rcm.2304. [DOI] [PubMed] [Google Scholar]
- 19.Kauppila TJ, Talaty N, Kuuranne T, Kotiaho T, Kostiainen R, Cooks RG. The Analyst. 2007;132:868–875. doi: 10.1039/b703524a. [DOI] [PubMed] [Google Scholar]
- 20.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Analytical Chemistry. 2007;79:8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 21.Justin MW, Satu MP, Zoltan T, Cooks RG, Richard MC. Angewandte Chemie International Edition. 2005;44:7094–7097. [Google Scholar]
- 22.Garcia-Reyes JF, Jackson AU, Molina-Diaz A, Cooks RG. Analytical Chemistry. 2008;81:820–829. doi: 10.1021/ac802166v. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AU, Tata A, Wu C, Perry RH, Haas G, West L, Cooks RG. The Analyst. 2009;134:867–874. doi: 10.1039/b823511b. [DOI] [PubMed] [Google Scholar]
- 24.Ifa DR, Manicke NE, Dill AL, Cooks RG. Science. 2008;321:805. doi: 10.1126/science.1157199. [DOI] [PubMed] [Google Scholar]
- 25.Wiseman JM, Ifa DR, Zhu Y, Kissinger CB, Manicke NE, Kissinger PT, Cooks RG. Proceedings of the National Academy of Sciences. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ifa DR, Wiseman JM, Song Q, Cooks RG. International Journal of Mass Spectrometry. 2007;259:8–15. [Google Scholar]
- 27.Justin MW, Demian RI, Qingyu S, Cooks RG. Angewandte Chemie International Edition. 2006;45:7188–7192. [Google Scholar]
- 28.Manicke NE, Wiseman JM, Ifa DR, Cooks RG. Journal of the American Society for Mass Spectrometry. 2008;19:531–543. doi: 10.1016/j.jasms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu C, Ifa DR, Manicke NE, Cooks RG. Analytical Chemistry. 2009;81:7618–7624. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qizhi H, Nari T, Robert JN, Cooks RG. Rapid Communications in Mass Spectrometry. 2006;20:3403–3408. doi: 10.1002/rcm.2757. [DOI] [PubMed] [Google Scholar]
- 31.Dill AL, Ifa DR, Manicke NE, Costa AB, Ramos-Vara JA, Knapp DW, Cooks RG. Analytical Chemistry. 2009;81:8758–8764. doi: 10.1021/ac901028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juan FG-R, Fabio M, Jason DH, Nicholas AC, Sheran O, Zheng O, Giovanni S, Cooks RG. Rapid Communications in Mass Spectrometry. 2009;23:3057–3062. doi: 10.1002/rcm.4220. [DOI] [PubMed] [Google Scholar]
- 33.Na N, Zhao M, Zhang S, Yang C, Zhang X. Journal of the American Society for Mass Spectrometry. 2007;18:1859–1862. doi: 10.1016/j.jasms.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Xu W, Visbal-Onufrak MA, Ouyang Z, Cooks RG. The Analyst. doi: 10.1039/b923427f. [DOI] [PubMed] [Google Scholar]
- 35.Wiley JS, Garcia-Reyes JF, Harper JD, Charipar NA, Ouyang Z, Cooks RG. The Analyst. doi: 10.1039/b919493b. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Zheng O, Cooks RG. Angewandte Chemie International Edition. 2008;47:8646–8649. doi: 10.1002/anie.200803477. [DOI] [PubMed] [Google Scholar]
- 37.Langmuir DB, Langmuir RV, Shelton H, Wuerker RF. THOMPSON RAMO WOOLDRIDGE INC United States. 1962;3065640 [Google Scholar]
- 38.Ouyang Z, Wu G, Song Y, Li H, Plass WR, Cooks RG. Anal. Chem. 2004;76:4595–4605. doi: 10.1021/ac049420n. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Cooks RG, Ouyang Z. International Journal of Mass Spectrometry. 2005;241:119–132. [Google Scholar]
- 40.Gao L, Song Q, Patterson GE, Cooks RG, Ouyang Z. Anal. Chem. 2006;78:5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 41.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Anal. Chem. 2008 doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 42.Liang G, Qingyu S, Robert JN, Jason D, Cooks RG, Zheng O. Journal of Mass Spectrometry. 2007;42:675–680. doi: 10.1002/jms.1201. [DOI] [PubMed] [Google Scholar]
- 43.Gao L, Cooks RG, Ouyang Z. Anal. Chem. 2008;80:4026–4032. doi: 10.1021/ac800014v. [DOI] [PubMed] [Google Scholar]
- 44.Huang G, Gao L, Duncan J, Harper JD, Sanders NL, Ouyang Z, Cooks RG. Journal of the American Society for Mass Spectrometry. 21:132–135. doi: 10.1016/j.jasms.2009.09.018. [DOI] [PubMed] [Google Scholar]