Abstract
Diagnosis of respiratory viruses traditionally relies on culture or antigen detection. We aimed to demonstrate capacity of the reverse transcription polymerase chain reaction/electrospray ionization mass spectrometry (RT-PCR/ESI-MS) platform to identify clinical relevant respiratory viruses in nasopharyngeal aspirate (NPA) samples and compare the diagnostic performance characteristics relative to conventional culture- and antigen-based methods. An RT-PCR/ESI-MS respiratory virus surveillance kit designed to detect respiratory syncytial virus, influenza A and B, parainfluenza types 1–4, Adenoviridae types A–F, Coronaviridae, human bocavirus, and human metapneumovirus was evaluated using both mock-ups and frozen archived NPA (N = 280), 95 of which were positive by clinical virology methods. RT-PCR/ESI-MS detected 74/95 (77.9%) known positive samples and identified an additional 13/185 (7%) from culture-negative samples. Viruses that are nondetectable with conventional methods were also identified. Viral load was semiquantifiable and ranged from 2400 to >320 000 copies/mL. Time to results was 8 h. RT-PCR/ESI-MS showed promise in rapid detection of respiratory viruses and merits further evaluation for use in clinical settings.
Abbreviations: Electrospray, ionization mass spectrometry RT-PCR (RT-PCR/ESI-MS); Nasopharyngeal, aspirate; Reverse, transcription PCR (RT-PCR); Respiratory, tract infections (RTIs); Limit, of detection (LOD); Respiratory, syncytial virus (RSV)
Keywords: Electrospray ionization mass spectrometry, Respiratory tract infections, Viruses, Diagnosis, Surveillance
1. Background
Acute respiratory tract infections (RTIs), which are considered the “forgotten pandemic”, remain the leading cause of all-cause mortality in children worldwide (Bryce et al., 2005). In spite of public health prevention measures and advances in therapeutics, incidence rates of RTIs in the United States have varied little over the past 70 years (Monto and Ullman, 1974), and etiologic diagnosis remains challenging. The lack of broad-based rapid and accurate diagnostic tools leads to overprescribing of antibiotics, delayed definitive diagnosis with potential for increased complications, and ineffective epidemic control.
Molecular diagnostic assays have the greatest potential for impacting clinical practice for infectious agents, such as viruses, where conventional microbiologic methods (i.e., culture) do not provide timely results (Ratcliff et al., 2007). While nucleic acid amplification tests have been developed for multiple individual viruses (Liolios et al., 2001), the utility in clinical settings has been further extended by the availability of single platform systems, which can simultaneously detect multiple pathogens (Liao et al., 2009, Pabbaraju et al., 2008, Raymond et al., 2009, Wu and Tang, 2009).
Electrospray ionization mass spectrometry following broad-range reverse transcription polymerase chain reaction (RT-PCR/ESI-MS), one of the single system platforms, has the potential to rapidly detect and semiquantify different pathogens simultaneously. To date, studies with RT-PCR/EMI-MS have been restricted to evaluation with individual respiratory bacteria and viruses [i.e., streptococcus (Ecker et al., 2005), coronavirus (SARS) (Sampath et al., 2005), adenovirus (Russell et al., 2006), and influenza viruses (Sampath et al., 2007)], or detailed characterization [e.g. resistance gene recognition (Ecker et al., 2006), genotyping of the organism (Ecker et al., 2005)]. The capacity of RT-PCR/EMI-MS for broad-range detection and rapid turnaround may provide a useful tool for clinicians in health care settings to aid in early diagnosis of RTIs.
We aimed to 1) demonstrate capacity of the RT-PCR/ESI-MS platform to identify and subtype multiple respiratory viruses in nasopharyngeal aspirates (NPAs) 2) and examine the performance characteristics of the RT-PCR/ESI-MS platform in a pilot hospital-based retrospective proof-of-concept study.
2. Materials and methods
2.1. Viral species and mock-ups
Seven clinically relevant viral pathogens [coronavirus, respiratory syncytial virus (RSV), influenza A and B, parainfluenza types 2 and 3, adenovirus, and coronavirus (SARS)] were obtained from Zeptometrix with given concentrations (Buffalo, NY). Pooled negative NPAs tested by RT-PCR/ESI-MS were used for the mock-up experiments. To determine the limit of detection (LOD), serial of 2-fold dilutions from 1000 to 1 genome copy/well (1.3 × 105 to 133 genome copies/mL) were spiked into the negative NPAs. Each concentration was repeated 5 times independently to ensure the precision. The LOD was determined by the least concentration for which 5 out of 5 repeats were detected by RT-PCR/ESI-MS.
2.2. Clinical samples
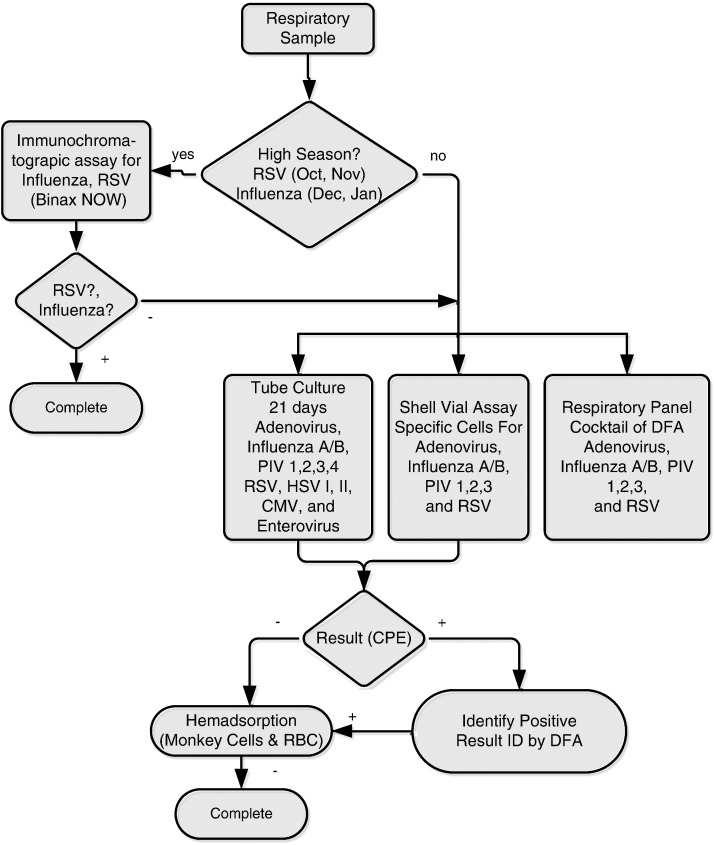
Clinical samples were derived from patients who presented with suspected RTI in a tertiary inner-city teaching hospital according to a standardized collection procedure from May 2006 to November 2007. “Excess” clinical samples were stored at −80 °C until nucleic acid extraction. The microbiology database was queried for standard clinical virologic results including rapid immunochromatographic tests for influenza and RSV (Binex Now, Inverness, Bedford, UK), direct fluorescent antibody staining tests (D3 ultra DFA respiratory virus ID kit, Diagnostic HYBRIDS, Athens, OH), rapid shell vial culture identification (R-mix too, Diagnostic HYBRIDS, Athens, OH), roller tube culture, and hemadsorption tests (Fig. 1 ). Routine PCR testing was not performed in the clinical virology laboratory. Two negative NPAs by clinical virology were obtained to match each positive sample in the same month of collection. The study was approved by the Johns Hopkins University Institutional Review Board.
Fig. 1.
Diagnostic algorithm of clinical virology laboratory for respiratory samples. Diagnostic algorithm used in clinical virology laboratory to detect respiratory viruses mainly divided into respiratory season or nonrespiratory season. Respiratory season defined as October to the following January (October to November for RSV, December to January for influenza). Immunochromatographic assays were used in respiratory season as the sole screening test for RSV and influenza, which will stop the testing algorithm if results are positive. Respiratory panel cocktail DFA tests serve for adenovirus, influenza A and B, parainfluenza types 1–3, and RSV, which will stop the testing algorithm if results are positive in nonrespiratory season or for those screened negative by immunochromatographic assays in respiratory season as well. RSV = respiratory syncytial virus; Flu = influenza A or B viruses; PIV = parainfluenza virus; HSV = herpes simplex virus; CMV = cytomegalovirus; hMPV = human metapneumovirus; DFA = direct fluorescent antibody test; CPE = cytopathic effect; RBC = red blood cells; ID = identified.
2.3. Sample processing
Samples were processed for total nucleic acid extraction using the Thermo King-Fisher (Waltham, MA) robot according to an Ambion (ABI, Foster City, CA) MagMAX viral kit extraction protocol. All samples were processed by a dedicated investigator (KC) who was masked to the clinical virology laboratory results at the time of processing.
2.4. Reverse transcription PCR
One-step RT-PCR was performed in a 5-μL reaction mix consisting of 4 U of AmpliTaq Gold (Applied Biosystems, Foster City, CA); 20 mmol/L Tris, pH 8.3; 75 mmol/L KCl; 1.5 mmol/L MgCl2; 0.4 mol/L betaine; 800 mmol/L mix of dATP, dGTP, dCTP, and dTTP (Bioline USA, Randolph, MA); 10 mmol/L dithiothreitol; 100 ng sonicated polyA DNA (Sigma, St. Louis, MO); 40 ng random hexamers for the RT (Invitrogen, Carlsbad, CA); 1.2 U Superasin (Ambion, Austin, TX); 400 ng T4 gene 32 protein (Roche Diagnostics, Indianapolis, IN); 2 U Superscript III (Invitrogen); 20 mmol/L sorbitol (Sigma); and 250 nmol/L of each specific PCR primer. RT-PCR cycling conditions were 60 °C for 5 min, 4 °C for 10 min, 55 °C for 45 min, 95 °C for 10 min, followed by 8 cycles of 95 °C for 30 s, 48 °C for 30 s, and 72 °C for 30 s, with the 48 °C annealing temperature increasing 0.9 °C each cycle. The RT-PCR was continued for 37 additional cycles of 95 °C for 15 s, 56 °C for 20 s, and 72 °C for 20 s. The RT-PCR cycle ended with a final extension of 2 min at 72 °C followed by a 4 °C hold. RT-PCR was used for both RNA and DNA virus amplification.
2.5. Respiratory virus surveillance panel
The assay was performed using the Ibis T5000 Respiratory Virus Surveillance II kit (Ibis Biosciences, Carlsbad, CA), designed to detect and subtype viruses from 7 groups: conventional viruses (RSV, influenza A and B, parainfluenza types 1–4, Adenoviridae types A–F) and viruses not conventionally identified in our clinical virology laboratory (Coronaviridae, human bocavirus, and human metapneumovirus). In this research-use-only kit, there were 16 primer pairs distributed in a 96-well plate, in which each well contained one pair of primers. Most viruses had 2 primer sets, but there were 4 sets of primers for influenza viruses. Each plate was able to test 6 patient samples with each specimen being tested in 16 wells.
2.6. Mass spectrometry for base composition analysis and pathogen semiquantification
The Ibis T5000 platform performed automated post-PCR desalting, ESI-MS signal acquisition, spectral analysis, and data reporting to analyze RT-PCR product as described previously (Sampath et al., 2005). Briefly, the steps were as follows: 15-μL aliquots of each PCR reaction were desalted and purified using a weak anion exchange protocol as described elsewhere (Ward et al., 2004). Accurate mass (61 ppm), high-resolution (M/dM.100 000 full-width half-maximum) mass spectra were acquired for each sample using high-throughput ESI-MS protocols described previously (Sampath et al., 2007). For each sample, approximately 1.5 μL of analyte solution was consumed during the 74-s spectral acquisition. Raw mass spectra were post-calibrated with an internal mass standard and deconvolved to monoisotopic molecular masses. Unambiguous base compositions were derived from the exact mass measurements of the complementary single-stranded oligonucleotides. Semiquantitative results of pathogens were obtained by comparing the peak heights with the internal PCR calibration internal mass standard present in every PCR well at 100 molecules, which was also treated as the internal positive control in each well. A negative control was implemented in each batch of processing with sterile viral transport media as well.
2.7. Evaluation of sample for which virology and RT-PCR/ESI-MS did not agree
Our Clinical Virology Laboratory does not perform PCR tests routinely for all respiratory viruses. Accordingly, those samples for which clinical virology laboratory and RT-PCR/ESI-MS did not agree and which had enough remaining volume (200 μL) were sent to Viracor (Lee's Summit, MO) for identification by another PCR-based platform (Luminex Respiratory Assay, Austin, TX), which is able to detect all the viruses RT-PCR/ESI-MS could detect except for human bocavirus (Pabbaraju et al., 2008).
2.8. Throughput determination
Sample throughput for RT-PCR/ESI-MS, which included using 1 King/Fisher extraction robot, 1 JANUS automated dispensing robot, 4 Eppendorf thermocyclers, and 1 T5000 cleanup and injection automation system, was evaluated.
2.9. Statistical analysis
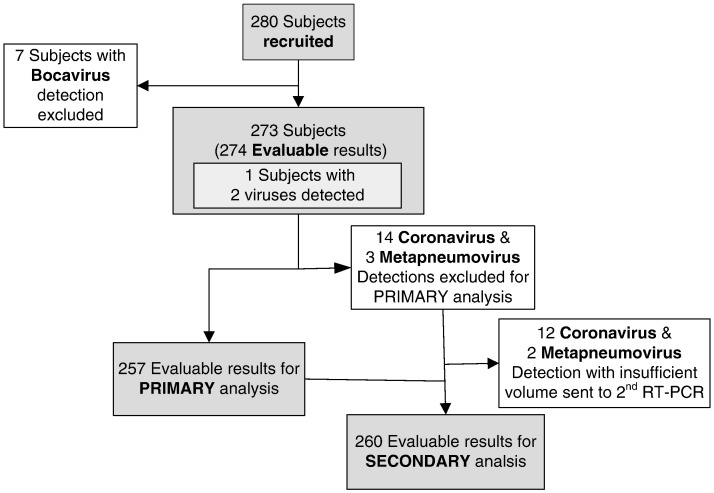
For the primary analysis evaluating performance of RT-PCR/ESI-MS, standard clinical virology laboratory results were used as the reference test. Fig. 2 describes the number of subjects, each of which had one NPA sample evaluated. The number of “evaluable results” was operationally defined for purposes of primary and secondary performance evaluation as the number of individual evaluable results that could be compared to one another because each NPA sample could yield test results (by culture or RT-PCR/MS-ESI) of “negative”, single positive, or multiple viral detections (Fig. 2). In the secondary analysis, we combined clinical virology laboratory results and the secondary (i.e., Luminex) PCR-based results as the reference test. Samples containing viruses for which the hospital's clinical virology laboratory had no protocol available (i.e., bocavirus and coronavirus detection) were excluded from the primary analysis but included in the secondary analysis if the viral agent could be detected by the other (Luminex) RT-PCR–based method (i.e., all viruses except bocavirus). Confidence intervals (CIs) for sensitivity and specificity were based on exact binomial probabilities. Two-sample Wilcoxon Mann–Whitney test was used for comparing the viral load in the clinical virology-positive group with the negative group.
Fig. 2.
Flow diagram of patient recruitment and analysis. Bocavirus that were not detectable in both clinical virology laboratory and another PCR-based platform were excluded (n = 7). After further exclusion of 14 coronavirus and 3 human metapneumovirus detections that clinical virology laboratory had no protocol for detection, 257 were included in the primary performance analysis as in Table 1. Twenty-five samples for which clinical virology laboratory and RT-PCR/ESI-MS did not agree or clinical virology laboratory has no protocol to detect with sufficient volume left were sent to another PCR-based assay for secondary analysis.
3. Results
3.1. Mocked NPA samples
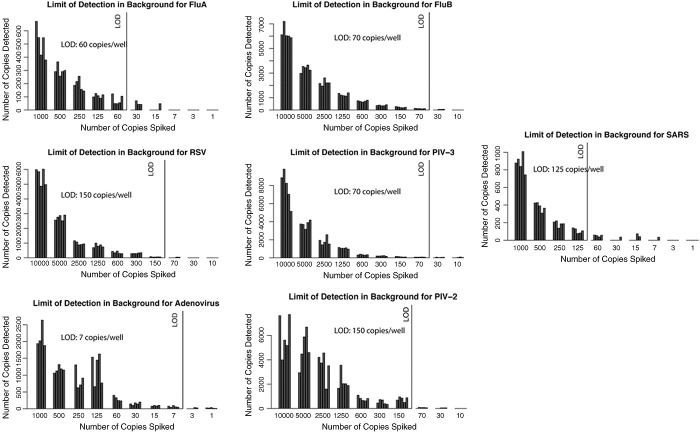
All the mocked NPA samples in 7 viruses groups were detected successfully by the RT-PCR/ESI-MS to the level of 150 copies/well (Fig. 3 ). The RT-PCR/ESI-MS was most sensitive in detecting adenovirus and least sensitive in detecting RSV (LOD: 7 and 150 genome copies/well, respectively).
Fig. 3.
LOD of RT-PCR/ESI-MS for respiratory viruses in NPA mock-up samples. Seven clinically relevant viral pathogens [coronavirus, respiratory syncytial virus (RSV), influenza A and B, parainfluenza types 2 and 3, adenovirus, and coronavirus (SARS)] were spiked into pooled negative NPAs tested by RT-PCR/ESI-MS to determine the LOD with 5 repeats of serial of 2-fold dilutions from 1000 to 1 copies/well (1.3 × 105 to 133 copies/mL). The RT-PCR/ESI-MS was most sensitive in detecting adenovirus (LOD: 7 copies/well) and least sensitive in detecting RSV (LOD: 150 copies/well). One copy/well is approximate to 133 copies/mL.
3.2. Clinical NPA samples
3.2.1. Primary analysis: comparison of RT-PCR/ESI-MS and clinical virology diagnostic algorithm
A total of 274 examinable results from 280 NPA samples collected from clinical virology previously frozen repository from March 2006 to November 2007 were identified, after excluding bocavirus detection unexaminable in both clinical virology diagnostic algorithm and the secondary PCR-based assay (Fig. 2). After exclusion, overall agreement was 87.9% (95% CI, 83.3–91.7%); sensitivity and specificity, excluding those nonconventional viruses for which Johns Hopkins University (JHU) has no identification protocols (14 coronavirus and 3 metapneumovirus), were 77.9% (95% CI, 68.2–85.8%) and 92.1% (95% CI, 86.9–95.7%), respectively (Table 1 ). Among 257 samples for which results were available for the first accuracy analysis, 34 samples were found to disagree between RT-PCR/ESI-MS and clinical virology laboratory. For individual pathogens identified in clinical virology, RT-PCR/ESI-MS detected 98.9% RSV, 77.8% adenovirus, 70.3% parainfluenza, and 59.1% influenza viruses (Table 2 ). RT-PCR/ESI-MS also successfully subtyped 8 influenza A, 5 influenza B, 2 parainfluenza type 1, and 14 parainfluenza type 3 that were identified by clinical virology, as well as 7 adenovirus types B and C that clinical virology had no protocol to subtype, but mis-subtyped 1 parainfluenza type 2 and 1 parainfluenza type 3, which were identified by the virology laboratory as parainfluenza type 3 and parainfluenza type 4, respectively. The median storage time was 10 months (range 7–19).
Table 1.
Overall performance of the RT-PCR/ESI-MS platform compared to clinical virologic methods
| JHU virology reference tests (257a) |
|||
|---|---|---|---|
| Positive | Negative | ||
| RT-PCR/ ESI-MS | Positive | 74 | 13 |
| Negative | 21 | 149 | |
Overall agreement, sensitivity, and specificity, excluding those nonconventional viruses for which JHU has no identification protocols, were 87.9%, 77.9%, and 92.1%, respectively.
Excluding 24 viruses (14 coronavirus, 7 bocavirus, and 3 metapneumovirus), which were detected by RT-PCR/ESI-MS for which clinical virology had no identification protocols.
Table 2.
Performance of the RT-PCR/ESI-MS platform for individual pathogens compared to clinical virologic methods
| Pathogens | RT-PCR/ESI-MS (+) AND clinical virology (+) | RT-PCR/ESI-MS (+) AND clinical virology (−) | RT-PCR/ESI-MS (−) AND clinical virology (+) | RT-PCR/ESI-MS (+) AND clinical virology (+) OR second RT-PCR (+) |
|---|---|---|---|---|
| RSV | 34 | 3 | 2 | 36 |
| Parainfluenza | 19 | 6 | 8 | 21 |
| Influenza | 14 | 4 | 9 | 17 |
| Adenovirus | 7 | 0 | 2 | 7 |
| Coronavirus | NA | NA | NA | 2 |
| Metapneumovirus | NA | NA | NA | 1 |
NA = not available.
Viral load was semiquantifiable by RT-PCR/ESI-MS and ranged from 2400 to >320 000 copies/mL. Viral loads of RT-PCR/ESI-MS–positive samples in the clinical virology-negative group were significantly lower than in the clinical virology-positive group (median 66 960 vs >320 000 copies/mL, respectively, P = 0.04).
3.2.2. Secondary analysis: comparison of RT-PCR/ESI-MS with clinical virology and secondary PCR-based assay
Total examinable results were 260 (Fig. 2). Among the 51 original samples with results in disagreement between RT-PCR/ESI-MS and conventional virology assays or for which JHU had no protocols, 25 (43%) had sufficient volume to be sent to for another PCR-based method, 12 had confirmed RT-PCR/ESI-MS results, including 7 positive conventional viruses (3 influenza A, 2 RSV, 2 parainfluenza). Two coronavirus and 1 metapneumovirus detection not detectable by conventional clinical virology protocols were confirmed as well. The overall agreement, sensitivity, and specificity after secondary analysis of available samples were 90.7% (95% CI, 86.5–94.0%), 83.2% (95% CI, 74.4–90.0%), and 95.6% (95% CI, 91.1–98.2%), respectively (Table 3 ).
Table 3.
Performance of the RT-PCR/ESI-MS platform compared to clinical virologic methods and another PCR-based method
| Combined reference tests (260a) |
|||
|---|---|---|---|
| Positive | Negative | ||
| RT-PCR/ ESI-MS | Positive | 84 | 7 |
| Negative | 17 | 152 | |
Combined reference tests: clinical virology assays and another PCR-based method.
Includes 2 additional coronavirus and 1 human metapneumovirus detections confirmed by another PCR-based method.
3.3. Throughput
Time to first result from sample preparation to detection of RT-PCR/ESI-MS was 8 h: 1 h of RNA extraction, 4 h of RT-PCR, and 3 h of processing in ESI-MS. The estimated throughput of RT-PCR/ESI-MS was 300 samples with 2 technicians and 24 working hours, which represented 1080 PCR reactions.
4. Discussion
This study was a hospital-based retrospective pilot proof-of-concept study designed to demonstrate the capacity and to determine the performance of the novel RT-PCR/ESI-MS platform on mock-up samples and previously frozen clinical samples compared to clinical virology assays. Our study showed that this novel assay was rapid and able to detect and subtype multiple respiratory pathogens in the hospital with 87.9% accuracy, compared to conventional clinical virology assays. Our study also indicated that pathogens not detectable by traditional clinical virology methods could be successfully detected by the RT-PCR/ESI-MS platform.
The RT-PCR/ESI-MS platform has several advantages including rapid turnaround time and a more detailed pathogen characterization (i.e., relative semiquantification, typing, and subtyping of species) versus conventional culture methods. The current common diagnostic methods in our clinical virology laboratory are culture-based, which may take days to yield results and are labor-intensive and expensive (Anzueto and Niederman, 2003). Although some rapid antigen tests offer faster detection times, their individual lower sensitivity and specificity limit their clinical utility as a sole methodology (Ginocchio, 2007). The RT-PCR/ESI-MS platform has the capability to detect multiple pathogens efficiently and with high sensitivity, as well as the ability to determine the quantity of pathogens, which would be potential biomarkers of infection course or severity. However, further prospective validation study will be needed to determine clinical performance of this assay.
While determining the performance of novel assays, similar problems have appeared for the discordant analysis of results between the new assays and reference standard tests. Some researchers confirmed result of the novel assays discordant to the conventional culture or rapid antigen test by another multiplex RT-PCR assay (Liolios et al., 2001, Pabbaraju et al., 2008). It remains controversial regarding how to examine a potentially more accurate novel assay versus the current “alloyed” standard. Doring et al. (2008) proposed that clinical evidence of infections should be included in the validation of the novel nucleic acid test; however, our study design did not permit detailed clinical data collection.
We recognize several limitations of our design. First, the operation of RT-PCR/ESI-MS was performed almost a year after the initial tests done in clinical virology. Utilizing “excess” NPA obtained from clinical virology resulted in some specimens not being processed until more than a year after collecting them from patients. Although the effect of length of storage of NPA has been shown to be minimal within 2 months (Ward et al., 2004), the impact of longer periods of storage remains unknown. Ward et al. (2004) demonstrated that multiple freezing–thawing cycles did not significantly alter the concentrations measured before freezing the influenza samples, while Frisbie et al. (2004) indicated influenza RNA could degrade in the −70 °C freezer and may result in some loss of sensitivity. The second limitation is that we could not send every specimen for secondary analyses because of insufficient volume of some samples. This is an inherent limitation of the retrospective design used here. However, because almost half of the discordant results was verified by another PCR-based method (12 out of 25), it might have little impact on the estimate. Future study is merited to perform parallel comparison on every sample by another reference test (e.g., another PCR-based method) to better characterize the performance of the platform. Third, we could not test the performance of RT-PCR/ESI-MS on every subtype of clinically relevant respiratory viruses because of the design of the study. For those viral subtypes not demonstrated in this pilot study (i.e., parainfluenza types 2 and 4, adenovirus types A and D–F), further study will be required to determine the performance of this novel platform. Lastly, we only performed 5 repeats in the LOD experiment, in which we utilized the 100% detection (5/5) criteria to determine the LOD but not the 95% detection rule that generally applied in LOD studies. Although the fewer repeats in our study may have limitation in precision of the LODs, the 100% detection still should be considered a conservative estimate in this pilot study that could provide some evidence for further studies.
In conclusion, we demonstrated that the innovative RT-PCR/ESI-MS technology could rapidly and accurately detect and subtype most viruses identified by conventional virologic methods. Detection of conventional viruses missed by clinical virology and unconventional viruses required additional confirmatory testing to further determine the performance characteristics. The RT-PCR/ESI-MS method is a promising diagnostic platform for the rapid identification of conventional and unconventional viruses and merits further prospective evaluation.
Footnotes
Funding: Middle Atlantic Regional Center of Excellence (MARCE) for Biodefense and Emerging Infectious Diseases, Public Health Response Project VI. NIH; U54 AI57168-01P1.
The results of this work in part were previously presented at the 48th Annual ICAAC/ IDSA 46th Annual Meeting, Washington DC, October 12–16, 2008.
The authors have a conflict of interest because they either work for the IBIS Company (Now Abbott) (Blyn, Sampath) or have received “free” kits for the performance of the assays for the T-5000 assay (Gaydos, Chen, Rothman, Ramachandran).
References
- Anzueto A., Niederman M.S. Diagnosis and treatment of rhinovirus respiratory infections. Chest. 2003;123:1664–1672. doi: 10.1378/chest.123.5.1664. [DOI] [PubMed] [Google Scholar]
- Bryce J., Boschi-Pinto C., Shibuya K., Black R.E. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- Doring G., Unertl K., Heininger A. Validation criteria for nucleic acid amplification techniques for bacterial infections. Clin Chem Lab Med. 2008;46:909–918. doi: 10.1515/CCLM.2008.152. [DOI] [PubMed] [Google Scholar]
- Ecker D.J., Sampath R., Blyn L.B., Eshoo M.W., Ivy C., Ecker J.A., Libby B., Samant V., Sannes-Lowery K.A., Melton R.E., Russell K., Freed N., Barrozo C., Wu J., Rudnick K., Desai A., Moradi E., Knize D.J., Robbins D.W., Hannis J.C., Harrell P.M., Massire C., Hall T.A., Jiang Y., Ranken R., Drader J.J., White N., McNeil J.A., Crooke S.T., Hofstadler S.A. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc Natl Acad Sci U S A. 2005;102:8012–8017. doi: 10.1073/pnas.0409920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J.A., Massire C., Hall T.A., Ranken R., Pennella T.T., Agasino Ivy C., Blyn L.B., Hofstadler S.A., Endy T.P., Scott P.T., Lindler L., Hamilton T., Gaddy C., Snow K., Pe M., Fishbain J., Craft D., Deye G., Riddell S., Milstrey E., Petruccelli B., Brisse S., Harpin V., Schink A., Ecker D.J., Sampath R., Eshoo M.W. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–2932. doi: 10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbie B., Tang Y.W., Griffin M., Poehling K., Wright P.F., Holland K., Edwards K.M. Surveillance of childhood influenza virus infection: what is the best diagnostic method to use for archival samples? J Clin Microbiol. 2004;42:1181–1184. doi: 10.1128/JCM.42.3.1181-1184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginocchio C.C. Detection of respiratory viruses using non-molecular based methods. J Clin Virol 40 Suppl. 2007;1:S11–S14. doi: 10.1016/S1386-6532(07)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R.S., Tomalty L.L., Majury A., Zoutman D.E. Comparison of viral isolation and multiplex real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J Clin Microbiol. 2009;47:527–532. doi: 10.1128/JCM.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liolios L., Jenney A., Spelman D., Kotsimbos T., Catton M., Wesselingh S. Comparison of a multiplex reverse transcription-PCR-enzyme hybridization assay with conventional viral culture and immunofluorescence techniques for the detection of seven viral respiratory pathogens. J Clin Microbiol. 2001;39:2779–2783. doi: 10.1128/JCM.39.8.2779-2783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S., Ullman B.M. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- Pabbaraju K., Tokaryk K.L., Wong S., Fox J.D. Comparison of the Luminex xTAG respiratory viral panel with in-house nucleic acid amplification tests for diagnosis of respiratory virus infections. J Clin Microbiol. 2008;46:3056–3062. doi: 10.1128/JCM.00878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R.M., Chang G., Kok T., Sloots T.P. Molecular diagnosis of medical viruses. Curr Issues Mol Biol. 2007;9:87–102. [PubMed] [Google Scholar]
- Raymond F., Carbonneau J., Boucher N., Robitaille L., Boisvert S., Wu W.K., De Serres G., Boivin G., Corbeil J. Comparison of automated microarray detection with real-time PCR assays for detection of respiratory viruses in specimens obtained from children. J Clin Microbiol. 2009;47:743–750. doi: 10.1128/JCM.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell K.L., Broderick M.P., Franklin S.E., Blyn L.B., Freed N.E., Moradi E., Ecker D.J., Kammerer P.E., Osuna M.A., Kajon A.E., Morn C.B., Ryan M.A. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006;194:877–885. doi: 10.1086/507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath R., Hofstadler S.A., Blyn L.B., Eshoo M.W., Hall T.A., Massire C., Levene H.M., Hannis J.C., Harrell P.M., Neuman B., Buchmeier M.J., Jiang Y., Ranken R., Drader J.J., Samant V., Griffey R.H., McNeil J.A., Crooke S.T., Ecker D.J. Rapid identification of emerging pathogens: coronavirus. Emerg Infect Dis. 2005;11:373–379. doi: 10.3201/eid1103.040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath R., Russell K.L., Massire C., Eshoo M.W., Harpin V., Blyn L.B., Melton R., Ivy C., Pennella T., Li F., Levene H., Hall T.A., Libby B., Fan N., Walcott D.J., Ranken R., Pear M., Schink A., Gutierrez J., Drader J., Moore D., Metzgar D., Addington L., Rothman R., Gaydos C.A., Yang S., St George K., Fuschino M.E., Dean A.B., Stallknecht D.E., Goekjian G., Yingst S., Monteville M., Saad M.D., Whitehouse C.A., Baldwin C., Rudnick K.H., Hofstadler S.A., Lemon S.M., Ecker D.J. Global surveillance of emerging Influenza virus genotypes by mass spectrometry. PLoS ONE. 2007;2:e489. doi: 10.1371/journal.pone.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C.L., Dempsey M.H., Ring C.J., Kempson R.E., Zhang L., Gor D., Snowden B.W., Tisdale M. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J Clin Virol. 2004;29:179–188. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Tang Y.W. Emerging molecular assays for detection and characterization of respiratory viruses. Clin Lab Med. 2009;29:673–693. doi: 10.1016/j.cll.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]